Abstract
Background
The accord 11/0402 trial demonstrated that folfirinox (5-fluorouracil, leucovorin, irinotecan, oxaliplatin) is significantly more efficacious than gemcitabine monotherapy in the first-line treatment of metastatic pancreatic cancer (mpc). The present study assessed the cost-effectiveness of first-line folfirinox compared with first-line gemcitabine for public payers in Canada.
Methods
A Markov model simulated the movement of mpc patients from first-line treatment until death. Overall survival (os) and progression-free survival (pfs) data were derived from accord. Published utility data and Canadian costs were applied based on time in each health state and on treatment-related adverse event (ae) rates. Costs included first- and second-line therapy, monitoring, and costs to treat aes. Two separate analyses were performed. Analysis 1 was based on trial data [first-line folfirinox followed by second-line gemcitabine compared with first-line gemcitabine followed by second-line platinum-based chemotherapy, with use of granulocyte colony–stimulating factor (g-csf) allowed], and analysis 2 used Ontario treatment patterns before folfirinox funding (first-line folfirinox followed by second-line gemcitabine compared with first-line gemcitabine followed by best supportive care, no use of g-csf).
Results
Compared with first-line gemcitabine, first-line folfirinox resulted in more life-years and quality adjusted life-years (qalys). Probabilistic sensitivity analysis results showed that, for analyses 1 and 2 respectively, folfirinox has a greater than 85% probability and an approximately 80% probability of being cost-effective at the $100,000 threshold.
Conclusions
Compared with gemcitabine, first-line folfirinox significantly prolongs median os. Given the favourable cost per qaly, the improvement in clinical efficacy, and the limited available treatment options, folfirinox represents an attractive cost-effective treatment for mpc.
Keywords: folfirinox, gemcitabine, cost-effectiveness, pancreatic cancer, accord trial, chemotherapy, quality-adjusted life year, qaly
1. INTRODUCTION
Metastatic pancreatic cancer (mpc) is one of the most aggressive and lethal forms of cancer in both men and women. At present, the disease is the fourth leading cause of cancer-related death in North America and Europe1–3. More than 80% of patients presenting with symptoms of pancreatic carcinoma have unresectable, locally advanced, or metastatic disease at diagnosis, rendering them ineligible for the potentially curative surgery that is available to patients with early-stage disease1,4. Importantly, the disease progresses rapidly and claims the lives of most diagnosed patients: in 2012, an estimated 4500 Canadians (9 per 100,000) were expected to be diagnosed with pancreatic cancer, and 4200 (9 per 100,000), to die of the disease5. Those numbers translate into a 5-year relative survival ratio of approximately 6%, one of the lowest rates for all known cancers2.
Gemcitabine has long been the standard of care for treatment of mpc6. Numerous randomized clinical trials conducted since the early 1990s have attempted to build on single-agent therapy, with some improvements in survival7. Nonetheless, given poor prognosis and limited effective treatment options, there is a significant unmet medical need for an improved treatment regimen.
The accord 11/0402 phase ii/iii clinical trial, conducted by the prodige group of the Fédération Nationale des Centres de Lutte Contre le Cancer, generated results that provide support for folfirinox chemotherapy [5-fluorouracil (5fu), leucovorin, irinotecan, oxaliplatin] as first-line treatment in mpc patients8. A substantial impact of folfirinox was demonstrated with respect to the trial’s primary endpoint of os and secondary endpoints of progression-free survival (pfs) and quality of life (qol). Median os was 11.1 months in patients treated with folfirinox, compared with 6.8 months in patients treated with gemcitabine monotherapy (p < 0.0001)8.
The accord trial produced the longest-ever survival advantage observed in a clinical trial for mpc8. Additionally, a median pfs of 6.4 months was achieved for patients treated with folfirinox, compared with 3.3 months for patients receiving gemcitabine8. Moreover, despite the increased toxicity of folfirinox, patients experienced longer preservation of qol8–10. The additional toxicities associated with the more aggressive chemotherapy therefore appear to be mitigated by improved effective control of cancer-related symptoms for a longer time9.
In addition to the efficacy data for folfirinox, and considering the potential of this regimen to become the new standard of care, there was an interest in the cost-effectiveness of folfirinox compared with gemcitabine. In 2011, an analysis similar to the one presented here was conducted and submitted to Ontario public decision-makers to inform their decision with respect to funding the regimen as an option for the first-line treatment of mpc. In November 2011, Cancer Care Ontario issued a New Drug Funding Program update stating that the Ontario public drug programs had approved funding for folfirinox for the first-line treatment of mpc in patients with a good performance status—that is, an Eastern Cooperative Oncology Group score of 0–1, with no cardiac ischemia and normal or nearly normal bilirubin levels. The analysis presented at that time has been updated to reflect 2013 prices and to incorporate data that were not available in 2011.
The purpose of the present report was to estimate the cost-effectiveness of first-line folfirinox compared with first-line gemcitabine in mpc patients.
2. METHODS
One of the key motivations for conducting this cost-effectiveness analysis was to provide evidence to Ontario public decision-makers evaluating whether to fund folfirinox in the first line for mpc. Two analyses were therefore conducted:
Analysis 1 was based as closely as possible on accord.
Analysis 2 reflected Ontario practice patterns and funding restrictions before the time of provincial submission.
These analyses had two key differences in practice patterns. First, upon progression with gemcitabine, a proportion of patients in accord received a platinum-based chemotherapy. In Ontario, patients who progressed on gemcitabine primarily received best supportive care (bsc). Second, patients in accord received granulocyte colony–stimulating factor (g-csf) for the treatment of neutropenia after chemotherapy. Because of funding restrictions on the use of g-csf in a noncurative setting, Ontario physicians controlled neutropenia primarily through chemotherapy dose reductions. In analysis 2, costs were therefore adjusted to reflect Ontario practice patterns at the time of submission; however, efficacy data in the absence of g-csf use were not available, and that aspect of the analysis could not be adjusted.
Both analyses took the perspective of the Ontario public payer. Costs and outcomes were discounted at a rate of 5% per year.
2.1. Patient Population
The model was populated with data from accord, which included mpc patients naïve to chemotherapy. Patients were between 18 and 75 years of age, had a good performance status (Eastern Cooperative Oncology Group performance score of 0 or 1), no cardiac ischemia, and adequate bone marrow, liver (bilirubin levels ≤1.5 the upper limit of normal), and renal function8.
2.2. Treatment Regimens from ACCORD
Patients received gemcitabine intravenously for 30 minutes at a dose of 1000 mg/m2 body surface area, weekly for 7 weeks. During week 8, patients rested. The schedule then continued on a 4-week rotation in which patients received treatment for 3 weeks and rested during week 4. Patients received the combination folfirinox regimen every 2 weeks. Oxaliplatin was delivered intravenously (85 mg/m2) for 2 hours, followed immediately by an intravenous infusion of leucovorin (400 mg/m2) for 2 hours. Thirty minutes after the leucovorin administration, an additional intravenous infusion of irinotecan was administered (180 mg/m2) for 90 minutes. The irinotecan was immediately followed by 5fu, first in an intravenous bolus infusion (400 mg/m2) and then in a continuous intravenous infusion (2400 mg/m2) over a 46-hour period. Responding patients were recommended to receive a 6-month period of chemotherapy (that is, 12 cycles) and were followed every 3 months until death8.
2.3. Decision Analytic Model
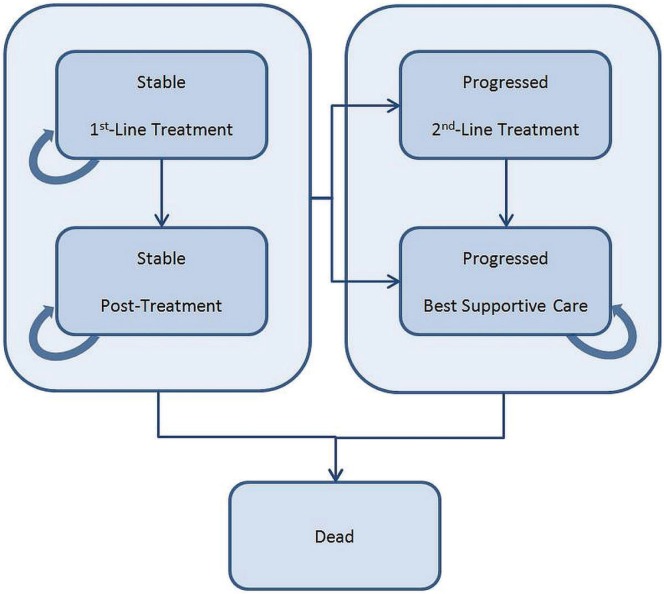
A Markov state transition model (Figure 1) simulated the movement of a hypothetical cohort of mpc patients from commencement of first-line treatment to death. One arm of the model considered treatment starting with first-line folfirinox, with a proportion of the patients receiving second-line gemcitabine after progression. The other arm considered treatment starting with first-line gemcitabine, with a proportion of the patients receiving either second-line platinum-based chemotherapy (analysis 1) or bsc after progression (analysis 2).
FIGURE 1.
Markov economic model. Simulation represents the transitions of the hypothetical cohort of metastatic pancreatic cancer patients through various health states from commencement of first-line treatment to death. Treatment arms in the model consisted of first-line folfirinox (5-fluorouacil, leucovorin, oxaliplatin, irinotecan) and first-line gemcitabine respectively, and both arms considered second-line treatment. Each model cycle is 1 week.
Patients began in the Stable–1st-Line Treatment state. During each model cycle (1 week), patients could reside in one of the Stable states, move to one of the Progressed states, or move to the Dead state (Figure 1). The process was repeated until the entire initial cohort resided in the Dead state. Patients were attributed survival, quality-adjusted survival, and costs for each cycle spent in a given state. Adverse event costs and utility decrements were also incorporated into the model.
The pfs data from accord informed the movement of patients between the Stable and Progressed states. Movement from any living state (that is, Stable or Progressed) to the Dead state was based on os data. The number of patients in the Progressed state were calculated by subtracting the number of patients in the Dead state from the number of patients in the Stable state. The time horizon for the model was a lifetime.
2.4. Effectiveness Assessment
Treatment effectiveness was summarized in terms of quality adjusted life-years (qalys) so as to capture both survival and health-related qol. The qaly is a composite measure that combines the length of a patient’s life with the qol that a patient experiences. Effectiveness was also summarized in terms of life-years (lys), which does not take into account qol.
2.4.1. OS and PFS
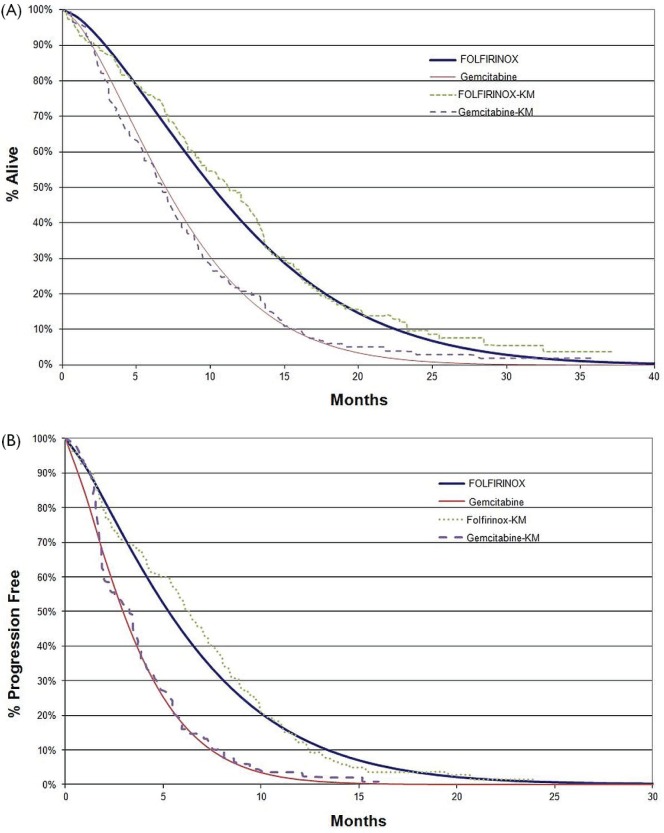
The os and pfs were based on fitted Weibull curves generated from accord (Figure 2). Four curve types—Weibull, Gompertz, logistic, and exponential—were fitted to the data. Reliability data were summarized by plotting the cumulative distribution function estimates against time using a log–log scale and fitting a straight line. The Weibull curve was chosen based on the values for R2 (0.9956) and residual sum of squares (0.2392). The Weibull curves for folfirinox were close to the original Kaplan–Meier curves from accord, with a slight skewing to the left, which underestimates os and pfs for folfirinox. That closeness was confirmed by an analysis of the area under the curve for os in both treatment groups, with the difference between folfirinox and gemcitabine being 3.65 months and 4.04 months for the Weibull and Kaplan–Meier curves respectively. Similarly, the difference in pfs between the treatment groups was very minimal—2.87 months and 2.89 months for the Weibull and Kaplan–Meier curves respectively.
FIGURE 2.
(A) Overall and (B) progression-free survival curves for first-line treatment of metastatic pancreatic cancer with gemcitabine and folfirinox (5-fluorouacil, leucovorin, oxaliplatin, irinotecan). Kaplan–Meier (KM) estimates come from the accord trial; fitted Weibull curves are also presented.
2.4.2. QOL Associated with Disease States
Patients in the Stable state are assumed to have better qol than those in the Progressed state. Utility values are preference weights that can be used to quantify qol (as a value between 0 and 1) in each state.
Utility values were not collected in accord and are therefore derived from the available literature. Values were obtained from Romanus et al.11, who reported utilities for both stable and progressed pancreatic cancer patients treated with gemcitabine. In that study, patients with advanced pancreatic cancer participating in the Cancer and Leukemia Group B multicentre double-blind randomized 80303 trial, which compared os in two treatment arms (gemcitabine plus bevacizumab, gemcitabine plus placebo), were evaluated on their health-related qol using the EuroQol instrument (EQ-5D) at baseline and at 8 weeks. The utility value for patients with stable disease was 0.79 at baseline and 0.81 at 8 weeks. For patients with progressive disease, the values were 0.77 and 0.7311. Our analysis therefore made use of the average utility value for stable disease (that is, 0.80) and the lower value from the range reported for progressive disease (that is, 0.73), given that the 8-week value would be representative of the patients in our model, being carried through progressive disease until death. Similarly, Romanus et al.11 reported the utility value for a partial response as 0.83, and that value was also incorporated into our model (Table i).
TABLE I.
Summary of key input parameters
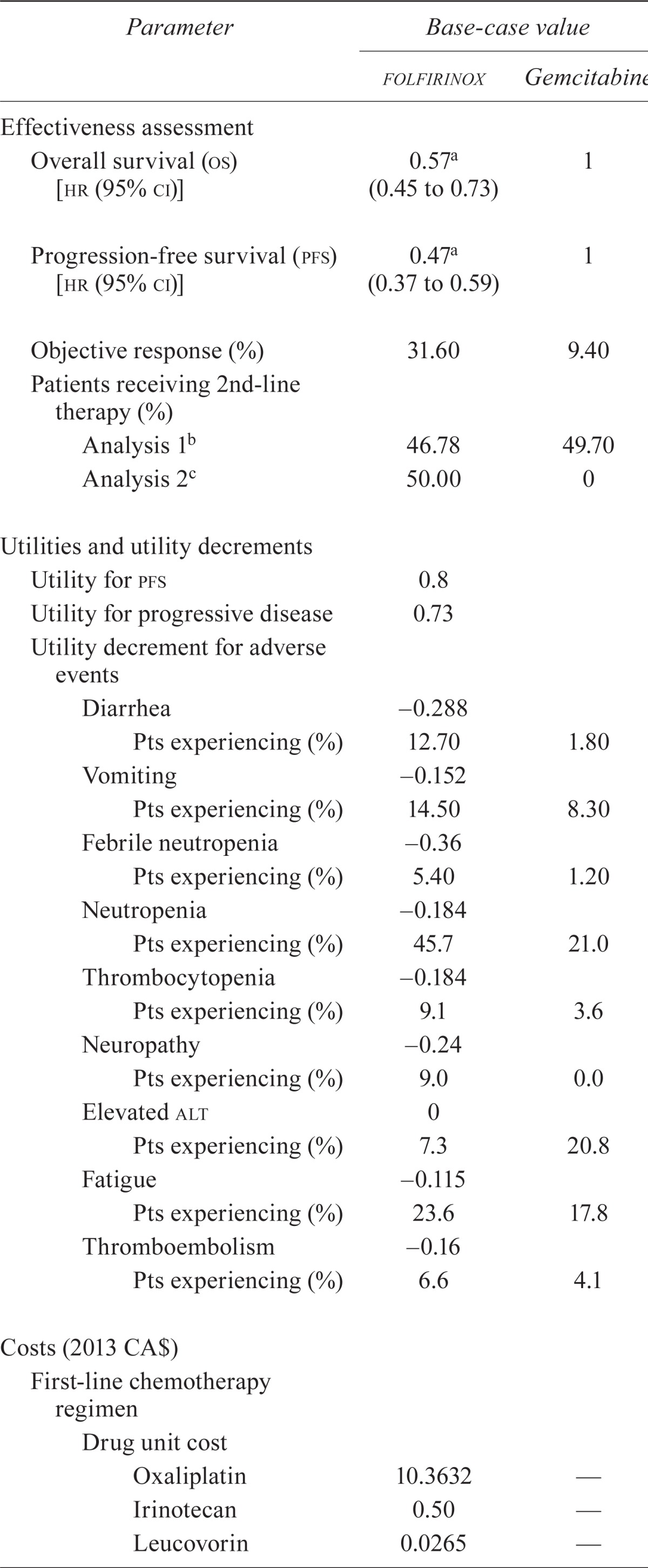
| Parameter |
Base-case value
|
|
|---|---|---|
| folfirinox | Gemcitabine | |
| Effectiveness assessment | ||
| Overall survival (os) [hr (95% ci)] | 0.57a (0.45 to 0.73) | 1 |
| Progression-free survival (pfs) [hr (95% ci)] | 0.47a (0.37 to 0.59) | 1 |
| Objective response (%) | 31.60 | 9.40 |
| Patients receiving 2nd-line therapy (%) | ||
| Analysis 1b | 46.78 | 49.70 |
| Analysis 2c | 50.00 | 0 |
| Utilities and utility decrements | ||
| Utility for pfs | 0.8 | |
| Utility for progressive disease | 0.73 | |
| Utility decrement for adverse events | ||
| Diarrhea | −0.288 | |
| Pts experiencing (%) | 12.70 | 1.80 |
| Vomiting | −0.152 | |
| Pts experiencing (%) | 14.50 | 8.30 |
| Febrile neutropenia | 0.36 | |
| Pts experiencing (%) | 5.40 | 1.20 |
| Neutropenia | −0.184 | |
| Pts experiencing (%) | 45.7 | 21.0 |
| Thrombocytopenia | −0.184 | |
| Pts experiencing (%) | 9.1 | 3.6 |
| Neuropathy | −0.24 | |
| Pts experiencing (%) | 9.0 | 0.0 |
| Elevated alt | 0 | |
| Pts experiencing (%) | 7.3 | 20.8 |
| Fatigue | −0.115 | |
| Pts experiencing (%) | 23.6 | 17.8 |
| Thromboembolism | 0.16 | |
| Pts experiencing (%) | 6.6 | 4.1 |
| Costs (2013 CA$) | ||
| First-line chemotherapy regimen | ||
| Drug unit cost | ||
| Oxaliplatin | 10.3632 | — |
| Irinotecan | 0.50 | — |
| Leucovorin | 0.0265 | — |
| Fluorouracil | 0.004 | — |
| Gemcitabine | — | 0.07 |
| Total drug costs per cycle | 1,737.18 | 122.50 |
| Relative dose index (rdi) | 0.8 | 1.0 |
| rdi adjusted drug cost | 1,389.74 | 122.50 |
| Co-medication drug costs | 52.92 | 10.90 |
| Monitoring and administration cost | 190.55 | 67.56 |
| Total cost per cycle | 1,633.21 | 200.96 |
| Second-line platinum-based chemotherapy regimen (per cycle) | ||
| folfox | 1,574.08 | |
| gemox | 1,936.06 | |
| 5-Fluorouracil/cisplatin | 92.31 | |
| folfirinox | 1,737.18 | |
| Weighted averaged | 1,378.03 | |
| Costs to treat adverse events (grade 3 or 4)e | ||
| Hospitalization for diarrheaf | 7,867 | |
| Hospitalization for vomitingg | 3,467 | |
| Hospitalization for thromboembolismh | 8,451 | |
| Hospitalization for febrile neutropeniai | 6,324 | |
| Cost of g-csf [2013 CA$ (% pts treated)] | ||
| Analysis 1 | 4,411j (42.5) | 4,411j (5.3) |
| Analysis 2 | — (0) | — (0) |
p < 0.001.
Based on accord intention-to-treat patient populations (n=171).
Based on current Ontario treatment patters and the hypothetical model cohort (n=1000).
Based on 56.0%, 20.0%, 18.7%, and 5.3% for folfox, gemox, 5-fluorouracil/cisplatin, and folfirinox respectively.
Percentage assumed based on clinical expert opinion.
Cost based on the assumption that 50% of patients with diarrhea are hospitalized12.
Cost based on the assumption that 10% of patients with vomiting are hospitalized12.
Cost based on the assumption that 25% of patients with thromboembolism are hospitalized12.
Cost based on the assumption that 100% of patients with febrile neutropenia are hospitalized13.
Represents the cost of granulocyte colony–stimulating factor for a mean of 21.44 days at a dose of 0.300 mg daily14.
folfirinox = 5-flourouracil, leucovorin, oxaliplatin, irinotecan; hr = hazard ratio; ci = confidence interval; pts = patients; alt = alanine aminotransferase; folfox = 5-flourouracil, leucovorin, oxaliplatin; gemox = gemcitabine, oxaliplatin; g-csf = granulocyte colony–stimulating factor.
2.4.3. QOL Associated with Adverse Events and Chemotherapy Toxicities
The effect of adverse events (aes) on qol was incorporated by applying utility decrements to patients who experienced grade 3 or 4 diarrhea, vomiting, febrile neutropenia, neutropenia, thrombocytopenia, peripheral neuropathy, fatigue, and elevated alanine aminotransferase. The proportion of patients experiencing aes was based on accord (Table i). Utility decrements associated with aes after chemotherapies for cancer were extracted from the literature. The utility decrements used in our model had previously been described in studies evaluating the disutilities associated with chemotherapy-induced aes in cancer patients using elicitation methods such as the standard gamble15–17 (Table i). The disutilities were applied conservatively to each arm for a duration of 20 weeks for folfirinox and 12 weeks for gemcitabine. The assumption is conservative, because aes are unlikely to persist for the entire treatment duration.
2.4.4. Objective Response
Based on the qol study reported by Romanus et al.11, objective response was assigned a utility advantage compared with stable disease (+0.03)11. The objective response for patients treated with folfirinox (31.6%) was significantly greater (p < 0.001) than that for patients treated with gemcitabine (9.4%)8.
2.5. Cost and Resource Utilization
Costs were obtained predominantly from Ontario and reported in 2013 Canadian dollars.
2.5.1. Costs of Chemotherapy Treatment
Chemotherapies were assumed to be administered in an outpatient clinic setting. Drug costs came from publically available data—that is, the Ontario Drug Benefit Program18, the Ontario Case Costing Initiative12, and public cost-effectiveness reports19, among others. Health care costs—laboratory work, physician visits, diagnostic tests, and so on—were obtained from the Ontario Health Insurance Plan Schedule of Benefits and Fees12,20.
Costs for each treatment arm were based on the treatment regimens delivered in accord and described in Conroy et al.8. The model was calibrated to predict a mean number of cycles of 9.65 for folfirinox and 6.93 for gemcitabine. Those values closely resemble the actual mean number of cycles administered in accord [that is, 9.47 and 6.93 respectively (Conroy T. Personal communication, 2013)]. The relative dose intensity (rdi) was reported to be 0.82 for 5fu, 0.81 for irinotecan, and 0.78 for oxaliplatin. An average rdi of 0.8 for all components of the folfirinox regimen was therefore conservatively applied. For gemcitabine, the reported rdi was 1.00. In addition to costs for the chemotherapy drug or drugs, the costs of intravenous infusion, oncologist visits, and monitoring tests and procedures were included. The per-cycle costs of first-line folfirinox and gemcitabine were therefore $1,633.21 and $200.96 respectively (Table i). A lack of wastage was assumed, given that, with an average body surface area of 1.75 m2, 99% of a vial would be used. The cost of the drug is therefore based on the actual dose required per patient, and any remaining drug was assumed to be stored and utilized for another patient.
For patients treated with a second-line platinum-based chemotherapy regimen, a weighted cost of $1,378.03 based on folfox (leucovorin, 5fu, oxaliplatin), gemox (gemcitabine, oxaliplatin), 5fu and cisplatin, and folfirinox as reported in accord8 was assumed per cycle. The rdis for those regimens were not available and were therefore assumed to be 0.8 (Table i).
2.5.2. Costs Associated with Adverse Events
Treatment-related grade 3 or 4 aes occurring in more than 5% of patients and considered clinically significant based on expert opinion were included (Table i). Those events were febrile neutropenia, neutropenia, thrombocytopenia, thromboembolism, neuropathy, fatigue, elevated alanine aminotransferase, diarrhea, and vomiting8. Clinical advice was sought to estimate the percentage of patients with febrile neutropenia, diarrhea, and vomiting who would be hospitalized. Only the costs associated with hospitalization for those aes were included12 (Table i). Based on clinical opinion, thrombocytopenia, peripheral neuropathy, fatigue, and elevated alanine aminotransferase would be treated in Ontario with chemotherapy dose reductions.
2.5.3. Costs Associated with G-CSF
The accord trial reported that 42.5% of folfirinox patients and 5.3% of gemcitabine patients received g-csf during the course of the trial8. It was assumed that most of this use was for treatment of neutropenia after chemotherapy. In Analysis 1, the cost of g-csf treatment was included in the cost of aes for 42.5% of folfirinox patients and 5.3% of gemcitabine patients (Table i). The median length of administration in the velour trial was 11.00 days (Conroy T. Personal communication, 2013). However, to reflect mean g-csf costs, the total cost of g-csf treatment used in the analysis was calculated for 21.44 days14 to reflect the mean length of administration in patients receiving g-csf in the velour trial. The use of the mean length of administration instead of the median is conservative, given that the mean appears to be skewed, and its use in the analysis might overestimate the cost of g-csf. In reality, the length of g-csf administration might fall between those two values. Accordingly, to provide a range of plausible icers, the total g-csf costs associated with the median length of administration were evaluated in a sensitivity analysis.
In Ontario, g-csf is not readily reimbursed in a noncurative setting as a treatment for chemotherapy-induced neutropenia. Chemotherapy dose adjustments are preferred, and the cost of g-csf treatment was therefore omitted in Analysis 2 (Table i).
2.6. Sensitivity Analyses
Sensitivity analyses were conducted to test the robustness of the cost-effectiveness model, and the impact of the input variables on the results. One-way sensitivity analyses were conducted on parameters such as rdi, g-csf treatment, duration of treatment, utilities, and costs (Table ii). In addition, alternative values for health state utilities were specifically evaluated to assess the effect of lower utilities for stable and progressive disease. Health state utility values reported by Mϋller–Nordhorn and colleagues from their study evaluating the EQ-5D utility and European Organization for Research and Treatment of Cancer (eortc) values for pancreatic cancer patients were therefore included in the sensitivity analysis for this model21. It is important to note that the eortc values were similar to the baseline eortc values reported by Gourgou–Bourgade and colleagues10 that describe the qol data from accord. An average of the male and female English utility values for stable disease from Mϋller–Nordhorn et al. was therefore used. Given that a value for progressive disease was not provided, a decrement similar to that observed in the study reported by Romanus et al. (that is, −0.07) was applied to obtain a value for progressive disease11.
TABLE II.
Deterministic results of cost-effectiveness
| Parameter (per patient) |
Base-case value
|
|||
|---|---|---|---|---|
| Analysis 1 | Analysis 2 | |||
|
|
|
|||
| folfirinox | Gemcitabine | folfirinox | Gemcitabine | |
| Life years (lys) | 0.974 | 0.670 | 0.974 | 0.670 |
| Stable disease | 0.540 | 0.301 | 0.540 | 0.301 |
| Progressive disease | 0.434 | 0.369 | 0.434 | 0.369 |
| Quality-adjusted life years (qalys) | 0.752 | 0.510 | 0.752 | 0.510 |
| Costs (2013 CA$) | ||||
| First-line therapy | 13,404 | 849 | 13,404 | 849 |
| First-line monitoring | 2,735 | 1,052 | 2,735 | 1,052 |
| First-line adverse events | 2,913 | 500 | 1,038 | 266 |
| Second-line therapy | 573 | 2,192 | 613 | 0 |
| Second-line monitoring | 316 | 379 | 338 | 0 |
| Second-line adverse events | 234 | 1,448 | 133 | 0 |
| bsc during progression | 927 | 787 | 927 | 787 |
| Total | 21,103 | 7,207 | 19,188 | 2,955 |
| Incremental (undiscounted) | ||||
| lys | 0.304 | 0.304 | ||
| qalys | 0.241 | 0.241 | ||
| Costs (2013 CA$) | 13,896 | 16,233 | ||
| Cost (2013 CA$) | ||||
| Per ly (undiscounted) | 45,653 | 53,331 | ||
| Per qaly (undiscounted) | 57,600 | 67,289 | ||
| Per ly (discounted) | 45,877 | 53,623 | ||
| Per qaly (discounted) | 57,858 | 67,626 | ||
folfirinox = 5-flourouracil, leucovorin, oxaliplatin, irinotecan; bsc = best supportive care.
Probabilistic sensitivity analyses were performed to simultaneously capture the uncertainty in model parameters. The pfs, os, disease state utilities, and cost inputs (that is, costs of ae management, first- and second-line treatments, and so on) were simultaneously varied using a second-order Monte Carlo simulation. The 95% confidence intervals around the hazard ratios for os and pfs (folfirinox compared with gemcitabine) were reported in accord, and a logged distribution was assumed. A beta distribution was assumed for the utility estimates. The weighted average and standard error for the reported utilities from Romanus et al.11 were used to derive the parameters for the beta distribution.
3. RESULTS
3.1. Effectiveness
The primary and secondary outcome measures from accord indicate that when folfirinox is compared with gemcitabine for first-line chemotherapy, both os and pfs show a significant increase (Figure 2)8. Those increases translate into gains, without discounting, of 0.304 lys and 0.241 qalys (Table ii). With discounting, the gains are 0.300 lys and 0.238 qalys.
Effectiveness results from analysis 1 and analysis 2 are identical, because the difference in the analyses pertain to treatment practices, which are assumed to affect only costs and not the effectiveness of the treatments.
3.2. Cost Outcomes
The cost estimates for analysis 1 and analysis 2 (Table ii) differ because of varying assumptions about treatment practices. Analysis 1 corresponds to the treatment provided in accord, and analysis 2 corresponds to treatment patterns at the time of submission in Ontario. As expected, the cost of first-line chemotherapy was not different in the two analyses. The cost of treating first-line aes differed because analysis 1 included the cost of g-csf. In analysis 2, no costs were associated with second-line therapy after gemcitabine because second-line therapy is bsc alone. For both analyses, bsc costs were applied for all patients in the progressed state until death in both the folfirinox and the gemcitabine arms.
For patients treated with first-line folfirinox, the largest cost was that for the chemotherapy drugs ($13,404), followed by monitoring and administration ($2,735). When g-csf was included (that is, in analysis 1), the cost of ae care was also a large contributor ($2,913). Second-line treatment for those patients is not a large cost because gemcitabine is not costly in Ontario.
For patients treated with first-line gemcitabine, the cost of first-line chemotherapy is approximately $1,000, plus an additional $1,052 for administration and monitoring. Should a platinum-based chemotherapy be a treatment option for patients after first-line gemcitabine (that is, as in analysis 1), the patient’s second-line treatment exceeds the cost of their first-line care.
3.3. Cost-Effectiveness
The base-case analysis demonstrated that first-line folfirinox was more effective than first-line gemcitabine, but also more costly. Table ii shows the cost per ly and qaly for first-line folfirinox and first-line gemcitabine. When mimicking treatment patterns in accord (analysis 1), the cost per qaly with folfirinox was $57,858. When mimicking practice patterns in Ontario (analysis 2), the cost per qaly was $67,626.
3.4. Sensitivity
3.4.1. Deterministic Sensitivity Analyses
Table iii summarizes the results of the sensitivity analyses. Most of the parameters tested suggest that the incremental cost per qaly for folfirinox compared with gemcitabine does not exceed $70,000.
TABLE III.
Deterministic sensitivity analyses
| Parameter |
Value used in the analysis
|
icer analysis (2013 CA$) | |||||
|---|---|---|---|---|---|---|---|
| Base case | Alternate | 1a | 2b | ||||
|
|
|
||||||
| A | B | Alternate | Alternate | ||||
| A | B | A | B | ||||
| Discounting (%) | 5 | 0 | 3 | 57,600 | 57,756 | 67,289 | 67,493 |
| Relative dose index | |||||||
| folfirinox | 0.8 | 1 | 0.7 | 69,604 | 51,985 | 81,666 | 60,606 |
| Gemcitabine | 1 | 0.9 | 0.8 | 57,975 | 58,092 | 67,727 | 67,828 |
| Maximum cycles (n), per the model, of | |||||||
| First-line folfirinox (2-week cycle) and gemcitabine (1-week cycle) | 13 and 9 | 12 and 26 | — | 52,004 | — | 61,741 | — |
| Second-line gemcitabine (after 1st-line folfirinox) | 10 | 9 | 6 | 57,487 | 56,372 | 67,229 | 66,039 |
| Patients receiving 2nd-line treatment (%) | |||||||
| folfirinox | 46.8/50 | 50 | 40 | 58,077 | 60,460 | 54,624 | 56,320 |
| Gemcitabine | 49.7/0 (analysis 1/2) | 50 | 40 | ||||
| Hazard ratio for overall survival | 0.57 | 0.45 | 0.73 | 38,420 | 105,004 | 44,928 | 122,678 |
| Alternate health state utilities | |||||||
| Stable | 0.8 | 0.65 | — | 64,192 | — | 75,029 | — |
| Progressed | 0.73 | 0.58 | — | ||||
| Adverse event disutilities | |||||||
| folfirinox | −0.119 | −0.143 | −0.095 | 57,954 | 57,763 | 67,738 | 67,515 |
| Gemcitabine | −0.016 | −0.020 (−20%) | −0.013 (+20%) | ||||
| Duration of g-csf administration (days) | 21.44 (mean) | 11.00 (median) | — | 56,180 | — | — | — |
Base-case cost per quality-adjusted life year: $57,858.
Base-case cost per quality-adjusted life year: $67,626.
icer = incremental cost-effectiveness ratio; folfirinox = 5-flourouracil, leucovorin, oxaliplatin, irinotecan; g-csf = granulocyte colony–stimulating factor.
3.4.2. Probabilistic Sensitivity Analysis
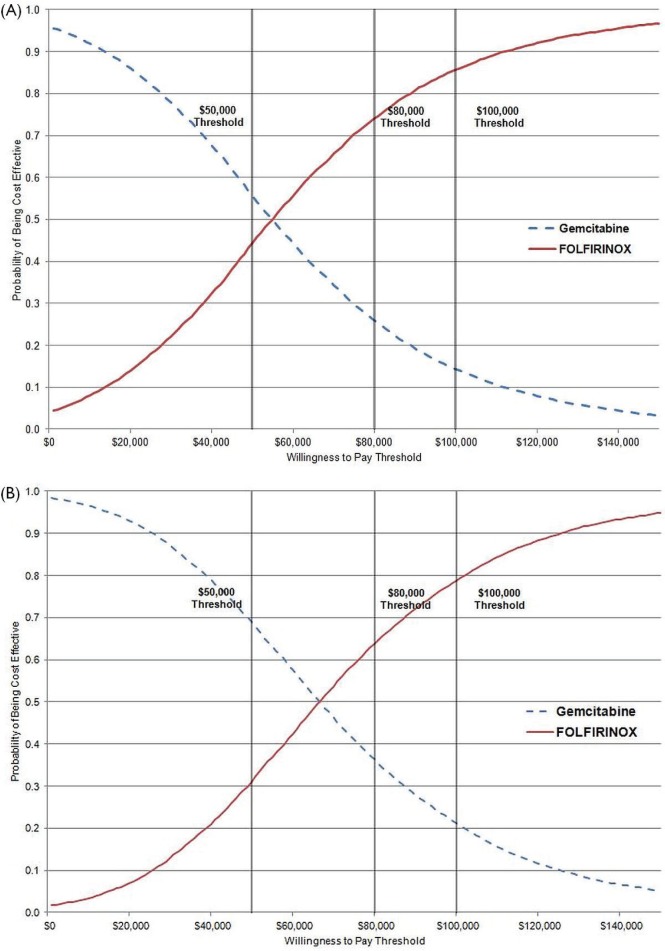
The cost-effectiveness acceptability curve used to represent the results of the Monte Carlo simulation shows the probability that a therapy is cost-effective (y axis) compared with the alternative for a range of maximum acceptable cost-effectiveness ratios (x axis). The cost-effectiveness acceptability curve for analysis 1 [Figure 3(A)] shows that folfirinox has a greater than 85% probability of being cost-effective at a threshold of $100,000. The cost-effectiveness acceptability curve for analysis 2 [Figure 3(B)] shows that folfirinox has an almost 80% probability of being cost-effective at a threshold of $100,000. The $100,000 threshold was chosen based on Canadian empirical evidence showing that cost-effectiveness ratios above that range start to negatively effect oncology drug funding recommendations—albeit with some exceptions22.
FIGURE 3.
Cost-effectiveness acceptability curves comparing folfirinox (5-fluorouacil, leucovorin, oxaliplatin, irinotecan) with gemcitabine. (A) Analysis 1. (B) Analysis 2.
4. DISCUSSION
Great excitement has surrounded the positive results of accord23–25, leading to rapid introduction of folfirinox into practice. Early adoption was observed after release of the preliminary results at the 2010 meeting of the American Society of Clinical Oncology, with a U.S. study estimating that oncologists had created prescribing plans that included folfirinox for 18% of patients23,26. But the criteria for adoption of a new therapy should consider both the clinical benefits and the economic value of the treatment. Given that the incremental icers for folfirinox (approximately $58,000 and $68,000 for analyses 1 and 2 respectively) fall below commonly quoted acceptable icer thresholds for oncology products, there is also strong economic support for adoption22.
The icers are driven by the important os differences observed. When variables in the model were adjusted by 20% in each direction, the model drivers were
the os hazard ratio,
the cost of the folfirinox drugs,
the rdi of folfirinox,
the number of cycles of folfirinox, and
the utility values.
Our analysis also demonstrated that, although Canadian costs and practice patterns might vary from those observed in other regions, assumptions about treatment patterns vary the results only marginally. However, it is important to note that, in analysis 2, omitting the costs for g-csf would favour the folfirinox regimen, and yet omitting the second-line post-gemcitabine costs and assuming bsc did not favour folfirinox; it had a more pronounced impact on the icer than the exclusion of g-csf costs.
Benefits of folfirinox have been observed in patients 76 years of age and younger with good performance status8. Because of the safety profile of folfirinox (which, compared with gemcitabine, is associated with a higher risk of toxicity), reserving treatment for patients with a good performance status is highly recommended. Nevertheless, compared with patients treated with gemcitabine, those treated with folfirinox experience significantly greater qol. Results from accord indicate that 31% of patients in the folfirinox group, compared with 66% in the gemcitabine group, experienced a definitive decrease in global health status and qol scores (hazard ratio: 0.47; 95% confidence interval: 0.30 to 0.70; p < 0.001)8. Similarly, Gourgou–Bourgade and colleagues10 reported a comparison of qol using the eortc qlq-C30 every 2 weeks. Results of the study indicated that qol impairment was significantly reduced with folfirinox than with gemcitabine, particularly with respect to time until definitive deterioration in overall global health status, physical, role, cognitive, and social functioning, plus 6 additional symptom domains (p < 0.001)10.
Key assumptions were made that may represent potential limitations of the analysis. For example, the analysis relied on the literature and expert opinion to provide estimates of utility values, resource use, and costs. Although the sensitivity analyses suggested that the results were robust to changes in those parameters, the inclusion of real-life data would strengthen the analysis. Recently, folfirinox has been funded, and studies are ongoing to further evaluate the way it is used and its efficacy and safety in clinical practice. In fact, a Canadian registry of folfirinox in advanced or metastatic pancreatic cancer is ongoing to collect and assess real-life data on folfirinox delivery, safety, and outcomes in clinical practice27. The present cost-effectiveness analysis should be repeated once the “real world” data become available. Because the difference in os was the key driver in the model, real-life data on the efficacy observed in light of possible changes in the administration of the folfirinox regimen (dose reductions, use of a bolus as opposed to a 5fu infusion) would provide an icer estimate that is better reflective of actual practice. Preliminary data indicate efficacy similar to that observed in the trial, thereby reducing uncertainty around the icer estimates in our analysis28,a. Rates of aes and rates of discontinuation with or without g-csf prophylaxis could further inform the cost-effectiveness and need for g-csf. Changes in the length of administration of g-csf were not a major driver in the model. When the median length of administration (11.00 days) instead of the mean (21.44 days) was used, the sensitivity analysis demonstrated that the icer in analysis 1 would decline to $56,180 per qaly. Finally, although not key drivers, real-life patient-level utility data could further strengthen confidence in the icer estimates.
The efficacy of folfirinox observed in accord has generated interest in its use in locally advanced pancreatic cancer and in adjuvant treatment. It will be interesting to evaluate the cost-effectiveness of the regimen in these new indications once supportive clinical data are available. Considering the icer for folfirinox in first-line mpc treatment, and assuming comparable efficacy in the new indications (generally associated with longer os), it could be hypothesized that the cost-effectiveness ratio might be even lower.
5. CONCLUSIONS
Gemcitabine monotherapy has been the standard of care for the first-line treatment of mpc, with a median life expectancy of approximately 6 months, but the use of first-line folfirinox significantly extends life expectancy by more than 4 months to a median os of 11.1 months. Given the favourable cost per qaly of approximately $58,000 and $68,000 and the impressive os and qol benefits of folfirinox over current treatment options, folfirinox represents an attractive cost-effective treatment for mpc in patients with good performance status. Collectively, the availability of an effective treatment at reasonable cost has contributed to the reimbursement of folfirinox in a number of jurisdictions worldwide.
6. ACKNOWLEDGMENTS AND CONFLICT OF INTEREST DISCLOSURES
This research was conducted by Cornerstone Research Group Inc., an independent research organization located in Burlington, Ontario. Sanofi Canada was solely responsible for the funding of all components of this project. Portions of this analysis were presented in a poster (Attard CL, Brown S, Alloul K, Moore MJ. Cost-effectiveness of folfirinox for first-line treatment of metastatic pancreatic cancer) at the American Society of Clinical Oncology 2012 Gastrointestinal Cancers Symposium; January 19–21, 2012; San Francisco, California.
Dr. Malcolm Moore (Princess Margaret Hospital, Toronto, ON) was contracted for advice on clinical issues pertinent to this evaluation. The advisor was contacted during the study process as needed. The advisor signed a confidentiality agreement with Sanofi Canada and was provided with an honorarium for his time. The authors thank Sarah S. Hollmann for her contribution to the analyses and manuscript writing.
Footnotes
Conroy T. Randomized phase iii trial comparing folfirinox versus gemcitabine as first-line treatment for metastatic pancreatic adenocarcinoma: final analysis results of the prodige 4/accord 11 trial. Presented at the 2010 American Society of Clinical Oncology Annual Meeting; Chicago, IL, U.S.A.; June 4–8, 2010.
7. REFERENCES
- 1.Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. J Nat Rev Clin Oncol. 2010;7:163–72. doi: 10.1038/nrclinonc.2009.236. [DOI] [PubMed] [Google Scholar]
- 2.Canadian Cancer Society’s Steering Committee on Cancer Statistics . Canadian Cancer Statistics 2011. Toronto, ON: Canadian Cancer Society; 2011. [Google Scholar]
- 3.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 4.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607–20. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canadian Cancer Society’s Steering Committee on Cancer Statistics . Canadian Cancer Statistics 2012. Toronto, ON: Canadian Cancer Society; 2012. [Google Scholar]
- 6.Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–13. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 7.Heinemann V, Boeck S, Hinke A, Labianca R, Louvet C. Meta-analysis of randomized trials: evaluation of benefit from gemcitabine-based combination chemotherapy applied in advanced pancreatic cancer. BMC Cancer. 2008;8:82. doi: 10.1186/1471-2407-8-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conroy T, Desseigne F, Ychou M, et al. folfirinox versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:2347–25. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 9.Ko AH, Cella D. Achieving the best of both worlds. J Clin Oncol. 2013;31:3–4. doi: 10.1200/JCO.2012.46.4891. [DOI] [PubMed] [Google Scholar]
- 10.Gourgou–Bourgade S, Bascoul–Mollevi C, Desseigne F, et al. Impact of folfirinox compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: results from the prodige 4/accord 1 randomized trial. Clin Oncol. 2013;31:23–9. doi: 10.1200/JCO.2012.44.4869. [DOI] [PubMed] [Google Scholar]
- 11.Romanus D, Kindler HL, Archer L, et al. Does health-related quality of life improve for advanced pancreatic cancer patients who respond to gemcitabine? Analysis of a randomized phase iii trial of the Cancer and Leukemia G roup B (calgb 80303) J Pain Symptom Manage. 2012;43:205–17. doi: 10.1016/j.jpainsymman.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ontario Ministry of Health and Long-Term Care (mohltc) Ontario Case Costing Initiative [Cost Analysis (CAT) Tool, Web resource] Toronto, ON: MOHLTC; 2011. [Available at: http://www.occp.com/mainPage.htm; cited March 15, 2013] [Google Scholar]
- 13.Lathia N, Mittmann N, DeAngelis C, et al. Evaluation of direct medical costs of hospitalization for febrile neutropenia. Cancer. 2010;116:742–8. doi: 10.1002/cncr.24773. [DOI] [PubMed] [Google Scholar]
- 14.Dryden DM, Fassbender K, Doucette K, et al. Granulocyte-Colony Stimulating Factor for Antiviral-Associated Neutropenia: Systematic Review and Economic Evaluation. Ottawa, ON: Canadian Agency for Drugs and Technologies in Health; 2008. [DOI] [PubMed] [Google Scholar]
- 15.Aballéa S, Chancellor JVM, Raikou M, et al. Cost-effectiveness analysis of oxaliplatin compared with 5-fluorouracil/leucovorin in adjuvant treatment of stage iii colon cancer in the US. Cancer. 2007;109:1082–9. doi: 10.1002/cncr.22512. [DOI] [PubMed] [Google Scholar]
- 16.Gould MK, Dembitzer AD, Doyle RL, Hastie TJ, Garber AM. Low-molecular-weight heparins compared with unfractionated heparin for treatment of acute deep venous thrombosis. A meta-analysis of randomized, controlled trials. Ann Intern Med. 1999;130:800–9. doi: 10.7326/0003-4819-130-10-199905180-00003. [DOI] [PubMed] [Google Scholar]
- 17.Lloyd A, Nafees B, Narewska J, Dewilde S, Watkins J. Health state utilities for metastatic breast cancer. Br J Cancer. 2006;95:683–90. doi: 10.1038/sj.bjc.6603326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ontario, Ministry of Health and Long-Term Care (mohltc) Ontario Public Drug Programs. Formulary: Drugs Funded by Ontario Drug Benefit (ODB) Program [Web resource] Toronto, ON: MOHLTC; 2009. [Google Scholar]
- 19.Health Quality Ontario KRAS testing for anti-egfr therapy in advanced colorectal cancer: an evidence-based and economic analysis. Ont Health Technol Assess Ser. 2010;10:1–49. [PMC free article] [PubMed] [Google Scholar]
- 20.Ontario, Ministry of Health and Long-Term Care (mohltc) Ontario Health Insurance (OHIP) Schedule of Benefits and Fees [choose Physician Services, Web resource] Toronto, ON: MOHLTC; 2009. [Google Scholar]
- 21.Müller–Nordhorn J, Roll S, Bohmig M, et al. Health-related quality of life in patients with pancreatic cancer. Digestion. 2006;74:118–25. doi: 10.1159/000098177. [DOI] [PubMed] [Google Scholar]
- 22.Ciapanna CC, Yunger S, Shum D, Milliken D, Longo CJ, Aissa F. Cost-effectiveness observations and oncology drug reimbursement recommendations in Canada by the Joint Oncology Review. Value Health. 2010;13:A51. doi: 10.1016/S1098-3015(10)72231-2. [DOI] [Google Scholar]
- 23.Oberstein PE, Saif MW. First-line treatment for advanced pancreatic cancer. Highlights from the “2011 asco Gastrointestinal Cancers Symposium.” San Francisco, CA, USA. January 20–22, 2011. JOP. 2011;12:96–100. [PubMed] [Google Scholar]
- 24.Ko AH. folfirinox: a small step or a great leap forward? J Clin Oncol. 2011;29:3727–9. doi: 10.1200/JCO.2011.37.3464. [DOI] [PubMed] [Google Scholar]
- 25.Kim R. folfirinox: a new standard treatment for advanced pancreatic cancer? Lancet Oncol. 2011;12:8–9. doi: 10.1016/S1470-2045(10)70237-0. [DOI] [PubMed] [Google Scholar]
- 26.Bendell JC, Britton S, Green MR, Willey J, Lemke KE, Marshall J. Immediate impact of the folfirinox phase iii data reported at the 2010 asco Annual Meeting on prescribing plans of American oncology physicians for patients with metastatic pancreas cancer (mpc) [abstract 286] J Clin Oncol. 2011;29 doi: 10.1200/JCO.2011.36.1980. [Available online at: http://meetinglibrary.asco.org/content/71496-103; cited December 4, 2013] [DOI] [Google Scholar]
- 27.Maroun J, Cripps C, Jonkers D, et al. A Canadian registry of folfirinox in advanced/metastatic pancreatic cancer. Ann Oncol. 2013;24(supp 4):iv82–3. doi: 10.1093/annonc/mdt203.161. [DOI] [Google Scholar]
- 28.Kris MG, Benowitz SI, Adams S, et al. Clinical cancer advances 2010: annual report on progress against cancer from the American Society of Clinical Oncology. J Clin Oncol. 2010;28:5327–47. doi: 10.1200/JCO.2010.33.2742. [DOI] [PubMed] [Google Scholar]