Abstract
Background
Delays in chemotherapy because of neutropenia may be associated with poorer outcomes. The purpose of the present study was to examine the effect that granulocyte colony–stimulating factors (g-csfs) have on survival.
Methods
We conducted a chart review of all outpatients diagnosed with metastatic colorectal cancer and treated with folfiri chemotherapy (irinotecan, 5-fluorouracil, leucovorin) with or without bevacizumab at Mount Sinai Hospital between 2007 and 2012. Multivariable Cox proportional hazards models were used to compare survival in neutropenic patients treated with g-csf, in neutropenic patients not so treated, and in patients without neutropenia.
Results
The review identified 93 patients, 31 of whom did not experience a neutropenic event. Of the 62 who experienced neutropenia, 18 were managed with g-csf support, and 44, with reductions or delays in dose. Compared with patients experiencing a neutropenic episode not treated with g-csf, those treated with g-csf experienced a nonsignificant increase in time to event [progression or death: hazard ratio (hr): 1.37; 95% confidence limits (cl): 0.72, 2.61], but compared with patients not having a neutropenic episode, the same patients experienced a significant increase in time to event (hr: 2.07; 95% cl: 1.03, 4.15).
Conclusions
In patients who experienced neutropenia, g-csf did not have a statistically significant impact on survival. Time to event was prolonged in g-csf–treated patients compared with patients who did not experience neutropenia.
Keywords: Colorectal cancer, g-csf, metastasis, neutropenia, progression, survival
1. INTRODUCTION
Myelosuppression is a common toxicity associated with chemotherapy, and it increases a patient’s susceptibility to serious infection. When neutropenia—an absolute neutrophil count less than 500/μL, or less than 1000/μL with a predicted decline to less than 500/μL over the next 48 hours—is accompanied by a single temperature reading of more than 38.2°C or a temperature consistently above 38°C for more than 1 hour, it is termed febrile neutropenia per the guidelines of the U.S. National Comprehensive Cancer Network1. Febrile neutropenia is considered a significant complication that increases mortality risk, hospitalization rates, and health care expenditures, and that might adversely affect quality of life2. Mortality rates associated with febrile neutropenia can range from 2% to 21% and are affected by patient-specific factors such as the malignancy type and comorbidities3,4. Kuderer et al. found that the presence of more than one major comorbidity such as heart failure or kidney disease was associated with a greater than 21% risk of mortality in patients with febrile neutropenia and that, as the number of comorbidities increased, so did the mortality risk. The mortality rate reached as high as 82% in patients with multiple comorbidities5.
Development of neutropenia while on chemotherapy may be dose-limiting and can result in dose reductions or treatment delays6. There is evidence to suggest that when at least 75% of the planned dose is given, benefit is seen in overall and progression-free survival7–11. However, when looking at survival outcomes, the evidence is conflicting, because other studies have not reported similar survival benefits12–14. When therapy is considered palliative, none of the three major guideline organizations—the American Society of Clinical Oncology, the European Organisation for Research and Treatment of Cancer, and the U.S. National Comprehensive Cancer Network—has made a definitive recommendation1,15,16.
One option to prevent neutropenia in cancer patients is the use of granulocyte colony–stimulating factors (g-csfs) such as filgrastim or pegfilgrastim. Use of g-csf has been proved to lower rates of febrile neutropenia; to reduce the incidence of infection, antibiotic use, and hospital admissions; and to allow for maintenance of dose intensity5,6,17–21. However, evidence about the benefit of these agents in overall and progression-free survival is conflicting5,20,22.
Therapy with g-csf is recommended as secondary prophylaxis in patients receiving chemotherapy with curative or adjuvant intent who have experienced dose delays or who have been hospitalized for neutropenia1,15,16,23. The American Society of Clinical Oncology, The European Organisation for Research and Treatment of Cancer, and the National Comprehensive Cancer Network all suggest that, when treatment is palliative, a less myelosuppressive chemotherapy dose or schedule should be considered.
Most of the available evidence supporting the use of g-csfs has occurred in the adjuvant setting for breast cancer and the first-line curative setting for lymphomas, where such use has lowered the risk of infection, associated complications, and length of hospital stay5,19–21. A recent study by Hecht et al.24 in patients with colorectal cancer showed that the use of pegfilgrastim (compared with placebo) significantly reduced the occurrence of grades 3 and 4 neutropenia and reduced the occurrence of dose delays or reductions, but provided no survival benefit.
The objective of the present study was to determine whether the use of g-csf to prevent chemotherapy dose reductions and delays has an impact on survival. Our hypothesis was that patients who received g-csf support as secondary prophylaxis would experience improved survival outcomes. That is, the time to progression or death would be longer in treated patients than in those not receiving g-csf support.
2. METHODS
2.1. Study Design and Population
This single-centre study was conducted at Mount Sinai Hospital, Toronto, Ontario, in the outpatient oncology clinic. Our retrospective chart review of all metastatic colorectal cancer patients managed by a single oncologist was approved by the Research Ethics Board of Mount Sinai Hospital. All metastatic colorectal cancer patients at our centre who were treated with folfiri chemotherapy (irinotecan, 5-fluorouracil, leucovorin) with or without bevacizumab25 during a 5-year period (January 1, 2007, to December 31, 2011) were included.
2.2. Outcomes and Measures
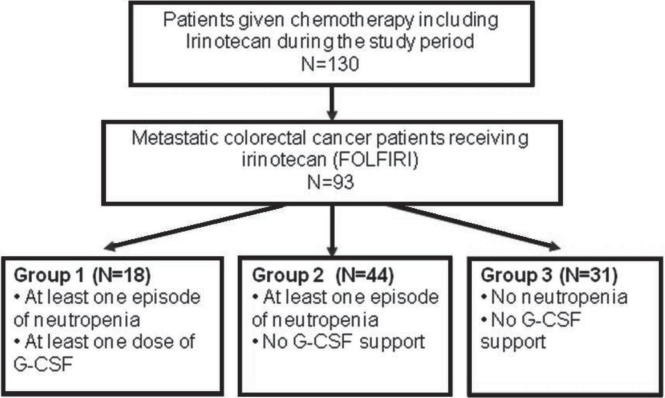
The study population was divided into three groups (Figure 1). Patients in group 1 had experienced at least 1 previous episode of neutropenia and were treated with g-csf. Patients in group 2 experienced a neutropenic episode but were not treated with g-csf (that is, they were managed with dose reductions or delays). Patients in group 3 did not experience any neutropenic episodes and therefore did not require g-csf support.
FIGURE 1.
Patients included in the study. folfiri = irinotecan, 5-f lourouracil, leucovorin; g-csf = granulocyte colony–stimulating factor.
In the initial screening, a list was generated of all patients treated during the study period with the chemotherapeutic agent irinotecan, as part of the folfiri regimen (with or without bevacizumab) that is commonly used as first- or second-line treatment in metastatic colorectal cancer. Date of chemotherapy treatment was determined by correlating the initial paper order with the electronic database. Treatment with g-csf (either filgrastim or pegfilgrastim) was determined by extracting data from the physician’s clinic notes and confirming with carbon copies of prescriptions in clinic charts. Eastern Cooperative Oncology Group (ecog) performance status (ps) scores26 were available in all patient files; Charlson age–comorbidity index scores27 were calculated by the data abstractor.
Days of event-free survival were calculated from the index date (first day of folfiri with or without bevacizumab) to the event date. The primary outcome of the study was a composite endpoint of time to progression or time to colorectal cancer–related death. Progression was determined by radiographically proven increases in lesion size or appearance of new lesions, at the discretion of the radiologist who compared computed tomography images taken at 2-month intervals (or sooner as indicated by signs and symptoms) at the discretion of the ordering physician. Death was defined as mortality caused directly by the malignancy or by complications secondary to the disease. People who died from other causes (for example, motor vehicle accident) were censored at the date of death. Patients who were lost to follow-up or who were alive at the end of the study period were censored at the date of their last clinic visit. Secondary outcomes included rates of neutropenia (defined as a neutrophil count less than 1.5×109/L) and febrile neutropenia, hospitalizations (any cause), infections requiring intervention (hospitalization or antibiotic therapy, or both), and maintenance of prescribed chemotherapy dose (measured by delay in days or by percentage decrease from the expected dose).
2.3. Data Collection
To maintain uniformity between abstractions, data were extracted from the paper and electronic medical records using a standardized case assessment form. The demographic data collected included age, sex, comorbidities, postal code (to assess socioeconomic status), family history, body surface area (height / weight), and allergy status. Clinical data collected included blood work on the index date, extent of disease (TNM staging), prior therapy, initial diagnosis and recurrence dates, dates of progression and death, neutropenic episodes, dose reductions or delays, g-csf use, bevacizumab use, and incidence of infection.
2.4. Data Analysis
All analyses were conducted using Stata/SE for Windows (version 11.2: StataCorp, College Station, TX, U.S.A.). After the data were assessed for abnormalities, chi-square tests, Student t-tests, and Kruskal–Wallis tests were used as appropriate to determine whether the baseline characteristics of the groups were comparable. Kaplan–Meier curves and median survival times were estimated by study group. To assess the associations between g-csf use, neutropenia, and risk of progression or death, we calculated hazard ratios (hrs) using Cox proportional hazards models and adjusting for other covariates. We reviewed the pattern of censoring to determine whether there was an association with survival time. All variables associated with survival at a p value of 0.20 or less on univariate analysis were entered into the full regression model, together with the grouping variable. Variables were removed, one at a time, based on p value, until the final, restricted model was determined. This process included the grouping variable and covariates associated at a p value of 0.05 or less. Any variable that produced a change in the hr of 20% or more, or that was significant when added back into the model one at a time, was retained. Although the number of folfiri cycles was associated with survival on univariate analysis, it was not included in the final model because of its dependence on outcome (days to progression or death). Continuous variables were split into categories to assess their linearity, and interaction terms were reviewed for inclusion in the model. Proportionality was assessed by plotting log[–log(survival)] by time for each stratum of each covariate28,29.
3. RESULTS
During the study period, 93 patients were diagnosed with metastatic colorectal cancer and began treatment with folfiri. Of those patients, 18 were treated with g-csf (group 1), 16 of whom had experienced at least 1 episode of neutropenia before folfiri treatment, including during prior chemotherapy. Another 44 patients experienced neutropenia, but were not treated with g-csf (group 2), and 31 had experienced no neutropenic episodes (group 3). The patients ranged in age from 28 to 86 years; ecog ps was 0 in 42 patients (45%), 1 in 45 patients (48%), 2 in 5 patients (5%), and 3 in 1 patient (1%). In 81 patients (87%), score on the Charlson age–comorbidity index was 10 or lower (indicating fewer concomitant illnesses). As shown in Table i, the groups were similar in age, sex, and other baseline variables.
TABLE I.
Baseline characteristics of the study patients
| Variable |
Neutropenia
|
p Value | ||
|---|---|---|---|---|
|
Yes
|
Yes
|
No
|
||
| g-csf | No g-csf | No g-csf | ||
| Patients (n) | 18 | 44 | 31 | |
| Mean age (years) | 62 (13) | 62 (13) | 60 (14) | 0.77 |
| Sex [n (%)] | ||||
| Men | 8 (44.4) | 26 (59.1) | 19 (61.3) | 0.48 |
| Women | 10 (55.6) | 18 (40.9) | 12 (38.7) | |
| Smoking history [n (%)] | 3 (16.7) | 17 (38.6) | 12 (38.7) | 0.21 |
| Positive family history [n (%)] | 3 (16.7) | 18 (40.9) | 13 (41.9) | 0.15 |
| Household income, 2006 ($) | ||||
| Median | 84,858 | 71,448 | 80,545 | 0.12 |
| Standard deviation | 20,874 | 25,545 | 28,231 | |
| Comorbidities | ||||
| Heart failure | 0 | 1 (2.3) | 0 | 1.00 |
| Lung disease | 1 (5.6) | 0 | 0 | 0.19 |
| Renal impairment | 2 (11.1) | 3 (6.8) | 1 (3.2) | 0.48 |
| Hepatic dysfunction | 0 | 1 (2.3) | 1 (3.2) | 1.00 |
| Diabetes | 4 (22.2) | 11 (25.0) | 3 (9.7) | 0.24 |
| Coronary artery disease | 0 | 5 (11.4) | 1 (3.2) | 0.17 |
| Gastroesophageal reflux disease | 0 | 1 (2.3) | 0 | 0.57 |
| Hypothyroidism | 0 | 3 (6.8) | 1 (3.2) | 0.67 |
| Atrial fibrillation | 0 | 2 (4.5) | 2 (6.5) | 0.56 |
| Venous thromboembolism | 1 (5.6) | 2 (4.5) | 6 (19.4) | 0.19 |
| Inflammatory bowel disease | 1 (5.6) | 2 (4.5) | 5 (16.1) | 0.17 |
| cci–age score > 10 | 2 (11.1) | 6 (13.6) | 4 (12.9) | 1.00 |
| ecog performance status | ||||
| 0 | 8 (44.4) | 17 (38.6) | 17 (54.8) | 0.38 |
| 1–3 | 10 (55.6) | 27 (61.4) | 14 (45.2) | |
| Tumour characteristics | ||||
| T2 | 1 (5.6) | 3 (6.8) | 4 (12.9) | 0.86 |
| T3 | 6 (33.3) | 24 (54.5) | 17 (54.8) | |
| T4 | 6 (33.3) | 14 (31.8) | 10 (32.3) | |
| N0 | 7 (38.9) | 15 (34.1) | 11 (35.5) | 0.89 |
| N1 | 3 (16.7) | 14 (31.8) | 10 (32.3) | |
| N2 | 5 (27.8) | 12 (27.3) | 10 (32.3) | |
| Locations of distant metastasis | ||||
| 1 | 8 (44.4) | 17 (38.6) | 10 (32.3) | 0.64 |
| 2 | 7 (38.9) | 23 (52.3) | 15 (48.4) | |
| ≥3 | 3 (16.7) | 4 (9.1) | 6 (19.4) | |
| Prior therapy | ||||
| Primary resection | 12 (66.7) | 27 (61.4) | 23 (74.2) | 0.51 |
| Adjuvant therapy | 6 (33.3) | 13 (29.5) | 13 (41.9) | 0.54 |
| folfiri as 1st-line therapy | 15 (83.3) | 36 (81.8) | 22 (71.0) | 0.48 |
| Bevacizumab use | 11 (61.1) | 17 (38.6) | 15 (48.4) | 0.26 |
| Outcome at study end | ||||
| Progressed | 11 (61.1) | 33 (75.0) | 22 (71.0) | 0.50 |
| Died before progression | 3 (16.7) | 8 (18.2) | 4 (12.9) | |
| Alive or lost to follow-up | 4 (22.2) | 3 (6.8) | 5 (16.1) | |
g-csf = granulocyte colony–stimulating factor; cci = Charlson comorbidity index; ecog = Eastern Cooperative Oncology Group; folfiri = irinotecan, 5-flourouracil, leucovorin.
The median duration of treatment was significantly longer for patients in groups 1 and 2 than for patients in group 3. Although patients given g-csf showed a trend toward a longer duration between initiation of chemotherapy and progression, the difference was not statistically significant (323 days in group 1 vs. 294 days in group 2, p = 0.47)
3.1. Survival Analysis
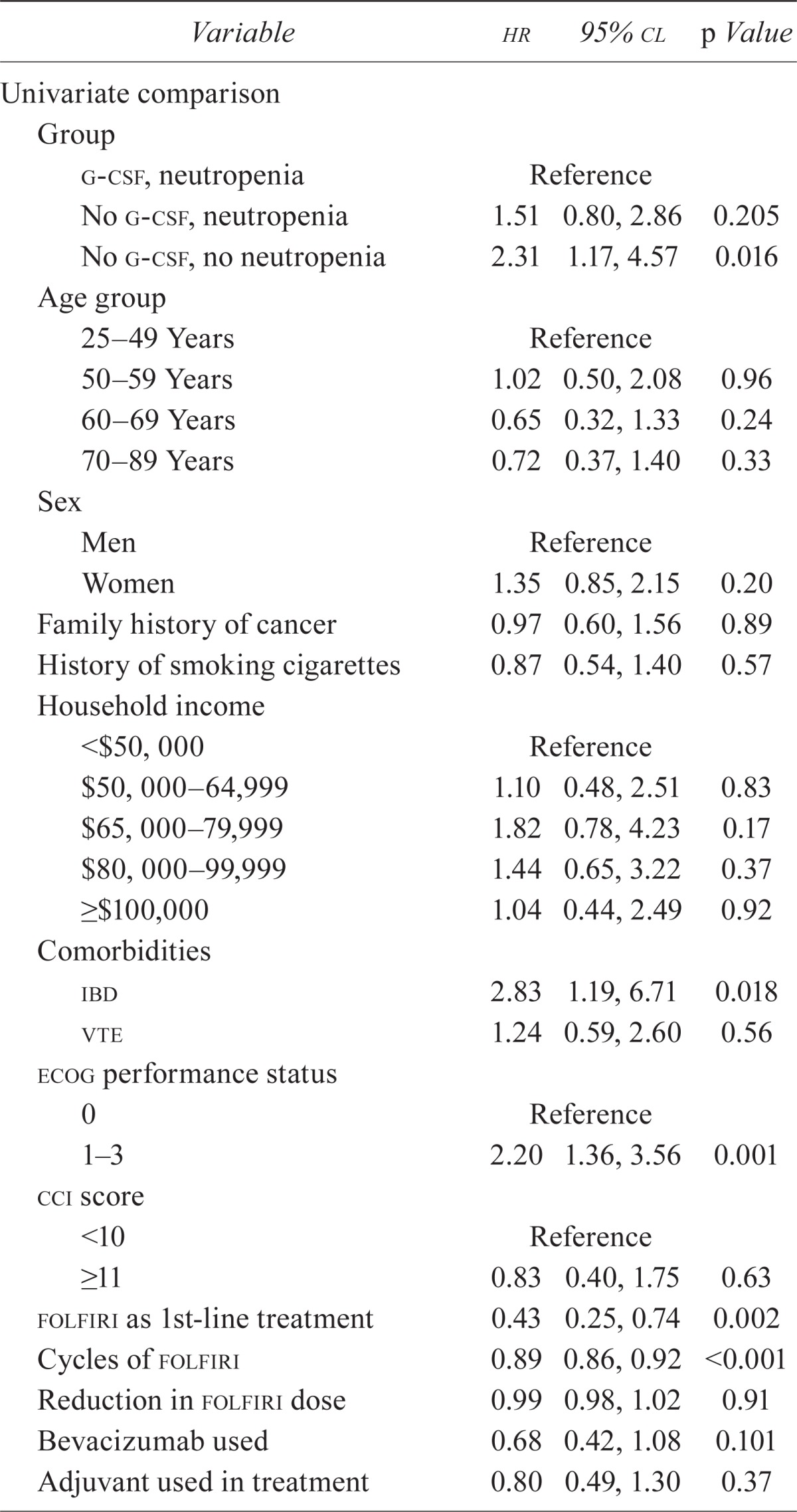
Median survival in these patients was 8.9 months (range: 0.9–43.3 months). On univariate analysis, no difference in the risk of an event (time to progression or death) was observed between patients in group 1 (hr: 1.00) and group 2 (hr: 1.51; 95% cl: 0.80, 2.86), but risk of an event was significantly higher for patients in group 3 (hr: 2.31; 95% cl: 1.17, 4.57) than for those in group 1.
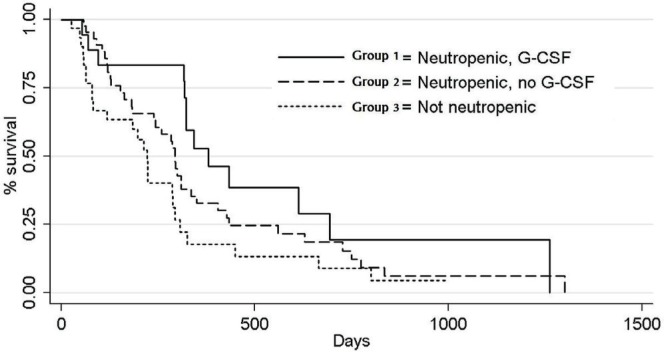
Figure 2 shows the Kaplan–Meier survival curves for the three groups. Groups 1 and 2 did not differ in time to the primary endpoint and neither did groups 2 and 3. But compared with group 3, group 1 had a significantly longer time to event. As Table ii shows, risk of an event was significantly higher in patients with inflammatory bowel disease than in those without (hr: 2.83; 95% cl: 1.19, 6.71), and patients with an ecog ps of 1 or more had a risk of an event that was more than double the risk for patients with a score of 0 (hr: 2.20; 95% cl: 1.36, 3.56). The risk of progression or death was 57% lower in patients who were treated with folfiri as first-line therapy (hr: 0.43; 95% cl: 0.25, 0.74) than in patients who did not receive that chemotherapy.
FIGURE 2.
Kaplan–Meier survival estimates. g-csf = granulocyte colony–stimulating factor.
TABLE II.
Cox proportional hazards analyses: univariate comparison and multivariable model of survival in patients with metastatic colorectal cancer
| Variable | hr | 95% cl | p Value |
|---|---|---|---|
| Univariate comparison | |||
| Group | |||
| g-csf, neutropenia | Reference | ||
| No g-csf, neutropenia | 1.51 | 0.80, 2.86 | 0.205 |
| No g-csf, no neutropenia | 2.31 | 1.17, 4.57 | 0.016 |
| Age group | |||
| 25–49 Years | Reference | ||
| 50–59 Years | 1.02 | 0.50, 2.08 | 0.96 |
| 60–69 Years | 0.65 | 0.32, 1.33 | 0.24 |
| 70–89 Years | 0.72 | 0.37, 1.40 | 0.33 |
| Sex | |||
| Men | Reference | ||
| Women | 1.35 | 0.85, 2.15 | 0.20 |
| Family history of cancer | 0.97 | 0.60, 1.56 | 0.89 |
| History of smoking cigarettes | 0.87 | 0.54, 1.40 | 0.57 |
| Household income | |||
| <$50, 000 | Reference | ||
| $50, 000 64,999 | 1.10 | 0.48, 2.51 | 0.83 |
| $65, 000 79,999 | 1.82 | 0.78, 4.23 | 0.17 |
| $80, 000 99,999 | 1.44 | 0.65, 3.22 | 0.37 |
| ≥$100,000 | 1.04 | 0.44, 2.49 | 0.92 |
| Comorbidities | |||
| ibd | 2.83 | 1.19, 6.71 | 0.018 |
| vte | 1.24 | 0.59, 2.60 | 0.56 |
| ecog performance status | |||
| 0 | Reference | ||
| 1–3 | 2.20 | 1.36, 3.56 | 0.001 |
| cci score | |||
| <10 | Reference | ||
| ≥11 | 0.83 | 0.40, 1.75 | 0.63 |
| folfiri as 1st-line treatment | 0.43 | 0.25, 0.74 | 0.002 |
| Cycles of folfiri | 0.89 | 0.86, 0.92 | <0.001 |
| Reduction in folfiri dose | 0.99 | 0.98, 1.02 | 0.91 |
| Bevacizumab used | 0.68 | 0.42, 1.08 | 0.101 |
| Adjuvant used in treatment | 0.80 | 0.49, 1.30 | 0.37 |
| Number of metastases | |||
| 1 | Reference | ||
| 2 | 0.95 | 0.58, 1.56 | 0.84 |
| ≥3 | 0.85 | 0.41, 1.74 | 0.65 |
| Tumour grade | |||
| 2 | Reference | ||
| 3 | 1.10 | 0.51, 2.37 | 0.81 |
| 4 | 0.74 | 0.32, 1.70 | 0.48 |
| Nodal involvement | |||
| 0 | Reference | ||
| 1 | 0.81 | 0.44, 1.49 | 0.50 |
| 2 | 1.18 | 0.68, 2.07 | 0.55 |
| Primary resection | 0.74 | 0.46, 1.20 | 0.22 |
| Hospitalized during treatment | 0.85 | 0.57, 1.27 | 0.42 |
| Any infection | 0.57 | 0.34, 0.94 | 0.029 |
| Hospitalized for febrile neutropenia | 0.85 | 0.31, 2.34 | 0.75 |
| Chemotherapy delay | 0.93 | 0.90, 0.97 | <0.001 |
| Multivariable model | |||
| Group | |||
| g-csf, neutropenia | Reference | ||
| No g-csf, neutropenia | 1.37 | 0.72, 2.61 | 0.34 |
| No g-csf, no neutropenia | 2.07 | 1.03, 4.15 | 0.04 |
| ecog performance status | |||
| 0 | Reference | ||
| 1–3 | 2.28 | 1.41, 3.70 | 0.001 |
| folfiri | |||
| Not used | Reference | ||
| Used as 1st-line treatment | 0.46 | 0.26, 0.80 | 0.006 |
hr = hazard ratio; cl = confidence limits; g-csf = granulocyte colony–stimulating factor; ibd = inflammatory bowel disease; vte = venous thromboembolism; ecog = Eastern Cooperative Oncology Group; cci = Charlson comorbidity index; folfiri = irinotecan, 5-flourouracil, leucovorin.
On multivariate analysis, risk of an event continued to be significantly higher in group 3 patients than in group 1 patients (hr: 2.07; 95% cl: 1.03, 4.15) after adjustment for the impact of ecog ps and of use of folfiri as first-line treatment (Table ii).
When the model was built and both ecog ps and folfiri as first-line therapy had been included, the probability of an event remained lower in the treatment group. Higher ecog ps was negatively correlated with survival, with the number of event-free days declining as ps scores increased (mean days to an event: 395 days for ecog 0 vs. 255 days for ecog 1 vs. 204 days for ecog 2 or 3).
3.2. Secondary Analyses
3.2.1. Neutropenia
Of the 93 study patients, 31 did not experience neutropenia. Of the remaining 62 patients, 18 received g-csf, and 44 did not. Of the 18 patients who received g-csf, 2 had experienced neutropenia while on a chemotherapy regimen before they started folfiri. Those 2 patients continued g-csf therapy when they were switched to folfiri, and they did not experience any further neutropenia. Of the remaining 16 patients supported with g-csf, only 2 (11.1%) went on to experience further neutropenia while receiving g-csf, resulting in a total treatment delay of 97 days (average of 6.1 days per patient per delay). In the group of 44 patients who experienced neutropenia and did not receive g-csf support, a significantly higher proportion of patients (23 of 44, 52.3%, p = 0.03) experienced further neutropenia leading to a total treatment delay of 1048 days (mean of 24 days per patient).
The mean number of neutropenic episodes before g-csf therapy was 2.06 (range: 1–4 episodes) in group 1, which declined to a mean of 0.31 episodes (range: 0–4 episodes) after the initiation of g-csf support. By comparison, the mean number of episodes was 2.29 in group 2 (p = 0.65).
3.2.2. Febrile Neutropenic Episodes
This study group experienced 6 episodes of febrile neutropenia. One episode occurred in a patient receiving g-csf therapy (1 o f 18, 5 .5%); the other 5 episodes occurred in patients not receiving g-csf support (5 of 44, 11.4%, p = 0.66).
3.2.3. Dose Reductions or Delays
For patients in group 1, the mean treatment delay declined from 13.7 days (range: 0–28 days) before g-csf therapy to 5.4 days (range: 0–69 days) after supportive therapy was started. Neither of the patients who experienced dose delay while on g-csf had their dose reduced. The mean number of neutropenic episodes was higher in patients without g-csf support (2.29 episodes) that in those with such support (0.28 episodes), dose reductions were greater (mean: 6.8% vs. 0%), and delay in treatment because of neutropenia was longer (mean: 21.4 days vs. 6.06 days).
3.2.4. Infections and Hospitalizations
The median number of infections did not differ between the groups, ranging from 0 to 5 documented infections requiring antibiotics. When patients who did not experience neutropenia were excluded, use of g-csf did not significantly affect the number of infections. The group treated with g-csf had a higher mean number of infections (0.87 vs. 0.48, p = 0.19), although the difference was not statistically significant.
The median number of hospital admissions also did not differ between the groups (Table iii).
TABLE III.
Survival and secondary analyses in all patients
| Variable |
Neutropenia
|
p Value | |||
|---|---|---|---|---|---|
| Yes | Yes | No | |||
|
|
|
|
|||
| g-csf (n=18) | No g-csf (n=44) | No g-csf (n=31) | |||
| Index to progression (days) | Median | 323 | 294 | 217 | 0.08a |
| iqr | 96–434 | 128–404 | 64–294 | ||
| Survival (days) | Median | 613 | 487 | 340 | 0.30 |
| iqr | 342–758 | 295–796 | 176–680 | ||
| Time to eventb (days) | Median | 337 | 284 | 197 | 0.006 |
| iqr | 317–539 | 119–415 | 64–288 | ||
| folfiri cycles (n) | Median | 10 | 12.5 | 17 | 0.09 |
| iqr | 4–16 | 6–20 | 11–22 | ||
| Treatment delay for any reason (days) | Median | 34 | 29 | 0 | 0.001 |
| iqr | 17–96 | 14–65 | 0–14 | ||
| Proportion of treatment reduction for any reason (%) | Mean | 7.5±13.0 | 9.5±11.2 | 4.5±8.5 | 0.14 |
| Range | 0–36 | 0–40 | 0–20 | ||
| Hospital admissions for any reason (n) | Median | 0 | 0 | 0 | 0.32 |
| Range | 0–2 | 0–2 | 0–1 | ||
| Infections for any reason (n) | Median | 0 | 0 | 0 | 0.08 |
| Range | 0–5 | 0–2 | 0–1 | ||
| Reductions in folfiri dose because of infection or neutropenia (n) | Mean | 4.2±8.1 | 6.8±9.9 | ||
| Range | 0–20 | 0–30 | |||
| Total episodes of neutropenia (n) | Mean | 1.83±1.46 | 2.29±1.85 | na | |
| Range | 0–6 | 0–9 | |||
| Neutropenic episodes before g-csf treatment (n) | Mean | 1.5±0.98 | |||
| Range | 0–4 | ||||
| Neutropenic episodes during g-csf treatment (n) | Mean | 0.28±0.96 | |||
| Range | 0–4 | ||||
Kruskal–Wallis.
Progression, death, or censoring.
g-csf = granulocyte colony–stimulating factor; iqr = interquartile range; folfiri = irinotecan, 5-flourouracil, leucovorin; na = not applicable.
4. DISCUSSION
To our knowledge, this study is the first to evaluate the impact on survival of g-csf used as secondary prophylaxis for neutropenia. The use of g-csf as secondary prophylaxis did not affect survival in metastatic colorectal cancer patients.
In a recently published study evaluating the use of g-csf as primary prophylaxis in colorectal cancer patients, whose patient population was similar to most patients with stage iv disease (100% of patients in our study), the use of g-csf was found to reduce grades 3 and 4 neutropenia (absolute neutrophil count < 1.0/mL) from 50% in untreated to 15.6% in treated patients receiving folfiri over 1–4 cycles24. Our study examined the rate of neutropenic episodes, which declined in group 1 patients to 0.31 from 2.06 episodes per patient. Of 6 episodes of febrile neutropenia, only 1 occurred in a patient on g-csf therapy. The secondary outcomes in the previously published study included progression-free survival and overall survival, which were primary outcomes in our study. Neither study demonstrated any benefit in survival.
Our study demonstrates that g-csf lowered the number of episodes of neutropenia and febrile neutropenia, which led to fewer dose delays and reductions. Compared with other groups, the group of patients who received g-csf therapy had a lower probability of an event (death or progression); compared with the group of patients who experienced no episodes of neutropenia, the difference was statistically significant. Because of the nature of their disease, all patients will experience an event, but it appears that experiencing neutropenia and receiving g-csf therapy does reduce the probability of such an event occurring. Our results are consistent with the available literature and with current guidelines for metastatic patients, in whom a chemotherapy dose delay or reduction remains a viable treatment option when neutropenia occurs.
In terms of our secondary outcomes, g-csf had no observable effect on hospitalizations or rates of infection. The lack of a difference in hospitalizations can be attributed to the small sample size and small number of hospitalizations observed in our study. In terms of infection rates, our results were skewed by 1 patient who experienced documented cellulitis on 5 occasions caused by scratches and bites from her pet cats. That patient received g-csf therapy, but was not neutropenic during any of her infections.
Being a retrospective chart review, our study has its own inherent limitations. The nonrandomized nature of the study meant that we were unable to control for all variables. Also, patients were seen only every 2 weeks in clinic for blood work and chemotherapy; neutropenic episodes might therefore have been missed. Routine imaging (by computed tomography) was performed every 2 months unless signs and symptoms suggestive of progressive disease were noted, which might possibly have influenced the recorded time to progression. Another limitation to be considered is the generalizability of the results, given that our data were collected at a single centre managed by one physician. Nevertheless, we believe that our patient population was representative of other patient populations studied. Despite the retrospective design, our study groups were also comparable at baseline. We used the validated Charlson age–comorbidity index to compare mortality risk based on the underlying comorbidities in the patients. A study published by Ouellette et al.28 in 2004 looked specifically at that index in colorectal cancer patients. Their study determined that, as the comorbidity index increased, so did the risk of overall cancer-related mortality. Specifically, for every 1 point increase on the scale, the risk of mortality increased by 32%. A score of 7 was used as a cut-off, and given that all of our patients had metastatic disease (which is worth 6 points on the scale), all would have scored higher than 7 on the index. The Ouellette et al. study also used a cut-off of 10 and found that patients whose comorbidity index was greater than 10 had a cancer-related mortality risk that was higher by a factor of 6.
The care from one oncologist at a single centre reduced variability in the way patients were managed. All of the patients seen in our clinic were offered similar, if not identical, treatment regimens based on their presentation. The study also used data from a real-world clinic and not a rigorously controlled clinical trial, which might provide results that are more generalizable.
Given our findings, it remains reasonable to pursue g-csf as secondary prophylaxis in this patient population, but the high cost of therapy (CA$1500–CA$3000 per cycle)a makes access to these medications an issue for many patients. It remains important to reassure patients that if they are unable to obtain g-csf, their survival will not be negatively affected.
An interesting finding in this study is that patients who did not experience neutropenia fared significantly worse that did patients who experienced neutropenia and received g-csf. Our initial hypothesis was that patients who received g-csf therapy to prevent dose reductions or delays would experience improved progression-free and overall survival. Our belief was that group 3 patients (no neutropenia) would experience survival similar to that in group 1 patients (neutropenia and receiving g-csf), based on the understanding that both groups would be receiving full-dose therapy. Our results showed the opposite, with the probability of death or progression being significantly less in group 1 patients than in group 3 patients. Once patients who did not experience neutropenia were removed from the analysis, no statistical differences were seen between the g-csf–treated and untreated groups in any predefined outcome (Table iv).
TABLE IV.
Survival and secondary analyses in neutropenic patients
| Variable |
Neutropenia
|
p Value | ||
|---|---|---|---|---|
| Yes | Yes | |||
|
|
|
|||
| g-csf (n=16) | No g-csf (n=44) | |||
| Index to progression (days) | Median | 323a | 294b | 0.47 |
| iqr | 317–380 | 127–427 | ||
| Survival (days) | Median | 613c | 487d | 0.48 |
| iqr | 342–1070 | 295–796 | ||
| folfiri cycles (n) | Mean | 19.2 | 16.4 | 0.52 |
| 95% cl | 10.0, 28.3 | 12.2, 20.6 | ||
| Treatment delay for any reason (days) | Mean | 88.9 | 56.3 | 0.27 |
| 95% cl | 24.4, 153.4 | 28.3, 84.4 | ||
| Proportion of treatment reduction for any reason (%) | Mean | 8.5 | 9.5 | 0.33 |
| 95% cl | 0.06, 0.57 | 0.30, 0.61 | ||
| Hospital admissions for any reason (n) | Mean | 0.44 | 0.39 | 0.80 |
| 95% cl | 0.05, 0.82 | 0.19, 0.58 | ||
| Infections for any reason (n) | Mean | 0.87 | 0.48 | 0.19 |
| 95% cl | 0, 1.78 | 0.27, 0.68 | ||
| Neutropenic episodes (n) | Mean | 2.06 | 2.29 | 0.65 |
| 95% cl | 1.32, 2.80 | 1.73, 2.86 | ||
| Dose delays for any reason (n) | Mean | 2.06 | 2.18 | 0.82 |
| 95% cl | 1.32, 2.80 | 1.60, 2.76 | ||
| Treatment delay because of infection or neutropenia (days) | Mean | 25.2 | 23.8 | 0.84 |
| 95% cl | 14.4, 36.1 | 15.8, 31.8 | ||
| Reduction in folfiri dose because of infection or neutropenia (%) | Mean | 4.7 | 6.8 | 0.45 |
| 95% cl | 0.2, 9.2 | 3.8, 9.8 | ||
| Treatment delay because of neutropenia (days) | Mean | 21.3 | 21.4 | 0.99 |
| 95% cl | 11.0, 31.6 | 13.8, 29.0 | ||
| Neutropenic episodes (n) | Mean | 2.06 | 2.29 | 0.65 |
| 95% cl | 1.32, 2.80 | 1.73, 2.86 | ||
Of 16 patients, 9 had documented progression.
Of 44 patients, 31 experienced progression during the study period.
Of 16 patients, 7 died before the study period ended.
Of 44 patients, 25 died before the study period ended.
A possible explanation for those results might be the concept of toxicity equating to efficacy, in which chemotherapy-induced neutropenia is understood to be a positive prognostic marker for survival. Evidence in the literature demonstrates a positive relationship between chemotherapy-induced toxicity and efficacy of the chemotherapy regimen. Examples include rash induced by epidermal growth factor receptor inhibitors29, and hypertension induced by vascular endothelial growth factor inhibitors30. A number of studies have also looked specifically at chemotherapy-induced neutropenia and survival. Studies in gastric, ovarian, breast, and lung cancers have all shown that survival was improved in patients who experienced neutropenia while on chemotherapy compared with patients who did not31–36.
5. CONCLUSIONS
Our finding that g-csf use does not appear to have a significant impact on survival in patients with metastatic colorectal cancer accords with the available literature. These agents lower the incidence of neutropenia and febrile neutropenia and prevent chemotherapy dose delays and reductions. Those results, coupled with evidence in the literature, suggest that chemotherapy-induced neutropenia may be a positive prognostic marker for survival.
Footnotes
Prescription for filgrastim 300 μg (10 vials), CA$2,181.24; prescription pegfilgrastim 6 mg×1 dose, CA$2,860.22. Data source: billing from a major drug store chain in Ontario on March 1, 2013.
6. CONFLICT OF INTEREST DISCLOSURES
The authors declare that they have no financial conflicts of interest.
7. REFERENCES
- 1.National Comprehensive Cancer Network (nccn) NCCN Clinical Practice Guidelines in Oncology: Myeloid Growth Factors. Fort Washington, PA: NCCN; 2013. Ver. 1.2011. [Current version available online at: http://www.nccn.org/professionals/physician_gls/pdf/myeloid_growth.pdf; cited October 12, 2011] [Google Scholar]
- 2.Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106:2258–66. doi: 10.1002/cncr.21847. [DOI] [PubMed] [Google Scholar]
- 3.Schwenkglenks M, Pettengell R, Jackisch C, et al. Risk factors for chemotherapy-induced neutropenia occurrence in breast cancer patients: data from the inc-eu Prospective Observational European Neutropenia Study. Support Care Cancer. 2011;19:483–90. doi: 10.1007/s00520-010-0840-y. [DOI] [PubMed] [Google Scholar]
- 4.Moreau M, Klastersky J, Schwarzbold A, et al. A general chemotherapy myelotoxicity score to predict febrile neutropenia in hematological malignancies. Ann Oncol. 2009;20:513–19. doi: 10.1093/annonc/mdn655. [DOI] [PubMed] [Google Scholar]
- 5.Kuderer NM, Dale DC, Crawford J, Lyman GH. Impact of primary prophylaxis with granulocyte colony–stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol. 2007;25:3158–67. doi: 10.1200/JCO.2006.08.8823. [DOI] [PubMed] [Google Scholar]
- 6.Khan S, Dhadda A, Fyfe D, Sundar S. Impact of neutropenia on delivering planned chemotherapy for solid tumours. Eur J Cancer Care (Engl) 2008;17:19–25. doi: 10.1111/j.1365-2354.2007.00797.x. [DOI] [PubMed] [Google Scholar]
- 7.Bonadonna G, Valagussa P. Dose–response effect of adjuvant chemotherapy in breast cancer. N Engl J Med. 1981;304:10–15. doi: 10.1056/NEJM198101013040103. [DOI] [PubMed] [Google Scholar]
- 8.Wood WC, Budman DR, Korzun AH, et al. Dose and dose intensity of adjuvant chemotherapy for stage ii, node-positive breast carcinoma. N Engl J Med. 1994;330:1253–9. doi: 10.1056/NEJM199405053301801. [Erratum in: N Engl J Med 1994;331:139] [DOI] [PubMed] [Google Scholar]
- 9.Budman DR, Berry DA, Cirrincione CT, et al. Dose and dose intensity as determinants of outcome in the adjuvant treatment of breast cancer. The Cancer and Leukemia Group B. J Natl Cancer Inst. 1998;90:1205–11. doi: 10.1093/jnci/90.16.1205. [DOI] [PubMed] [Google Scholar]
- 10.Husband DJ, Green JA. pomb/ace chemotherapy in non-seminomatous germ cell tumours: outcome and importance of dose intensity. Eur J Cancer. 1992;28:86–91. doi: 10.1016/0959-8049(92)90392-F. [DOI] [PubMed] [Google Scholar]
- 11.Wildiers H, Reiser M. Relative dose intensity of chemotherapy and its impact on outcomes in patients with early breast cancer or aggressive lymphoma. Crit Rev Oncol Hematol. 2011;77:221–40. doi: 10.1016/j.critrevonc.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Clahsen PC, van de Velde CJ, Welvaart K, van Driel OJ, Sylvester RJ. Ten-year results of a randomized trial evaluating prolonged low-dose adjuvant chemotherapy in node-positive breast cancer: a joint European Organization for Research and Treatment of Cancer-Dutch Breast Cancer Working Party Study. Cooperating Investigators. J Clin Oncol. 1995;13:33–41. doi: 10.1200/JCO.1995.13.1.33. [DOI] [PubMed] [Google Scholar]
- 13.Amador ML, Jimeno A, Hitt R, Cortés–Funes H, Colomer R. Dose and dose intensity effect of adjuvant anthracycline-based chemotherapy in early breast cancer: a retrospective analysis. Am J Clin Oncol. 2004;27:269–73. doi: 10.1097/01.coc.0000093082.79608.1a. [DOI] [PubMed] [Google Scholar]
- 14.Schaapveld M, de Vries EG, van der Graaf WT, Otter R, Willemse PH. Quality of adjuvant cmf chemotherapy for node-positive primary breast cancer: a population-based study. J Cancer Res Clin Oncol. 2004;130:581–90. doi: 10.1007/s00432-004-0583-6. [DOI] [PubMed] [Google Scholar]
- 15.Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 Update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;24:3187–205. doi: 10.1200/JCO.2006.06.4451. [DOI] [PubMed] [Google Scholar]
- 16.Aapro MS, Bohlius J, Cameron DA, et al. 2010 Update of eortc guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011;47:8–32. doi: 10.1016/j.ejca.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Vogel CL, Wojtukiewicz MZ, Carroll RR, et al. First and subsequent cycle use of pegfilgrastim prevents febrile neutropenia in patients with breast cancer: a multicenter, double-blind, placebo-controlled phase iii study. J Clin Oncol. 2005;23:1178–84. doi: 10.1200/JCO.2005.09.102. [DOI] [PubMed] [Google Scholar]
- 18.Thatcher N, Girling DJ, Hopwood P, Sambrook RJ, Qian W, Stephens RJ. Improving survival without reducing quality of life in small-cell lung cancer patients by increasing the dose-intensity of chemotherapy with granulocyte colony-stimulating factor support: results of a British Medical Research Council Multicenter Randomized Trial. Medical Research Council Lung Cancer Working Party. J Clin Oncol. 2000;18:395–404. doi: 10.1200/JCO.2000.18.2.395. [DOI] [PubMed] [Google Scholar]
- 19.Gupta S, Singh PK, Bhatt ML, Pant MC, Gupta R, Negi MP. Efficacy of granulocyte colony–stimulating factor as a secondary prophylaxis along with full-dose chemotherapy following a prior cycle of febrile neutropenia. Biosci Trends. 2010;4:273–8. [PubMed] [Google Scholar]
- 20.Crawford J, Ozer H, Stoller R, et al. Reduction by granulocyte colony–stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med. 1991;325:164–70. doi: 10.1056/NEJM199107183250305. [DOI] [PubMed] [Google Scholar]
- 21.Pettengell R, Gurney H, Radford JA, et al. Granulocyte colony–stimulating factor to prevent dose-limiting neutropenia in non-Hodgkin’s lymphoma: a randomized controlled trial. Blood. 1992;80:1430–6. [PubMed] [Google Scholar]
- 22.Lyman GH, Dale DC, Wolff DA, et al. Acute myeloid leukemia or myelodysplastic syndrome in randomized controlled clinical trials of cancer chemotherapy with granulocyte colony-stimulating factor: a systematic review. J Clin Oncol. 2010;28:2914–24. doi: 10.1200/JCO.2009.25.8723. [DOI] [PubMed] [Google Scholar]
- 23.Kourourksi CT, Chia S, Verma S, et al. Canadian supportive care recommendations for the management of neutropenia in patients with cancer. Curr Oncol. 2008;15:9–23. doi: 10.3747/co.2008.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hecht JR, Pillai M, Gollard R, et al. A randomized, placebo-controlled phase ii study evaluating the reduction of neutropenia and febrile neutropenia in patients with colorectal cancer receiving pegfilgrastim with every-2-week chemotherapy. Clin Colorectal Cancer. 2010;9:95–101. doi: 10.3816/CCC.2010.n.013. [DOI] [PubMed] [Google Scholar]
- 25.Sobrero A, Ackland S, Clarke S, et al. Phase iv study of bevacizumab in combination with infusional fluorouracil, leucovorin and irinotecan (folfiri) in first-line metastatic colorectal cancer. Oncology. 2009;77:113–19. doi: 10.1159/000229787. [DOI] [PubMed] [Google Scholar]
- 26.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55. doi: 10.1097/00000421-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 27.Ouellette JR, Small DG, Termuhlen PM. Evaluation of Charlson-Age Comorbidity Index as predictor of morbidity and mortality in patients with colorectal carcinoma. J Gastrointest Surg. 2004;8:1061–7. doi: 10.1016/j.gassur.2004.09.045. [DOI] [PubMed] [Google Scholar]
- 28.Stevenson M. An Introduction to Survival Analysis. Palmerston North, NZ: EpiCentre, Massey University; 2009. [Google Scholar]
- 29.Hosmer DW, Jr, Lemeshow S, May S. Applied Survival Analysis. Regression Modeling of Time to Event Data. Toronto, ON: John Wiley and Sons; 1999. [Google Scholar]
- 30.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–45. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 31.Scartozzi M, Galizia E, Chiorrini S, et al. Arterial hypertension correlates with clinical outcome in colorectal cancer patients treated with first-line bevacizumab. Ann Oncol. 2009;20:227–30. doi: 10.1093/annonc/mdn637. [DOI] [PubMed] [Google Scholar]
- 32.Banerji U, Ashley S, Coward J, et al. The association of chemotherapy induced neutropenia on treatment outcomes in small cell lung cancer. Lung Cancer. 2006;54:371–7. doi: 10.1016/j.lungcan.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Kishida Y, Kawahara M, Teramukai S, et al. Chemotherapy-induced neutropenia as a prognostic factor in advanced non-small-cell lung cancer: results from Japan Multinational Trial Organization LC00–03. Br J Cancer. 2009;101:1537–42. doi: 10.1038/sj.bjc.6605348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pallis AG, Agelaki S, Kakolyris A, et al. Chemotherapy-induced neutropenia as a prognostic factor in patients with advanced non-small cell lung cancer treated with front-line docetaxel–gemcitabine chemotherapy. Lung Cancer. 2008;62:356–63. doi: 10.1016/j.lungcan.2008.03.030. [DOI] [PubMed] [Google Scholar]
- 35.Lee CK, Gurney H, Brown C, et al. Carboplatin-paclitaxel–induced leucopenia and neuropathy predict progression-free survival in recurrent ovarian cancer. Br J Cancer. 2011;105:360–5. doi: 10.1038/bjc.2011.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamanaka T, Matsumoto S, Teramukai S, Ishiwata R, Nagai Y, Fukushima M. Predictive value of chemotherapy-induced neutropenia for the efficacy of oral fluoropyrimidine S-1 in advanced gastric carcinoma. Br J Cancer. 2007;97:37–42. doi: 10.1038/sj.bjc.6603831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han Y, Yu Z, Wen S, Zhang B, Cao X, Wang X. Prognostic value of chemotherapy-induced neutropenia in early-stage breast cancer. Breast Cancer Res Treat. 2012;131:483–90. doi: 10.1007/s10549-011-1799-1. [DOI] [PubMed] [Google Scholar]