Abstract
Background: Post-acute pancreatic collections (PAPCs) may require intervention when persistent, large or symptomatic. An open cystgastrostomy is an effective treatment option particularly for larger, solid predominant collections. A laparoscopic cystgastrostomy (LCG) as initially described, could be technically challenging. This report describes the evolution of the operative technique and the results from LCG in a tertiary referral centre.
Methods: Retrospective analysis of the unit’s prospectively populated database was conducted. All patients who underwent a surgical cystgastrostomy (SCG) were identified. Patient demographics, outcome and complications were collected and analysed.
Results: Forty-four patients underwent SCG: 8 open and 36 laparoscopic. Of the 36 LCG, 6 required open conversion, although with evolution of the technique all of the last 17 cases were completed laparoscopically. The median interquartile range (IQR) length of stay in patients completed laparoscopically was 6 (2–10) compared with 15.5 days (8–19) in those patients who were converted (P = 0.0351). The only peri-operative complication after a LCG was a self-limiting upper gastrointestinal bleed. With a median (IQR) follow-up of 891 days (527–1495) one patient required re-intervention for a residual collection with no recurrent collections identified.
Conclusion: LCG is a safe and effective procedure in patients with large, solid predominant PAPCs. With increased experience and technical expertise conversion rates can be lowered and outcome optimized.
Introduction
Acute peri-pancreatic fluid collections commonly occur after an attack of severe acute pancreatitis. They vary (liquid to solid) in content and are frequently associated with necrosis. The definitions of a collection have been recently clarified by the revised Atlanta criteria,1 which separates collections on the basis of the time from presentation (with a cut-off of 4 weeks) and a solid component (greater or less than 5%). The majority resolve, however, in around 15% of patients the acute collection persists beyond 4 weeks2 then being defined per the Atlanta Classification3 as late pancreatic collections: either a pancreatic pseudocyst (PP) or walled off necrosis (WON) depending on the presence of a significant solid component (Table 1), (Appendix A1).
Table 1.
Collections as defined in the Revised Atlanta Classification
| Revised Atlanta Classification terms | ||
|---|---|---|
| Interstitial Edematous Pancreatitis | Necrotizing Pancreatitis | |
| Acute inflammation of the pancreatic parenchyma and peripancreatic tissues, but without recognizable necrosis of pancreatic parenchyma or peripancreatic tissues. | Inflammation associated with pancreatic parenchymal necrosis and/or peripancreatic necrosis. | |
| AFC (Acute Fluid Collection) | ANC (Acute Necrotic Collection) | EARLY |
| Peripancreatic fluid associated with IEP with no associated peripancreatic necrosis. This term applies only to areas of peripancreatic fluid seen within the first 4 weeks after onset of IEP. | A collection containing variable amounts of both fluid and necrosis associated with necrotizing pancreatitis; the necrosis can involve the pancreatic parenchyma and/or the peripancreatic tissues. | (1st 4 weeks after onset of pancreatitis) |
CT Criteria:
|
CT Criteria:
|
|
| Pancreatic Pseudocyst | WON (Walled-Off Necrosis) | LATE |
| A complete encapsulated collection of fluid outside the pancreas with minimal (< 5%) or no necrosis usually requires more than 4 weeks after onset of IEP to mature and has a well defined inflammatory wall; rarely a pancreatic pseudocyst may develop in a patient with necrotizing pancreatitis after treatment by necrosectomy, usually related to disconnected duct syndrome. | A encapsulated collection of pancreatic and/or peripancreatic necrosis that persists for > 4 weeks after onset of necrotizing pancreatitis and has a well-defined inflammatory wall. | (≥4 weeks after onset of pancreatitis) |
CT Criteria:
|
CT Criteria:
|
|
IEP, interstitial edematous pancreatitis.
Surgical management of patients with pancreatic necrosis within the first 4–6 weeks is determined by the presence of sepsis and/or organ failure. Interventions for infected necrosis have traditionally required surgical debridement,4–7 with a trend towards utilizing minimally invasive techniques in the past decade. The recently published PANTER trial8 has suggested that in some cases simple percutaneous radiological drainage without necrosectomy may even be sufficient in selected patients.
Intervention may be required beyond this period for a symptomatic collection that fails to resolve spontaneously, for large collections owing to the high risk of sepsis and haemorrhage, or for symptomatic management (abdominal discomfort, anorexia, vomiting and general failure to thrive). The optimum management of these more mature PAPC is also debatable, being determined by the anatomy, timing and the extent of necrosis.
Conventional management of late pancreatic collections was by open pancreatic cystgastrostomy,9 but with developments in interventional radiology, therapeutic endoscopy and minimal access surgery, new techniques have been employed as alternatives to this approach. While all have proven feasible in small cohort series,10–12 evidence is limited as to the relative benefits of one method over another in the management of PAPC. Laparoscopic cystgastrostomy (LCG) should, in theory, allow a wide debridement of the cyst cavity with the advantages of a minimally invasive approach. Initial reports employed an endo-luminal intra-gastric approach12 utilizing ballooned laparoscopic ports to maintain intra-gastric access and a gas seal. However, the technical challenges of endoluminal surgery and the emergence of endoscopic ultrasound (EUS)-guided endoscopic cystgastrostomy led to the laparoscopic approach falling from favour over the past decade.
Endoscopic cystgastrostomy was initially reported for the management of a mature pancreatic abscess with minimal necrosis,13 but the technique has evolved in the past 10 years to become an established Natural Orifice (NOTES) procedure, with endoscopic transmural exploration and debridement of the retroperitoneum. The presence of significant walled off necrosis (WON) is no longer considered a contraindication, but concerns do remain regarding the adequacy of endoscopic drainage, particularly in solid predominant or larger collections.14 The risks of secondary sepsis and the requirement for repeated tract dilatation with the EUS-guided approach, coupled with parallel improvements in laparoscopic equipment and operative technique have re-focused interest on the potential of a single laparoscopic intervention. This paper discusses the evolution of the LCG technique from a laparoscopic endo-luminal approach to a technically less challenging trans-gastric technique, and describes a single-centre (tertiary referral) experience of LCG in the relatively small subset of patients with sterile WON.
Patients and methods
A prospective unit database (Microsoft Excel; Microsoft Corporation, Seattle, WA, USA) was used to identify patients who had undergone an open and laparoscopic cystgastrostomy over the 9-year period since the procedure was first conducted laparoscopically within our unit. Case-note review, operation note review, national radiology database (PACS) search and a regional electronic database (Clinical Portal) were used to supplement the data within the database. In the event of discharge or loss to follow-up, the General Practitioner was contacted to confirm the current status. The dataset of relevant clinical, surgical and radiological data included patient demographics, pre-operative imaging, operation reports, post-operative course, complications, hospital stay, post-operative imaging and clinical follow-up data. The diagnosis of a WON was made with either a computed tomography (CT) or magnetic resonance imaging scan. The decision to drain the WON was made after discussion at the multi-disciplinary team meeting, which included at least two pancreato-biliary consultants, a radiologist and a therapeutic endoscopist. Expertise in laparoscopic-, endoscopic- (with EUS guidance) and radiologically-assisted percutaneous drainage were available in the unit throughout this time period.
The group of patients considered in this series were those with no evidence of sepsis but a well-defined retro-gastric collection, who had recovered from the original ‘organ failure’ period after acute pancreatitis. EUS drainage was used as a first-line therapy for patients with pancreatic pseudocyst (PP), with the surgical procedure reserved for patients with large or solid predominant collections. Although LCG use in some units is reserved for failed EUS drainage, in our experience, laparoscopic access after a prior attempt at EUS drainage is challenging therefore one method is chosen over another from the outset depending on the collections characteristics. External drainage was conducted rarely in this particular group of patients, although patients with sepsis were treated with initial percutaneous drainage followed by a ‘step-up dilatation and percutaneous necrosectomy’ approach.
Surgical technique
Initial technique
According to the technique initially described by Way et al.,12 the unit’s early experience involved the use of radially expanding balloon trocars to facilitate a laparoscopic intraluminal cystgastrostomy. Three intra-gastric trocars were initially used: 2 × 12 mm and 1 × 5 mm. Saline was instilled into the duodenum as a ‘U-bend’ sump to prevent passage of insufflation gas down the gastrointestinal tract. The collection was located by laparoscopic ultrasound and a direct puncture achieved using laparoscopic scissors. Access was maintained with the scissors and a second suction trocar inserted into the cyst cavity. A stapled cystgastrostomy was then performed using an Endo GIA Stapler 30 mm (Covidien plc, Dublin, Ireland) with four to five firings. This procedure presented technical difficulties such as maintenance of cyst access after puncture, and difficult angulation for stapling and intra-gastric suturing (when required for haemostasis). The advantage to this approach lay in the use of only sealed gastrotomies, minimizing potential spillage of gastric contents.
Current technique
The current technique has evolved from the intra-gastric approach to a true laparoscopic trans-gastric approach. An open sub-umbilical cut down is employed. Blunt trocars are then inserted on the patient’s left and right side with the specific port site placement being determined by the position of the retro-gastric collection on cross-sectional imaging, thus optimizing triangulation over the cystgastrostomy site. Adhesions from recent inflammation are common and are divided to expose the anterior gastric wall. An anterior gastrotomy (5–10 cm long) is then performed using the harmonic scalpel (Ethicon Endo-Surgery, Inc, Cincinnati, OH, USA). The superior leaf of the opened stomach is lifted towards the anterior abdominal wall to maximize access and delineate the area of adherence between the cyst and the posterior aspect of the stomach. This is achieved by passing a straight needle 2/0 suture through the abdominal wall, the anterior stomach wall and back out of the abdomen.
The key advance has been the use of a ‘Step’ dilatation port system (Covidien plc) to achieve an initial cyst puncture, allow tract dilatation and maintain access until insertion of the initial staple device. The puncture trocar is inserted through the abdominal wall and having chosen an appropriate epigastric/stomach puncture site under ultrasonic guidance, the sharp trocar enters the collection via the exposed posterior gastric wall (Fig. 1). The port is dilated, allowing a 12-mm access to the cyst cavity, apposition of the posterior stomach wall and cyst being maintained by the radial resistance of the dilatation sleeve. After aspiration of the collection to relative dryness, the port is withdrawn leaving the suction instrument within the collection to maintain access, and a stapled cystgastrostomy is performed using 4–5 firings of the angulating Universal Endo GIA stapler (Covidien plc) (Fig. 2). Necrotic debris within the cavity is removed and placed in the fundus of the stomach (Fig. 3). Once adequate debridement and haemostasis have been assured, the anterior gastrotomy is closed using a running 3/0 monofilament suture (Biosyn™, Covidien plc), with the integrity of the closure then tested by insufflating the stomach through an oro-gastric tube, with the anastomoses under lavage fluid. Post-operative fluid and diet is allowed as tolerated. In this complex cohort of patients, suitability for hospital discharge is often multi-factorial, but may be within 36 h of surgery when dietary intake is adequate.
Figure 1.
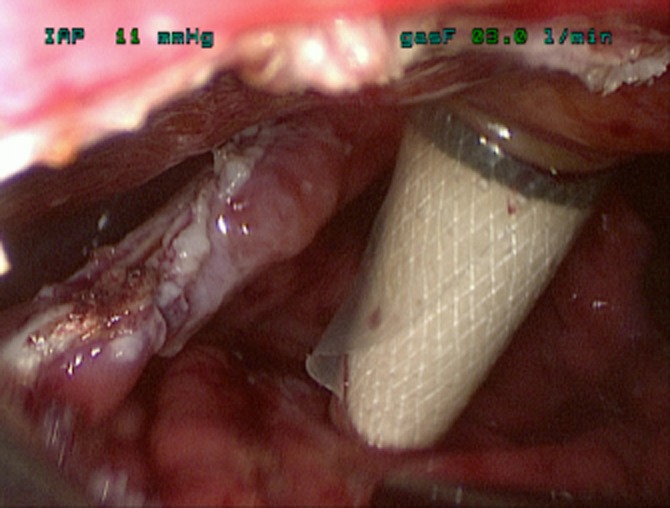
‘Step’ dilatation port system
Figure 2.
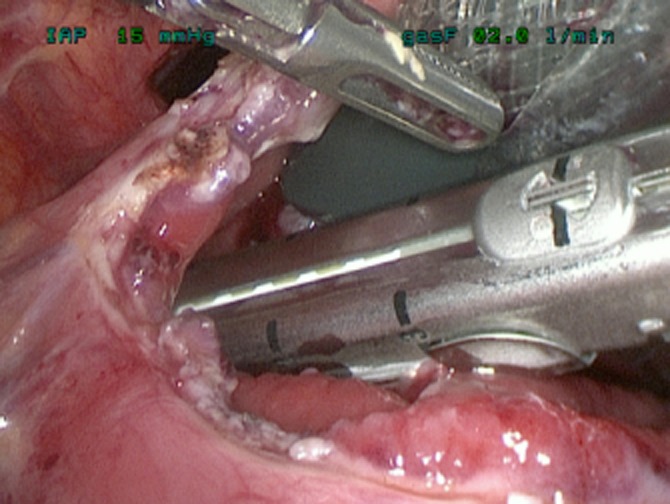
Stapled cystgastrostomy
Figure 3.
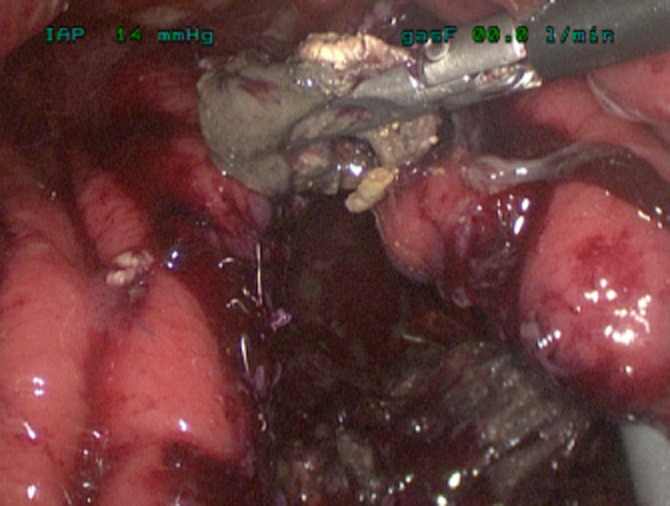
Removal of necrotic debris from within collection
Where gallstones are present, a simultaneous laparoscopic cholecystectomy is performed. Patient follow-up assessment was conducted routinely in the unit as an outpatient, however, if asymptomatic, patients from remote areas were reviewed in their local institution. Patients routinely underwent a post-operative CT scan to confirm resolution of the collection. In the event of a complication or symptom recurrence, patients were readmitted to the specialist unit for investigation.
Statistical analysis
Data are expressed as median [interquartile range (IQR)]. Statistical significance (P < 0.05) was determined using the Mann–Whitney U-test for continuous variables and Fisher’s exact test for categorical variable.
Results
Demographics
Some 44 patients (29 males, 15 females) with PAPC underwent surgical intervention over the 9-year period. The median (IQR) age was 54 years (39–63). The majority of patients had alcohol (14) or gallstones (25) as the aetiology of acute pancreatitis. Pancreatitis also occurred secondary to trauma (1) and endoscopic retrograde cholangiopancreatography (1) with 3 cases being idiopathic. The median (IQR) time from first diagnosis at the base hospital to procedure at the specialist unit was 71 days (43–139). The median (IQR) maximum diameter of the cysts was 15 cm (10.4–17).
Procedures
Eight patients underwent a planned OCG. The indications for this were availability of necessary laparoscopic operative experience (3), associated pancreatic ascites (1) or a small bowel fistula requiring intervention (1), a defunctioning ileostomy required (1) and ‘rescue’ after a complication of EUS drainage (1). One patient had an OCG having sustained intra-abdominal trauma leading to a pancreatic transection.
Thirty-six patients underwent planned LCG. Of these, 30 were completed laparoscopically with 6 converted. The reason for conversion in all cases was failure to progress safely owing to a combination of technical difficulties with visualization, angulation or an inability to attain a safe cyst puncture angle, rather than intra-abdominal bleeding or another complication. Conversion rates decreased over the study period as operative experience increased, and the new technique was utilized (Table 2 and Fig. 3). As a result of technical challenges associated with the intraluminal technique, few procedures were conducted between 2004 and 2006, owing to the preference within the unit to use EUS-guided cystgastrostomy at this time.
Table 2.
Epidemiology of procedures per annum
| Year | Procedures | Laparoscopic | Conversions | Open |
|---|---|---|---|---|
| 2002 | 3 | 1 | 2 | 0 |
| 2003 | 4 | 1 | 1 | 2 |
| 2005 | 3 | 2 | 0 | 1 |
| 2007 | 5 | 4 | 1 | 0 |
| 2008 | 10 | 5 | 2 | 3 |
| 2009 | 8 | 7 | 0 | 1 |
| 2010 | 8 | 7 | 0 | 1 |
| 2011 (to March) | 3 | 3 | 0 | 0 |
Outcome
The median (IQR) length of stay after a cystgastrostomy was 8 days (3–19.5) with 22 days (20–65.5) for the open procedure and 7 days (3–12.5) for planned laparoscopic procedures. The median (IQR) length of stay in those patients completed laparoscopically was 6 (2–10) versus 15.5 days (8–19) in those patients who were converted (P = 0.0351). In 12 patients additional procedures were conducted at cystgastrostomy: laparoscopic cholecystectomy (10), open cholecystectomy (1) and ileostomy (1).
Early complications
Only two patients suffered peri-operative (within 6 weeks of the procedure) complications after a cystgastrostomy. One patient had a post-operative leak into the lesser sac after OCG and another had a self-limiting upper GI bleed after LCG. This did not require intervention. No deaths occurred in the peri-operative period.
Late complications
Patients have been followed up for a median of 891 days (527–1495) post discharge. In no case was a repeat intervention required for a recurrent cyst. One patient required a laparoscopic cystjejunostomy for a residual collection.
Two patients, both initially converted, required further intervention for on-going complications of pancreatitis. One required both a laparotomy and subsequent gastro-duodenal artery embolization for resolution of sepsis and haemorrhage, and another required a hepatico-jejunostomy and cholecystectomy for a late biliary obstruction.
To date, 3 of the 44 patients have died with no procedure-related mortality. Deaths occurred in patients who had an OCG (2) and LCG (1) at 243 (pneumonia), 317 (acute renal failure and uro-sepsis) and 952 (cholangiocarcinoma) days post-procedure, respectively.
Discussion
This paper presents the second largest series of LCG in the English literature. Moreover, it also describes a technique modification that considerably simplifies the procedure, now making it a practical alternative to endoscopic cystgastrostomy. Palinavelu et al.15 presented a larger series of 90 patients, also demonstrating low morbidity and a high success rate. The two series are however not comparable owing to differences in the respective populations, the Palinavelu series including PP of any aetiology, and not limited to WON. The patients in our series represent a selected group in whom EUS-guided drainage was not deemed appropriate given the solid content of the cyst. This series illustrates that LCG can be conducted effectively and safely in patients with a large solid predominant PAPC.
Many early studies of PP management have reported the success of a specific interventional approach applied to a consecutive cohort of patients, encompassing PAPC across the spectrum of evolution. It is now recognized that severe acute pancreatitis is a dynamic process, with progressive organization and liquefaction of pancreatic and peri-pancreatic collections over a period that may extend to several months. Sterile necrosis, transitional necrosis (4–10 weeks from onset) and WON (usually sterile or secondarily infected post-intervention) are fundamentally different from ‘true’ infected pancreatic necrosis. Patients with infected acute necrotic collections require intervention early in the disease process, often in the presence of significant organ dysfunction and when the infected necrosum is adherent and poorly organized. It is now well recognized that the majority of sterile collections do not require intervention, at least in the early phase of disease, and that the mortality and morbidity after an intervention are time dependent, falling to almost 0% by the stage of sterile WON. The indication for an early intervention for infected necrosis is now limited to sepsis control, and there is increasing consensus that within this group some form of minimally invasive approach may enhance the outcome.
LCG is utilized in mature symptomatic collections. It facilitates complete drainage of the collection with a minimal requirement for re-intervention. It also allows simultaneous definitive management of gallstones. The operative technique has changed considerably over time. Initially ports were inserted trans-gastrically; however, the use of a true laparoscopic technique with an anterior gastrotomy has in our experience improved access leading to better ergonomics. The benefits of this approach have been validated by other previous studies.16 The initial experience in our series was with direct diathermy-assisted puncture of the collection via the posterior gastric wall; however, the use of the ‘Step port’ dilating system has improved the ease of access to the collection facilitating the initial (and most troublesome) stapling. Further changes such as the use of the Harmonic scalpel (Ethicon Endo-Surgery Inc.) and intra-operative ultrasound have improved haemostasis and access to the collection, respectively. These technical progressions have led to a better outcome with fewer conversions and no major morbidity.
The overall success rate of LCG in this series was excellent with only a single patient (3.3%) requiring further intervention for a residual rather than a recurrent cyst. This is comparable to the best results from other large series: Palinavelu reported re-intervention for pseudocyst in 1 of 90 patients (1.1%); 15 Melman et al. reported a primary success rate of 87.5% with the laparoscopic approach17 and Hindmarsh et al. required a further intervention in 2/15 (13.3%) cysts owing to recurrence.18
In our series there was only one complication attributable to LCG, a minor GI bleed that did not require intervention; this low morbidity compares favourably to other published series.17,18 Patients did have late (> 6 months) morbidity and mortality, however, these were secondary to complications related to cardio-respiratory co-morbidity, pancreatitis or metachronous de novo disease rather than the procedure per se.
No direct comparisons were made between those patients who underwent a laparoscopic and open procedures as any difference is probably owing to a selection bias; however, it is interesting that patients who were converted to an open procedure had a significantly longer length of stay (LOS) than those completed laparoscopically. LOS in this study (6 days) is comparable to that in other series of laparoscopic cystgastrostomy.15,17 Palinavelu reported a mean LOS of 5.6 days17 and Melman of 6.9 days.18 In this group of patients with severe pancreatitis, LOS is commonly driven by the pathology itself, however, the laparoscopic approach does allow for patients to be discharged the day after the procedure when clinically appropriate. No procedures were conducted as a day case.
The overall conversion rate in this group of patients is similar to that in other published series,18 although some groups have produced lower figures.15,17 Of note, in recent years, the conversion rate in our series fell to that in those latter studies with no conversions in the last 17 patients (3 years). This is attributed to increased experience, technical improvements and better patient selection.
Tertiary referral units for severe pancreatitis should have EUS-guided, laparoscopic and open cystgastrostomy available, as each approach has its own indications. OCG is utilized when an intervention is required on additional intra-abdominal pathology (e.g. enteric stricture or fistula) or where collection anatomy precludes other approaches. LCG allows larger collections to be managed by a one-step intervention, and the solid necrosis to be more effectively drained. Importantly, definitive management of gallstones can be achieved. However, the present concept that EUS-guided drainage, the least invasive approach, may be of most benefit in fluid predominant collections, requires evaluation within a study format, as experience has shown some PAPC with significant necrosis may resolve completely using only this approach.
The optimum management of collections with intermediate (size and fluid content) characteristics is not clear and there may be clinical equipoise regarding whether a laparoscopic or endoscopic cystgastrostomy should be used as a preferred approach. A well-conducted randomized controlled study is required to determine which method is most effective in this particular group of patients.
Appendix
Appendix A1
Changes in classification schemes for acute pancreatitis
| Atlanta Classification – 1992 | Revised Atlanta Classification – 2011 |
|---|---|
| Interstitial pancreatitis | Interstitial edematous pancreatitis (IEP) |
| Pancreatic necrosis | Necrotizing pancreatitis
|
| <4 weeks after onset of pancreatitis | <4 weeks after onset of pancreatitis |
| Acute fluid collection | Acute fluid collection (AFC) Acute necrotic collection (ANC)
|
| >4 weeks after onset of pancreatitis | >4 weeks after onset of pancreatitis |
| Pancreatic pseudocyst | Pancreatic pseudocyst |
| Pancreatic abscess | Walled-off necrosis (WON)
|
Conflict of interest
No conflict of interest to disclose (all authors).
References
- 1.Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, et al. Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis – 2012: revision of the Atlanta classification and definitions by International Concensus. Gut. 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 2.Aljarabah M, Ammori BJ. Laparoscopic and endoscopic approaches for drainage of pancreatic pseudocysts: a systematic review of published series. Surg Endosc. 2007;21:1936–1944. doi: 10.1007/s00464-007-9515-2. [DOI] [PubMed] [Google Scholar]
- 3.Bradley EL., 3rd A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11 through 13, 1992. Arch Surg. 1993;128:586–590. doi: 10.1001/archsurg.1993.01420170122019. Review. [DOI] [PubMed] [Google Scholar]
- 4.Fernández-del Castillo C, Rattner DW, Makary MA, Mostafavi A, McGrath D, Warshaw AL. Débridement and closed packing for the treatment of necrotizing pancreatitis. Ann Surg. 1998;228:676–684. doi: 10.1097/00000658-199811000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradley EL., 3rd Management of infected pancreatic necrosis by open drainage. Ann Surg. 1987;206:542–550. doi: 10.1097/00000658-198710000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter CR, McKay CJ, Imrie CW. Percutaneous necrosectomy and sinus tract endoscopy in the management of infected pancreatic necrosis: an initial experience. Ann Surg. 2000;232:175–180. doi: 10.1097/00000658-200008000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connor S, Ghaneh P, Raraty M, Sutton R, Rosso E, Garvey CJ, et al. Minimally invasive retroperitoneal pancreatic necrosectomy. Dig Surg. 2003;20:270–277. doi: 10.1159/000071184. [DOI] [PubMed] [Google Scholar]
- 8.Besselink MG, van Santvoort HC, Nieuwenhuijs VB, Boermeester MA, Bollen TL, Buskens E, et al. Minimally invasive ‘step-up approach’ versus maximal necrosectomy in patients with acute necrotising pancreatitis (PANTERtrial): design and rationale of a randomised controlled multicentertrial [ISRCTN13975868] BMC Surg. 2006;6:6. doi: 10.1186/1471-2482-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradley RL, Klein MM. Pancreatic cystogastrostomy. Am J Surg. 1959;97:718–720. doi: 10.1016/0002-9610(59)90336-8. [DOI] [PubMed] [Google Scholar]
- 10.Andersson R, Cwikiel W. Percutaneous cystogastrostomy in patients with pancreatic pseudocysts. Eur J Surg. 2002;168:345–348. doi: 10.1080/11024150260284851. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs M, Reimann FM, Gaebel C, Ludwig D, Stange EF. Treatment of infected pancreatic pseudocysts by endoscopic ultrasonography-guided cystogastrostomy. Endoscopy. 2000;32:654–657. doi: 10.1055/s-2000-4660. [DOI] [PubMed] [Google Scholar]
- 12.Mori T, Abe N, Sugiyama M, Atomi Y, Way LW. Laparoscopic pancreatic cystgastrostomy. J HepatobiliaryPancreat Surg. 2000;7:28–34. doi: 10.1007/s005340050150. [DOI] [PubMed] [Google Scholar]
- 13.Baron TH, Thaggard WG, Morgan DE, Stanley RJ. Endoscopic therapy for organized pancreatic necrosis. Gastroenterology. 1996;111:755–764. doi: 10.1053/gast.1996.v111.pm8780582. [DOI] [PubMed] [Google Scholar]
- 14.Fockens P. EUS in drainage of pancreatic pseudocysts. Gastrointest Endosc. 2002;56:S93–S97. doi: 10.1016/s0016-5107(02)70095-3. [DOI] [PubMed] [Google Scholar]
- 15.Palanivelu C, Senthilkumar K, Madhankumar MV, Rajan PS, Shetty AR, Jani K, et al. Management of pancreatic pseudocyst in the era of laparoscopic surgery – experience from a tertiary centre. Surg Endosc. 2007;21:2262–2267. doi: 10.1007/s00464-007-9365-y. [DOI] [PubMed] [Google Scholar]
- 16.Hamza N, Ammori BJ. Laparoscopic drainage of pancreatic pseudocysts: a methodological approach. J Gastrointest Surg. 2010;14:148–155. doi: 10.1007/s11605-009-1048-7. [DOI] [PubMed] [Google Scholar]
- 17.Melman L, Azar R, Beddow K, Brunt LM, Halpin VJ, Eagon JC, et al. Primary and overall success rates for clinical outcomes after laparoscopic, endoscopic, and open pancreatic cystgastrostomy for pancreatic pseudocysts. Surg Endosc. 2009;23:267–271. doi: 10.1007/s00464-008-0196-2. [DOI] [PubMed] [Google Scholar]
- 18.Hindmarsh A, Lewis MP, Rhodes M. Stapled laparoscopic cystgastrostomy: a series with 15 cases. Surg Endosc. 2005;19:143–147. doi: 10.1007/s00464-004-9042-3. [DOI] [PubMed] [Google Scholar]


