Abstract
Mismatch-repair–deficient colorectal cancers often contain kinase-activating V600E BRAF mutations, but no clinical utility has yet been demonstrated in this setting for monotherapy using oral braf kinase inhibitors such as vemurafenib or dabrafenib. Recent studies have indicated that tumour resistance to braf inhibition is mediated by upregulated epidermal growth factor receptor (egfr) signalling, disruption of which is a routine treatment strategy in KRAS wild-type colorectal cancer. In this report, we describe the clinical course of a heavily pretreated patient who elected to receive off-label dual-targeted braf- and egfr-inhibitory therapy with good tolerance and apparent clinical benefit.
Keywords: Colorectal neoplasms, combination drug therapy, personalized oncology, precision medicine, genomic profiling
1. INTRODUCTION
Melanomas containing missense V600E BRAF mutations may respond dramatically to braf kinase inhibitors such as vemurafenib1 or dabrafenib2, but the survival benefit has proved to be relatively modest3, in part reflecting rapid selection for resistance4 because of braf inhibitor–induced upregulation of wild-type signalling pathways5–8, such as those regulated by platelet-derived growth factor9 or hepatocyte growth factor10. Activating V600E BRAF mutations are also present in up to 10% of unselected colorectal cancers, with this proportion rising to more than 60% in tumours with microsatellite instability because of reduced expression of mismatch repair enzymes11. These BRAF mutations are mutually exclusive with oncogenic KRAS mutations12, but imply a similar treatment-refractory prognosis13. In contrast to melanomas, fewer than 10% of BRAFV600E-mutated colorectal cancers have responded to braf kinase inhibitor monotherapy in early-phase trials14, raising the possibility that effective treatment may require blockade of one or more additional pathways15. Conversely, the clinical response of colorectal cancers to regimens including epidermal growth factor receptor (egfr) inhibitors appears to require both wild-type BRAF and KRAS status16–18.
Because recent laboratory studies have confirmed that de novo resistance to braf inhibitors in BRAFV600E-mutated colorectal cancer is caused by egfr signalling19,20, we hypothesised that dual blockade of the braf and egfr pathways might be therapeutically useful, as has been suggested by preclinical data15. Moreover, a previous randomized trial of cetuximab combined with the nonspecific Raf inhibitor sorafenib indicated a doubling of the response rate in patients with metastatic colon cancer21. Here, we report an end-stage colorectal cancer patient benefiting from combination noncytotoxic drug therapy simultaneously targeting the egfr and braf signalling pathways.
2. CASE REPORT
A 78-year-old man presented in 2006 with anemia caused by an obstructive mucinous colorectal cancer of the hepatic flexure. The 75-mm tumour was resected en bloc. Vascular invasion and perineural infiltration were present, and 9 of 21 nodes contained metastases. The mismatch repair proteins mlh1 and pms2 were not detectable by immunohistochemistry.
After surgery and biweekly oxaliplatin-based (folfox) adjuvant chemotherapy, our patient remained well until 2010, when rising serum carcinoembryonic antigen (cea) and fluorodeoxyglucose positron-emission tomography heralded recurrence in the right external iliac and inguinal nodes, with involvement of the anterior abdominal wall. Palliative use of oral capecitabine was poorly tolerated because of severe diarrhea and hand–foot syndrome, and it failed to stem the rising cea. Treatment was changed to raltitrexed, but 3 cycles of that regimen also proved ineffective.
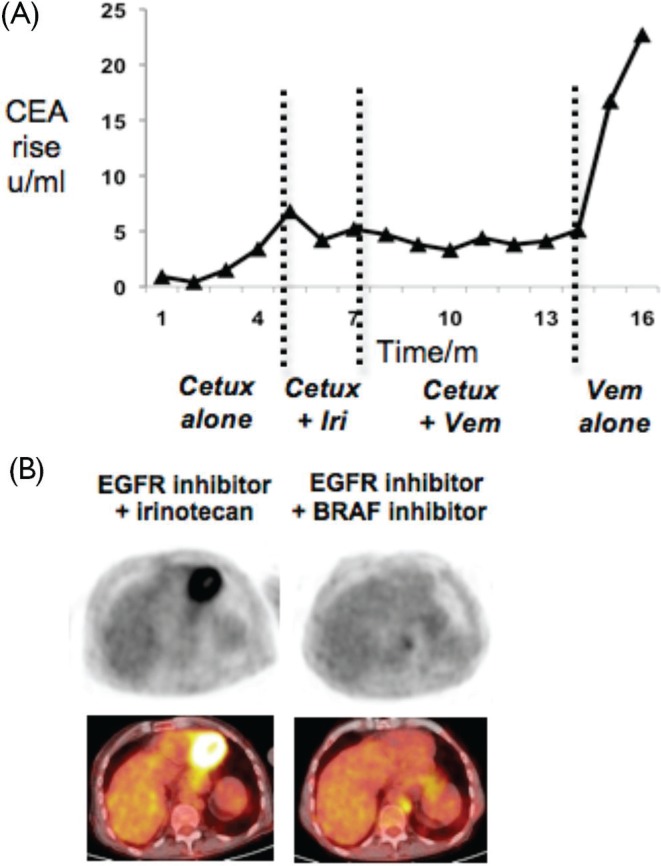
By early 2012, disease progression had supervened again. Tumour gene sequencing—by exonic polymerase chain reaction amplification followed by direct sequencing—showed KRAS (exon 1, codons 12–13) wild-type status and a classical BRAF exon 15 V600E mutation. Monotherapy with cetuximab failed to slow the rising cea [Figure 1(A)]. Irinotecan chemotherapy was added, but plasma cea rose again within 2 months, and the patient’s quality of life was impaired by diarrhea.
Figure 1.
Control of BRAF-mutant colorectal cancer with vemurafenib (Vem) plus cetuximab (Cetux). (A) Time course in months of plasma carcinoembryonic antigen (cea), charted here as absolute rise above normal range, during treatment with cetuximab alone, cetuximab plus irinotecan (Iri), cetuximab plus vemurafenib, and after cessation of cetuximab. (B) Fluorodeoxyglucose (fdg)-avid abdominal node metastasis shown by positron-emission tomography imaging (left top panel) and computed tomography–magnetic resonance imaging (left bottom panel) during treatment with cetuximab and irinotecan. Regression of fdg-avid disease after 2 months treatment with combined cetuximab and vemurafenib (right panels). egfr = epidermal growth factor receptor.
All standard treatment options now being exhausted, and with symptoms and iatrogenic toxicities compromising the patient’s quality of life, we initiated detailed discussions about off-label substitution of vemurafenib for irinotecan. Having thus obtained the patient’s informed consent, vemurafenib was commenced at 240 mg daily, with weekly escalation as tolerated. Cetuximab was continued throughout, according to the standard schedule. Skin reaction was florid at first, requiring interruption of vemurafenib therapy at the initial lowest dose, but the target dose of 960 mg daily was achieved within 6 weeks. By this time, plasma cea had stabilized, a palpable right groin mass had regressed, and positron-emission tomography showed reduced fluorodeoxyglucose avidity of the abdominal adenopathy [Figure 1(B)]. Apart from one new skin cancer on the scalp requiring radiotherapy, the combination was well tolerated and yielded symptom stabilization for 6–7 months—similar to the average duration of clinical response to vemurafenib monotherapy in melanoma.
In view of the inconvenience of weekly intravenous infusions, and to clarify whether most of the clinical benefit could be attributed to vemurafenib alone, the decision was made to discontinue cetuximab while continuing oral vemurafenib. However, cetuximab cessation was followed by rapid disease progression [Figure 1(A)]. Reintroduction of combined therapy was attempted, but failed to restore disease control, and the patient was managed palliatively from that point until demise.
3. DISCUSSION
Mismatch-repair–deficient colorectal cancers are widely believed to be suboptimally responsive to fluoropyrimidines in both the adjuvant and the palliative settings, presumably reflecting a failure of the incorporated antimetabolite to trigger apoptosis. Hence, options for effective therapy in microsatellite-unstable disease are limited. Because activating BRAF mutations occur more often in such disease, the present case raises the possibility that braf kinase inhibitors might come to merit consideration in some of these patients. Moreover, in a departure from current teachings, it is plausible that the combination of an egfr inhibitor with a braf inhibitor could transform the natural history of such cases to a prognosis superior to that of “undruggable” KRAS-mutant disease.
A single case report such as ours cannot definitively clarify whether the apparent clinical benefit was specifically attributable to the therapeutic combination, especially given the failure of the reintroduced combined therapy to restore disease control. Early-phase trials of vemurafenib monotherapy indicated a minor response rate in colorectal cancer cases. In the present report, our clinical impression before commencement of dual therapy was that single-agent cetuximab was failing to control the patient’s disease, and yet cessation of cetuximab from the dual-therapy protocol was immediately followed by disease progression. However, given the interpretational weaknesses of such temporal correlations, only larger controlled trials will be able to establish the clinical safety and efficacy of combined therapy.
In the era of personalized medicine, cases such as this raise important ethical and safety issues relating to off-label prescribing. Previous reports of similar off-label therapy using cetuximab and sorafenib have been published with acceptable tolerance and evident benefit22. In the present case, care was taken to inform the patient of the unproven safety and efficacy of combined braf and egfr blockade, as well as of the out-of-pocket costs involved, balanced against a lack of standard treatment options in this heavily-pretreated clinical context.
4. CONCLUSIONS
Relatively few reports have assessed the utility of combining cancer drugs with distinct molecular targets, and some combinations—for example, bevacizumab and cetuximab—have proved to be detrimental. A positive precedent was recently set by studies combining braf and mek inhibitors with improved therapeutic:toxic ratios in melanoma23,24. The present case suggests that formal clinical trials to assess the safety and efficacy of combined braf and egfr blockade in BRAFV600E colorectal cancer are now timely.
5. CONFLICT OF INTEREST DISCLOSURES
All authors declare no financial conflicts of interest.
6. REFERENCES
- 1.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–65. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 3.Livingstone E, Zimmer L, Piel S, Schadendorf D. PLX4032: does it keep its promise for metastatic melanoma treatment? Expert Opin Investig Drugs. 2010;19:1439–49. doi: 10.1517/13543784.2010.527945. [DOI] [PubMed] [Google Scholar]
- 4.Cichowski K, Janne PA. Drug discovery: inhibitors that activate. Nature. 2010;464:358–9. doi: 10.1038/464358a. [DOI] [PubMed] [Google Scholar]
- 5.Hatzivassiliou G, Song K, Yen I, et al. Raf inhibitors prime wild-type Raf to activate the mapk pathway and enhance growth. Nature. 2010;464:431–5. doi: 10.1038/nature08833. [DOI] [PubMed] [Google Scholar]
- 6.Halaban R, Zhang W, Bacchiocchi A, et al. PLX4032, a selective BRAFV600E kinase inhibitor, activates the Erk pathway and enhances cell migration and proliferation of braf melanoma cells. Pigment Cell Melanoma Res. 2010;23:190–200. doi: 10.1111/j.1755-148X.2010.00685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su F, Bradley WD, Wang Q, et al. Resistance to selective braf inhibition can be mediated by modest upstream pathway activation. Cancer Res. 2012;72:969–78. doi: 10.1158/0008-5472.CAN-11-1875. [DOI] [PubMed] [Google Scholar]
- 8.Joseph EW, Pratilas CA, Poulikakos PI, et al. The Raf inhibitor PLX4032 inhibits erk signaling and tumor cell proliferation in a V600E BRAF-selective manner. Proc Natl Acad Sci U S A. 2010;107:14903–8. doi: 10.1073/pnas.1008990107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nazarian R, Shi H, Wang Q, et al. Melanomas acquire resistance to B-RAFV600E inhibition by RTK or N-RAS upregulation. Nature. 2010;468:973–7. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson TR, Fridlyand J, Yan Y, et al. Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature. 2012;487:505–9. doi: 10.1038/nature11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parsons MT, Buchanan DD, Thompson B, Young JP, Spurdle AB. Correlation of tumour BRAF mutations and MLH1 methylation with germline mismatch repair (mmr) gene mutation status: a literature review assessing utility of tumour features for mmr variant classification. J Med Genet. 2012;49:151–7. doi: 10.1136/jmedgenet-2011-100714. [DOI] [PubMed] [Google Scholar]
- 12.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 13.Rebersek M, Boc M, Cerkovnik P, et al. Efficacy of first-line systemic treatment in correlation with BRAF V600E and different KRAS mutations in metastatic colorectal cancer—a single institution retrospective analysis. Radiol Oncol. 2011;45:285–91. doi: 10.2478/v10019-011-0039-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falchook GS, Long GV, Kurzrock R, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet. 2012;379:1893–901. doi: 10.1016/S0140-6736(12)60398-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang H, Higgins B, Kolinsky K, et al. Antitumor activity of braf inhibitor vemurafenib in preclinical models of BRAF-mutant colorectal cancer. Cancer Res. 2012;72:779–89. doi: 10.1158/0008-5472.CAN-11-2941. [DOI] [PubMed] [Google Scholar]
- 16.Di Nicolantonio F, Martini M, Molinari F, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008;26:5705–12. doi: 10.1200/JCO.2008.18.0786. [DOI] [PubMed] [Google Scholar]
- 17.Laurent–Puig P, Cayre A, Manceau G, et al. Analysis of PTEN, BRAF, and EGFR status in determining benefit from cetuximab therapy in wild-type KRAS metastatic colon cancer. J Clin Oncol. 2009;27:5924–30. doi: 10.1200/JCO.2008.21.6796. [DOI] [PubMed] [Google Scholar]
- 18.De Roock W, Claes B, Bernasconi D, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11:753–62. doi: 10.1016/S1470-2045(10)70130-3. [DOI] [PubMed] [Google Scholar]
- 19.Prahallad A, Sun C, Huang S, et al. Unresponsiveness of colon cancer to BRAFV600E inhibition through feedback activation of egfr. Nature. 2012;483:100–3. doi: 10.1038/nature10868. [DOI] [PubMed] [Google Scholar]
- 20.Corcoran RB, Ebi H, Turke AB, et al. egfr-mediated re-activation of mapk signaling contributes to insensitivity of BRAF mutant colorectal cancers to Raf inhibition with vemurafenib. Cancer Discov. 2012;2:227–35. doi: 10.1158/2159-8290.CD-11-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galal KM, Khaled Z, Mourad AM. Role of cetuximab and sorafenib in treatment of metastatic colorectal cancer. Indian J Cancer. 2011;48:47–54. doi: 10.4103/0019-509X.75825. [DOI] [PubMed] [Google Scholar]
- 22.Al-Marrawi MY, Saroya BS, Brennan MC, Yang Z, Dykes TM, El-Deiry WS. Off-label use of cetuximab plus sorafenib and panitumumab plus regorafenib to personalize therapy for a patient with V600E BRAF-mutant metastatic colon cancer. Cancer Biol Ther. 2013;14:703–10. doi: 10.4161/cbt.25191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flaherty KT, Infante JR, Daud A, et al. Combined braf and mek inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367:1694–703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Catalanotti F, Solit DB, Pulitzer MP, et al. Phase ii trial of mek inhibitor selumetinib (AZD6244, ARRY-142886) in patients with BRAFV600E/K-mutated melanoma. Clin Cancer Res. 2013;19:2257–64. doi: 10.1158/1078-0432.CCR-12-3476. [DOI] [PMC free article] [PubMed] [Google Scholar]