Abstract
Objectives: The estimation of liver volume (LV) has been widely studied in normal liver, the density of which is considered to be equivalent to 1 kg/l. In cirrhosis, volumetric evaluation and its correlation to liver mass remain unclear. The aim of this study was to evaluate the accuracy of computed tomography (CT) scanning to assess LV in patients with cirrhosis.
Methods: Liver volume was evaluated by CT (CTLV) and correlated to the explanted liver weight (LW) in 49 patients. Liver density (LD) and its association with clinical features were analysed. Commonly used formulae for estimating LV were also evaluated. The real density of cirrhotic liver was prospectively measured in explant specimens.
Results: Wide variations between CTLV (in ml) and LW (in g) were found (range: 3–748). Cirrhotic livers in patients with hepatitis B virus infection presented significantly increased LD (P = 0.001) with lower CTLV (P = 0.005). Liver volume as measured by CT was also decreased in patients with Model for End-stage Liver Disease scores of >15 (P = 0.023). Formulae estimating LV correlated poorly with CTLV and LW. The density of cirrhotic liver measured prospectively in 15 patients was 1.1 kg/l.
Conclusions: In cirrhotic liver, LV assessed by CT did not correspond to real LW. Liver density changed according to the aetiology and severity of liver disease. Commonly used formulae did not accurately assess LV.
Introduction
Liver volume (LV) has been widely studied and its assessment has become an essential part of preoperative evaluation in many fields of hepatic surgery.1–21 Its use in the evaluation and matching of livers by size prior to liver transplantation1–7,20 and its role as a tool for predicting postoperative outcomes prior to liver resection9–21 make it an inevitable parameter in modern liver surgery.
The reference standard methods used to assess LV in vivo include computed tomography (CT) scanning and formulae based on morphological characteristics. The reliability of measurements obtained in CT has been validated in many studies.22–25 Of the various formulae based on body weight or body surface area (BSA) that have been developed,24–33 Vauthey’s formula represents the standard for use in Western countries.27,34
However, LV has been studied mainly in non-cirrhotic liver.26,30,35 Initial studies measuring LV aimed to establish a relationship between LV and morphological characteristics (body weight or BSA), and were mostly based on measurements of cadaveric healthy livers in autopsy series.26,30,35 When liver density (LD) was calculated, by dividing liver mass by LV, it was found to be very near to 1 kg/l.30 Usually, density itself was not measured and the authors presented only correlation curves showing a linear relationship between liver weight and volume.23 Since these studies, LV has been assumed to equal liver mass.23,24
In the presence of cirrhosis, the evaluation of LV and its correlation to liver mass have been poorly analysed. Clinical and surgical practice show that a cirrhotic liver may be either hypo-or hypertrophic and, in the context of extended fibrosis, the liver parenchyma may exhibit variations in density.
The primary aim of this study was to evaluate the correlation between volume measurements obtained using CT and real liver mass in patients with cirrhosis undergoing liver transplantation, and its potential variations. A secondary goal was to evaluate the applicability and accuracy of the most commonly used formulae in the setting of cirrhosis. The final goal was to prospectively measure the density of cirrhotic liver.
Materials and methods
Evaluation of the correlation between LV based on CT and liver weight using retrospective data
Patient selection
From November 2009 to February 2011 (a 16-month period), all patients undergoing liver transplantation at the study centre were registered in this study. Of the 94 patients initially selected, patients undergoing retransplantation (n = 12), patients who had undergone a previous hepatic resection (n = 7) and patients transplanted for fulminant hepatitis or acute liver failure (n = 2) were excluded.
Pre-transplant patient characteristics, such as aetiology of cirrhosis, Model for End-stage Liver Disease (MELD) score and Child–Pugh class, were recorded. In addition, morphological characteristics were collected, including weight, height, body mass index (BMI), and BSA (calculated from weight and height using the Mosteller formula).36
All of the data collected referred to those valid at the time of entry to the waiting list so that the time periods that elapsed between the measurements of the various parameters were equivalent across the patient cohort.
Explant weighing and pathological characteristics
Explanted cirrhotic livers were weighed at the time of transplantation by pathology staff; other pathological characteristics, such as parenchyma micro-or macronodular architecture, the presence and degree of steatosis, cholestasis or haemosiderin deposition, were noted. Pathology data for three patients were unavailable; these patients were excluded from the study.
Volume assessment by CT scan
In each patient, LV was measured in a pre-transplant abdominal CT scan performed at the time of entry to the waiting list. This volumetric measurement was obtained in all patients by the same member of the surgical team, who had been previously trained in the study centre’s radiology department by a radiologist experienced in hepatobiliary radiology.
On each CT slice, the liver shape was delineated manually using the mouse; the gallbladder, attached ligaments, vena cava and portal structures were excluded. Semi-automatic ADW 4.5 GE software (GE Healthcare, Little Chalfont, UK) was used to calculate the total LV on 3 mm – thick slices.
Pre-transplant CT scans were not available for 21 patients because these patients had been assessed outwith the study centre; these patients were excluded from analysis.
Estimation of LV using formulae based on morphological characteristics
Seven recognized formulae were selected and applied in each patient; results were then compared with LV measurements obtained using CT scans. The formulae tested were taken from the work of Schiano et al., Urata et al., Heinemann et al., Vauthey et al., Yoshizumi et al., Chan et al. and DeLand and North.24–28,31,35 Volume was calculated using either body weight (Schiano et al., Vauthey et al., Chan et al.24,27,31) or BSA (Urata et al., Heinemann et al., Yoshizumi et al., DeLand and North25,26,28,35).
Evaluation of LD in cirrhotic livers in a prospective cohort
From December 2011 to June 2012, 48 patients underwent liver transplantation at the study centre. Most of the following data were collected prospectively at the time of transplantation: patient age and morphological characteristics (weight, BMI, BSA), aetiology of cirrhosis and MELD score. Immediately after liver explantation, the explant was weighed in the operating room and its volume was measured precisely using the 25 °C water displacement technique before any fixative was applied for the pathology examination. Patients undergoing retransplantation or transplantation for fulminant hepatitis were excluded.
A total of 15 patients were included in this second part of the study.
Liver density was calculated from the weights and volumes of explants.
Statistical analysis
Descriptive and comparative statistical analyses were realized using SPSS Version 20 (SPSS, Inc., IBM Corp., Armonk, NY, USA).
The correlation between CT-assessed LV (CTLV) and liver weight (LW) was assessed by linear regression. Liver density was calculated from CTLV and LW (LD = LW/CTLV).
The correlations between LVs calculated by the different formulae and CTLV were also assessed using linear regression.
Non-parametric tests (Mann–Whitney U-test) were used to analyse the variations in CTLV and LD depending on the aetiology of cirrhosis, pathological features and MELD score.
Results
Part 1: evaluation in a retrospective cohort (n = 49)
Population characteristics
A total of 49 patients who underwent a first liver transplantation between November 2009 and February 2011 at the study centre were included in the study. A flow chart of these patients is displayed in Fig. 1.
Figure 1.
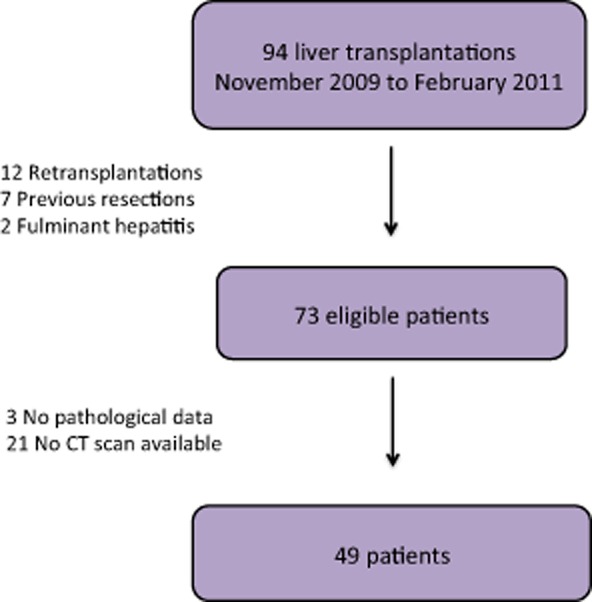
Flow chart showing patients enrolled in part 1 of the study. CT, computed tomography
Patient characteristics are represented in Table 1.
Table 1.
Characteristics of the 49 patients analysed retrospectively
| Characteristic | |
|---|---|
| Age, years, median (range) | 56 (15–69) |
| Gender, n (%) | |
| Male | 31 (63.3%) |
| Female | 18 (36.7%) |
| Median BSA, m2, median (range) | 1.84 (1.37–2.22) |
| Body weight, kg, median (range) | 70 (45–105) |
| BMI, kg/m2, median (range) | 24 (17–36) |
| Aetiology, n (%) | |
| Alcohol | 23 (46.9%) |
| Hepatitis C virus infection | 18 (36.7%) |
| Hepatitis B virus infection | 5 (10.2%) |
| Biliary diseases | 8 (16.3%) |
| NASH | 5 (10.2%) |
| MELD score, median (range) | 15 (6–40) |
| Time between CT scan and LT, days. median (range) | 122 (1–549) |
BSA, body surface area; BMI, body mass index; NASH, non-alcoholic steatohepatitis; MELD, Model for End-stage Liver Disease; CT, computed tomography; LT, liver transplant.
Correlation between explant weight and CTLV
Results are displayed in Table 2. The median CTLV was 1298 ml (range: 530–3126 ml); mean CTLV was 1394 ± 490 ml. Median LW was 1300 g (range: 600–2500 g); mean LW was 1321 ± 437 g. The median difference between CTLV (in ml) and LW (in g) was 145, corresponding to a mean variation of 14% (range: 0.4–38.4%) between the two values. This variation between CTLV and LW was >20% in 14 of the 49 (28.6%) patients and >10% in 26 of the 49 (53.1%) patients (Fig. 2).
Table 2.
Liver volume measured by computed tomography (CTLV) and liver weight (LW) in 49 patients
| Value | |
|---|---|
| CT liver volume, ml, median (range) | 1298 (530–3126) |
| Liver weight, g, median (range) | 1300 (600–2500) |
| Difference between CTLV (in ml) and liver weight (in g), median (range) | 145 (3–748) |
| Liver density, kg/1, median (range) | 0.92 (0.7–1.6) |
Figure 2.
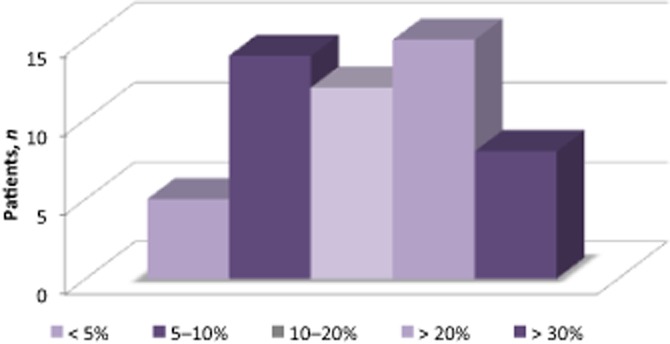
Variation between computed tomography liver volume (CTLV) and liver weight, %
Correlation between CTLV and LV estimated by formulae
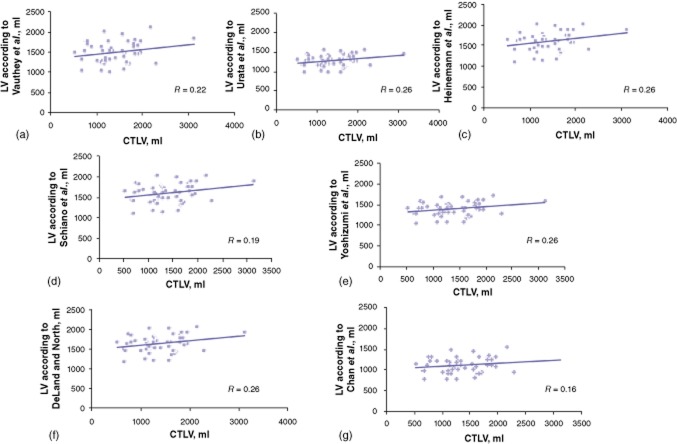
Correlation curves for CTLV and LV measurements obtained using the various formulae are displayed in Fig. 3.
Figure 3.
Correlations between computed tomography liver volume (CTLV) and liver volume (LV) estimated according to seven commonly used formulae described by (a) Vauthey et al., (b) Urata et al., (c) Heinemann et al., (d) Schiano et al., (e) Yoshizumi et al., (f) DeLand and North and (g) Chan et al
None of the seven formulae applied showed a satisfactory correlation with CTLV or LW. The correlation coefficients ranged from 0.16 (Chan et al. formula31) to 0.26 (Heinemann et al., Yoshizumi et al., DeLand and North formulae26,28,35).
Variations in CTLV and LD according to aetiology of cirrhosis and severity of liver disease
In cirrhotic liver, the median estimated LD was 0.92 kg/l (range: 0.7–1.6 kg/l); mean LD was 0.97 ± 0.18 kg/l (Table 3).
Table 3.
Liver volume measured by computed tomography (CTLV), liver weight and liver density in 49 patients according to aetiology of cirrhosis and Model for End-stage Liver Disease (MELD) score
| CTLV, ml, median (range) | Liver weight, g, median (range) | Liver density, kg/1, median (range) | |
|---|---|---|---|
| All patients (n = 49) | 1298 (530–3126) | 1300 (600–2500) | 0.92 (0.70–1.60) |
| Alcohol-related cirrhosis (n = 23) | 1273 (676–2158) | 1300 (600–2100) | 0.92 (0.72–1.62) |
| HCV infection (n = 18) | 1437 (730–2298) | 1425 (700–2000) | 0.92 (0.72–1.34) |
| HBV infection (n = 5) | 757 (530–1202)a | 830 (600–1950) | 1.11 (1.06–1.62)a |
| NASH (n = 5) | 1807 (797–3126) | 1600 (800–2500) | 0.88 (0.79–1.00) |
| Biliary diseases (n = 8) | 1628 (684–1963) | 1550 (600–1900) | 0.90 (0.79–0.97) |
| MELD score > 15 (n = 18) | 1198 (530–2146)a | 1100 (600–1950)a | 0.98 (0.72–1.16) |
P < 0.050, Mann–Whitney U-test.
HCV, hepatitis C virus; HBV, hepatitis B virus; NASH, non-alcoholic steatohepatitis.
Median MELD scores did not differ statistically significantly among the different aetiologies [alcohol-related, hepatitis B virus (HBV) infection, HCV infection, biliary diseases, non-alcoholic steatohepatitis (NASH)].
In HBV-infected patients with cirrhosis (n = 5), median CTLV was significantly lower than in patients without HBV infection (757 ml versus 1370 ml; P = 0.005) and LD was significantly increased (1.11 kg/l versus 0.91 kg/l; P = 0.001). In patients with NASH (n = 5), CTLV tended to be higher (1807 ml versus 1293 ml; P = 0.211) and LD lower (0.88 kg/l versus 0.94 kg/l; P = 0.223) than in non-NASH patients, but these differences did not reach statistical significance. The same trend was observed for CTLV in patients with and without biliary diseases (1628 ml versus 1275 ml; P = 0.244). In the NASH group, BMI, BSA and patient weight were significantly increased in comparison with those in patients with the other aetiologies (median BMI: 30.5 kg/m2 versus 23.6 kg/m2; P < 0.001); in consequence, LVs calculated using the various formulae were all significantly increased (data not shown).
Patients presenting with a MELD score of >15 had a significantly lower CTLV compared with patients with MELD scores of ≤15 (1198 ml versus 1572 ml; P = 0.023).
Part 2: evaluation of cirrhotic LD in a prospective cohort (n = 15)
Patient characteristics are presented in Table 4. Of the 15 patients, seven (46.7%) had alcohol-related cirrhosis, four (26.7%) had biliary diseases, three (20.0%) had HCV cirrhosis, and one (6.7%) had NASH. The median MELD score was 12 (range: 8–20). The calculated median LD of cirrhotic liver was 1.09 kg/l (range: 1.07–1.14 kg/l).
Table 4.
Characteristics of the prospective patient cohort (n = 15)
| Characteristic | Value |
|---|---|
| Median age, years, median (range) | 60 (41–69) |
| Gender, n (%) | |
| Male | 12 (80.0%) |
| Female | 3 (20.0%) |
| BMI, kg/m2, median (range) | 23 (17–33) |
| Aetiology, n (%) | |
| Alcohol-related | 7 (46.7%) |
| Hepatitis C virus infection | 3 (20.0%) |
| Hepatitis B virus infection | 0 |
| Biliary diseases | 4 (26.7%) |
| NASH | 1 (6.7%) |
| MELD score, median (range) | 12 (8–20) |
| Liver weight, g, median (range) | 1479 (805–1985) |
| Liver volume, ml, median (range) | 1370 (750–1800) |
| Liver density, kg/1, median (range) | 1.09 (1.07–1.14) |
BMI, body mass index; NASH, non-alcoholic steatohepatitis; MELD, Model for End-stage Liver Disease.
Discussion
This study revealed the discrepancy between the real weight of cirrhotic liver and cirrhotic liver volume estimated according to CT scans. Wide variations were found between these two values: variations were found to exceed 30% in one third of patients and to exceed 20% in half of the patient population. Obviously, the volume of cirrhotic liver is not equivalent to its mass. Moreover, the formulae usually applied to estimate LV based on morphological characteristics were correlated to neither LV according to CT evaluation nor LW. Overall, the present experience indicates that the standard methods used to evaluate LV in patients with cirrhosis are not reliable. Finally, the prospective evaluation performed in the current study showed that LD was 1.1 kg/l, which differs from the 1 kg/l usually reported for LD in normal liver.
A first main finding of this study was a significant variation in LV according to both the aetiology and severity of cirrhosis. Cirrhotic livers are known to present wide variations in volume and weight.37–41 Indeed, previous studies found that livers affected by alcohol-related cirrhosis tend to be hypertrophic,24,41 and livers affected by HBV infection tend to be hypotrophic.24,39 These changes may be related to the fibrotic process resulting from chronic inflammation. The present results confirmed that HBV cirrhotic livers were significantly lower in volume compared with livers with cirrhosis of other aetiologies. Interestingly, this atrophy was also found in severely cirrhotic livers in patients with MELD scores of >15 whatever the aetiology. This latter result highlights the relationship between the severity of cirrhosis and the morphological changes usually seen in advanced liver disease. Although the methodology used to assess density could be considered questionable (LV assessed by CT scan and not measured by water displacement), the present study shows that LD was significantly increased in HBV cirrhosis, confirming that the volume of a liver is not equivalent to its mass. This latter finding led to the prospective measurement of LD using the water displacement technique. In this series, the volumetric assessment of cirrhotic liver did not correspond to the quantity of parenchyma and consequently represents a source of under-or overestimation. Interestingly, patients with biliary diseases and NASH-related cirrhosis tended to have higher LVs. This hypertrophy observed in ‘surcharge’ diseases (fatty surcharge in NASH, or cholestatic surcharge in biliary diseases) may reflect a pathogenesis that differs from that in primarily hepatocellular diseases (alcohol-related and viral hepatitis).
A second significant finding was the prospective confirmation that LD differs between patients with and without cirrhosis. In fact, the complementary measurement of exact LD in patients with cirrhosis using water displacement revealed a median value of 1.1 kg/l. Previous studies conducted on cadaveric healthy livers or in living donors in order to evaluate volumetric assessment (by CT scan or formulae) have always considered LD to be 1 kg/l. Few studies have consistently calculated LD. Two of these studies measured the density of cadaveric healthy livers and found densities of, respectively, 1.04 kg/l (Yu et al., 24 autopsies)30 and 1.08 kg/l (Heinemann et al., 33 autopsies).26 However, postmortem tissue changes make these data very difficult to interpret. The study that most reliably determined LD in healthy livers was conducted by Fu-Gui et al. and based on 115 right lobe grafts from living donors measured before implantation.33 Liver density was found to be 1.001 kg/l. Finally, LD in cirrhotic livers has been poorly investigated. Indeed, Van Thiel et al.23 reported findings in 99 explanted livers in which volume and weight were measured during transplantation and which demonstrated a linear relationship between LW and LV (R = 0.99). However, the authors made no calculation of density and the liver diseases were mostly (>70%) not ‘primarily hepatocellular’ (viral or alcoholic) diseases.23 To the present authors’ knowledge, the current study reports the first prospective measurement of LD in cirrhotic livers.
These results may have important implications in clinical practice. Indeed, short-term outcomes after liver resection are related to the quantity of parenchyma retained [i.e. the future liver remnant (FLR)].12–21 The popularized ‘standardized FLR’ is calculated from a CT measurement of the FLR and ‘standardized total liver volume (TLV)’ which is calculated using the BSA-based formula: FLR (%) = (FLR volume from CT)/(TLV from formula).19 Cut-off FLR values have been estimated in order to avoid postoperative liver failure, allowing liver resections to be safer. In normal liver, Truant et al. proposed a cut-off value of ≥0.5% for the ratio of FLR to body weight, which appeared to be more specific and sensitive in predicting postoperative course after extended hepatectomy than the ratio of FLR to TLV.42 Finally, in the setting of liver transplantation, the amount of liver parenchyma required for adequate postoperative liver function is based on the ratio of LW to body weight. The optimal liver quantity needed represents about 1% of body weight. The MD Anderson Cancer Center team proposed three FLR benchmarks for safe liver resection: >20% of TLV in patients with normal liver; >30% of TLV in patients with diseased liver, and >40% of TLV in patients with (well-compensated) cirrhosis.14,18,21 All these measurements are based on LV or were determined only in healthy liver. The present study showed that these values may not be reliable or representative of the real quantity and quality of the parenchyma in patients with cirrhosis. Consequently, volumetric discrepancies that are related to aetiology and severity may represent a source of error in the evaluation of the FLR. As in liver transplantation and liver resection of normal liver, LW rather than LV should be considered and the fact that the mass of a cirrhotic liver is 1.1 times higher than its volume should be noted. This refines the evaluation of hepatic function in cirrhosis, which depends on both the quantity and quality of the parenchyma, and thus introduces LD as an important, aetiology-related parameter with which to achieve a better evaluation of liver functional capacity in cirrhosis.
Conflicts of interest
None declared.
References
- 1.Yanaga K, Honda H, Ikeda Y, Nishizaki AT, Yamamoto K, Sugimachi K. Significance of liver size in hepatic surgery. HBP Surg. 1997;10:195–200. doi: 10.1155/1997/34842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kawasaki S, Makuuchi M, Ishizone S, Matsunami H, Terada M, Kawarazaki H. Liver regeneration in recipients and donors after transplantation. Lancet. 1992;339:580. doi: 10.1016/0140-6736(92)90867-3. [DOI] [PubMed] [Google Scholar]
- 3.Kawasaki S, Makuuchi M, Matsunami H, Hashikura Y, Ikegami T, Chisuwa H, et al. Preoperative measurement of segmental liver volume of donors for living related liver transplantation. Hepatology. 1993;18:1115–1120. [PubMed] [Google Scholar]
- 4.Kawasaki S, Makuuchi M, Matsunami H, Hashikura Y, Ikegami T, Nakazawa Y, et al. Living related liver transplantation in adults. Ann Surg. 1998;227:269–274. doi: 10.1097/00000658-199802000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiuchi T, Kasahara M, Uryuhara K, Inomata Y, Uemoto S, Asonuma K, et al. Impact of graft size mismatching on graft prognosis in liver transplantation from living donors. Transplantation. 1999;67:321–327. doi: 10.1097/00007890-199901270-00024. [DOI] [PubMed] [Google Scholar]
- 6.Lo CM, Fan ST, Liu CL, Chan JK, Lam BK, Lau GK, et al. Minimum graft size for successful living donor liver transplantation. Transplantation. 1999;68:1112–1116. doi: 10.1097/00007890-199910270-00009. [DOI] [PubMed] [Google Scholar]
- 7.Sakamoto S, Uemoto S, Uryuhara K, Kim I, Kiuchi T, Egawa H, et al. Graft size assessment and analysis of donors for living donor liver transplantation using right lobe. Transplantation. 2001;71:1407–1413. doi: 10.1097/00007890-200105270-00009. [DOI] [PubMed] [Google Scholar]
- 8.Leelaudomlipi S, Sugawara Y, Kaneko J, Matsui Y, Ohkubo T, Makuuchi M. Volumetric analysis of liver segments in 155 living donors. Liver Transpl. 2002;8:612–614. doi: 10.1053/jlts.2002.33731. [DOI] [PubMed] [Google Scholar]
- 9.Okamoto E, Kyo A. Prediction of the safe limits of hepatectomy by combined volumetric and functional measurements in patients with impaired hepatic function. Surgery. 1984;95:586–592. [PubMed] [Google Scholar]
- 10.Soyer P, Roche A, Elias D, Levesque M. Hepatic metastases from colorectal cancer: influence of hepatic volumetric analysis on surgical decision making. Radiology. 1992;184:695–697. doi: 10.1148/radiology.184.3.1509051. [DOI] [PubMed] [Google Scholar]
- 11.Ogasawara K, Une Y, Nakajima Y, Uchino J. The significance of measuring liver volume using computed tomographic images before and after hepatectomy. Surg Today. 1995;25:43–48. doi: 10.1007/BF00309384. [DOI] [PubMed] [Google Scholar]
- 12.Kubota K, Makuuchi M, Kusaka K, Kobayashi T, Miki K, Hasegawa K, et al. Measurement of liver volume and hepatic functional reserve as a guide to decision making in resectional surgery for hepatic tumours. Hepatology. 1997;26:1176–1181. doi: 10.1053/jhep.1997.v26.pm0009362359. [DOI] [PubMed] [Google Scholar]
- 13.Shirabe K, Shimada M, Gion T, Hasegawa H, Takenaka K, Utsunomiya T, et al. Postoperative liver failure after major hepatic resection for hepatocellular carcinoma in the modern era with special reference to remnant liver volume. J Am Coll Surg. 1998;188:304–309. doi: 10.1016/s1072-7515(98)00301-9. [DOI] [PubMed] [Google Scholar]
- 14.Vauthey JN, Chaoui A, Do KA, Bilimoria MM, Fenstermacher MJ, Charnsangavej C, et al. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical association. Surgery. 2000;127:512–519. doi: 10.1067/msy.2000.105294. [DOI] [PubMed] [Google Scholar]
- 15.Shoup M, Gonen M, D’Angelica M, Jarnagin WR, DeMatteo RP, Schwartz LH, et al. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg. 2003;7:325–330. doi: 10.1016/s1091-255x(02)00370-0. [DOI] [PubMed] [Google Scholar]
- 16.Abdalla EK, Denys A, Chevalier P, Nemr RA, Vauthey JN. Total and segmental liver volume variations: implications for liver surgery. Surgery. 2004;135:404–410. doi: 10.1016/j.surg.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 17.Chun YS, Ribero D, Abdalla EK, Madoff DC, Mortenson MM, Wei SH, et al. Comparison of two methods of future liver remnant volume measurement. J Gastrointest Surg. 2008;12:123–128. doi: 10.1007/s11605-007-0323-8. [DOI] [PubMed] [Google Scholar]
- 18.Kishi Y, Abdalla EK, Chun YS, Zorzi D, Madoff DC, Wallace MJ, et al. Three hundred and one consecutive extended right hepatectomies. Evaluation of outcomes based on systematic liver volumetry. Ann Surg. 2009;250:540–548. doi: 10.1097/SLA.0b013e3181b674df. [DOI] [PubMed] [Google Scholar]
- 19.Ribeiro D, Chun YS, Vauthey JN. Standardized liver volumetry for portal vein embolization. Sem Interv Radiol. 2008;25:104–109. doi: 10.1055/s-2008-1076681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Redvanly RD, Nelson RC, Stieber AC, Dodd GD., 3rd Imaging in the preoperative evaluation of adult liver transplant candidates: goals, merits of various procedures, and recommendations. AJR Am J Roentgenol. 1995;164:611–617. doi: 10.2214/ajr.164.3.7863881. [DOI] [PubMed] [Google Scholar]
- 21.Abdalla EK, Adam R, Bilchik AJ, Jaeck D, Vauthey JN, Mahvi D. Improving resectability of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol. 2006;13:1271–1280. doi: 10.1245/s10434-006-9045-5. [DOI] [PubMed] [Google Scholar]
- 22.Heymsfield SB, Fulenwider T, Nordlinger B, Barlow R, Sones P, Kutner M. Accurate measurement of liver, kidney, and spleen volume mass by computerized axial tomography. Ann Intern Med. 1979;90:185–187. doi: 10.7326/0003-4819-90-2-185. [DOI] [PubMed] [Google Scholar]
- 23.Van Thiel DH, Hagler NG, Schade RR, Skolnick ML, Heyl AP, Rosenblum E, et al. In vivo hepatic volume determination using sonography and computed tomography. Gastroenterology. 1985;88:1812–1817. doi: 10.1016/0016-5085(85)90005-8. [DOI] [PubMed] [Google Scholar]
- 24.Schiano TD, Bodian C, Schwartz ME, Glajchen N, Min AD. Accuracy and significance of computed tomographic scan assessment of hepatic volume in patients undergoing liver transplantation. Transplantation. 2000;69:545–550. doi: 10.1097/00007890-200002270-00014. [DOI] [PubMed] [Google Scholar]
- 25.Urata K, Kawasaki S, Matsunami H, Hashikura Y, Ikegami T, Ishizone S, et al. Calculation of child and adult standard liver volume for liver transplantation. Hepatology. 1995;21:1317–1321. [PubMed] [Google Scholar]
- 26.Heinemann A, Wischhusen F, Püschel K, Rogiers X. Standard liver volume in the Caucasian population. Liver Transpl. 1999;5:366–368. doi: 10.1002/lt.500050516. [DOI] [PubMed] [Google Scholar]
- 27.Vauthey JN, Abdalla EK, Doherty DA, Gertsch P, Fenstermacher MJ, Loyer EM, et al. Body surface area and body weight predict total liver volume in Western adults. Liver Transpl. 2002;8:233–240. doi: 10.1053/jlts.2002.31654. [DOI] [PubMed] [Google Scholar]
- 28.Yoshizumi T, Gondolesi GE, Bodian CA, Jeon H, Schwartz ME, Fishbein TM, et al. A simple new formula to assess liver weight. Transpl Proc. 2003;35:1415–1420. doi: 10.1016/s0041-1345(03)00482-2. [DOI] [PubMed] [Google Scholar]
- 29.Choukèr A, Martignoni A, Dugas M, Eisenmenger W, Schauer R, Kaufmann I, et al. Estimation of liver size for liver transplantation: the impact of age and gender. Liver Transpl. 2004;10:678–685. doi: 10.1002/lt.20113. [DOI] [PubMed] [Google Scholar]
- 30.Yu HC, You H, Lee H, Jin ZW, Moon JI, Cho BH. Estimation of standard liver volume for liver transplantation in the Korean population. Liver Transpl. 2004;10:779–783. doi: 10.1002/lt.20188. [DOI] [PubMed] [Google Scholar]
- 31.Chan SC, Liu CL, Lo CM, Lam BK, Lee EW, Wong Y, et al. Estimating liver weight of adults by body weight and gender. World J Gastroenterol. 2006;12:2217–2222. doi: 10.3748/wjg.v12.i4.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hashimoto T, Sugawara Y, Tamura S, Hasegawa K, Kishi Y, Kokudo N, et al. Estimation of standard liver volume in Japanese living liver donors. J Gastroenterol Hepatol. 2006;21:1710–1713. doi: 10.1111/j.1440-1746.2006.04433.x. [DOI] [PubMed] [Google Scholar]
- 33.Fu-Gui L, Lu-Nan Y, Bo L, Yong Z, Tian-Fu W, Ming-Qing X, et al. Estimation of standard liver volume in Chinese adult living donors. Transpl Proc. 2009;41:4052–4056. doi: 10.1016/j.transproceed.2009.08.079. [DOI] [PubMed] [Google Scholar]
- 34.Johnson TN, Tucker GT, Tanner MS, Rostami-Hodjegan A. Changes in liver volume from birth to adulthood: a meta-analysis. Liver Transpl. 2005;12:1481–1493. doi: 10.1002/lt.20519. [DOI] [PubMed] [Google Scholar]
- 35.DeLand FH, North WA. Relationship between liver size and body size. Radiology. 1968;91:1195–1198. doi: 10.1148/91.6.1195. [DOI] [PubMed] [Google Scholar]
- 36.Mosteller RD. Simplified calculation of body surface area. N Engl J Med. 1987;317:1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 37.Ludwig J, Elveback LR. Parenchyma weight changes in hepatic cirrhosis. Lab Invest. 1972;26:338–343. [PubMed] [Google Scholar]
- 38.Matsui Y, Tu W, Kitade H, Nakagawa A, Kamiya T, Kwon AH, et al. Hepatocyte volume as an indicator of hepatic functional reserve in cirrhotic patients with liver tumours. J Gastroenterol Hepatol. 1996;11:540–545. doi: 10.1111/j.1440-1746.1996.tb01699.x. [DOI] [PubMed] [Google Scholar]
- 39.Li WX, Zhao XT, Chai WM, Zhu NY, Du LJ, Huang W, et al. Hepatitis B virus-induced liver fibrosis and cirrhosis: the value of liver and spleen volumetry with multidetector spiral CT. J Dig Dis. 2010;11:215–223. doi: 10.1111/j.1751-2980.2010.00441.x. [DOI] [PubMed] [Google Scholar]
- 40.Honda H, Onitsuka H, Masuda K, Nishitani H, Nakata H, Watanabe K. Chronic liver disease: value of volumetry of liver and spleen with computed tomography. Radiat Med. 1990;8:222–226. [PubMed] [Google Scholar]
- 41.Lin XZ, Sun YN, Liu YH, Sheu BS, Cheng BN, Chen CY, et al. Liver volume in patients with or without chronic liver diseases. Hepatogastroenterology. 1998;45:1069–1074. [PubMed] [Google Scholar]
- 42.Truant S, Oberlin O, Sergent G, Lebuffe G, Gambiez L, Ernst O, et al. Remnant liver volume to body weight ratio ≥0.5%: a new cut-off to estimate postoperative risks after extended resection in non-cirrhotic liver. J Am Coll Surg. 2007;204:22–33. doi: 10.1016/j.jamcollsurg.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 43.Carles J, El Kohen J, Midy D, Saric J, Videu J. Estimation of liver volume as a function of the individual’s morphology. Bull Assoc Anat (Nancy) 1993;77:9–13. [PubMed] [Google Scholar]