Abstract
Background
Some studies report neurobehavioral symptoms in neonates exposed to serotonin reuptake inhibitors (SRIs) in utero. However, maternal psychiatric illness during the last trimester of pregnancy, as a confounding factor, has not always been assessed.
Aims
In this prospective study we compared neurobehavioral complications among neonates who were born to euthymic women who either took or did not take an SRI during the last trimester of pregnancy.
Study design
Exposed and unexposed infants were assessed for: 1) temperament as measured by the Neonatal Behavioral Assessment Scale (NBAS); 2) activity via Actiwatch electronic monitoring; 3) sleep state using trained observer ratings; and 4) perinatal complications through medical record review. T-tests, Fisher's exact tests, and analyses of covariance were used to assess the relationship between clinical and neurobehavioral factors and exposure status.
Subjects
67 infants (61 controls and 6 exposed to SRIs).
Outcome measures
Neonatal Assessment Behavioral Scale, APGAR scores, infant sleep state (% sleep, % wakeful), startles and tremulousness, gestational age, birth weight, and head circumference.
Results
Infants exposed to SRIs in the third trimester had poorer motor development, lower 5-minute APGAR scores, and shorter mean gestational age as compared to unexposed infants.
Conclusion
Results of this study show differences in autonomic and gross motor activity between neonates who were or were not exposed to SRIs in utero after controlling for active maternal psychiatric illness. Future longitudinal work should compare longer term outcomes of exposed and unexposed infants of depressed mothers.
Keywords: Selective serotonin reuptake inhibitors, Perinatal depression
1. Introduction
Approximately 13% of pregnant women take an antidepressant, typically a serotonin reuptake inhibitor (SRI), to either treat an ongoing psychiatric disorder or prevent recurrence of a mood or anxiety disorder [1]. Thus, the safety of antidepressants in pregnancy and effects on infants is an issue of high priority. Several studies have reported that SRIs are associated with a “perinatal syndrome” in the neonate that may result from either serotonergic overstimulation [2] or withdrawal of the serotonergic agent [3–7]. In these studies, complications found at a higher rate among SRI-exposed compared to non-SRI exposed neonates include lower APGAR scores [2,8,12], respiratory distress [4], restlessness and tremor [2,3,9], rigidity, increased muscle tone [2,10] and startle [3,9]. Studies that have used biobehavioral measures to assess infant function are fewer in number and few have controlled for maternal illness that may confound outcomes. The few studies that included data on the use of serotonin reuptake inhibitors as well as psychiatric diagnoses were either small [11,12] or used registry data for diagnoses [13]. The present study expands upon the existing literature through an in depth examination of the sleep, activity, and temperament of infants exposed to in utero SRI and compares it to the infants not exposed to SRIs in utero.
The aim of this report was to determine whether there is a higher rate of neurobehavioral complications among offspring of euthymic women exposed to selective serotonin reuptake inhibitors (SRIs) during the third trimester of pregnancy compared to offspring who were neither exposed to major depressive disorder nor SRIs during pregnancy. We hypothesized that offspring exposed to SRIs during the third trimester would have a higher rate of motor activity and symptoms of serotonin hyperactivity (tremulousness, startles) and fewer (sleep/awake) state changes than controls with neither exposure.
2. Method
2.1. Recruitment and assessment procedures
Subjects in this analysis were eligible infants born to women enrolled in the Yale Pink and Blue Study, a longitudinal cohort study investigating the effects of depression and antidepressant treatment on birth outcomes. Pregnant women were recruited from 137 clinicians' offices or hospital-based clinics in Connecticut and Western Massachusetts between 2004 and 2008. Respondents were screened by research staff who obtained consent. A structured screening questionnaire collected information about pregnancy dates, mood and antidepressant treatment. We invited women to participate in the larger Pink and Blue Study if they reported depressed mood or treatment for major depressive disorder within the prior five years or experienced a trauma and suffered from flashbacks. We also randomly selected one of every three women who had none of these experiences to participate as “non-exposed” controls.
Mothers were administered selected modules from the World Mental Health Composite International Diagnostic Interview v2.1 (WMH-CIDI) [14], that assessed major depressive disorder (MDD), generalized anxiety disorder (GAD) or panic disorder. The CIDI is a valid and reliable lay interview instrument which was administered at two points in pregnancy, before 17 weeks, at 28 weeks (+/− 2 weeks), and once at six weeks (+/− 2 weeks) postpartum. A history or current episode of post traumatic stress disorder (PTSD) was diagnosed from the PTSD module of the Mini International Neuropsychiatric Interview 5.0 (MINI) [15]. Depressive symptoms were assessed using the Edinburgh Postnatal Depression Scale (EPDS) [16]. The interviews captured information about antidepressant and other pharmacotherapy monthly during pregnancy. Mothers were asked to provide the medication bottle to the interviewer such that type of medication and dosage could be accurately recorded. We obtained confirmation of antidepressant medication name and dosage by the woman's treating physician when possible. Also collected were data about medical complications, use of alcohol and or drugs, race/ethnicity and socioeconomic status. Mothers were recruited during pregnancy to obtain accurate prospective exposure information and to allow for an infant evaluation that would occur after delivery. Written consent was obtained during a face-to-face interview that was conducted before 17 completed weeks of pregnancy and again at the time of delivery for the evaluation of the infant. Approval for the study was obtained from the Human Investigation Committee at Yale University School of Medicine and from Baystate Medical Center.
Infants were eligible to participate in the study on infant outcomes of SRI exposure if: 1.) the mother was not depressed in pregnancy yet was taking an SRI in the 3rd trimester (exposed group); 2.) the mother was neither depressed nor taking antidepressant medications in pregnancy (non exposed group). Depression status in the mother was determined based upon diagnosis of a major depressive episode in pregnancy (by trimester) according to the CIDI. The one month time period of antidepressant use was stipulated because the mechanism of action of antidepressants could evolve over a period of days to weeks to ensure that the drug reached its full pharmacologic potential, we limited mothers who were considered exposed to those who had taken SRIs for at least one month in the last trimester of pregnancy. We restricted enrollment to infants born at Yale New Haven Hospital or Baystate Medical Center since these sites were of easy access to the study team. Women were ineligible if they did not speak either English or Spanish or delivered before 37 weeks gestation. This restriction was used because the infant behavioral assessment, the Neonatal Behavioral Assessment Scale (NBAS), had not yet been widely validated for use in neonates born prior to 37 weeks [17]. Women who regularly used antipsychotic agents during the last trimester as well as benzodiazepine anxiolytics or drugs of abuse were excluded since this can confound behavioral assessments. Also excluded were infants born to mothers who required general anesthesia for delivery.
2.2. Infant assessment
After delivery, a trained assessor who was blind to maternal antidepressant use evaluated neonates. Neonates were assessed at approximately 24 h age (+/− 8 h) post-delivery so that maternal anesthesia would not complicate evaluations. Due to the lack of availability of study personnel, infants were not assessed on weekends. We utilized methods outlined by Zeskind and Stephens [3] and Laine et al. [2] that included use of: (1) a personal digital assistant device (PDA) tomonitor sleep state; (2) an Actiwatch activity monitor to evaluate activity and (3) the Neonatal Behavioral Assessment Scale (NBAS) [17] to assess the infant's response to the environment. All infants were assessed in an open crib in a dimly lit room by study staff blind to the infant and mother's exposure status. Specifics on each method of assessment are outlined below.
2.2.1. Neonatal Behavioral Assessment Scale (NBAS)
Developed in 1973, the NBAS is used to evaluate neurobehavioral outcomes in the infant. The NBAS measures 28 behavioral and 18 reflex items across a broad range of behaviors including reflexes, state changes, attention, arousal, and regulatory capacities. Four research staff (PhD and master level nurses and clinical researchers) were trained and certified by the Brazelton Institute to administer the NBAS. The NBAS was typically administered during the second day after delivery. Individual item and cluster scores based on the scoring method developed by Brazelton et al. [18] were compared for exposed and unexposed infants.
2.2.2. Infant sleep state
Bachelor and master level research staff was trained over a three day period by a study investigator (L.M.) with expertise in neurobehavioral assessment. The training involved each rater satisfactorily completing observations on three infants and achieving excellent interrater reliability with study personnel on an additional three infants. Sleep was assessed using a handheld personal digital assistant (PDA) device, using Hand dB database software, version 4.1.5 (DDH Software, Wellington, FL). Infants were monitored in 30 second epochs over the course of 25 min to 1 h for tremulousness (on a 3-point scale), startle (total number) and changes of state (on a 6-point scale according to the NBAS classification system). All data were recorded on the PDA device with the option to pause and resume assessment if significant disturbance to the environment occurred (e.g. entrance of a healthcare provider). Data were exported directly from Hand dB into SAS statistical software. One of the four raters performed 79% of the infant sleep observations, including those for all of the exposed infants. Among the control infants, the raters did not differ significantly in their assessments of sleep state or tremulousness (p-values >0.05) but did differ in assessment of average startles per minute (p = 0.03).
2.5. Infant motor activity
Infant motor activity was measured for 30 to 60 min using infant activity monitors, Minimeter Actiwatch model number AW-64, placed on the infant's ankle. The Actiwatch device provided information on percent of sleep and wake states along with the number of wake bouts during the 1-hour observation period.
2.6. Medical record review
Medical records were reviewed and information on other infant exposures and APGAR scores was abstracted by trained medical record reviewers.
2.7. Statistical methods
Analyses were performed using SAS 9.1 (SAS Institute Inc., Cary, NC). Mothers without depression in pregnancy and infants exposed to SRI use for more than one month in their third trimester were compared to mothers without depression and infants without SRI exposure at any time in pregnancy. Fisher's exact test for categorical variables and Student's t-test for continuous variables were used to compare the exposed and unexposed groups with statistically significant differences defined by a p-value of <0.05. Key infant characteristics examined included gestational age, birth weight, length, head circumference, APGAR scores (1 and 5 min), and admission to the neonatal intensive care unit. Additional infant outcome measures included NBAS scores, the degree of neonatal startles and tremulousness per minute, and mean length of intervals between two startles and two tremors as captured by observations with the PDA device. Potential confounding of infant neurobehavioral outcomes by gestational age at birth was assessed using analysis of covariance.
3. Results
3.1. Study recruitment and selection statistics
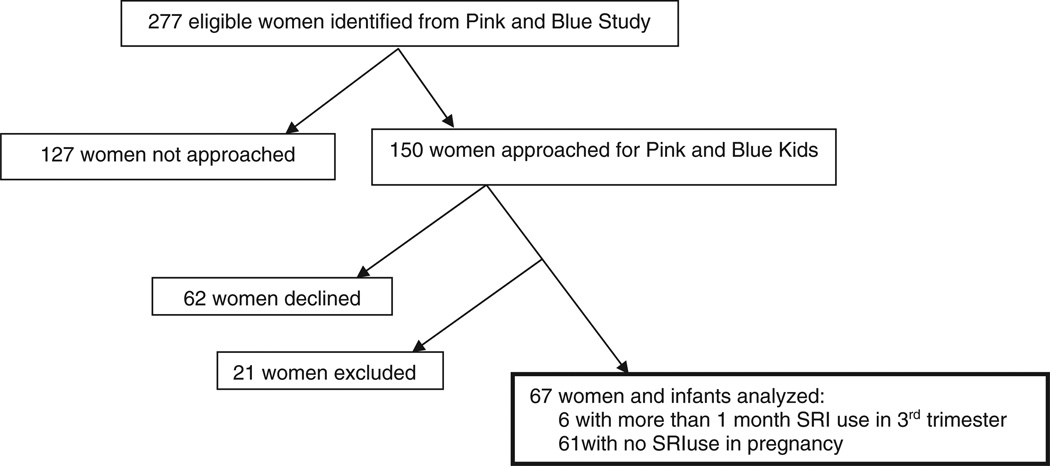
Study recruitment is illustrated in Fig. 1. Of the 2793 women who participated in the Yale Pink and Blue Study, 277 had a live birth at one of the two hospitals participating in the evaluation of infants and were determined to be eligible for the study. Of the eligible infants, 150 mothers were approached to participate in the study. Eighty-eight subjects agreed to participate in the study and 67 were included in the analysis. Sixteen women were excluded for depression (MDD, minor depressive disorder, or dysthymia reported anytime in pregnancy or six months prior to pregnancy). Two women were excluded due to SRI use of one month or less and two women were excluded for SRI use in the first or second trimesters but not the third trimester. One infant was determined to be ineligible due to a gestational age that was less than 37 weeks. We tracked hospital procedures such as time to discharge and circumcision (for male infants) as potential contributors to participation bias. However, the only significant baseline differences between the 150 women who were and those who were not approached (n = 127) for participation in the study was that women who were approached were slightly older than the women that were not approached (31.11 ± 5.61 years and 29.75 ± 5.85 years, p = 0.05). The 88 women who agreed to participate were more likely to have had a lifetime history of a psychiatric disorder than were women who declined to participate (18.18% and 6.45%, p = 0.05). The average EPDS score in mothers in the unexposed group was 5.18 (4.86) and 7.67 (6.47) in the exposed group (p = 0.25).
Fig. 1.
Consort diagram.
The exposed group consisted of six infants of mothers who did not meet diagnostic criteria for MDD in pregnancy and who took an SRI for more than one month in their third trimester. Use of SRIs in the exposed group began within six months before pregnancy, with exposure in each trimester and continuation after delivery. Although women may have taken medication prior to pregnancy and throughout the pregnancy, to be included in the exposed group, they must have taken SRIs for more than one month in their third trimester. SRIs taken during the third trimester included fluoxetine at a maximum of 20 mg/day, citalopram at an average dose of 20 mg/day, and sertraline at a maximum dose of 100 mg/day. Use of SRIs by this group began within six months before pregnancy, with exposure in each trimester and continuation after delivery. The unexposed group consisted of 61 control infants of mothers who were neither exposed to MDD nor SRIs in pregnancy. On average, women were exposed to SRIs for 8.8 months (35.5 weeks) of pregnancy. All women in the exposure group began SRI use prior to pregnancy. Four of the exposed women took SRIs throughout pregnancy, 1 discontinued use in her first month of pregnancy, but resumed use in the sixth month of gestation.
3.2. Demographic and clinical characteristics
The baseline demographic and clinical characteristics of the mothers and infants participating in the study (n = 67 infants) are summarized in Table 1. The majority of study participants were white, married, college graduates and their ages ranged from 18 to 40 years. Mothers exposed to SRI use for more than one month in their third trimester were more likely to have been diagnosed with GAD, panic disorder, or PTSD during pregnancy (50.00% exposed, 4.92% unexposed, p = 0.01). Specific to infant outcomes, birth weight, length, head circumference, neonatal intensive care admission, and 1 minute APGAR scores did not significantly differ between the exposed and unexposed infants (Table 1). However, gestational age and the 5 minute APGAR score were lower for the infants exposed to SRIs in utero than for unexposed infants (p = 0.02 and p = 0.01, respectively). Gestational age ranged from 37 to 39 weeks in exposed and from 37 to 41 weeks in unexposed infants. The mean 5 minute APGAR score was 8.50 in the exposed and 8.97 in the unexposed infants.
Table 1.
Demographics of study subjects.
| Control (n=61) |
More than 1 month SRI use in 3rd trimester (n=6) |
p-Valuea | |
|---|---|---|---|
| Maternal | |||
| Age, y (se) | 31.15 (5.19) | 32.66 (4.26) | 0.49 |
| Education, n (%) | 0.75 | ||
| <High school | 3 (4.92%) | 0 | |
| High school grad | 8 (13.11%) | 1 (16.67%) | |
| Some college | 11 (18.03%) | 0 | |
| College grad | 39 (63.93%) | 5 (83.33%) | |
| Marital status, n (%) | 1.00 | ||
| Married | 46 (75.41%) | 5 (83.33%) | |
| Living with partner | 9 (14.75%) | 1 (16.67%) | |
| Never married | 6 (9.84%) | 0 | |
| Ethnicity, n (%) | 0.83 | ||
| Asian | 4 (6.56%) | 0 | |
| Black | 4 (6.56%) | 0 | |
| Hispanic | 11 (18.03%) | 0 | |
| Other | 1 (1.64%) | 0 | |
| White | 41 (67.21%) | 6 (100%) | |
| Alcohol (4+ per week Tri1,2) | 1 (1.64%) | 0 | 1.00 |
| Any smoking | 8 (13.11%) | 0 | 1.00 |
| Any illicit drugs | 2 (3.28%) | 0 | 1.00 |
| Prior pregnancy | 40 (65.57%) | 4 (66.67%) | 1.00 |
| Psychiatric comorbidity in pregnancy | |||
| Generalized anxiety disorder | 0 | 1 (16.67%) | 0.09 |
| Panic | 1 (1.64%) | 1 (16.67%) | 0.17 |
| Post-traumatic stress disorder | 2 (3.28%) | 1 (16.67%) | 0.25 |
| Average EPDS score | 5.18 (4.86) | 7.67 (6.47) | 0.25 |
| C-section | 26 (42.62%) | 2 (33.33%) | 1.00 |
| Major medical complications | 6 (9.84%) | 1 (16.67%) | 0.50 |
| Baby | |||
| Gestational age, weeks (se) | 39.00 (0.97) | 38.00 (0.89) | 0.02 |
| Weight, g (se) | 3357.80 (426.76) | 3004.00 (569.10) | 0.06 |
| Length, cm (se) | 50.91 (2.61) | 49.75 (1.50) | 0.39 |
| Head circumference, cm (se) | 33.83 (1.67) | 33.40 (2.19) | 0.60 |
| Apgar | |||
| 1 min | 8.49 (0.92) | 8.33 (1.21) | 0.70 |
| 5 min | 8.97 (0.26) | 8.50 (1.22) | 0.01 |
| NICU | 4 (6.56%) | 1 (16.67%) | 0.38 |
Fisher's Exact test for categorical variables and t-test for continuous variables.
3.3. Neonatal Behavioral Assessment Scale (NBAS)
Inter-rater reliability for the NBAS was assessed on 25% of the participants, resulting in Spearman correlation coefficients ranging from 0.80 to 0.99. NBAS motor cluster scores were significantly lower in infants with more than one month of SRI exposure (25.2) in the third trimester compared to control infants (28.9) with no SRI exposure in pregnancy (p = 0.02) (Table 2). Additionally, the specific motor item scores for pull-to-sit were lower in the exposed infants compared to the unexposed infants, while specific motor item scores for general tone, motor maturity, defensive movement and activity did not differ significantly between the two groups. The pull-to-sit score measures traction, head control, and neck muscle strength of the infant with exposed infants exhibiting less head control than the unexposed infants (4.00 exposed, 5.56 control, p = 0.04). The significance of the motor finding became marginal when controlling for gestational age of the infant (p = 0.05), while the specific motor item scores for activity indicated that exposed infants were less active compared to control infants after controlling for gestational age (4.00 exposed, 4.59 control, p = 0.03). Based upon our small sample size, gestational age cannot be considered a mediator since it was not significantly associated with NBAS motor outcomes given SRI exposure status (p = 0.73, not shown) and taken alone, was not a significant predictor of NBAS motor outcomes (p = 0.21, not shown). The exposed infants did not differ significantly from the unexposed infants in NBAS scores for the state organization cluster or for the regulation of state cluster.
Table 2.
Neonatal Behavioral Assessment Scale results.
| NBAS (46) | Control (N=41) |
More than 1 month SRI in Tri3 (N=5) |
p-Value | p-Value with gest age |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | p | p | |
| Motor | 28.900 (3.055) | 25.200 (3.962) | 0.02 | 0.05 |
| Tonus | 5.718 (0.647) | 5.200 (0.837) | 0.11 | 0.39 |
| Maturity | 6.475 (0.847) | 5.800 (0.837) | 0.10 | 0.06 |
| Pull | 5.556 (1.463) | 4.000 (2.000) | 0.04 | 0.10 |
| Defense | 5.973 (1.323) | 6.200 (1.304) | 0.72 | 0.49 |
| Activity | 4.588 (0.609) | 4.000 (0.707) | 0.06 | 0.03 |
| Organization | 14.781 (2.636) | 15.667 (1.528) | 0.57 | 0.99 |
| Excitement | 3.675 (0.829) | 4.200 (0.447) | 0.17 | 0.38 |
| Buildup | 3.927 (1.191) | 3.200 (1.304) | 0.21 | 0.35 |
| Irritability | 4.553 (1.224) | 5.400 (0.894) | 0.14 | 0.20 |
| Lability | 2.939 (1.029) | 3.667 (0.577) | 0.24 | 0.29 |
| Regulation | 20.077 (3.862) | 19.200 (3.564) | 0.63 | 0.77 |
| Cuddly | 6.325 (1.421) | 6.400 (0.894) | 0.91 | 0.90 |
| Consolability | 4.744 (1.666) | 5.200 (1.304) | 0.56 | 0.88 |
| Quiet | 4.282 (1.276) | 3.200 (1.643) | 0.09 | 0.20 |
| Hand | 4.800 (1.964) | 4.400 (2.608) | 0.68 | 0.91 |
3.4. Activity assessment
There were no statistically significant differences in sleep patterns recorded by the Actiwatch between infants with more than one month of SRI exposure in the third trimester and control infants with no SRI exposure in pregnancy (Table 3). The proportion of sleep and wake states (88.96% sleep controls, 84.76% sleep exposed, p = 0.63) and the number of wake bouts (2.17 controls, 2.67 exposed, p = 0.71) were similar for infants in both groups. Differences in sleep and wake states (p = 0.60) and in wake bouts (p = 0.93) remained insignificant after controlling for gestational age.
Table 3.
Activity assessment findings.
| Actiwatch (26) | Control (N=23) |
More than 1 month SRI in Tri3 (N=3) |
p-Value | p-Value with gest age |
| Mean (SD) | Mean (SD) | |||
| # of wake bouts | 2.174 (2.167) | 2.667 (1.155) | 0.71 | 0.93 |
| % sleep | 88.956 (12.880) | 84.762 (21.450) | 0.63 | 0.60 |
| % wake | 11.044 (12.880) | 15.238 (21.450) | 0.63 | 0.60 |
| Palm (34) | Control (N=29) |
More than 1 month SRI in Tri3 (N=5) |
p-Value | p-Value with gest age |
| Ave # of startlesa | 0.004 (0.003) | 0.004 (0.002) | 0.75 | 0.69 |
| Ave # of tremulousa | 0.009 (0.010) | 0.003 (0.001) | 0.16 | 0.05 |
| Mean length of interval between two episodes of startles | 266.996 (308.261) | 315.317 (262.942) | 0.75 | 0.45 |
| Mean length of interval between two episodes of tremulous | 162.087 (220.745) | 289.953 (114.489) | 0.22 | 0.09 |
Average number per minute.
3.5. Sleep assessment
The average number of startles per minute was the same for both control (0.004) and exposed infants (0.004). The average number of tremulousness episodes per minute did not differ significantly between control and exposed infants (p = 0.16). After adjusting for gestational age, a greater average number of tremulousness episodes per minute (0.009) in control infants compared to exposed infants (0.003) approached significance (p = 0.05). The mean length of interval between two episodes of tremulousness (290 s exposed, 162 s control, p = 0.22) and mean length of interval between startles (315 s exposed, 267 s control, p = 0.75) was higher for infants in the exposed group, but these differences were not statistically significant even after adjusting for gestational age (p = 0.09 and p = 0.45, respectively).
4. Discussion
The majority of studies that investigated possible effects of prenatal SRI exposure on infants have focused on neonatal withdraw syndrome, with few examining a broad spectrum of infant outcomes including motor development. The present prospective study of prenatal SRI use in the last trimester of pregnancy on infant neurobehavior used a diverse set of assessment measures to examine infant sleep, temperament, and activity. Results of our study suggest that SRI exposure in the third trimester may have subtle effects on motor development and motor control. Specifically, we found that infants exposed to SRIs but not major depressive disorder in the third trimester scored lower on the motor module of the NBAS and had lower 5-minute APGAR scores as compared to infants exposed to neither. We did not find statistically significant differences between control and exposed infants in measures of startle.
Serotonin has been hypothesized to coordinate sensory and autonomic functions with gross motor activity [19]. SRIs have been hypothesized to cause a significant motor delay by possibly increasing the serotonin concentration in the cerebral cortex. This increase in cerebral serotonin could have an inhibitory effect on motor control [20]. Although some studies have not found a statistically significant effect for motor activity when gestational age of infant was controlled [3] others with larger sample sizes have shown significant differences between exposed and unexposed infants in motor activity and development [21,22]. At present it is not clear whether this is a transient or reversible effect or permanent effect of antidepressant exposure on fetal brain development [13].
It is unclear if our findings represent transient observations or provide the basis for subsequent neurobehavioral problems that may be detected at a later age. Initial delay in motor development was seen in rat pups exposed prenatally to SRIs [20], but this delay appeared transient. In human studies, the work of several groups suggests that poor motor development even when controlling for major depressive disorder persists [21,22] in some cases up to 40 months of age [8]. Although motor activity also reflects differences in maturation and gestational age, [3], we found that the differences in motor development remain after controlling for infants gestational age. Additional longitudinal examinations of infant's neurobehavior into childhood and adolescence may help to address these issues.
Our finding that exposed infants had lower 5-minute APGAR scores as compared to unexposed infants is similar to that found by others with larger sample sizes [8,12]. Although our finding of differences between the APGAR scores may be statistically different, the finding is most likely not clinically significant [23]. However, our finding on the differences in gestational age is likely to be clinically meaningful in terms of long-term infant outcomes [24]. We found no differences between groups in measures of startle and a small, borderline significant finding for tremulousness when adjusted for gestational age. This differs from case reports and longitudinal studies finding heightened tremulousness and exaggerated startle reflexes in infants who were exposed prenatally to maternal SRI use [2,3,6,12,25,26]. Additionally, we found no difference between states in response to sources of stimulation on the NBAS. Change in behavioral states reflects the infant's ability to regulate arousal and response to endogenous and exogenous sources of stimulation. Ideally, an infant transitions smoothly between states and uses a wide range of states in response to sources of external stimulation; however, SRI exposed infants have shown impairment in this domain. Zeskind and Stephens [3] used the NBAS cluster of autonomic stability similar to the one used in the present study and found that SRI exposed infants showed fewer transitions to different states and exhibited a narrower range of states than unexposed infants. However, they did not control for major depressive disorder and the analysis was not limited to exposure in the third trimester.
Our study has three main limitations. First, a larger sample size would provide greater statistical power to detect differences, allow for the use of additional covariates in our analysis (e.g. over-the-counter and other prescription medications), and likely increase the generalizability of our findings. Due to the sample size, we did not have the ability to assess the effect of SRI on infant outcomes as compared to other maternal disorders (e.g. anxiety disorders). Despite our small sample size, our study has several advantages including the ability to prospectively determine timing and duration of prenatal exposure and thoroughly assess a wide range of neurobehavioral outcomes in individual infants. Second, our study might have been subject to selection bias resulting from potential differences in women who were approached and those who were not approached to participate in the study. This is highly unlikely since the main reason women were not offered study participation was that their delivery occurred on a weekend when study personnel were not available to conduct assessments. This occurrence is therefore most likely random in nature and not an indication of systematic bias. Third, we did not have sufficient information on dosage of SRI across pregnancy to comment on the relationship between SRI dose and infant outcome.
The long range public health significance of our findings is that if in utero antidepressant use increases difficulties with motor development, surveillance methods in primary care settings (through well child visits) and systems of care (early intervention) may be implemented to monitor the long term consequences of early exposure. Future longitudinal study should examine long term outcomes of last trimester antidepressant exposed and unexposed infants compared to nonexposed infants of depressed mothers and dually exposed infants (SRIs and depressed).
Acknowledgments
This work was supported by a grant from NARSAD, the Brain and Behavior Research Fund R01 HD045735 from National Institutes of Child Health and Human Development, and K12 DA031050 (PI) from the National Institute on Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health.
The study sponsors did not have a role in the design, collection, analysis, or interpretation of the data.
Footnotes
Conflict of interest
Dr. Yonkers has the following conflicts to disclose: she has received study medication from Pfizer for an NIMH trial, she has received support from Eli Lilly for an investigator-initiated grant and has received royalties from Up to Date. All other investigators do not have any conflicts of interest.
References
- 1.Cooper WO, Willy ME, Pont SJ, Ray WA. Increasing use of antidepressants in pregnancy. Am J Obstet Gynecol. 2007;196(6):544.e1. doi: 10.1016/j.ajog.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 2.Laine K, Heikkinen T, Ekblad U, Kero P. Effects of exposure to selective serotonin reuptake inhibitors during pregnancy on serotonergic symptoms in newborns and cord blood monoamine and prolactin concentrations. Arch Gen Psychiatry. 2003;60:720–726. doi: 10.1001/archpsyc.60.7.720. [DOI] [PubMed] [Google Scholar]
- 3.Zeskind P, Stephens L. Maternal selective serotonin reuptake inhibitor use during pregnancy and newborn neurobehavior. Pediatrics. 2004;113(2):368–375. doi: 10.1542/peds.113.2.368. [DOI] [PubMed] [Google Scholar]
- 4.Costei A, Kozer E, Ho T, Ito S, Koren G. Perinatal outcome following third trimester exposure to paroxetine. Arch Pediatr Adolesc Med. 2002;156:1129–1132. doi: 10.1001/archpedi.156.11.1129. [DOI] [PubMed] [Google Scholar]
- 5.Sanz E, De-las-Cuevas C, Kiuru A, Bate A, Edwards R. Selective serotonin reuptake inhibitors in pregnant women and neonatal withdrawal syndrome: a database analysis. Lancet. 2005;365:482–487. doi: 10.1016/S0140-6736(05)17865-9. [DOI] [PubMed] [Google Scholar]
- 6.Boucher N, Bairam A, Beaulac-Baillargeon L. A new look at the neonate's clinical presentation after in utero exposure to antidepressants in late pregnancy. J Clin Psychopharmacol. 2008;28(3):334–339. doi: 10.1097/JCP.0b013e318173aa2e. [DOI] [PubMed] [Google Scholar]
- 7.Gentile S. On categorizing gestational birth, and neonatal complications following late pregnancy exposure to antidepressants: the prenatal antidepressant exposure syndrome. CNS Spectr. 2010;15(3):167–185. doi: 10.1017/s1092852900027449. [DOI] [PubMed] [Google Scholar]
- 8.Casper RC, Fleisher BE, Lee-Ancajas J, Gilles A, Gaylor E, DeBattista A, et al. Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. J Pediatr. 2003;142:402–408. doi: 10.1067/mpd.2003.139. [DOI] [PubMed] [Google Scholar]
- 9.Ferreira E, Carceller AM, Agogue C, Martin BZ, St-Andre M, Francoeur D, et al. Effects of selective serotonin reuptake inhibitors and venlafaxine during pregnancy in term and preterm neonates. Pediatrics. 2007;119(1):52–59. doi: 10.1542/peds.2006-2133. [DOI] [PubMed] [Google Scholar]
- 10.Levinson-Castiel R, Merlob P, Linder N, Sirota L, Klinger G. Neonatal abstinence syndrome after in utero exposure to selective serotonin reuptake inhibitors in term infants. Arch Pediatr Adolesc Med. 2006;160:173–176. doi: 10.1001/archpedi.160.2.173. [DOI] [PubMed] [Google Scholar]
- 11.Suri R, Altshuler L, Hellemann G, Burt VK, Aquino A, Mintz J. Effects of antenatal depression and antidepressant treatment on gestational age at birth and risk of preterm birth. Am J Psychiatry. 2007;164(8):1206–1213. doi: 10.1176/appi.ajp.2007.06071172. [DOI] [PubMed] [Google Scholar]
- 12.Wisner KL, Sit DK, Hanusa BH, Moses-Kolko EL, Bogen DL, Hunker DF, et al. Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes. Am J Psychiatry. 2009;166(5):557–566. doi: 10.1176/appi.ajp.2008.08081170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oberlander TF, Warburton W, Misri S, Aghajanian J, Hertzman C. Neonatal outcomes after prenatal exposure to selective serotonin reuptake inhibitor antidepressants and maternal depression using population-based linked health data. Arch Gen Psychiatry. 2006;63(8):898–906. doi: 10.1001/archpsyc.63.8.898. [DOI] [PubMed] [Google Scholar]
- 14.Wittchen H-U. Reliability and validity studies of the WHO Composite International Diagnostic Interview (CIDI): a critical review. J Psychiatr Res. 1994;28:57–84. doi: 10.1016/0022-3956(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 15.Sheehan D, Lecrubier Y, Sheehan K, Amorim P, Janava J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(S20):22–33. [PubMed] [Google Scholar]
- 16.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 17.Brazelton T, Nugent J. Neonatal Behavioral Assessment Scale. 3rd ed. London: Cambridge University Press; 1995. [Google Scholar]
- 18.Lester B, Als H, Brazelton T. Regional obstetric anesthesia and newborn behavior: a reanalysis toward synergistic effects. Child Dev. 1982;53:687–692. [PubMed] [Google Scholar]
- 19.Oberlander T, Gingrich J, Ansorge M. Sustained neurobehavioral effects of exposure to SSRI antidepressants during development: molecular to clinical evidence. Clin Pharmacol Ther. 2009;86(6):672–677. doi: 10.1038/clpt.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bairy K, Madhyastha S, Ashok K, Bairy I, Malini S. Developmental and behavioral consequences of prenatal fluoxetine. Pharmacology. 2007;79:1–11. doi: 10.1159/000096645. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen L, Henriksen T, Olsen J. Fetal exposure to antidepressants and normal milestone development at 6 and 19 months of age. Pediatrics. 2010;125:e600–e608. doi: 10.1542/peds.2008-3655. [DOI] [PubMed] [Google Scholar]
- 22.Oberlander TF, Bonaguro RJ, Misri S, Papsdorf M, Ross CJD, Simpson EM. Infant serotonin transporter (SLC6A4) promoter genotype is associated with adverse neonatal outcomes after prenatal exposure to serotonin reuptake inhibitor medications. Mol Psychiatry. 2008;13(1):65–73. doi: 10.1038/sj.mp.4002007. [DOI] [PubMed] [Google Scholar]
- 23.College of Obstetricians and Gynecologists and Committee on Obstetric Practice American Academy of Pediatrics CoFaN, American. The APGAR score. Pediatrics. 2006;117(4):1144–1447. [Google Scholar]
- 24.Institute of Medicine. Preterm birth: causes, consequences, and prevention. Washington, D.C.: National Academies Press; 2006. [Google Scholar]
- 25.Stiskal J, Kulin N, Koren G, Ho T, Ito S. Neonatal paroxetine withdrawal syndrome. Arch Dis Child Fetal Neonatal Ed. 2001;84:F134–F135. doi: 10.1136/fn.84.2.F134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dahl M, Olhager E, Ahlner J. Paroxetine withdrawal syndrome in a neonate. Br J Psychiatry. 1997;171:391–392. doi: 10.1192/bjp.171.4.391c. [DOI] [PubMed] [Google Scholar]