Abstract
Objectives: Isolated intrahepatic recurrence is noted in up to 40% of patients following curative liver resection for colorectal liver metastases (CLM). The aims of this study were to analyse the outcomes of repeat hepatectomy for recurrent CLM and to identify factors predicting survival.
Methods: Data for all liver resections for CLM carried out at one centre between 1998 and 2011 were analysed.
Results: A total of 1027 liver resections were performed for CLM. Of these, 58 were repeat liver resections performed in 53 patients. Median time intervals were 10.5 months between the primary resection and first hepatectomy, and 15.4 months between the first and repeat hepatectomies. The median tumour size was 3.0 cm and the median number of tumours was one. Six patients had a positive margin (R1) resection following first hepatectomy. There were no perioperative deaths. Significant complications included transient liver dysfunction in one and bile leak in two patients. Rates of 1-, 3-and 5-year overall survival following repeat liver resection were 85%, 61% and 52%, respectively, at a median follow-up of 23 months. R1 resection at first hepatectomy (P = 0.002), a shorter time interval between the first and second hepatectomies (P = 0.02) and the presence of extrahepatic disease (P = 0.02) were associated with significantly worse overall survival.
Conclusions: Repeat resection of CLM is safe and can achieve longterm survival in carefully selected patients. A preoperative knowledge of poor prognostic factors helps to facilitate better patient selection.
Introduction
Colorectal cancer (CRC) is the third most common malignancy and the second most common cause of cancer-related deaths worldwide.1 Up to 70% of patients with CRC develop either synchronous (15–25%) or metachronous (20–40%) liver metastases.2,3 Despite the recent advances in chemotherapeutic agents, liver resection remains the only potentially curative treatment for colorectal liver metastases (CLM).4–8 Reported 5-year overall survival rates following curative liver resection lie in the range of 35–58%.9–12 However, about two thirds of patients develop tumour recurrence and the recurrent disease is limited to the liver in up to 40%.13–16 Advances in surgical techniques and perioperative management have enabled some centres to perform repeat hepatectomy in selected groups of patients.17–20 Experiences with repeat resections are limited and the published literature is confined to a few retrospective studies.21–24 The aims of this study were to analyse the outcomes of repeat hepatectomy performed for recurrent CLM and to identify factors predicting survival.
Materials and methods
Following approval of the study protocol by the institutional review board, all patients who underwent surgery for CLM between January 1998 and January 2011 were identified. Data were collected retrospectively using the prospectively maintained liver unit database. Only patients who underwent more than one liver resection were included in the study. Patients with recurrent liver disease treated with radiofrequency ablation (RFA) were excluded. Information collected included data on patient demographics, clinicopathological features of the primary and metastatic tumours, operative data and postoperative complications in both the single hepatectomy group and the repeat liver resection group. Rates of 1-, 3-and 5-year overall survival were calculated from the time of first hepatectomy and were compared with those in the single hepatectomy group. A further analysis was performed to identify factors influencing survival in the repeat liver resection group.
Preoperative staging included contrast-enhanced, triple-phase computed tomography and liver-specific magnetic resonance imaging. All imaging was discussed at a multidisciplinary meeting. Neoadjuvant chemotherapy was considered in patients with borderline resectable disease or a clinical risk score of >3.25
The criteria for consideration for repeat liver resection were similar to those for initial liver resection: patients were required to show the presence of resectable liver disease, the absence of unresectable extrahepatic disease and the likelihood of an adequate functional liver remnant following resection. Intermittent inflow occlusion was used selectively and parenchymal transection was performed using the Cavitron Ultrasonic Surgical Aspirator (CUSA; Valleylab™, Covidien, Inc., Boulder, CO, USA) and/or a harmonic scalpel according to the surgeon’s preference. A thoracic epidural infusion of bupivacaine and fentanyl was used routinely to provide postoperative pain relief.
Liver metastases that were diagnosed within 3 months of the primary tumour were defined as synchronous, whereas those diagnosed >3 months later were classed as metachronous lesions.26 The extent of liver resection was classified according to the Brisbane 2000 Guidelines. Resections involving more than three Couinaud segments were regarded as major.
Perioperative mortality was defined as death during the same hospital admission or within 90 days of surgery. Postoperative complications were graded using the modified Dindo–Clavien system of classification.27
Patients were followed up by clinical evaluation and measurement of serum carcinoembryonic antigen (CEA) every 3 months during the first year, every 4 months during the second, every 6 months during the third and annually thereafter until the fifth anniversary. Computed tomography was performed at 6 and 18 months following surgery and if clinically indicated.
Statistical analysis
Data were analysed using spss Version 17 (SPSS, Inc., Chicago, IL, USA). Descriptive statistics were presented as percentages or median values. Non-parametric Mann–Whitney U-tests and chi-squared tests were used to analyse differences between the single and repeat hepatectomy groups. Survival was calculated from the time of both the first and second hepatectomies in the repeat hepatectomy group and survival analysis was performed according to the Kaplan–Meier method. Factors associated with survival were assessed using the Cox regression test. A P-value of <0.05 was considered to indicate statistical significance in all tests.
Results
A total of 1027 liver resections were performed for CLM during the study period. Of these, 58 (6%) represented repeat hepatectomies performed in 53 patients. Five patients underwent a third liver resection. Single hepatectomy was performed in 916 patients. The median age at presentation in the repeat hepatectomy group was 63 years (range: 38–81 years). A total of 29 (55%) of the 53 patients in the repeat hepatectomy group were male.
Primary tumours were staged according to the Dukes classification system as stage A in three patients, stage B in 15, stage C in 18 and stage D in 17 patients. Hepatic metastases at index presentation were synchronous in 17 and metachronous in 36 patients. Thirty-one patients received chemotherapy prior to first liver resection and three received chemotherapy before the repeat liver resection. The majority of chemotherapy regimens were 5-fluorouracil (5-FU) and oxaliplatin-based The median time interval between the colectomy and the first hepatectomy was 10.5 months (range: 1.9–65.0 months); median intervals were 15.4 months (range: 4.3–60.1 months) between the first and second hepatectomies, and 12.2 months (range: 7.7–33.8 months) between the second and third liver resections.
Operative data
In the 53 patients submitted to repeat liver resection, the first hepatectomy was major in 25 and minor in 28 cases. The repeat liver resection was major in 10 and minor in 43 patients. All of the five third hepatectomies were non-anatomical wedge resections. Types of liver resection in the repeat hepatectomy group are summarized in Table 1.
Table 1.
Type of liver resection in the repeat hepatectomy group (n = 53)
| Type | At first hepatectomy, n (%) | At second hepatectomy, n (%) |
|---|---|---|
| Right hepatectomy | 13 (25%) | 7 (13%) |
| Extended right hepatectomy | 4 (8%) | 2 (4%) |
| Left hepatectomy | 5 (9%) | 1 (2%) |
| Extended left hepatectomy | 3 (5%) | 0 |
| Left lateral segmentectomy | 8 (15%) | 2 (4%) |
| Non-anatomical resections | 20 (38%) | 41 (77%) |
Blood products were used in 13 patients during the first hepatectomy and in 10 patients during the repeat procedure. None of the patients undergoing third hepatectomies required transfusions.
Metastatic tumour characteristics at first hepatectomy (n = 53)
The median size of the tumour was 3.0 cm (range: 1.0–11.0 cm) and the median number of lesions was one (range: 1–9). A bilobar distribution was noted in 12 patients. One patient showed the regional spread of tumour to the hilar lymph node. Postoperative histology showed residual microscopic tumour at the resection margin (R1 resection) in six patients. No R2 resections were identified.
Metastatic tumour characteristics at repeat hepatectomy (n = 53)
The median size of the tumour was 3.0 cm (range: 1.0–7.5 cm) and the median number of lesions was one (range: 1–3). Four patients had bilobar disease. Extrahepatic disease was noted in six patients. Three patients had local diaphragmatic invasion, one had hilar lymph node metastasis, one had a pre-sacral mass and one patient had extracapsular tumour infiltration. Final histology revealed an R1 resection in six specimens. No patients had R2 resection.
Postoperative outcome and survival
No in-hospital deaths occurred after repeat liver resection. Two patients developed bile leak from the cut surface of the liver and required percutaneous drainage. One patient developed a chest infection and another had transient liver dysfunction; both were treated conservatively.
The median hospital stay was 6 days (range: 4–17 days) and the median intensive care unit stay was 1 day (range: 1–5 days). None of the five patients who underwent third hepatectomy had any significant morbidity.
At a median follow-up of 32 months (range: 5–103 months), 16 patients were found to have died of tumour recurrence. Eleven of these 16 patients had developed further recurrent metastatic disease in the liver. Rates of 1-, 3-and 5-year survival calculated from the date of first hepatectomy were 98%, 83% and 59%, respectively, with a median survival of 53 months (range: 11–101 months). Rates of 1-, 3-and 5-year survival calculated from the date of the second liver resection were 85%, 61% and 52%, respectively, with a median survival of 45 months (range: 6–98 months). The present results are compared with those of previously published studies in Table 2.
Table 2.
Comparison of the present results with those of published studies
| Authors (year) | Patients, n | Mortality, % | Median survival, months | 3-year survival, % | 5-year survival, % |
|---|---|---|---|---|---|
| Fernández-Trigo et al. (1995)42 | 170 | – | 34 | 45% | 32% |
| (Liver Met Registry) | |||||
| Adam et al. (2003)43 | 139 | 2.5% | – | 54% | 35% |
| Shaw et al. (2006)24 | 66 | 0% | 56% | 68% | 44% |
| de Jong et al. (2009)44 | 246 | 0.4% | – | – | 32.6% |
| (multi-institution data) | |||||
| Current studya | 53 | 0% | 45 | 61% | 52% |
In the current study, 3-and 5-year survival rates were calculated from the date of first hepatectomy.
Comparison with the single hepatectomy group
Patients in the single hepatectomy group (n = 916) and the repeat liver resection group (n = 53) were comparable with respect to age, Dukes stage of the primary tumour, the presence of synchronous liver lesions, the time interval between the resections of the primary and metastatic lesions, and the size and number of liver metastases (Table 3). Significantly more major liver resections were undertaken in the single hepatectomy group compared with the first repeat resection group. A comparative analysis of intraoperative blood product requirements and postoperative complication rates between the two groups revealed no significant variables. A detailed summary is presented in Table 4.
Table 3.
Data on patient demographics and metastatic tumours in the present series
| Demographics | Single hepatectomy (n = 916) | First repeat hepatectomy (n = 53) | P-value |
|---|---|---|---|
| Age, years, median (range) | 66 (21–87) | 63 (38–81) | 0.882 |
| Sex, male/female, n | 619/297 | 29/24 | |
| Dukes stage of primary tumour, n (%) | |||
| A | 30 (3%) | 3 (6%) | 0.471 |
| B | 270 (30%) | 15 (28%) | 0.530 |
| C | 377 (41%) | 18 (34%) | 0.311 |
| D | 239 (26%) | 17 (32%) | 0.334 |
| Synchronous tumour | 239 (26%) | 17 (32%) | 0.334 |
| Time interval, months, median (range) | |||
| Primary resection to first hepatectomy | 11.5 (0–188.1) | 10.5 (1.9–65) | 0.396 |
| First to second hepatectomy | – | 15.4 (4.3–60.1) | |
| Second to third hepatectomy | – | 12.2 (7.7–33.8) | |
| Size of liver tumour, cm, median (range) | 3.5 (1.0–18.5) | 3.0 (1.0–11.0) | 0.743 |
| Number of liver lesions, n, median (range) | 1 (1–9) | 1 (1–8) | 0.912 |
Table 4.
Intraoperative variables and postoperative complications graded using the Clavien–Dindo classification
| Single hepatectomy (n = 916) | First liver resection (n = 53) | P-value | Second live r resection (n = 53) | P-value | |
|---|---|---|---|---|---|
| Type of resection, n (%) | |||||
| Major | 746 (82%) | 25 (47%) | 0.001 | 10 (19%) | 0.003 |
| Minor | 171 (12%) | 28 (53%) | 43 (81%) | ||
| Perioperative transfusions | |||||
| Blood | 0 (0–35 units) | 0 (0–18 units) | 0.439 | 0 (0–19 units) | 0.818 |
| Fresh frozen plasma | 0 (0–22 units) | 0 (0–10 units) | 0 (0–24 units) | ||
| Platelets | 0 (0–22 units) | 0 | 0 (0–2 units) | ||
| Patients with blood production, % | 31% | 24% | 19% | ||
| 30-day mortality, n | 45 | 0 | 0 | ||
| Liver dysfunctiona, n (%) | 9 (2%) [Grade 5: 1] | 1 (2%) | 0.159 | 1 (2%) | 1.0 |
| Bleeding, n (%) | 11 (1%) [Grade 3a: 3] | – | 1.0 | 0 | 1.0 |
| Bile leak, n (%) | 55 (6%) [Grade 3a: 17] | 4 (7%) [Grade 3a: 1] | 0.551 | 2 (4%) [Grade 3a: 2] | 0.628 |
| Pulmonary complications, n (%) | 52 (6%) [Grade 3a: 2; Grade 4: 4] | 2 (4%) | 0.768 | 1 (2%) | 1.0 |
| AF/dysrhythmia, n (%) | 40 (4%) | – | 0.061 | – | 1.0 |
| Abdominal collections, n (%) | 3 (<1%) [Grade 3a: 2] | – | 0.153 | – | 1.0 |
| Wound infection, n (%) | 17 (2%) | – | 0.610 | – | 1.0 |
| Wound dehiscence, n (%) | 3 (<1%) [Grade 3b: 2] | – | 0.151 | – | 1.0 |
Postoperative liver dysfunction was defined using the 50–50 criteria (serum prothrombin time <50% of normal, bilirubin >50 μmol/l on postoperative day 5.45
Significant complications requiring surgical, endoscopic or radiological intervention and life-threatening complications are shown in parentheses.
AF, atrial fibrillation.
Rates of 1-, 3-and 5-year overall survival after a single hepatectomy were 95%, 58% and 43%, respectively. The difference in survival rates between the single hepatectomy and the repeat hepatectomy groups was not significant. Survival curves are presented in Fig. 1.
Figure 1.
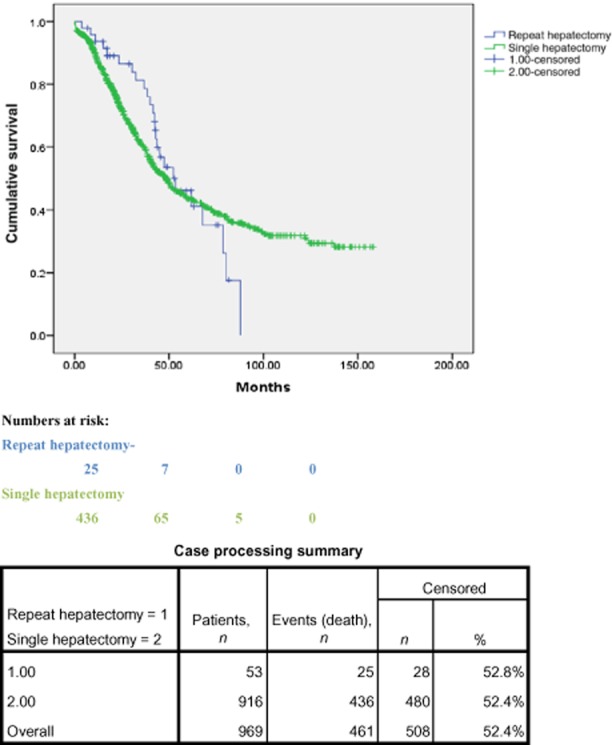
Kaplan–Meier curves showing survival in the single hepatectomy and repeat hepatectomy groups
Predictors of survival
Cox regression analysis demonstrated that R1 resection at repeat hepatectomy (P = 0.002), a shorter time interval between the first and second hepatectomies (P = 0.02) and the presence of extrahepatic disease (P = 0.02) were associated with significantly poorer survival in patients who underwent repeat hepatectomy. The details of the analysis are shown in Table 5.
Table 5.
Cox regression analysis: factors predicting survival in the repeat liver resection group
| Factors | P-value | HR (95% CI) |
|---|---|---|
| Time interval between first and second resections | 0.021 | 1.095 (3.37–5.14) |
| Type of resection (major versus minor) | 0.783 | 1.747 (0.15–2.50) |
| Tumour distribution (unilateral versus bilateral) | 0.443 | 0.629 (0.46–12.40) |
| R1 resection at second hepatectomy | 0.002 | 11.054 (2.90–3.70) |
| Presence of extrahepatic disease | 0.025 | 1.847 (1.60–2.50) |
| Age | 0.637 | 0.736 |
HR, hazard ratio; 95% CI, 95% confidence interval.
Discussion
A large proportion of patients with CRC with liver metastases develop tumour recurrence despite surgical treatment with curative intent. In an estimated 25–30% of these patients, recurrence is confined to the liver.28 Modern systemic chemotherapy regimens have achieved a median survival of 20 months in patients with colorectal metastases. However, data on the efficacy of these treatments in patients with recurrence in the liver following hepatectomy are lacking.22,29 Improvements in surgical technique and perioperative management have encouraged a few centres to adopt an aggressive surgical approach in selected patients with metastatic recurrence.22–24,30
Repeat liver resection is made challenging by a combination of factors, such as adhesions from previous surgery, chemotherapy-induced liver injury and the alterations to the anatomy caused by regeneration. This has led to perceptions that increased mortality and morbidity are associated with such resections.31,32
The incidence of repeat liver resection in the present study was 6%; that in a large UK-based study published by Shaw et al. was 7%.24
In the present series, no perioperative mortality occurred following repeat liver surgery. Intraoperative blood product usage, postoperative morbidity and length of hospital stay were comparable between the repeat and single hepatectomy groups. However, the majority of repeat hepatectomies in the study group were minor, as they were in other published series.31–35 Over the years, the unit at which this study was conducted has adopted a strict technique for first hepatectomy that involves the mobilizing of only the lobes with target lesions, minimal retrohepatic dissection and the use of parenchyma-preserving resection when possible. This approach is intended to minimize adhesions and perihepatic fibrosis and thus to facilitate any future repeat hepatectomies.
Five-year survival in patients who underwent a repeat hepatectomy was 59% in the present study; this was slightly better than the 43% achieved in patients undergoing single hepatectomy. The survival benefit to be derived from repeat liver resection is also indicated by the results of other studies reported in the literature, which quote 5-year survival rates ranging from 26% to 44%.24 The enhanced survival noted in the present group is likely to relate to the fact that this was a highly selected patient population with favourable tumour biology.
Complete tumour clearance at index hepatectomy is crucial for a good prognosis.36 Accurate preoperative staging to identify all tumour nodules is a key factor in achieving clearance. Early tumour recurrence usually reflects unidentified metastatic disease or a positive microscopic resection margin. The Cox regression analysis in the present study showed that early recurrence (or a shorter time interval between the first and second hepatectomies) was associated with a poor prognosis. An R1 resection and the presence of extrahepatic disease were also associated with a poor prognosis. Aggressive surveillance to identify residual disease and early recurrence could potentially extend the benefits of repeat hepatectomy to a wider population and thus improve outcomes. Neoadjuvant chemotherapy may be considered in patients affected by poor prognostic factors.37
The role of RFA as a primary treatment modality for recurrent CLM is currently unclear. It has been shown to confer an acceptable survival benefit in patients with CLM: for example, Solbiati et al. demonstrated a 3-year survival rate of 46% in patients treated with RFA.38 Although the results were encouraging, the role of RFA in recurrent CLM has not been thoroughly studied.39 Published studies have tended to include patients who were not fit for surgical intervention and therefore it is not possible to compare the short-and longterm outcomes of RFA and surgery, respectively, for recurrent CLM. Further, studies have also reported local intrahepatic recurrence in up to 40% of patients following ablation treatments.40 At the present centre, the low morbidity and good longterm survival rates associated with surgery have restricted the use of RFA to its administration in combination with surgery or as a second-line treatment in patients who are unfit for surgery.
A small proportion of patients (10%) with recurrent CLM isolated to the liver are amenable to repeat liver resection. Published experience in repeat hepatectomy for recurrent CLM is derived from small retrospective studies that support the role of repeat hepatectomy as a curative treatment in carefully selected patients.41 The present results concur with current opinion. The roles of more conservative modalities, such as chemotherapy or local ablative treatments (alone or in combination), should be addressed in randomized controlled trials.
In conclusion, repeat hepatectomy is safe and provides survival outcomes comparable with those of single hepatectomy in selected patients with recurrent CLM. Both technical and oncological factors should be considered at patient selection. The presence of a positive resection margin, extrahepatic disease and a shorter time interval between the first and repeat hepatectomies are associated with a significantly poorer outcome.
Conflicts of interest
None declared.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Stangl R, Altendorf-Hofmann A, Charnley RM. Factors influencing the natural history of colorectal liver metastases. Lancet. 1994;343:1405–1410. doi: 10.1016/s0140-6736(94)92529-1. [DOI] [PubMed] [Google Scholar]
- 3.Scheele J, Stangl R, Altendorf-Hofmann A. Hepatic metastases from colorectal cancer: impact of surgical resection on the natural history. Br J Surg. 1990;77:1241–1246. doi: 10.1002/bjs.1800771115. [DOI] [PubMed] [Google Scholar]
- 4.Altendorf-Hofmann A, Scheele J. A critical review of the major indicators of prognosis after resection of hepatic metastases from colorectal carcinoma. Surg Oncol Clin N Am. 2003;12:165–192. doi: 10.1016/s1055-3207(02)00091-1. [DOI] [PubMed] [Google Scholar]
- 5.Pawlik T, Schulick R, Choti M. Expanding criteria for resectability of colorectal liver metastases. Oncologist. 2008;13:51–64. doi: 10.1634/theoncologist.2007-0142. [DOI] [PubMed] [Google Scholar]
- 6.Khatri VP, Petrelli NJ, Belghiti J. Extending the frontiers of surgical therapy for hepatic colorectal metastases: is there a limit? J Clin Oncol. 2005;23:8490–8499. doi: 10.1200/JCO.2004.00.6155. [DOI] [PubMed] [Google Scholar]
- 7.Chuanq SC, Su YC, Lu CY, Hsu HT, Sun LC, Shih YL, et al. Risk factors for the development of metachronous liver metastasis in colorectal cancer patients after curative resection. World J Surg. 2011;35:424–429. doi: 10.1007/s00268-010-0881-x. [DOI] [PubMed] [Google Scholar]
- 8.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–321. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdalla EK, Vauthey JN, Ellis LM. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–827. doi: 10.1097/01.sla.0000128305.90650.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma S, Camci C, Jabbour N. Management of hepatic metastasis from colorectal cancers: an update. J Hepatobiliary Pancreat Surg. 2008;15:570–580. doi: 10.1007/s00534-008-1350-x. [DOI] [PubMed] [Google Scholar]
- 11.Jamison RL, Donohue JH, Nagorney DM. Hepatic resection for metastatic colorectal cancer results in cure in some patients. Arch Surg. 1997;132:505–510. doi: 10.1001/archsurg.1997.01430290051008. [DOI] [PubMed] [Google Scholar]
- 12.Hughes KS, Simon R, Songhorabotdi S, Adson MA, Ilstrup DM, Fortner JG. Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of patterns of recurrence. Surgery. 1986;100:278–284. [PubMed] [Google Scholar]
- 13.Sa Cunha A, Laurent C, Rault A, Couderc P, Rullier E, Saric J. A second liver resection due to recurrent colorectal liver metastases. Arch Surg. 2007;142:1144–1149. doi: 10.1001/archsurg.142.12.1144. [DOI] [PubMed] [Google Scholar]
- 14.Yamashita Y, Adachi E, Toh Y, Ohgaki K, Ikeda O, Oki E, et al. Risk factors for early recurrence after curative hepatectomy for colorectal liver metastases. Surg Today. 2011;41:526–532. doi: 10.1007/s00595-010-4471-1. [DOI] [PubMed] [Google Scholar]
- 15.Steele G, Jr, Bleday R, Mayer RJ, Lindblad A, Petreli N, Weaver D. A prospective evaluation of hepatic resection for colorectal carcinoma metastases to the liver: Gastrointestinal Tumour Study Group Protocol 6584. J Clin Oncol. 1991;9:1105–1112. doi: 10.1200/JCO.1991.9.7.1105. [DOI] [PubMed] [Google Scholar]
- 16.Ohlsson B, Stenram U, Tranberg KG. Resection of colorectal liver metastases: 25-year experience. World J Surg. 1998;22:268–277. doi: 10.1007/s002689900381. [DOI] [PubMed] [Google Scholar]
- 17.Brachet D, Lermite E, Rouquette A, Lrimier G, Hamy A, Arnaud JP. Prognostic factors of survival in repeat liver resection for recurrent colorectal metastases: review of sixty-two cases treated at a single institution. Dis Colon Rectum. 2009;52:475–483. doi: 10.1007/DCR.0b013e31819d12bc. [DOI] [PubMed] [Google Scholar]
- 18.Yan T, Sim J, Black D, Niu R, Morris D. Systematic review on safety and efficacy of repeat hepatectomy for recurrent liver metastases from colorectal carcinoma. Ann Surg Oncol. 2007;14:2069–2077. doi: 10.1245/s10434-007-9388-6. [DOI] [PubMed] [Google Scholar]
- 19.Que FG, Nagorney DM. Resection of ‘recurrent’ colorectal metastases to the liver. Br J Surg. 1994;81:255–258. doi: 10.1002/bjs.1800810234. [DOI] [PubMed] [Google Scholar]
- 20.Fowler WC, Hoffman JP, Eisenberg BL. Redo hepatic resection for metastatic colorectal carcinoma. World J Surg. 1993;17:658–661. doi: 10.1007/BF01659136. [DOI] [PubMed] [Google Scholar]
- 21.Petrowsky H, Gonen M, Jarnagin W. Second liver resections are safe and effective treatment for recurrent hepatic metastases from colorectal cancer: a bi-institutional analysis. Ann Surg. 2002;235:863–871. doi: 10.1097/00000658-200206000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riesener KP, Kasperk R, Winkeltau G, Schumpelick V. Repeat resection of recurrent hepatic metastases – improvement in prognosis? Eur J Surg. 1996;162:709–715. [PubMed] [Google Scholar]
- 23.Nordlinger B, Vaillant JC, Guiguet M. Survival benefit of repeat liver resections for recurrent colorectal metastases: 143 cases. Association Française de Chirurgie. J Clin Oncol. 1994;12:1491–1496. doi: 10.1200/JCO.1994.12.7.1491. [DOI] [PubMed] [Google Scholar]
- 24.Shaw IM, Rees M, Welsh FK, Bygrave S, John TG. Repeat hepatic resection for recurrent colorectal liver metastases is associated with favourable longterm survival. Br J Surg. 2006;93:457–464. doi: 10.1002/bjs.5323. [DOI] [PubMed] [Google Scholar]
- 25.Charnsangavej C, Clary B, Fong Y, Grothey A, Pawlik T, Choti M. Selection of patients for hepatic colorectal metastases: a Consensus Statement. Ann Surg Oncol. 2006;13:1261–1268. doi: 10.1245/s10434-006-9023-y. [DOI] [PubMed] [Google Scholar]
- 26.Ng WW, Cheung YS, Wong J, Lee KF, Lai PB. A preliminary analysis of combined liver resection with new chemotherapy for synchronous and metachronous colorectal liver metastasis. Asian J Surg. 2009;32:189–197. doi: 10.1016/S1015-9584(09)60394-8. [DOI] [PubMed] [Google Scholar]
- 27.Dindo D, Demartines N, Clavien PA. Classification of surgical complications. A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan KM, Chiang JM, Lee CF, Yu MC, Lee WC, Chen JS, et al. Outcomes of resection for colorectal cancer hepatic metastases stratified by evolving eras of treatment. World J Surg Oncol. 2011;9:174. doi: 10.1186/1477-7819-9-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishiguro S, Akasu T, Fujimoto Y. Second hepatectomy for recurrent colorectal liver metastasis: analysis of preoperative prognostic factors. Ann Surg Oncol. 2006;13:1579–1587. doi: 10.1245/s10434-006-9067-z. [DOI] [PubMed] [Google Scholar]
- 30.Pessaux P, Lermite E, Brehant O, Tuech JJ, Lorimier G, Arnaud JP. Repeat hepatectomy for recurrent colorectal liver metastases. J Surg Oncol. 2006;93:1–7. doi: 10.1002/jso.20384. [DOI] [PubMed] [Google Scholar]
- 31.Jakab F, Mersich T. Repeat resection of the liver – a challenge in modern oncologic surgery. Magy Seb. 2010;63:3–8. doi: 10.1556/MaSeb.63.2010.1.1. [DOI] [PubMed] [Google Scholar]
- 32.Aramaki M, Kawano K, Kai T, Sasaki A, Ohno T, Yoshida T, et al. Postoperative complications of repeat hepatectomy for liver metastasis from colorectal carcinoma. Hepatogastroenterology. 2000;47:478–480. [PubMed] [Google Scholar]
- 33.Suzuki S, Sakaguchi T, Yokoi Y. Impact of repeat hepatectomy on recurrent colorectal liver metastases. Surgery. 2001;129:421–428. doi: 10.1067/msy.2001.112486. [DOI] [PubMed] [Google Scholar]
- 34.Zacharias T, Jaeck D, Oussoultzoglou E. First and repeat resection of colorectal liver metastasis in elderly patients. Ann Surg. 2004;240:858–865. doi: 10.1097/01.sla.0000143272.52505.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruiz-Tovar J, Lopez Hervas P. Repeated liver resection for recurrence of colorectal cancer metastases. Clin Transl Oncol. 2010;12:634–638. doi: 10.1007/s12094-010-0569-6. [DOI] [PubMed] [Google Scholar]
- 36.Mise Y, Imamura H, Hashimoto T, Seyama Y, Aoki T, Hasegawa K, et al. Cohort study of the survival benefit of resection for recurrent hepatic and/or pulmonary metastases after primary hepatectomy for colorectal metastases. Ann Surg. 2010;251:902–909. doi: 10.1097/SLA.0b013e3181c9868a. [DOI] [PubMed] [Google Scholar]
- 37.Hebbar M, Pruvot FR, Romano O, Triboulet JP, de Gramont A. Integration of neoadjuvant and adjuvant chemotherapy in patients with resectable liver metastases from colorectal cancer. Cancer Treat Rev. 2009;35:668–675. doi: 10.1016/j.ctrv.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 38.Solbiati L, Livraghi T, Goldberg SN, Ierace T, Meloni F, Dellanoce M, et al. Percutaneous radiofrequency ablation of hepatic metastases from colorectal cancer: longterm results in 117 patients. Radiology. 2001;221:159–166. doi: 10.1148/radiol.2211001624. [DOI] [PubMed] [Google Scholar]
- 39.Elias D, De Baere T, Smayra T, Ouellet JF, Roche A, Lasser P. Percutaneous radiofrequency thermoablation as an alternative to surgery for treatment of liver tumour recurrence after hepatectomy. Br J Surg. 2002;89:752–756. doi: 10.1046/j.1365-2168.2002.02081.x. [DOI] [PubMed] [Google Scholar]
- 40.Tsai S, Pawlik TM. Outcomes of ablation versus resection for colorectal liver metastases: are we comparing apples with oranges? Ann Surg Oncol. 2009;16:2422–2428. doi: 10.1245/s10434-009-0491-8. [DOI] [PubMed] [Google Scholar]
- 41.de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250:440–448. doi: 10.1097/SLA.0b013e3181b4539b. [DOI] [PubMed] [Google Scholar]
- 42.Fernández-Trigo V, Shamsa F, Sugarbaker PH. Repeat liver resections from colorectal metastasis. Repeat Hepatic Metastases Registry. Surgery. 1995;117:296–304. doi: 10.1016/s0039-6060(05)80205-3. [DOI] [PubMed] [Google Scholar]
- 43.Adam R, Pascal G, Azoulay D, Tanaka K, Castaing D, Bismuth H. Liver resection for colorectal metastases: the third hepatectomy. Ann Surg. 2003;238:871–883. doi: 10.1097/01.sla.0000098112.04758.4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Jong MC, Mayo SC, Pulitano C, Lanella S, Ribero D, Strub J, et al. Repeat curative intent liver surgery is safe and effective for recurrent colorectal liver metastasis: results from an international multi-institutional analysis. J Gastrointest Surg. 2009;13:2141–2151. doi: 10.1007/s11605-009-1050-0. [DOI] [PubMed] [Google Scholar]
- 45.Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, et al. The ‘50–50 criteria’ on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824–828. doi: 10.1097/01.sla.0000189131.90876.9e. [DOI] [PMC free article] [PubMed] [Google Scholar]


