Abstract
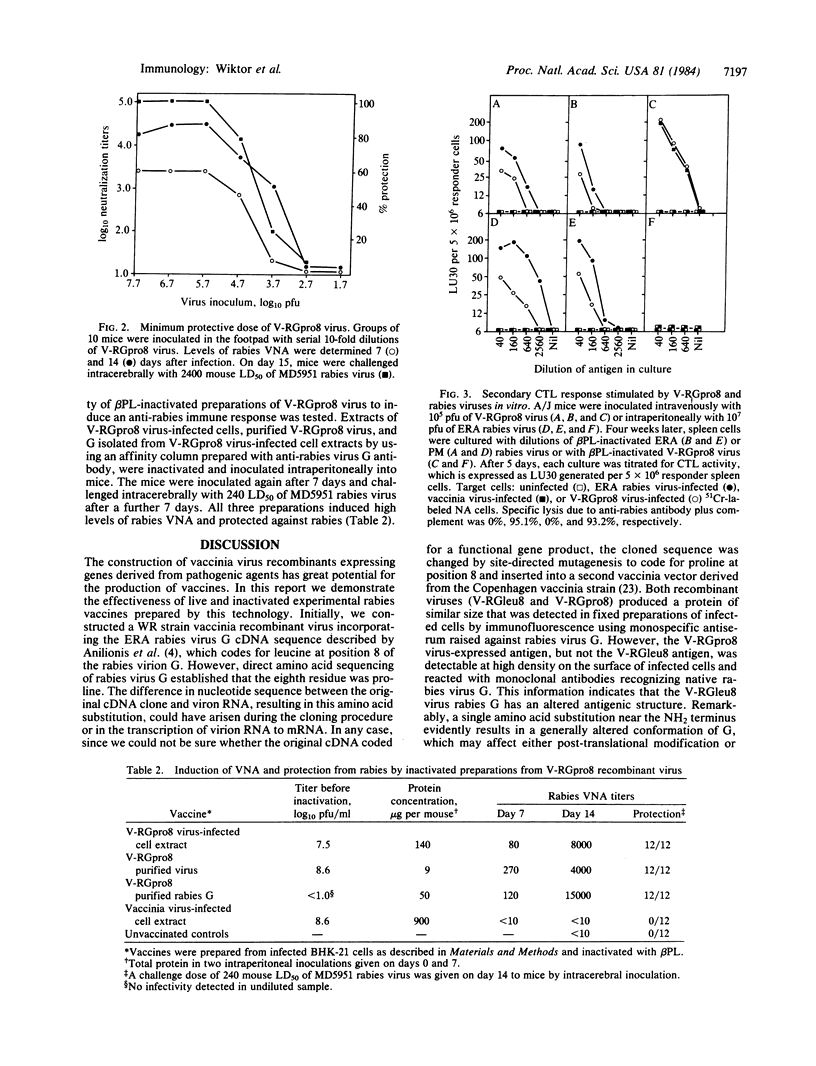
Inoculation of rabbits and mice with a vaccinia-rabies glycoprotein recombinant (V-RG) virus resulted in rapid induction of high concentrations of rabies virus-neutralizing antibodies and protection from severe intracerebral challenge with several strains of rabies virus. Protection from virus challenge also was achieved against the rabies-related Duvenhage virus but not against the Mokola virus. Effective immunization by V-RG depended on the expression of a rabies glycoprotein that registered proline rather than leucine as the eighth amino acid from its NH2 terminus (V-RGpro8). A minimum dose required for effective immunization of mice was 10(4) plaque-forming units of V-RGpro8 virus. beta-propiolactone-inactivated preparations of V-RGpro8 virus also induced high levels of rabies virus-neutralizing antibody and protected mice against intracerebral challenge with street rabies virus. V-RGpro8 virus was highly effective in priming mice to generate a secondary rabies virus-specific cytotoxic T-lymphocyte response following culture of lymphocytes with either ERA or PM strains of rabies virus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anilionis A., Wunner W. H., Curtis P. J. Structure of the glycoprotein gene in rabies virus. Nature. 1981 Nov 19;294(5838):275–278. doi: 10.1038/294275a0. [DOI] [PubMed] [Google Scholar]
- Bramhall S., Noack N., Wu M., Loewenberg J. R. A simple colorimetric method for determination of protein. Anal Biochem. 1969 Oct 1;31(1):146–148. doi: 10.1016/0003-2697(69)90251-6. [DOI] [PubMed] [Google Scholar]
- Clark H. F. Rabies serogroup viruses in neuroblastoma cells: propagation, "autointerference," and apparently random back-mutation of attenuated viruses to the virulent state. Infect Immun. 1980 Mar;27(3):1012–1022. doi: 10.1128/iai.27.3.1012-1022.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J. H., Dietzschold B., Schneider L. G. Rabies virus glycoprotein. II. Biological and serological characterization. Infect Immun. 1977 Jun;16(3):754–759. doi: 10.1128/iai.16.3.754-759.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzschold B., Wiktor T. J., Macfarlan R., Varrichio A. Antigenic structure of rabies virus glycoprotein: ordering and immunological characterization of the large CNBr cleavage fragments. J Virol. 1982 Nov;44(2):595–602. doi: 10.1128/jvi.44.2.595-602.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzschold B., Wiktor T. J., Wunner W. H., Varrichio A. Chemical and immunological analysis of the rabies soluble glycoprotein. Virology. 1983 Jan 30;124(2):330–337. doi: 10.1016/0042-6822(83)90349-5. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. The purification fo four strains of poxvirus. Virology. 1962 Sep;18:9–18. doi: 10.1016/0042-6822(62)90172-1. [DOI] [PubMed] [Google Scholar]
- Lafon M., Wiktor T. J., Macfarlan R. I. Antigenic sites on the CVS rabies virus glycoprotein: analysis with monoclonal antibodies. J Gen Virol. 1983 Apr;64(Pt 4):843–851. doi: 10.1099/0022-1317-64-4-843. [DOI] [PubMed] [Google Scholar]
- Lane J. M., Ruben F. L., Neff J. M., Millar J. D. Complications of smallpox vaccination, 1968: results of ten statewide surveys. J Infect Dis. 1970 Oct;122(4):303–309. doi: 10.1093/infdis/122.4.303. [DOI] [PubMed] [Google Scholar]
- Lathe R. F., Kieny M. P., Schmitt D., Curtis P., Lecocq J. P. M13 bacteriophage vectors for the expression of foreign proteins in Escherichia coli: the rabies glycoprotein. J Mol Appl Genet. 1984;2(4):331–342. [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J Virol. 1984 Mar;49(3):857–864. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith C. D., Prossouw A. P., Koch H. van P. An unusual case of human rabies thought to be of chiropteran origin. S Afr Med J. 1971 Jul 17;45(28):767–769. [PubMed] [Google Scholar]
- Moss B., Smith G. L., Gerin J. L., Purcell R. H. Live recombinant vaccinia virus protects chimpanzees against hepatitis B. Nature. 1984 Sep 6;311(5981):67–69. doi: 10.1038/311067a0. [DOI] [PubMed] [Google Scholar]
- Panicali D., Davis S. W., Weinberg R. L., Paoletti E. Construction of live vaccines by using genetically engineered poxviruses: biological activity of recombinant vaccinia virus expressing influenza virus hemagglutinin. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5364–5368. doi: 10.1073/pnas.80.17.5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti E., Lipinskas B. R., Samsonoff C., Mercer S., Panicali D. Construction of live vaccines using genetically engineered poxviruses: biological activity of vaccinia virus recombinants expressing the hepatitis B virus surface antigen and the herpes simplex virus glycoprotein D. Proc Natl Acad Sci U S A. 1984 Jan;81(1):193–197. doi: 10.1073/pnas.81.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin S. A., Wiktor T. J., Koprowski H., Rosanoff E. I., Tint H. Immunization schedules for the new human diploid cell vaccine against rabies. Am J Epidemiol. 1976 Jan;103(1):75–80. doi: 10.1093/oxfordjournals.aje.a112207. [DOI] [PubMed] [Google Scholar]
- Reagan K. J., Wunner W. H., Wiktor T. J., Koprowski H. Anti-idiotypic antibodies induce neutralizing antibodies to rabies virus glycoprotein. J Virol. 1983 Dec;48(3):660–666. doi: 10.1128/jvi.48.3.660-666.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOKER M., MACPHERSON I. SYRIAN HAMSTER FIBROBLAST CELL LINE BHK21 AND ITS DERIVATIVES. Nature. 1964 Sep 26;203:1355–1357. doi: 10.1038/2031355a0. [DOI] [PubMed] [Google Scholar]
- Shope R. E., Murphy F. A., Harrison A. K., Causey O. R., Kemp G. E., Simpson D. I., Moore D. L. Two African viruses serologically and morphologically related to rabies virus. J Virol. 1970 Nov;6(5):690–692. doi: 10.1128/jvi.6.5.690-692.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. L., Mackett M., Moss B. Infectious vaccinia virus recombinants that express hepatitis B virus surface antigen. Nature. 1983 Apr 7;302(5908):490–495. doi: 10.1038/302490a0. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Murphy B. R., Moss B. Construction and characterization of an infectious vaccinia virus recombinant that expresses the influenza hemagglutinin gene and induces resistance to influenza virus infection in hamsters. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7155–7159. doi: 10.1073/pnas.80.23.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. S., McCelland C. L., Reid F. L., Baer G. M. Dual role of the immune response in street rabiesvirus infection of mice. Infect Immun. 1982 Jan;35(1):213–221. doi: 10.1128/iai.35.1.213-221.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor T. J., Aaslestad H. G., Kaplan M. M. Immunogenicity of rabies virus inactivated by -propiolactone, acetylethyleneimine, and ionizing irradiation. Appl Microbiol. 1972 May;23(5):914–918. doi: 10.1128/am.23.5.914-918.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor T. J. Cell-mediated immunity and postexposure protection from rabies by inactivated vaccines of tissue culture origin. Dev Biol Stand. 1978;40:255–264. [PubMed] [Google Scholar]
- Wiktor T. J., Doherty P. C., Koprowski H. In vitro evidence of cell-mediated immunity after exposure of mice to both live and inactivated rabies virus. Proc Natl Acad Sci U S A. 1977 Jan;74(1):334–338. doi: 10.1073/pnas.74.1.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor T. J., György E., Schlumberger D., Sokol F., Koprowski H. Antigenic properties of rabies virus components. J Immunol. 1973 Jan;110(1):269–276. [PubMed] [Google Scholar]
- Wiktor T. J., Koprowski H. Monoclonal antibodies against rabies virus produced by somatic cell hybridization: detection of antigenic variants. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3938–3942. doi: 10.1073/pnas.75.8.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor T. J. Laboratoty techniques in rabies: tissue culture methods. Monogr Ser World Health Organ. 1973;(23):101–123. [PubMed] [Google Scholar]
- Wunner W. H., Dietzschold B., Curtis P. J., Wiktor T. J. Rabies subunit vaccines. J Gen Virol. 1983 Aug;64(Pt 8):1649–1656. doi: 10.1099/0022-1317-64-8-1649. [DOI] [PubMed] [Google Scholar]
- Wybier-Franqui J., Gomard E., Levy J. P. Accessory cells in the in vitro generation of type C virus-specific T-killer lymphocytes. III. Two methods of antigen presentation of cytolytic T-lymphocyte precursors. Cell Immunol. 1982 Apr;68(2):287–301. doi: 10.1016/0008-8749(82)90113-7. [DOI] [PubMed] [Google Scholar]
- Yelverton E., Norton S., Obijeski J. F., Goeddel D. V. Rabies virus glycoprotein analogs: biosynthesis in Escherichia coli. Science. 1983 Feb 11;219(4585):614–620. doi: 10.1126/science.6297004. [DOI] [PubMed] [Google Scholar]