Abstract
Objectives: Jaundice impairs cellular immunity, an important defence against the dissemination of cancer. Jaundice is a common mode of presentation in pancreatic head adenocarcinoma. The purpose of this study was to determine whether there is an association between preoperative jaundice and survival in patients who have undergone resection of such tumours.
Methods: Thirty possible survival risk factors were evaluated in a database of over 400 resected patients. Univariate analysis was used to determine odds ratio for death. All factors for which a P-value of <0.30 was obtained were entered into a multivariate analysis using the Cox model with backward selection.
Results: Preoperative jaundice, age, positive node status, poor differentiation and lymphatic invasion were significant indicators of poor outcome in multivariate analysis. Absence of jaundice was a highly favourable prognostic factor. Interaction emerged between jaundice and nodal status. The benefit conferred by the absence of jaundice was restricted to patients in whom negative node status was present. Five-year overall survival in this group was 66%. Jaundiced patients who underwent preoperative stenting had a survival advantage.
Conclusions: Preoperative jaundice is a negative risk factor in adenocarcinoma of the pancreas. Additional studies are required to determine the exact mechanism for this effect.
Introduction
Adenocarcinoma of the head of the pancreas is an aggressive cancer with a poor prognosis. In 1886, Nicolas Senn, one of the fathers of pancreatic surgery, described the association of jaundice with cancer of the head of the pancreas in his book Surgery of the Pancreas.1 He wrote: ‘It is demonstrated that jaundice is an invariable symptom of primary scirrhus [referring to cancer] of the head of the pancreas, while it is uncommon when the disease affects the body or tail of the organ.’1 Jaundice is still recognized as one of the most common presenting signs in patients with ductal adenocarcinoma of the pancreatic head, and occurs in approximately 75% of this population.2 Jaundice has been shown to cause immunosuppression in animals and humans.3–6 It also reduces sinusoidal blood flow within the liver, which is associated with increased rolling and sticking of leukocytes.7 As a result, jaundice may promote tumour growth or metastatic implantation in the liver. The purpose of this study was to determine whether there is an association between preoperative jaundice and longterm survival in patients who have undergone resection of such tumours.
Materials and methods
Patients and database
All patients who underwent pancreaticoduodenectomy or total pancreatectomy for adenocarcinoma of the head of the pancreas during the period from 1 February 1995 to 28 February 2010 were selected from an institutional pancreaticoduodenectomy database. Since 2007, the database has been prospectively maintained. For the period prior to 2007, the database was populated from medical records. The database and studies derived from it are approved by the institutional review board. The database contains the variables listed in Table 1. The presence of ‘jaundice’ simply means that the medical record stated that clinical jaundice was observed before referral to the surgical service or developed after referral but prior to surgery. In almost all patients, the former was true. Results for serum bilirubin were the highest values available. However, in many cases patients were stented prior to referral and bilirubin levels at the primary institution were not retrospectively retrievable. Therefore, the bilirubin levels available were not necessarily the highest recorded at the time a patient presented with jaundice. The cut-off values for laboratory tests and tumour sizes were selected prior to analysis and were not altered based on results.
Table 1.
Variables tested in 412 patients
| Variable | Missing, n | |
|---|---|---|
| Age at surgery, years, mean ± SD | 65.4 ± 10.4 | |
| Gender, male, n (%) | 214 (51.9%) | |
| Race, | ||
| White, n (%) | 371 (90.1%) | |
| Other, n (%) | 41 (10.0%) | |
| Weight loss, n (%) | 265 (70.3%) | Missing 35 |
| Jaundice, n (%) | 333 (81.0%) | Missing 1 |
| Hypertension, n (%) | 218 (53.0%) | Missing 1 |
| Diabetes mellitus, n (%) | 132 (32.1%) | Missing 1 |
| Chronic pancreatitis, n (%) | 35 (8.5%) | Missing 1 |
| Tobacco use, n (%) | Missing 4 | |
| Current | 106 (26.0%) | |
| Past | 157 (35.8%) | |
| Never | 156 (38.4%) | |
| Family history of, n (%) | ||
| Pancreatic cancer | 36 (9.5%) | Missing 34 |
| Other gastrointestinal cancer | 58 (15.3%) | Missing 33 |
| Non-gastrointestinal cancer | 186 (48.4%) | Missing 28 |
| Haematocrit <25, n (%) | 8 (2.0%) | Missing 4 |
| Platelet count <250 000/mm3, n (%) | Missing 9 | |
| Yes | 185 (45.9%) | |
| No | 218 (54.1%) | |
| AST >100 IU, n (%) | 97 (24.6%) | Missing 17 |
| Bilirubin >20 mg/dla, n (%) | 15 (3.8%) | Missing 13 |
| Albumin <3.0 mg/dl, n (%) | 34 (8.5%) | Missing 10 |
| CA 19-9 >100 IU, n (%) | 106 (46.9%) | Missing 186 |
| ERCP stent, n (%) | 274 (79.0%) | Missing 65 |
| Surgery type, n (%) | Missing 7 | |
| Standard | 336 (83.0%) | |
| Pylorus-sparing Whipple | 41 (10.1%) | |
| Total pancreatectomy | 28 (6.9%) | |
| Vascular resection, n (%) | 131 (31.9%) | Missing 1 |
| Estimated blood loss ≤500 ml, n (%) | 167 (41.7%) | Missing 11 |
| Tumour size, n (%) | Missing 17 | |
| <2.0 cm | 94 (23.8%) | |
| 2.0–2.9 cm | 147 (37.2%) | |
| 3.0–3.9 cm | 97 (24.6%) | |
| ≥4 cm | 57 (14.4%) | |
| Histological grade, n (%) | Missing 7 | |
| Good | 24 (5.9%) | |
| Moderate | 197 (48.6%) | |
| Poor | 184 (45.4%) | |
| Lymphatic invasion, n (%) | 227 (62.5%) | Missing 49 |
| Venous invasion, n (%) | 177 (49.3%) | Missing 53 |
| Perineural invasion, n (%) | 314 (84.0%) | Missing 38 |
| Surgical margins, n (%) | Missing 3 | |
| R0 | 294 (71.8%) | |
| R1 | 115 (28.1%) | |
| N-stage, n (%) | Missing 4 | |
| N0 | 119 (29.2%) | |
| N1 | 289 (70.8%) | |
| M-stage, n (%) | ||
| M0 | 408 (98.9%) | |
| M1 | 4 (1.1%) |
Normal range: 0.1–1.0 mg/dl. Other patients may have had bilirubin levels as high as this before being stented at outside institutions. Such results, if they occurred, were unavailable.
SD, standard deviation; AST, aspartate transaminase; CA 19-9, carbohydrate antigen 19-9; ERCP, endoscopic retrograde cholangiopancreatography; N-stage, nodal stage; M-stage, metastatic stage.
Operative procedures
A Whipple procedure with antrectomy was the standard procedure performed. Pylorus-sparing pancreatoduodenectomy and total pancreatectomy were performed occasionally. Frozen sections of the pancreatic neck and bile duct margins were obtained routinely in the resected specimen. If the pancreatic neck margin was positive, additional pancreas was resected until a negative margin was obtained, or total pancreatectomy was performed. This was the main indication for total pancreatectomy.
Pathological analysis
Surgical specimens were inked in the operating room in the presence of a pathologist. Four coloured inks were used for the pancreatic neck margin, mesenteric vein margin, uncinate margin and posterior margin, respectively. When a venous resection was performed, the intimal surface of the vein was not inked (i.e. the intravascular surface of the vein was not considered to be a margin). All margins were microscopically evaluated and graded as R0 (microscopically negative) or R1 (microscopically positive at margin or tumour within 1 mm of the margin). Specimens were also evaluated by microscopy for lymphatic, venous and nerve invasion. Cancers that arose in an intraductal papillary mucinous neoplasm (IPMN) or a mucinous cystic neoplasm, or which were described as having mucinous features were not included in the analysis. This was true whether these pathologies were identified preoperatively by imaging and in the pathological specimen or in the pathological specimen only. In the latter, the pathologist recorded that the tumour had mucinous features or appeared to arise in an IPMN.
Survival status
Survival status and date of death, if death had occurred, were determined from available medical records, institutional or otherwise, and in some patients by searching the Social Security Death Index (SSDI).
Statistical analysis
The primary outcome was overall survival (OS), which was defined as the time from surgery to the date of death from any cause. Survivors were censored at the date of last contact. Distributions of patient and clinical characteristics were compared between jaundiced and non-jaundiced patients using the chi-squared test or t-test as appropriate. Survival curves were estimated using the Kaplan–Meier product-limit method. Univariate Cox proportional hazard models were fit to identify factors significantly related to OS. Variables with almost homogeneous status (i.e. one of two groups comprising <5% of the total sample) were omitted. For example, with reference to metastasis (M) stage, the M1 population represented <1% of the total number of patients and this variable was not entered. To assess whether jaundice was an independent predictor of survival, a multivariate Cox model was constructed via a backward selection procedure, using all variables that had attained a P-value of <0.30 in the univariate analyses. Initially, only those patients for whom complete datasets were available were included for analysis. Two-way interaction terms between jaundice and other factors in the multivariate Cox model were also assessed. As a sensitivity analysis, a multivariate Cox model was also constructed after the missing values were imputed using multivariate regression models. All analyses were two-sided and significance was set at a P-value of 0.05. Statistical analyses were performed using sas Version 9.2 (SAS Institute, Inc., Cary, NC, USA).
Results
A total of 412 patients underwent resection of a carcinoma of the head of the pancreas during the study period. The median number of patients undergoing resection per year was 31 (range: 16–41). Summary statistics are shown in Table 1. An additional 30 patients who demonstrated adenocarcinomas arising in cysts underwent pancreatoduodenectomy during the period under study and were excluded from further analysis. The Kaplan–Meier curve for OS in the 412 patients is shown in Fig. 1. At the time of analysis, 346 (84%) patients had died and 66 (16%) remained alive. The median length of survival (follow-up) in patients who died was 1.18 years (time from surgery to date of death). The median follow-up of patients who remained alive at the time of analysis was 4.83 years.
Figure 1.
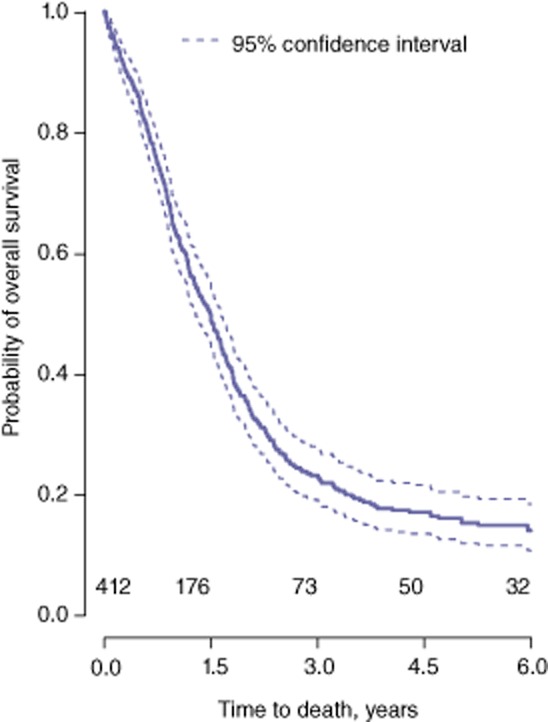
Kaplan–Meier curve for overall survival in the entire set of patients (n = 412)
Univariate analysis (Table 2) showed OS to be significantly related (P < 0.05) to 10 different variables. Three variables including jaundice were not significant at the P < 0.05 level, but did achieve a P-value of <0.30. Therefore, these 13 variables were included in a backward selection procedure for multivariate analysis to identify independent predictors of OS. The exclusion of patients for whom datasets were incomplete (Table 1) left data for 319 (77%) patients available for multivariate analysis.
Table 2.
Univariate analysis of factors affecting overall survival
| P-value | HRa | 95% CI of HR | ||
|---|---|---|---|---|
| Variables with a P-value of ≤0.05 (all included in later multivariate analysis)b | ||||
| Nodal status N1 versus N0 | <0.0001 | 1.89 | 1.47–2.46 | |
| Lymphatic invasion | <0.0001 | 1.72 | 1.34–2.20 | |
| Histological grade (differentiation)c | 0.0002 | |||
| Moderate versus good | 0.032 | 1.83 | 1.05–3.18 | |
| Poor versus good | 0.0008 | 2.58 | 1.48–4.49 | |
| Venous invasion | 0.0096 | 1.37 | 1.08–1.73 | |
| Age | 0.047 | 1.02 | 1.01–1.03 | |
| Surgical margins | 0.010 | 1.37 | 1.08–1.75 | |
| Tumour sizec | 0.0073 | |||
| 2.0–2.9 cm versus <2.0 cm | 0.082 | 1.30 | 0.97–1.77 | |
| 3.0–3.9 cm versus <2.0 cm | 0.0083 | 1.55 | 1.12–2.13 | |
| ≥4.0 cm versus <2.0 cm | 0.0014 | 1.83 | 1.26–2.64 | |
| Perineural invasion | 0.048 | 1.39 | 1.003–1.93 | |
| ERCP stent | 0.075 | 0.68 | 0.51–0.90 | |
| Surgery type | 0.031 | |||
| Pylorus-sparing versus standard | 0.91 | 1.02 | 0.71–1.48 | |
| Total pancreatectomy versus standard | 0.0091 | 1.76 | 1.15–2.68 | |
| Variables with a P-value of >0.05 but <0.30 (included in later multivariate analysis) | ||||
| Jaundice on presentation | 0.11 | 1.28 | 0.98–1.74 | |
| Gender (female) | 0.20 | 0.86 | 0.70–1.08 | |
| Vascular resection | 0.23 | 1.16 | 0.91–1.46 | |
| Variables with a P-value of >0.30a (NOT included in later multivariate analysis) | ||||
| Diabetes mellitus | 0.34 | |||
| Family history of non-GI cancer | 0.36 | |||
| Hypertension | 0.36 | |||
| Family history of pancreatic cancer | 0.42 | |||
| Weight loss | 0.42 | |||
| Race | 0.49 | |||
| CA 19-9 level | 0.58 | |||
| Estimated blood loss | 0.61 | |||
| Tobacco use | 0.83 | |||
| Aspartate transaminase | 0.89 | |||
| Platelet count | 0.96 | |||
| Family history of other GI cancer | 0.97 | |||
Hazard ratio and 95% confidence limits given for variables with P < 0.30. A positive value indicates that the variable is associated with a decreased probability of survival.
Low haematocrit, bilirubin >20 mg/dl and M status not evaluated by univariate analysis because of low numbers (<5% of total) in one group.
P-values for the question of whether levels of risk for all groups are equal (type III test).
HR, hazard ratio; 95% CI, 95% confidence interval; ERCP, endoscopic retrograde cholangiopancreatography; GI, gastrointestinal; CA 19-9, carbohydrate antigen 19-9.
The results are shown in Table 3. Significant predictors were jaundice, node status, histological grade, lymphatic invasion and age. The multivariate analysis showed the interaction between jaundice and N-stage to be the only significant two-way interaction. The influence of jaundice in N0 patients was found to differ from that in N1 patients. In N0 patients, those with jaundice had a much worse prognosis than those without jaundice [hazard ratio (HR) = 3.01; P = 0.01] (Table 3). However, no significant relationship between the presence or absence of jaundice and survival emerged in the group of N1 patients. The survival curves for the four groups of patients categorized according to node status and the presence or absence of jaundice (N0J0, N1J0, N0J1 and N1J1) are shown in Fig. 2. Note that 5-year OS in N0J0 patients was 66% [95% confidence interval (CI) 45–81%] and that this curve differed significantly from the other three curves. The analysis was repeated in all 412 patients with imputation of missing values; similar results were obtained (Table 3).
Table 3.
Significant variables in the multivariate analyses
| Variables | HR (95% CI) | P-valuea | P-valueb |
|---|---|---|---|
| Jaundice and node status | |||
| Non-jaundiced, N0 | 1 | ||
| Non-jaundiced, N1 | 4.92 (2.01–12.05) | <0.001 | <0.001 |
| Jaundiced, N0 | 3.01 (1.29–7.01) | 0.011 | 0.012 |
| Jaundiced, N1 | 4.02 (1.79–9.27) | 0.001 | <0.001 |
| Age (per 1-year increase) | 1.02 (1.01–1.03) | 0.004 | <0.001 |
| Differentiation | |||
| Good | 1 | ||
| Moderate | 1.39 (0.78–2.49) | 0.262 | 0.277 |
| Poor | 1.93 (1.08–3.47) | 0.028 | 0.017 |
| Lymphatic invasion | |||
| No | 1 | ||
| Yes | 1.37 (1.05–1.80) | 0.023 | 0.031 |
Using 319 patients without missing values.
Using all 412 patients with imputation of missing values.
HR, hazard ratio; 95% CI, 95% confidence interval.
Figure 2.
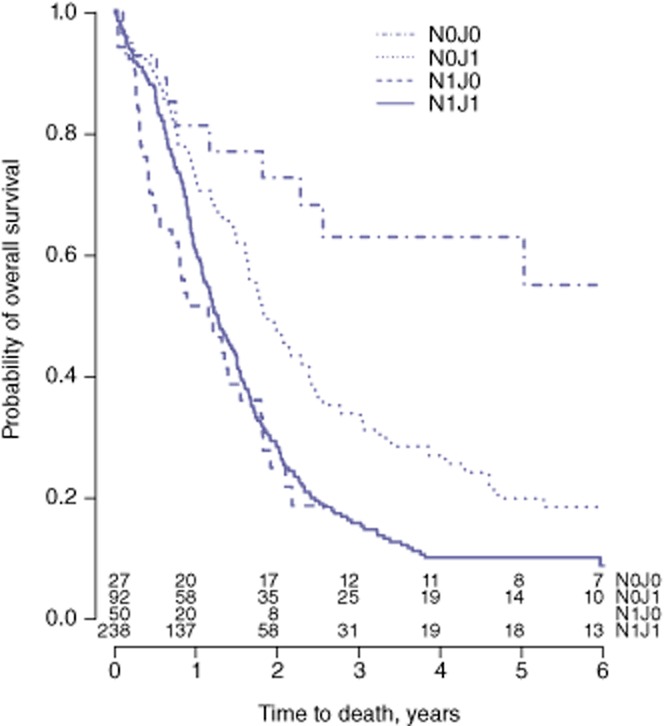
Kaplan–Meier curves for overall survival in subgroups of patients categorized according to nodal (N) status and the presence or absence of jaundice (P < 0.001). N1, lymph node metastasis present; N0, lymph node metastasis absent; J1, jaundice present; J0, jaundice absent
As jaundice was found to be a risk factor for OS, the effect on survival of preoperative stenting in jaundiced patients was analysed. Of the 333 patients who presented with jaundice, information regarding preoperative stenting was available in 296 (89%). Of these, 259 (88%) were stented and 37 (13%) were not. Survival probability was significantly greater in the stented group (HR = 0.59, 95% CI 0.41–0.85; P = 0.005) (Fig. 3). The difference remained significant after adjusting for the other independent risk factors identified earlier (HR = 0.50, 95% CI 0.33–0.75; P = 0.001).
Figure 3.
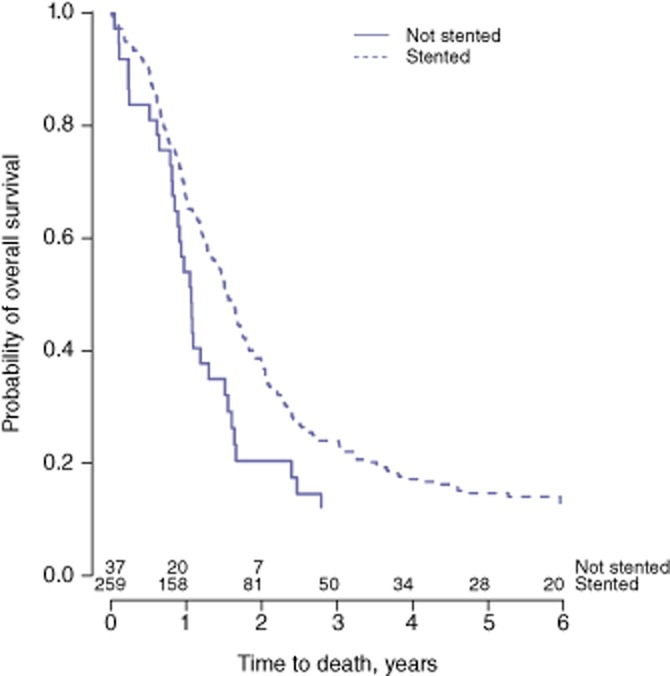
Kaplan–Meier curves for overall survival in stented (in endoscopic retrograde cholangiography) and non-stented patients (P = 0.005)
Discussion
This study examined the relationship between jaundice and survival in resected patients with adenocarcinoma of the head of the pancreas. The statistics for age, gender, comorbidities and pathological variables resemble those in prior large case series.8–12 There are two major findings. Firstly, patients with adenocarcinoma of the head of the pancreas who presented without clinical jaundice and who underwent pancreatoduodenectomy had a better prognosis than patients who were jaundiced on presentation. However, this benefit accrued only to patients who did not have lymph node metastases. Secondly, patients who presented with jaundice and who were stented prior to surgery had a better prognosis than patients who were not stented; this advantage seems to be independent of other prognostic factors.
There is some evidence in prior studies that preoperative jaundice negatively affects outcomes in patients in whom pancreatic cancers are resected. Cleary et al. performed a multi-institution review of 117 patients with pancreatic adenocarcinoma, in 110 of whom disease was located in the head of the pancreas.9 Preoperative jaundice was identified as a significant negative risk factor on univariate analysis. However, this did not hold true on multivariate analysis, in which only tumour stage, grade and degree of differentiation were independently associated with survival.9 A study from the Cleveland Clinic reviewed 179 consecutive pancreatoduodenectomies for pancreatic adenocarcinoma.2 This investigation found that an elevated bilirubin level was a significant negative predictor of OS on univariate analysis. Nevertheless, upon multivariate analysis serum bilirubin concentration was not independently prognostic. Instead, elevated liver function tests [defined in the Cleveland Clinic study2 as elevated alkaline phosphatase and/or bilirubin and/or aspartate transaminase (AST)] were significantly associated with decreased survival. A recent study of 164 patients from Korea with resected pancreatic adenocarcinoma, the majority of whom underwent pancreatoduodenectomy, revealed that preoperative bilirubin of <7 mg/dl was associated with improved survival upon multivariate analysis.13 Jaundice was defined in the current study by the documentation of clinical jaundice prior to surgery rather than by serum bilirubin level because of the retrospective nature of the study and thus the availability of results. Although all patients with a bilirubin level of >7 mg/dl would be expected to display jaundice, some of those with levels of <7 mg/dl would also exhibit jaundice as defined in this study. A 20-year, single-institution experience with pancreatoduodenectomy for periampullary pathologies at Indiana University Hospital also investigated the prognostic role of jaundice.14 Upon multivariate analysis, hyperbilirubinaemia was predictive of longterm survival in the subset of patients with periampullary cancer.14 The study did not focus solely on patients with pancreatic adenocarcinoma, although these patients represented the majority of those in the periampullary cancer group. The current study differs from the prior investigations in that an important relationship between jaundice and nodal status was identified, whereby the survival benefit to be derived from the absence of jaundice was observed only in patients who were also free of nodal metastases. It is possible that the prior studies might have found a stronger correlation if that relationship had been known and accounted for.
Two studies have examined the effect on OS of the relief of jaundice by stenting.15,16 Smith et al. investigated the impact of jaundice on patients with pancreatic adenocarcinoma undergoing preoperative biliary stenting, and demonstrated that persistent jaundice at the time of surgery, despite preoperative biliary drainage, was a significant negative predictor of early (6–12 months) but not overall survival.15 The association between residual jaundice at the time of resection and early survival was independent of other prognostic factors.15 This study and the present one were not prospective evaluations of the effect of relief of jaundice on survival. Both have relatively small groups of patients who were not stented, so the benefit of stenting on long term survival in this regard should be considered to be suggestive at this time. Eshuis et al. performed a randomized controlled trial of early surgery without relief of jaundice versus preoperative biliary drainage in patients with periampullary tumors.16 About 60% of their patients had adenocarcinoma of the pancreas. Total serum bilirubin at randomization was a negative risk factor for survival in both univariate and multivariate analyses.16 Median survival was 21.6 months in the stented group and 17.8 months in the early surgery group, but this difference did not reach statistical significance.16 It is not possible to directly compare the findings of this previous study with those of the current study as only a proportion of the patients in the earlier study had adenocarcinoma of the pancreas. Although many other studies of perioperative parameters have compared outcomes in stented and non-stented patients, the present group were unable to find other studies reporting on longterm survival.
At least two mechanisms may account for a negative effect of jaundice on survival. Firstly, tumours that are cicatrizing, and thus more likely to occlude the bile duct, may be inherently more aggressive than those that are not. Because cystic tumours may be less cicatrizing and may have a better prognosis, such tumours were eliminated from the analysis. The second potential harmful mechanism refers to the possibility that jaundice has a negative effect on tumour defences or enables metastasis formation. Jaundice impairs cellular immunity3–6 and reduces liver blood flow, which is associated with increased rolling and sticking of leukocytes.7 Obviously direct evaluation of the effect of jaundice on parameters that support tumour growth and implantation in this patient population is required.
There are several limitations to the current study. Firstly, patients treated during 2007–2010 were entered into a prospectively maintained database, but data for patients treated prior to 2007 were entered into the database retrospectively by chart review. Consequently, data on some variables were missing in a number of patients; these were excluded from the original multivariate analysis. However, the results did not change when all patients were studied using imputation of missing results. Because of the inherently difficult nature of investigating accurate overall disease-specific survival over a 15-year timeframe, the present authors elected to use OS as the primary endpoint. As in any study examining OS, outcome may be confounded by variables such as comorbidities and patient age, which may influence survival. As overall disease-specific survival was not evaluated, the relationship of age with OS is probably a reflection of the fact that older patients are more likely to die of unrelated diseases. Finally, it would be desirable to use a quantitative measure that accounted for both the level and duration of jaundice, but it was not possible to do so in a retrospective study of this duration.
In conclusion, the absence of jaundice is a highly favourable predictor of OS in N0 patients with resectable adenocarcinoma in the head of the pancreas. Recent investigations suggest that jaundice impairs antitumour immunity and promotes metastasis. Additional study of the factors that favour tumour dissemination in this patient subset is required.
Conflicts of interest
None declared.
References
- 1.Senn N. Surgery of the Pancreas. Philadelphia, PA: W. J. Dornan; 1886. Transactions of the American Surgical Association. [Google Scholar]
- 2.Kim R, Tsao R, Tan A, Byrne M, Almhanna K, Lazaryan A, et al. A single institution review of adjuvant therapy outcomes for resectable pancreatic adenocarcinoma: outcome and prognostic indicators. J Gastrointest Surg. 2010;14:1159–1169. doi: 10.1007/s11605-010-1213-z. [DOI] [PubMed] [Google Scholar]
- 3.Katz SC, Ryan K, Ahmed N, Plitas G, Chaudhry UI, Kingham TP, et al. Obstructive jaundice expands intrahepatic regulatory T cells, which impair liver T lymphocyte function but modulate liver cholestasis and fibrosis. J Immunol. 2011;187:1150–1156. doi: 10.4049/jimmunol.1004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawarabayashi N, Seki S, Hatsuse K, Takigawa T, Tsujimoto H, Kawabata T, et al. Immunosuppression in the livers of mice with obstructive jaundice participates in their susceptibility to bacterial infection and tumour metastasis. Shock. 2010;33:500–506. doi: 10.1097/SHK.0b013e3181c4e44a. [DOI] [PubMed] [Google Scholar]
- 5.Roughneen PT, Didlake R, Kumar SC, Kahan BD, Rowlands BJ. Enhancement of heterotopic cardiac allograft survival by experimental biliary ligation. Transplantation. 1987;43:437–438. doi: 10.1097/00007890-198703000-00023. [DOI] [PubMed] [Google Scholar]
- 6.Treglia-Dal Lago M, Jukemura J, Machado MC, da Cunha JE, Barbuto JA. Phagocytosis and production of H2O2 by human peripheral blood mononuclear cells from patients with obstructive jaundice. Pancreatology. 2006;6:273–278. doi: 10.1159/000092688. [DOI] [PubMed] [Google Scholar]
- 7.Nehez L, Andersson R. Compromise of immune function in obstructive jaundice. Eur J Surg. 2002;168:315–328. doi: 10.1080/11024150260284815. [DOI] [PubMed] [Google Scholar]
- 8.Pawlik TM, Gleisner AL, Cameron JL, Winter JM, Assumpcao L, Lillemoe KD, et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery. 2007;141:610–618. doi: 10.1016/j.surg.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Cleary SP, Gryfe R, Guindi M, Greig P, Smith L, Mackenzie R, et al. Prognostic factors in resected pancreatic adenocarcinoma: analysis of actual 5-year survivors. J Am Coll Surg. 2004;198:722–731. doi: 10.1016/j.jamcollsurg.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, et al. Resected adenocarcinoma of the pancreas – 616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–579. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 11.Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Ann Surg. 2003;237:74–85. doi: 10.1097/00000658-200301000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuhlmann KF, de Castro SM, Wesseling JG, ten Kate FJ, Offerhaus GJ, Busch OR, et al. Surgical treatment of pancreatic adenocarcinoma; actual survival and prognostic factors in 343 patients. Eur J Cancer. 2004;40:549–558. doi: 10.1016/j.ejca.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Yoon KW. Factors affecting longterm survival after surgical resection of pancreatic ductal adenocarcinoma. J Korean Surg Soc. 2011;81:394–401. doi: 10.4174/jkss.2011.81.6.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt CM, Powell ES, Yiannoutsos CT, Howard TJ, Wiebke EA, Wiesenauer CA, et al. Pancreaticoduodenectomy: a 20-year experience in 516 patients. Arch Surg. 2004;139:718–725. doi: 10.1001/archsurg.139.7.718. [DOI] [PubMed] [Google Scholar]
- 15.Smith RA, Dajani K, Dodd S, Whelan P, Raraty M, Sutton R, et al. Preoperative resolution of jaundice following biliary stenting predicts more favourable early survival in resected pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2008;15:3138–3146. doi: 10.1245/s10434-008-0148-z. [DOI] [PubMed] [Google Scholar]
- 16.Eshuis WJ, van der Gaag NA, Rauws EA, van Eijck CH, Bruno MJ, Kuipers EJ, et al. Therapeutic delay and survival after surgery for cancer of the pancreatic head with or without preoperative biliary drainage. Ann Surg. 2010;252:840–849. doi: 10.1097/SLA.0b013e3181fd36a2. [DOI] [PubMed] [Google Scholar]


