Abstract
Background
The ratio of extracellular mass to body cell mass (ecm/bcm), determined by bioelectrical impedance analysis, has been found to be a potentially useful indicator of nutrition status. Subjective global assessment (sga) is a subjective method of evaluating nutrition status in head-and-neck cancer. The present study was conducted to investigate the association between ecm/bcm and sga in head-and-neck cancer.
Methods
Patients were classified as either well-nourished or malnourished by sga. Bioelectrical impedance analysis was conducted on a population of 75 patients with histologically confirmed head-and-neck cancer, and the ecm/bcm was calculated. Receiver operating characteristic curves were estimated using the nonparametric method to determine an optimal cut-off value of the ecm/bcm.
Results
Compared with malnourished patients, those who were well-nourished had a statistically significantly lower ecm/bcm (1.11 vs. 1.28, p = 0.005). An ecm/bcm cut-off of 1.194 was 76% sensitive and 63% specific in detecting malnutrition.
Conclusions
The ecm/bcm can be an indicator that detects malnutrition in patients with head-and-neck cancer. Further observations are needed to validate the significance of the ecm/bcm and to monitor nutrition interventions.
Keywords: Head-and-neck cancer, bioelectrical impedance analysis, extracellular–to–body cell mass ratio, subjective global assessment
1. INTRODUCTION
Worldwide, an estimated 644,000 new cases of head-and-neck cancer (hnc) are diagnosed each year, with two thirds of the cases occurring in developing countries. In the United States, hncs account for 3.2% (n = 39,750) of all new cancers, and 2.2% (n = 12,460) of all cancer deaths1.
Malnutrition is common in patients with hnc2. Deficits in nutrition have a significant impact on mortality, morbidity, and quality of life in patients with hnc2–4. Bioelectrical impedance analysis (bia) has been established as a valuable tool in the evaluation of body composition and nutrition status in many conditions, including cancer5–9. Parameters of nutrition status (for example, weight change, mid-arm muscle circumference, triceps skin fold thickness) or laboratory measurements are unstable in cancer patients in the clinical setting10. Some serum parameters (for example, serum albumin, transferrin) are likely to be influenced by many non-nutrition factors11. A more objective assessment is provided by bia, which evaluates body components such as the extracellular mass (ecm) or body cell mass (bcm)12,13.
The ecm includes all metabolically inactive tissues of the body; the bcm includes all the metabolically active tissues. The ecm/bcm ratio is a highly sensitive index of malnutrition10. A rising ecm/bcm ratio is an early warning sign of worsening nutrition status. This new parameter could possibly be another option for assessment of nutrition status in addition to the commonly used phase angle. The ecm/bcm ratio has never been studied in hnc patients.
Subjective global assessment (sga) is a clinical technique that combines data from subjective and objective aspects of medical history (change in weight, change in dietary intake, gastrointestinal symptoms, and changes in functional capacity) and physical examination (low levels of subcutaneous fat and muscle mass, ankle or sacral edema, and ascites)14. Patients are evaluated and categorized into three distinct classes: well-nourished (sga A), moderately malnourished (sga B), and severely malnourished (sga C). The sga has been extensively validated as an assessment technique for nutrition in oncology patients3,14.
The primary objective of the present study was to investigate the association between ecm/bcm ratio and sga in patients with hnc.
2. METHODS
2.1. Patients
Between October 2009 and October 2012, our study enrolled a population of 75 pre-surgical patients (8 women, 67 men) who had received a new, histologically confirmed diagnosis of hnc (28 tumours of larynx, 21 tumours of middle pharynx, 18 tumours of oral cavity, 8 tumours of inferior pharynx) and who were treated at the Otolaryngology Department, Head and Neck Surgery, Medical University of Lublin, Lublin, Poland. All tumours were plano-epithelial carcinomas. All patients who had already received or were receiving preoperative or postoperative radiotherapy and who had been surgically treated were excluded from the study.
2.2. Nutrition Assessment
All patients underwent a baseline nutrition assessment, which included laboratory measurements of total protein, serum albumin, and transferrin; sga; and bia. The patient’s nutrition status was defined as well-nourished (sga A), moderately malnourished (sga B), or severely malnourished (sga C). The bia was performed by a medical doctor using an SFB7 BioImp v1.55 bioimpedance analyzer (ImpediMed, Pinkenba, Australia). The test was conducted with the patient lying supine on a bed, legs apart and arms not touching the torso. All evaluations used 4 standard surface electrodes (“tetra polar” technique) applied at the hand and foot on the patient’s right side. Direct measurements of R and Xc in ohms at 50 kHz were obtained three times for each patient; the mean of those measurements were used in the analysis.
The bcm was calculated using the equation
where lbm is lean body mass, and ln(pa50) is natural logarithm of the phase angle measured at 50 kHz. The lbm was calculated from total body water (tbw) by assuming 73% hydration of the lbm15,16:
Fat-free mass (ffm) in kilograms was obtained directly from the SFB7 BioImp v1.55.
Extracellular mass (ecm) was calculated using the equation
The ecm to bcm ratio was then calculated.
2.3. Statistical Analysis
Statistical analyses were performed using the Statistica software application (version 8.0: StatSoft, Krakow, Poland). For the analysis reported here, patients were classified as either well-nourished (sga A) or malnourished (sga B and sga C). The sga B and sga C groups were merged because only 6 patients had been classified as sga C.
The ecm/bcm results are expressed as mean ± standard deviation. The ffm index was found to be non-normally distributed as demonstrated by a Shapiro–Wilks test. Median ecm/bcm values were compared in the two nutrition status categories using the nonparametric Mann–Whitney test. The accepted error was 5%, and statistical significance was accepted at p < 0.05. Receiver operating characteristic curves were estimated using the nonparametric method. The area under the curve was calculated to determine the accuracy of ecm/bcm as a tool for assessment of nutrition. We attempted to select an optimal ecm/bcm cut-off that would identify malnourished patients. Sensitivity was defined as the proportion of malnourished patients with an ecm/bcm smaller than the cut-off value—that is, the ability of the ecm/bcm cut-off to identify truly malnourished patients. Similarly, specificity was defined as the proportion of well-nourished patients with an ecm/bcm greater than or equal to the cut-off value—that is, the ability of the ecm/bcm cut-off to identify truly well-nourished patients.
The study was conducted according to the guidelines in the Declaration of Helsinki, and all procedures involving human subjects or patients were approved by the Research Ethics Committee of the Medical University of Lublin, Lublin, Poland. All patients gave written informed consent to participate in the study.
3. RESULTS
Tables i and ii show the baseline characteristics of the patient cohort. Compared with hnc patients who were moderately or severely malnourished according to the sga, those who were classified as well-nourished had significantly higher serum total protein (7.14 ± 0.57 mg/dL vs. 6.16 ± 0.76 mg/dL, Z = 6.64, p < 0.000001), serum albumin (4.03 ± 0.37 g/dL vs. 3.49±0.38 g/dL, Z = 6.68, p < 0.000001), and transferrin (202.47 ± 39.63 mg/dL vs. 170.29 ± 39.83 mg/dL, Z = 4.76, p = 0.000002). The ecm/bcm ratio was significantly lower in healthy controls than in patients with hnc (overall: 1.07 ± 0.20 vs. 1.18 ± 0.26, Z = –3.34, p = 0.0008), and it was significantly lower in hnc patients who were classified as well-nourished according to sga than in patients who were moderately or severely malnourished (1.11 ± 0.21 vs. 1.28 ± 0.29, Z = –2.82, p = 0.005).
TABLE I.
Baseline characteristics of patients with a new diagnosis of head-and-neck cancer
| Characteristic | Value [n (%)] |
|---|---|
| Patients | 75 |
| Sex | |
| Men | 67 (89.3) |
| Women | 8 (10.7) |
| Tumour type | |
| Larynx | 28 (37) |
| Middle pharynx | 21 (28) |
| Oral cavity | 18 (24) |
| Inferior pharynx | 8 (11) |
| Tumour stage at diagnosis | |
| Stage iii | 27 (36) |
| Stage iv | 48 (64) |
| Subjective global assessment category | |
| A (well-nourished) | 45 (60) |
| B (moderately malnourished) | 24 (32) |
| C (severely malnourished) | 6 (8) |
TABLE II.
Assessment of baseline characteristics in 75 patients by score on subjective global assessment (sga)
| Characteristic | Mean | Range | p Value |
|---|---|---|---|
| Age at diagnosis (years) | 56.88±8.21 | 37–80 | na |
| Total protein (mg/dL) | |||
| sga A | 7.14±0.57 | 5.50–8.30 | Z=6.64, |
| sga B+C | 6.16±0.76 | 5.80–6.60 | p<0.000001 |
| Albumin (g/dL) | |||
| sga A | 4.03±0.37 | 3.10–4.70 | Z=6.68, |
| sga B+C | 3.49±0.38 | 3.20–3.80 | p<0.000001 |
| Transferrin (mg/dL) | |||
| sga A | 202.47±39.63 | 140–312 | Z=4.76, |
| sga B+C | 170.29±39.83 | 141–200 | p=0.000002 |
| Extracellular-to-body cell mass ratio | |||
| Overall | |||
| Healthy patients | 1.07±0.20 | 0.82–1.76 | Z=−3.34, |
| hnc before surgery | 1.18±0.26 | 0.64–1.97 | p=0.0008 |
| sga A | 1.11±0.21 | 0.76–1.82 | Z=−2.82, |
| sga B+C | 1.28±0.29 | 0.64–1.97 | p=0.005 |
na = not applicable; hnc = head-and-neck cancer.
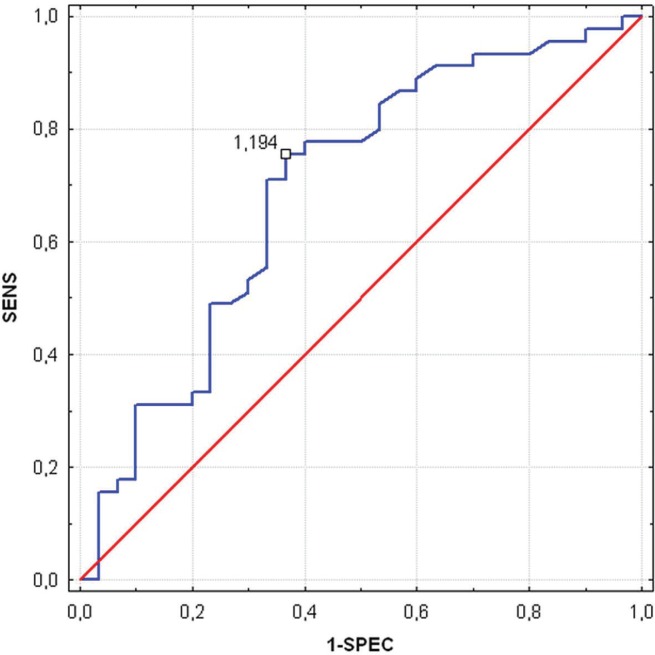
The optimal ecm/bcm cut-off for detecting malnourished patients was estimated to be 1.194 sensitivity: 76%; specificity: 63%). Figure 1 shows the receiver operating characteristic curve for the ecm/bcm ratio, revealing that it provides modest diagnostic accuracy in distinguishing well-nourished and malnourished individuals (area under the curve: 0.7; 95% confidence interval: 0.57 to 0.82; p = 0.005).
FIGURE 1.
Receiver operating characteristic curve assessing the optimal cut-off of extracellular—to—body cell mass ratio as a marker for malnutrition defined by the subjective global assessment (n = 75). sens = sensitivity; 1-spec = specificity.
4. DISCUSSION
Malnutrition has been associated with adverse outcomes in cancer patients. Patients who have been or are being treated for hnc are characterized by compromised nutrition status17.
The ecm/bcm ratio describes nutrition status16. The bcm is the overall cell mass responsible for metabolism; the ecm includes connective tissues such as collagen, elastin, skin, tendons, bones, and interstitial water (ascites, pleural effusion, and so on). In healthy individuals, the bcm is always distinctly higher than the ecm, and so the ratio is less than 118.
A rising ecm/bcm is an early warning sign of worsening nutrition status. The ecm/bcm ratio proved to be a useful tool for assessment of nutrition in patients with pancreatic cancer and an independent predictor of long-term survival in peritoneal dialysis patients10,19. For every 10% increase in the ecm/bcm, the relative risk of death increased by about 35% in peritoneal dialysis patients19. In a study by Pelzer et al.10, parental nutrition support for patients with pancreatic cancer lowered the ecm/bcm to 1.5 from 1.7, which signalled improved nutrition status. In another study, a declining ecm/bcm was associated with recovery in patients who had malnutrition because of non-malignant gastrointestinal diseases20.
To the best of our knowledge, our study is the first to evaluate the ecm/bcm as an indicator of malnutrition among patients with hnc. The study was restricted to newly diagnosed patients, and the results we observed provided valuable information about the nutrition status of patients before surgery. Other methods of assessing nutrition status in this population—such as sga—might not be sensitive enough to detect deficiency.
Limitations associated with the bia technique for predicting body composition in patients with cancer include the assumption of constant hydration and ffm composition, which can be different in obesity, various diseases, various age groups, and various ethnic groups21,22. In our opinion, further research with a larger sample size could potentially support our results, providing an avenue for early nutrition intervention and corrective nutritive replacement, which, combined with oncology intervention, might ultimately lead to increased survival in this patient population.
Evaluating the ecm/bcm in pre-surgical hnc patients could be a quick, simple, and reproducible means of determining nutrition status. This quick assessment can allow for early corrective intervention. Further research is needed to investigate the value of the ecm/bcm in Polish cancer patients to determine survival, validate the prognostic significance of the ratio, and monitor nutrition and therapeutic interventions.
5. CONCLUSIONS
The ecm/bcm can be considered an indicator of nutrition status in patients with cancer. The ecm/bcm cut-off of 1.194 might be a new parameter that could be used to detect malnutrition in patients with hnc. Further observations are needed to implement the ecm/bcm ratio as a prognostic marker of nutrition in clinical practice.
6. CONFLICT OF INTEREST DISCLOSURES
The authors declare that they have no financial conflicts of interest or any relationship with pharmaceutical companies or other entities that could be perceived to represent a financial conflict of interest.
7. REFERENCES
- 1.Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [Erratum in: CA Cancer J Clin 2005;55:259] [DOI] [PubMed] [Google Scholar]
- 2.van Bokhorst–de van der Schuer, van Leeuwen PA, Kuik DJ, et al. The impact of nutritional status on the prognoses of patients with advanced head and neck cancer. Cancer. 1999;86:519–27. doi: 10.1002/(SICI)1097-0142(19990801)86:3<519::AID-CNCR22>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 3.Naber TH, Schermer T, de Bree A, et al. Prevalence of malnutrition in nonsurgical hospitalized patients and its association with disease complications. Am J Clin Nutr. 1997;66:1232–9. doi: 10.1093/ajcn/66.5.1232. [DOI] [PubMed] [Google Scholar]
- 4.Hammerlid E, Wirbland B, Sandin C, et al. Malnutrition and food intake in relation to quality of life in head and neck cancer patients. Head Neck. 1998;20:540–8. doi: 10.1002/(SICI)1097-0347(199809)20:6<540::AID-HED9>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 5.Ward LC, Dylke E, Czerniec S, Isenring E, Kilbreath SL. Confirmation of the reference impedance ratios used for assessment of breast cancer-related lymphedema by bioelectrical impedance spectroscopy. Lymphat Res Biol. 2011;9:47–51. doi: 10.1089/lrb.2010.0014. [DOI] [PubMed] [Google Scholar]
- 6.Büntzel J, Krauss T, Büntzel H, et al. Nutritional parameters for patients with head and neck cancer. Anticancer Res. 2012;32:2119–23. [PubMed] [Google Scholar]
- 7.Małecka–Massalska T, Smolen A, Morshed K. Altered tissue electrical properties in squamous cell carcinoma in head and neck tumors: preliminary observations. Head Neck. 2013;35:1101–5. doi: 10.1002/hed.23091. [DOI] [PubMed] [Google Scholar]
- 8.Desport JC, Preux PM, Bouteloup–Demange C, et al. Validation of bioelectrical impedance analysis in patients with amyotrophic lateral sclerosis. Am J Clin Nutr. 2003;77:1179–85. doi: 10.1093/ajcn/77.5.1179. [DOI] [PubMed] [Google Scholar]
- 9.Simons JP, Schols AM, Westerterp KR, ten Velde GP, Wouters EF. The use of bioelectrical impedance analysis to predict total body water in patients with cancer cachexia. Am J Clin Nutr. 1995;61:741–5. doi: 10.1093/ajcn/61.4.741. [DOI] [PubMed] [Google Scholar]
- 10.Pelzer U, Arnold D, Goevercin M, et al. Parenteral nutrition support for patients with pancreatic cancer. Results of a phase ii study. BMC Cancer. 2010;10:86. doi: 10.1186/1471-2407-10-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waitzberg DL, Correia MI. Nutritional assessment in the hospitalized patient. Curr Opin Clin Nutr Metab Care. 2003;6:531–8. doi: 10.1097/00075197-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Kyle UG, Bosæus I, De Lorenzo AD, et al. on behalf of the ESPEN Working Group. Bioelectrical impedance analysis—part i: review of principles and methods. Clin Nutr. 2004;23:1226–43. doi: 10.1016/j.clnu.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Kyle UG, Bosæus I, De Lorenzo AD, et al. on behalf of ESPEN Bioelectrical impedance analysis—part ii: utilization in clinical practice. Clin Nutr. 2004;23:1430–53. doi: 10.1016/j.clnu.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Detsky AS, McLaughlin JR, Baker JP, et al. What is subjective global assessment of nutritional status? JPEN J Parenter Enteral Nutr. 1987;11:8–13. doi: 10.1177/014860718701100108. [DOI] [PubMed] [Google Scholar]
- 15.Dittmar M, Reber H. New equations for estimating body cell mass from bioimpedance parallel models in healthy older Germans. Am J Physiol Endocrinol Metab. 2001;281:E1005–14. doi: 10.1152/ajpendo.2001.281.5.E1005. [DOI] [PubMed] [Google Scholar]
- 16.Pace H, Rathburn EN. Studies in body composition. iii. The body water and chemically combined nitrogen content in relation to fat content. J Biol Chem. 1945;158:685–91. [Google Scholar]
- 17.Chasen RM, Bhargava R. A descriptive review of the factors contributing to nutritional compromise in patients with head and neck cancer. Support Care Cancer. 2009;17:1345–51. doi: 10.1007/s00520-009-0684-5. [DOI] [PubMed] [Google Scholar]
- 18.Talluri T, Lietdke RJ, Evangelisti A, Talluri J, Maggia G. Fat-free mass qualitative assessment with bioelectric impedance analysis (bia) Ann N Y Acad Sci. 1999;873:94–8. doi: 10.1111/j.1749-6632.1999.tb09454.x. [DOI] [PubMed] [Google Scholar]
- 19.Avram MM, Fein PA, Borawski C, Chattopadhyay J, Matza B. Extracellular mass/body cell mass ratio is an independent predictor of survival in peritoneal dialysis patients. Kidney Int Suppl. 2010;(117):S37–40. doi: 10.1038/ki.2010.192. [DOI] [PubMed] [Google Scholar]
- 20.Adami GF, Marinari G, Gandolfo P, Cocchi F, Friedman D, Scopinaro N. The use of bioelectrical impedance analysis for monitoring body composition changes during nutritional support. Surg Today. 1993;23:867–70. doi: 10.1007/BF00311363. [DOI] [PubMed] [Google Scholar]
- 21.Isenring E, Bauer J, Capra S, Davies PS. Evaluation of foot-to-foot bioelectrical impedance analysis for the prediction of total body water in oncology outpatients receiving radiotherapy. Eur J Clin Nutr. 2004;58:46–51. doi: 10.1038/sj.ejcn.1601744. [DOI] [PubMed] [Google Scholar]
- 22.Ward LC. Bioelectrical impedance validation studies: alternative approaches to their interpretation. Eur J Clin Nutr. 2013;67(suppl 1):S10–13. doi: 10.1038/ejcn.2012.159. [DOI] [PubMed] [Google Scholar]