Abstract
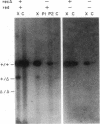
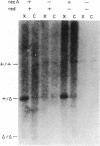
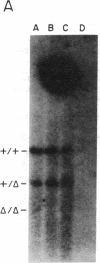
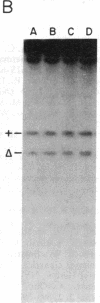
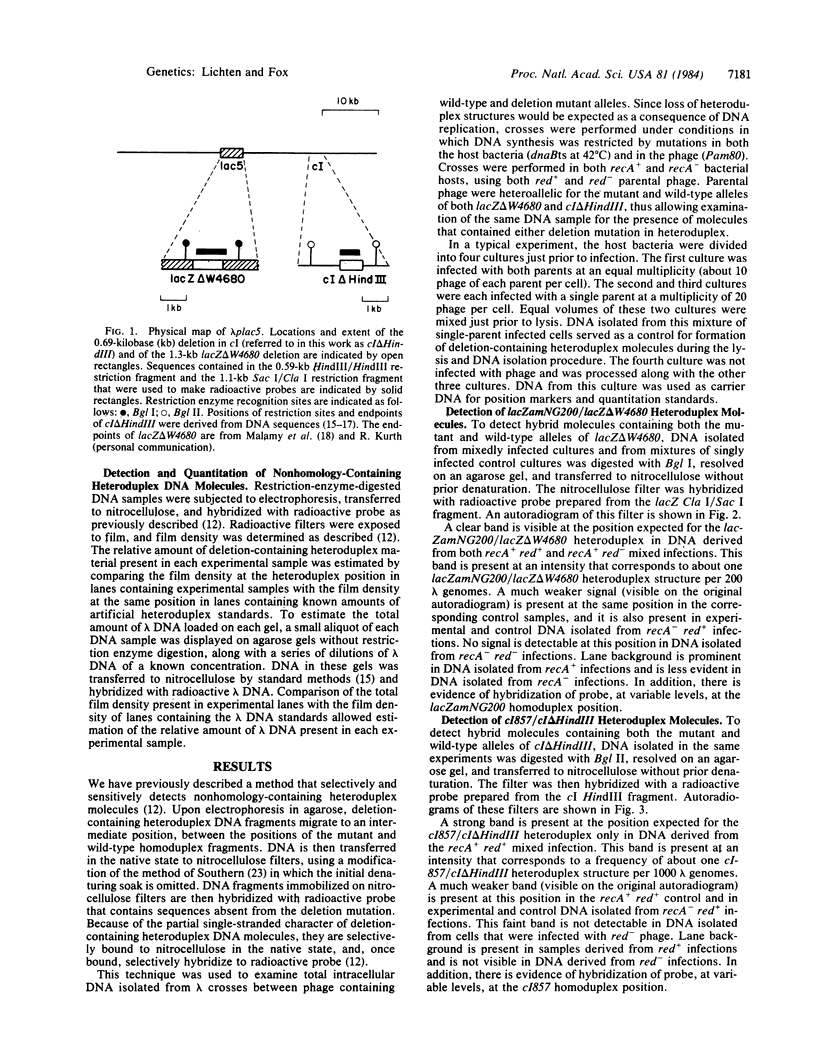
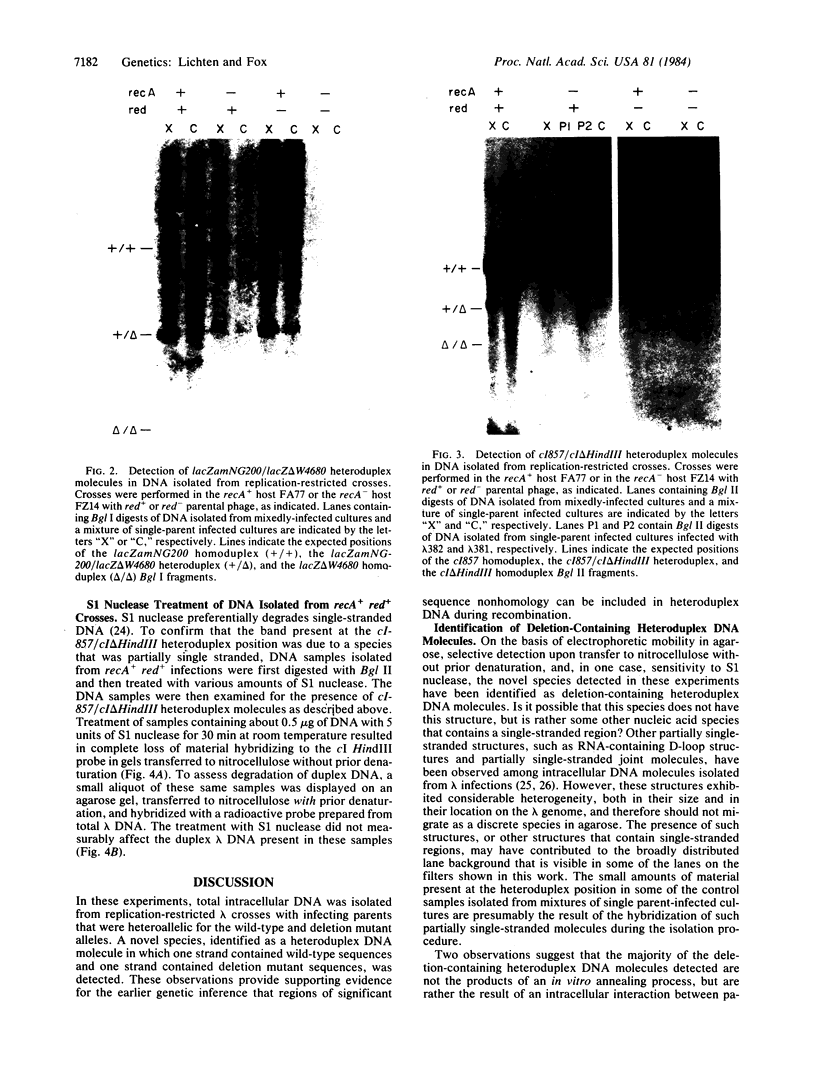
Total intracellular DNA was isolated from replication-restricted bacteriophage lambda crosses in which the infecting parents were heteroallelic for wild-type and deletion mutant alleles. This DNA was examined for the presence of heteroduplex DNA molecules that contained wild-type sequences in one strand and deletion-mutant sequences in the other. Molecules hybrid for a 689-nucleotide deletion in the immunity region of lambda were detected at significant levels only in crosses in which both the red recombination system of lambda and the rec recombination system of Escherichia coli were active. Molecules hybrid for a 1300-nucleotide deletion in the central portion of the lambda genome were detected at significant levels in DNA isolated from both red+ and red- crosses in which recA function was present.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backman K., Ptashne M. Maximizing gene expression on a plasmid using recombination in vitro. Cell. 1978 Jan;13(1):65–71. doi: 10.1016/0092-8674(78)90138-1. [DOI] [PubMed] [Google Scholar]
- Berger H., Warren A. J. Effects of deletion mutations on high negative interference in T4D bacteriophage. Genetics. 1969 Sep;63(1):1–5. doi: 10.1093/genetics/63.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi M. E., Radding C. M. Insertions, deletions and mismatches in heteroduplex DNA made by recA protein. Cell. 1983 Dec;35(2 Pt 1):511–520. doi: 10.1016/0092-8674(83)90185-x. [DOI] [PubMed] [Google Scholar]
- Broker T. R., Lehman I. R. Branched DNA molecules: intermediates in T4 recombination. J Mol Biol. 1971 Aug 28;60(1):131–149. doi: 10.1016/0022-2836(71)90453-0. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Chou J., Cohen S. N. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980 Aug;143(2):971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champoux J. J. Renaturation of complementary single-stranded DNA circles: complete rewinding facilitated by the DNA untwisting enzyme. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5328–5332. doi: 10.1073/pnas.74.12.5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattoraj D. K., Stahl F. W. Evidence of RNA in D loops of intracellular lambda DNA. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2153–2157. doi: 10.1073/pnas.77.4.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doermann A. H., Parma D. H. Recombination in bacteriophage T4. J Cell Physiol. 1967 Oct;70(2 Suppl):147–164. doi: 10.1002/jcp.1040700411. [DOI] [PubMed] [Google Scholar]
- FOX M. S., ALLEN M. K. ON THE MECHANISM OF DEOXYRIBONUCLEATE INTEGRATION IN PNEUMOCOCCAL TRANSFORMATION. Proc Natl Acad Sci U S A. 1964 Aug;52:412–419. doi: 10.1073/pnas.52.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink G. R., Styles C. A. Gene conversion of deletions in the his4 region of yeast. Genetics. 1974 Jun;77(2):231–244. doi: 10.1093/genetics/77.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel S., Mortimer R., Lusnak K., Tavares F. Meiotic gene conversion: a signal of the basic recombination event in yeast. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1325–1341. doi: 10.1101/sqb.1979.043.01.152. [DOI] [PubMed] [Google Scholar]
- Gudas L. J., Pardee A. B. DNA synthesis inhibition and the induction of protein X in Escherichia coli. J Mol Biol. 1976 Mar 15;101(4):459–477. doi: 10.1016/0022-2836(76)90240-0. [DOI] [PubMed] [Google Scholar]
- Hotchkiss R. D. Molecular basis for genetic recombination. Genetics. 1974 Sep;78(1):247–257. doi: 10.1093/genetics/78.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalnins A., Otto K., Rüther U., Müller-Hill B. Sequence of the lacZ gene of Escherichia coli. EMBO J. 1983;2(4):593–597. doi: 10.1002/j.1460-2075.1983.tb01468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lataste H., Claverys J. P., Sicard A. M. Physical and genetic characterization of deletions in Streptococcus pneumoniae. J Bacteriol. 1980 Oct;144(1):422–424. doi: 10.1128/jb.144.1.422-424.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence C. W., Sherman F., Jackson M., Gilmore R. A. Mapping and gene conversion studies with the structural gene for iso-1-cytochrome C in yeast. Genetics. 1975 Dec;81(4):615–629. doi: 10.1093/genetics/81.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichten M. J., Fox M. S. Detection of non-homology-containing heteroduplex molecules. Nucleic Acids Res. 1983 Jun 25;11(12):3959–3971. doi: 10.1093/nar/11.12.3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichten M., Fox M. S. Effects of nonhomology on bacteriophage lambda recombination. Genetics. 1983 Jan;103(1):5–22. doi: 10.1093/genetics/103.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makin G. J., Szybalski W., Blattner F. R. Asymmetric effects of deletions and substitutions on high negative interference in coliphage lambda. Genetics. 1982 Nov;102(3):299–317. doi: 10.1093/genetics/102.3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy M. H., Fiandt M., Szybalski W. Electron microscopy of polar insertions in the lac operon of Escherichia coli. Mol Gen Genet. 1972;119(3):207–222. doi: 10.1007/BF00333859. [DOI] [PubMed] [Google Scholar]
- Meselson M. S., Radding C. M. A general model for genetic recombination. Proc Natl Acad Sci U S A. 1975 Jan;72(1):358–361. doi: 10.1073/pnas.72.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meselson M. Formation of hybrid DNA by rotary diffusion during genetic recombination. J Mol Biol. 1972 Nov 28;71(3):795–798. doi: 10.1016/s0022-2836(72)80040-8. [DOI] [PubMed] [Google Scholar]
- Orosz L., Páy A., Dallmann G. Heterozygosis of phage 16-3 of Rhizobium meliloti: moderate level of mismatch repair or gene conversion. Mol Gen Genet. 1980;179(1):163–167. doi: 10.1007/BF00268459. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Hong G. F., Hill D. F., Petersen G. B. Nucleotide sequence of bacteriophage lambda DNA. J Mol Biol. 1982 Dec 25;162(4):729–773. doi: 10.1016/0022-2836(82)90546-0. [DOI] [PubMed] [Google Scholar]
- Sigal N., Alberts B. Genetic recombination: the nature of a crossed strand-exchange between two homologous DNA molecules. J Mol Biol. 1972 Nov 28;71(3):789–793. doi: 10.1016/s0022-2836(72)80039-1. [DOI] [PubMed] [Google Scholar]
- Sodergren E. J., Fox M. S. Effects of DNA sequence non-homology on formation of bacteriophage lambda recombinants. J Mol Biol. 1979 Jun 5;130(4):357–377. doi: 10.1016/0022-2836(79)90428-5. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stahl F. W., McMilin K. D., Stahl M. M., Crasemann J. M., Lam S. The distribution of crossovers along unreplicated lambda bacteriophage chromosomes. Genetics. 1974 Jul;77(3):395–408. doi: 10.1093/genetics/77.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl F. W., McMilin K. D., Stahl M. M., Malone R. E., Nozu Y., Russo V. E. A role for recombination in the production of "free-loader" lambda bacteriophage particles. J Mol Biol. 1972 Jul 14;68(1):57–67. doi: 10.1016/0022-2836(72)90262-8. [DOI] [PubMed] [Google Scholar]
- Syvanen M. Processing of bacteriophage lambda DNA during its assembly into heads. J Mol Biol. 1975 Jan 15;91(2):165–174. doi: 10.1016/0022-2836(75)90157-6. [DOI] [PubMed] [Google Scholar]
- Szpirer J., Brachet P. Relations physiologiques entre les phages tempérés lambda et phi80. Mol Gen Genet. 1970;108(1):78–92. doi: 10.1007/BF00343187. [DOI] [PubMed] [Google Scholar]
- Takahashi S. Joint molecules of lambda DNA as an intermediate of genetic recombination. Mol Gen Genet. 1977 Jan 7;150(1):43–52. doi: 10.1007/BF02425324. [DOI] [PubMed] [Google Scholar]
- Thompson B. J., Camien M. N., Warner R. C. Kinetics of branch migration in double-stranded DNA. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2299–2303. doi: 10.1073/pnas.73.7.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triman K. L., Chattoraj D. K., Smith G. R. Identity of a Chi site of Escherichia coli and Chi recombinational hotspots of bacteriophage lambda. J Mol Biol. 1982 Jan 15;154(2):393–399. doi: 10.1016/0022-2836(82)90072-9. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Warner R. C., Fishel R. A., Wheeler F. C. Branch migration in recombination. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):957–968. doi: 10.1101/sqb.1979.043.01.105. [DOI] [PubMed] [Google Scholar]
- White R. L., Fox M. S. On the molecular basis of high negative interference. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1544–1548. doi: 10.1073/pnas.71.4.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]