Abstract
Pheochromocytomas (pheos) and paragangliomas (pgls) are rare tumours of the autonomic nervous system, originating from paraganglia, which are dispersed neuroendocrine organs characterized by catecholamine and peptide-producing cells derived from the neural crest. Medical textbooks have traditionally suggested that 10% of pheos are heritable. However, the frequency of heritable pheo has been underestimated. Three syndromic conditions—Von Hippel–Lindau (vhl), multiple endocrine neoplasia type 2 (men2), and neurofibromatosis type 1 (nf1)—and three genes—subunits of the succinate dehydrogenase (SDH) complex: SDHB, SDHC, and SDHD—are established causes of hereditary pheo-pgl. In the last few years, four new genes (SDHA, SDHAF2, MAX, and TMEM127) have been found to be associated with predisposition to these tumours. The present review, illustrated by three case reports, gives an update of the genetic basis of pheo–pgl and of the parent-of-origin effect implicated in the transmission of SDHD and SDHAF2. We discuss the referral criteria that should guide the decision to offer genetic testing to affected patients. We also specify the genes that are most likely implicated—based on particular features such as malignancy, bilateralism, or childhood-onset—to help geneticists efficiently order appropriate genetic tests. Finally, we review the screening recommendations for carriers of a pheo–pgl predisposition mutation.
Keywords: Pheochromocytoma, paraganglioma, genetics, predisposition, management, screening, testing, parent-of-origin effect
1. INTRODUCTION
1.1. Case 1
A 67-year-old man of English origin was in generally good health. He did not report any history of hypertension, perspiration, or pallor. He was referred to genetics because of a family history of pheochromocytoma (pheo): his maternal cousin’s daughter had been diagnosed with an abdominal extra-adrenal paraganglioma (pgl). She and her mother underwent genetic testing and were both found to be positive for a SDHB mutation, c.343C>T, p.Arg115*. Our patient’s deceased maternal uncle was presumed to be a carrier, because his wife (the affected woman’s maternal grandmother) was tested and did not carry the mutation. The patient was tested and was found to be a carrier of the familial mutation, as were his three unaffected children.
1.2. Case 2
A 49-year-old French Canadian man was diagnosed with bilateral neck pgls. He did not report ataxia, skin problems, or other tumours, and the family history was negative for relevant illnesses. Magnetic resonance imaging showed bilateral masses in the carotid spaces. On the left side, the tumour extended from the post-styloid parapharyngeal space, splaying the carotid arteries anteriorly and the internal jugular vein posteriorly. The mass, which arose from the vagus nerve, measured 72×38×46 mm. On the right side, the tumour, which arose from the carotid body, was located in the right carotid bifurcation. It measured 11×14 mm. Because surgery was not possible, the patient was treated with radiotherapy.
The genetic work-up included SDHB and SDHD sequencing, the results of which were normal. A deletion and duplication analysis of SDHB, SDHC, and SDHD by multiplex ligation-dependent probe amplification was also normal. On a research basis, SDHAF2 and SDHC sequencing were then done. We identified a SDHC mutation, c.397C>T, p.Arg133*, which is a probable French Canadian founder mutation1.
1.3. Case 3
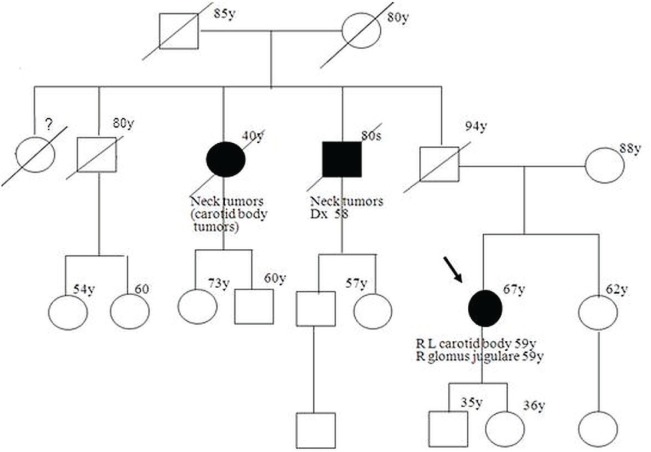
A 67-year-old woman of Ashkenazi Jewish inheritance had a history of a mass in the neck for about 20 years, but was otherwise asymptomatic. Computed tomography imaging revealed 3 pgls, all measuring between 3 cm and 4 cm. Two tumours were in the internal carotid region, one on the right and the other on the left; the third was in the region of the left jugular foramen. The woman was treated with surgery and radiation. Her family history revealed a paternal uncle and a paternal aunt who also had neck tumours (Figure 1). The patient had no information about her father because she had not had contact with him for more than 20 years.
FIGURE 1.
Pedigree of patient 3. Squares = men; circles = women; filled symbols = tumour-affected individuals (diagnoses indicated); diagonal line = deceased person; arrow = proband; number(y) = age or age at diagnosis in years; Dx = diagnosed at; R = right; L = left.
We confirmed the tumour pathology (a carotid body tumour) for the patient’s paternal aunt. The patient underwent genetic investigation and was found to carry a mutation in the SDHD gene (c.54_55dupC, p.Leu19ProfsX50).
2. DISCUSSION
2.1. Basics of PHEOs and PGLs
The rare tumours of the autonomic nervous system called pheos and pgls originate from paraganglia, which are dispersed neuroendocrine organs characterized by catecholamine and peptide-producing cells derived from the neural crest. The tumours arise from chromaffin cells of the adrenal medulla (80%–85%) or extra-adrenal chromaffin cells (15%–20%) and can originate in either the parasympathetic or sympathetic ganglia2.
The combined incidence of pheo and pgl in all sites is about 1 in 300,000 per year3. An increased frequency is noted in people subjected to chronic hypoxia, as in higher-altitude regions or in the presence of respiratory or heart diseases2. Pheochromocytoma is by far the most frequent tumour, with an annual incidence of 2–8 per million. Other tumours—such as head-and-neck pgl (hnpgl), abdominal pgl, and pelvic pgl—are much rarer (0.5 per million).
Paragangliomas can be divided into two broad categories: sympathoadrenal and parasympathetic (described in the 2004 World Health Organization’s classification of tumours of endocrine organs). Paragangliomas are therefore defined by location and whether they are hormonally active (Table i).
TABLE I.
Characteristics of the two broad categories of paragangliomas (World Health Organization classification)3–8
| Characteristic | Sympathoadrenal | Parasympathetic |
|---|---|---|
| Chromaffin status | Chromaffin paragangliomas | Non-chromaffin paragangliomas, often called chemodectomas or glomus tumours |
| Location | Adrenal medulla (most common) | Head and neck, also upper mediastinum |
| Distribution | Also symmetrically distributed along the prevertebral and paravertebral axis (thoracoabdominal and pelvic paraganglia) | Found in 20 distinct anatomic locations: carotid body (major paraganglion, the most common tumour location), jugular foramen, Jacobsen tympanic plexus, and vagal and aortic paraganglia (upper mediastinum) |
| Hormonal activity | Usually, but not always, hormonally active (catecholamine producing: noradrenalin, adrenalin, dopamine, l-dopa) | Hormonally inactive in 95% of cases (only rarely associated with catecholamine production) |
The term “pheochromocytoma” can be used to refer only to tumours in the adrenal gland, or it can be applied to all secreting tumours occurring below the neck. According to the World Health Organization classification, non-adrenal tumours should be described as “extra-adrenal sympathetic paragangliomas” and not as pheos4.
The role of normal paraganglia is homeostasis, either by acting directly as chemical sensors or by secreting catecholamines in response to stress. Most patients with parasympathetic pgl present with an asymptomatic slow-growing mass (as in our cases 2 and 3); patients with pheo and abdominal pgl present with hypertensive crises or symptoms induced by high levels of circulating catecholamines. Symptoms can also be caused by mass effect.
The prevalence of pheo in patients with hypertension is 0.1%–0.4%5,9. The age of onset for sporadic cases is approximately 40–50 years; for hereditary cases, it is usually before 40 years. More than 85% are benign; about 15% are malignant at diagnosis9. The risk for malignancy is greater with extra-adrenal sympathetic pgl (24% vs. 7% with pheo)2. There is no way to determine malignancy by histologic evaluation; only confirmation of metastasis to lymph nodes, bone, liver, or lung suffices5,9. However, pathology criteria such as size, weight, presence of tumour necrosis, Ki-67 index greater than 4%, and absence of PS100 have been linked to a greater risk of malignancy10. About 90% of pheos are unilateral, but bilateral tumours are seen in higher proportion in syndromic cases (about one third)11.
Among all adrenal lesions found incidentally, only 4% will be pheo9, but up to 25% of pheos–pgls are discovered incidentally during imaging studies for unrelated disorders. The other 75% are discovered after the development of symptoms such as hypertension, tachycardia, pallor, headaches, and feelings of panic or anxiety3,5. They can also cause metabolic effects such as hyperglycemia, lactic acidosis, and weight loss; however, normal blood pressure and even hypotension are common among patients with dopamine-producing pgls. Because symptoms are vague and frequent, there is an average delay of 3 years between the onset of symptoms and final diagnosis5.
2.2. Genetics of PHEO–PGL: Syndromic Causes
Medical textbooks—such as older editions of Harrison’s Principles of Internal Medicine—have traditionally suggested that 10% of pheos are heritable. However, the frequency of heritable pheo has been underestimated. In one population-based study, 25% of patients with unrelated, apparently sporadic, presentations (no syndromic features or family history) had mutations in one of the 4 main associated genes12. It is currently estimated that up to one third of cases are caused by a genetic predisposition6.
Three cancer predisposition syndromes are associated with the development of pheo–pgl: multiple endocrine neoplasia type 2 [men2 (RET gene)], von Hippel–Lindau [vhl (VHL gene)], and neurofibromatosis type 1 [nf1 (NF1 gene)]. Very rarely, men1 syndrome can be associated with the development of pheo7.
2.2.1. MEN2
An autosomal dominant condition, men2 has an estimated prevalence of 1 in 30,000. It is caused by activating mutations in the RET proto-oncogene (10q11.2), which encodes a transmembrane receptor tyrosine kinase involved in the regulation of cell proliferation and apoptosis. Nearly all patients have a positive family history. The three subtypes of men2 have strong genotype–phenotype correlations, and the risk of pheo is associated with specific mutations (typically in exons 10, 11, 13, and 16; Table ii). Because these mutations have also a high penetrance for medullary thyroid carcinoma, the combination of molecular and biochemical testing (calcitonin concentration) has been reported to be an efficient tool for the early diagnosis of men213.
TABLE II.
The three subtypes of men2 and their associated tumour risk
| Tumour feature | Risk (%) associated with | ||
|---|---|---|---|
| men2a (90%) | men2b (5%) | fmtc | |
| Medullary thyroid carcinoma | 95 | 100 | 100 |
| Pheochromocytoma | 50 | 50 | — |
| Primary hyperparathyroidism | 15–30 | — | — |
| Other features | Marfanoid habitus, gastroenteric mucosal ganglioneuromas, mucocutaneous neuromas, and medullated corneal nerves | ||
fmtc = familial medullary thyroid carcinoma.
Pheochromocytoma is the first clinical presentation in only 10%–30% of patients, with a lifetime penetrance of about 50% for the subtypes men2a and men2b. The contribution of RET mutations to apparently nonsyndromic pheo is only 5%14. Tumours associated with men2 are usually adrenal; on rare occasions, extra-adrenal pgls or hnpgls are seen. They usually produce norepinephrine and epinephrine and are often bilateral (50%–80%). They are very rarely malignant (fewer than 5%) and usually present between the ages of 30 and 40 years (mean age: 36 years)6.
2.2.2. VHL Syndrome
An autosomal dominant condition, vhl syndrome is linked to the VHL gene (3p25–26), which regulates hypoxia-inducible genes, fibronectin matrix assembly, and angiogenesis. The incidence of this condition is estimated at 1 in 36,000 live births. About 80% of patients have an affected parent; 20% of cases arise de novo. Von Hippel–Lindau syndrome is characterized by a variety of tumours, including cerebellar and spinal hemangioblastomas; retinal hemangiomas; clear cell renal carcinomas; pheos; pancreatic neuroendocrine tumours; and renal, pancreatic, and epididymal cysts. About 20% of patients will have a pheo (rarely a pgl)8. Age of onset is young, usually in the range 18–30 years, and vhl is implicated in 40% of pediatric patients with pheo14. Interfamilial variation is large, and multiple subtypes are known:
Type 1: no pheo (or less than 10% risk)
- Type 2: with pheo (40%–60% risk)
- Type 2A: without renal cancer (rare)
- Type 2B: with renal cancer (common)
- Type 2C: only pheo, without any other manifestations of vhl syndrome (rare, but there is a frequent founder mutation for this subtype which is believed to have originated in the Black Forest region of Germany)
Tumours associated with vhl syndrome produce norepinephrine, but not epinephrine. They are often bilateral or multicentric (50%), but rarely malignant (5%). They are most commonly pheos or extraadrenal sympathetic pgls7.
2.2.3. NF1 Syndrome
The nf1 syndrome is also an autosomal dominant condition, with a high birth incidence (about 1 in 3000). It arises de novo in 50% of cases, and penetrance is said to be complete, even if wide expressivity is present. However, genotype–phenotype correlation has been observed (Table iii).
TABLE III.
Clinical diagnostic criteria for neurofibromatosis type 1 (nf1) (note that pheochromocytoma is not listed)
| Criterion (must fulfil at least 2) | Proportion of nf1 patients (%) |
|---|---|
| Six or more café-au-lait spots | 86.7 |
| 1.5 cm or larger in postpubertal individual | |
| 0.5 cm or larger in prepubertal individual | |
| Two or more neurofibromata of any type or one or more plexiform neurofibromata | 89 |
| Freckling in the axilla, neck, or groin | 83 |
| Optic glioma | — |
| Two or more Lisch nodules | 63 |
| Distinctive bony lesion (dysplasia of sphenoid bone or long-bone cortex) | — |
| A first-degree relative with nf1 | 71 |
In nf1, pheo-pgl is a rare manifestation, estimated to occur in 0.1%–5.7% of people with nf1; however, in an autopsy series, 13% of nf1 patients had a pheo6. These tumours are more prevalent in the population of nf1 patients with hypertension (20%–50%)7. The mean age for development of the tumour is 40 years (similar to that in the general population). These mostly unilateral tumours (84%) are primarily adrenal tumours (less commonly, extra-adrenal). About 12% will be malignant6. Neurofibromatosis type 1 syndrome can be generally eliminated with a normal physical exam. In studies, all patients with nf1 and pheo had also cutaneous skin findings—such as café au lait macules, neurofibromas, and intertriginous freckling—that could be seen in a physical exam15.
2.3. Genetics of PHEO–PGL: Non-Syndromic Causes
In addition to the syndromic forms, many genes are associated with a predisposition to pheo–pgl. Over the last few years, mutations in the succinate dehydrogenase (SDH) gene complex (SDHB, SDHC, and SDHD) have been linked to an increased risk of tumour development—namely, hereditary pgl and pheo. More recently, SDHA, SDHAF2, TMEM127, and MAX have been also associated with predisposition to pheo–pgl.
The SDH complex is a tetramer composed of 4 proteins: SDHA, SDHB, SDHC, and SDHD. The succinate dehydrogenase for which these genes encode has a role in the oxidation of succinate to fumarate, and in mitochondrial complex ii of the electron transport chain2.
In the SDH complex, SDHA/B is the catalytic subunit. Mutations in the latter two genes cause complex ii destabilization and might activate the hypoxic–angiogenic pathway. The transmembrane proteins sdhc and sdhd anchor complex ii. Mutations in these genes result in destabilization of complex ii and a subsequent increase in oxygen free-radical production. Tumour formation results from stabilization of hypoxia-inducible factor 1 and activation of transforming growth factor β, platelet-derived growth factor receptor β, and a ligand for epidermal growth factor receptor16. All four are tumour suppressor genes, and the resulting tumours show loss of heterozygosity of the wild-type allele.
Biallelic mutations of the SDHA gene (5p15) were already known to be a cause of Leigh syndrome. It was only in 2010 that mutations in SDHA were identified to be a cause of pheo–pgl17. A mutation was first described in an abdominal pgl and has also been associated with hnpgl. In one series, mutations in SDHA represented approximately 3% of the germline mutations found in apparently sporadic pheo–pgl18. However, few patients have been described so far, and the exact prevalence and rate of malignancy are unknown6.
Mutations in the SDHB gene (1p36.1p35) cause familial pgl4, transmitted by autosomal dominant inheritance. The syndrome is associated mainly with extra-adrenal, abdominal, and pelvic tumours, but tumours can potentially be found anywhere (hnpgl, pheo)6. The classical presentation is a single tumour14, but one third are multifocal16. Mean age at onset is 25–30 years (range: 6–77 years)6,16. The penetrance is unclear, but is thought to be lower than those for the other SDHx genes14. Some studies reported a penetrance of 80%–100% by age 702,19, but others reported a much lower range (25%–40%) when the affected proband was excluded and asymptomatic relatives were included8. In a study of 295 patients, the penetrance of non-hnpgl was 52% by age 60 (mean age of diagnosis: 27 years), and the risk of hnpgl was 29% by age 60 (mean age: 42 years)20.
Mutation in this gene is associated with an increased malignant risk (30%–70%). The SDHB gene is implicated in 50% of all malignant extra-adrenal pgls, and the morbidity associated with these tumours is related mainly to metastatic disease, rather than to the hypercatecholaminemia16. The SDHB gene has recently been associated with other tumours such as gist (gastrointestinal stromal tumour), neuroblastoma, papillary thyroid cancer, and various types of renal cell cancer (clear cell and papillary cell types)3,6,8. The risk of renal cell cancer was up to 14% in one study (295 patients) compared with 1.5% in the general population20.
The SDHC gene (1q23.3) has been associated with familial pgl3, which is an autosomal dominant condition. Mutations in SDHC are less common than those in SDHB or SDHD, accounting for 0%–6.6% of all pheo–pgl patients6 and for 4% of all patients with hnpgl8. There is a possibility of a French Canadian founder mutation, based on its discovery in 5 different patients, one of whom is our case 21.
Mean age at diagnosis is 38 years (range: 17–70 years)2,6. The classical presentation is a solitary hnpgl, but rare extra-adrenal pgls and even pheos have also been described6,8. Ther risk of malignancy is very low. The exact penetrance is unknown, but according to some studies, “mutations carriers often develop the disease”2,14. However, that postulate does not seem to be the case with the French Canadian founder mutation, given the absence of family history and the fact that hnpgls are rare in the province of Quebec, even in the presence of a likely old founder mutation. Isolated reports suggest an association with gist, but the absolute risk is unknown2,3,5.
The SDHD gene (11q23) is implicated in familial pgl1. Founder mutations are known in the Netherlands, China, and Spain2,3. The typical presentation of this condition is multiple hnpgls8, with a mean age of onset of 28–31 years19. In one study, the penetrance reached 90% or greater by age 702, and fewer than 5% of the tumours were malignant6. Other studies found that the hnpgl presentation has a 71% penetrance by age 60, with a mean age of 40 years. Other extra-adrenal tumours have also been described in this condition, with a penetrance of 29% by 60 years of age, and a mean age of 21 years6. Adrenal pheo can also be seen, but more often in the context of multiple tumours throughout the body, including the adrenals.
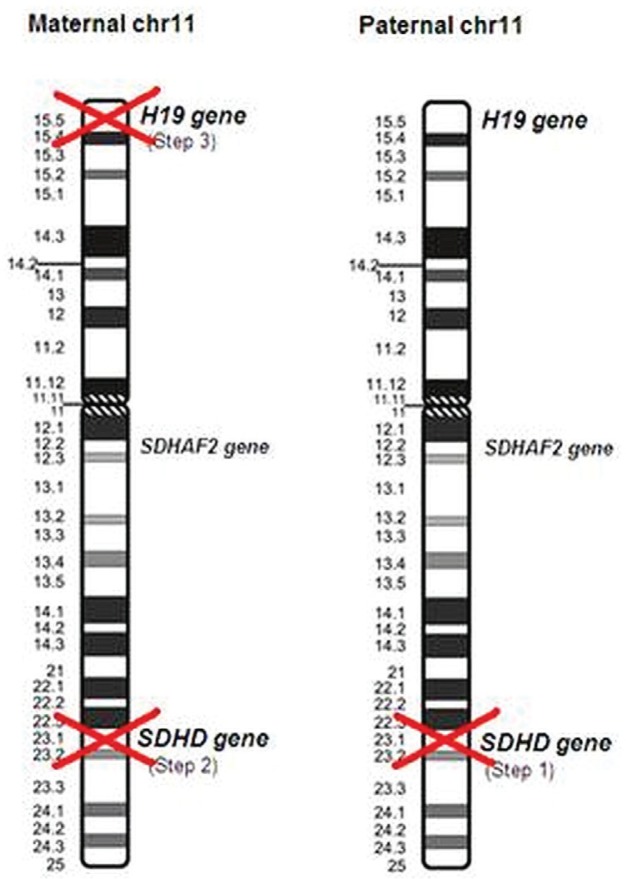
There was a suspicion of autosomal dominant inheritance, with maternal imprinting (meaning that a child could be affected only when the mutation is inherited from the father). It is now known that the inheritance is more complicated than was previously thought. The process is more like a parent-of-origin than an imprinting effect, because SDHD is not an imprinted gene. Effectively, it has been shown that the gene has biallelic expression in a number of nonparaganglionic tissues21. The current hypothesis is of a three-hit model for development of the tumour (Figure 2):
Step 1: SDHD mutation
Step 2: Loss or mutation of the wild-type allele
Step 3: Loss of a further imprinted (paternally silenced and maternally active) tumour suppressor gene from chr11 (thought to be the H19 gene)22
FIGURE 2.
Transmission of the SDHD gene and the parent-of-origin effect. The inactivation of both SDHD genes can be explained by a germline mutation (transmitted by a parent—usually the father, but in rare instances, the mother) in one allele and by a somatic mutation or loss (present only in the tumour) of the other allele. Loss of the maternal 11p15 region is also a somatic event.
In this hypothesis, steps 2 and 3 were thought to be explained solely by the obligate loss of the maternal copy of chromosome 11 (both losses generated by one event: the complete loss of the entire maternal chromosome) and that the three-hit model was therefore caused by only two events. That scenario was concordant with the parent-of-origin effect observed, with the mutation transmitted (step 1) on the paternal allele. However, 3 cases of affected individuals who received their mutations from their mothers have been published22. In one of those cases, the tumour analysis explained the phenomenon, because in addition to the maternal mutated allele, there was evidence of loss of the wild-type paternal SDHD and loss of the maternal 11p region (including the 11p15 H19 gene).
Those cases suggest two independent recombination events in the tumour (3 distinct events for the three-hit hypothesis). Even if the phenomenon is rare, development of tumours in SDHD carriers does not imply an obligate loss of maternal chromosome 11 in its entirety. However, for geneticists and genetic counsellors, that scenario does not majorly affect the information transmitted to patients and their relatives, because the risk of pgl in patients who inherit a mutation from their mothers is still very low (although not zero).
More recently, authors have suggested other hypotheses to explain the preferential paternal transmission, including a quantitative imprinting model with overexpression of the SDHD paternal allele relative to the maternal allele in paraganglionic tissues. This model is supported by the discovery of a tissue-specific differentially methylated CpG island that serves as an alternative promoter for a large intergenic noncoding rna located 200 kb downstream of the SDHD gene21. In the model, only a paternally transmitted mutation could deplete the gene product enough to trigger the hypoxic stimulation and tumour formation.
The SDHAF2 gene (11q12.2), also known as SDH5, was found in 2009 to be the cause of familial pgl223. This gene has a role in the flavination of the sdha protein. Familial pgl2 is rare; only 3 families have been described16. The first family described (in the 1980s) included 24 carriers; a penetrance of 100% by age 50 was reported for hnpgl. However, that penetrance has to be recalculated now that molecular testing is possible. Those families also showed that 91% of affected individuals had more than one hnpgl and that the average age of onset was 33 years (range: 22–47 years)24. No malignant tumours have been reported; only hnpgl has been described3.
As for SDHD, there is also autosomal dominant transmission with a parent-of-origin effect, because the cancer susceptibility appears only with paternal transmission. It is probably significant that SDHAF2 is also located on 11q8. Although this gene is nowadays generally included in clinical genetic panels for patients with hnpgl, it has to be offered with discretion because, in one study, no mutation was found in 201 patients with hnpgl who were negative for SDHD, SDHC, and SDHB25.
The TMEM127 gene (2q11.2) was identified as a pheo–pgl susceptibility gene in 201026, and it is thought to be involved in the mammalian target of rapamycin signalling pathway8. Transmission is autosomal dominant. The typical presentation is a unilateral adrenal pheo in patients with no prior family history. Recently, however, bilateral pheo, extraadrenal pgl, and hnpgl have been described27,28. The penetrance is unknown. The prevalence is low (2% of all cases negative for other genes, 990 individuals in total)29. The average age of onset appears to be 42–45 years, and the risk of malignancy is very low8.
The MAX gene (14q23) is the gene most recently described to be associated with pgl–pheo. It was found in 2011 after the whole-exome sequencing of three unrelated patients30. The max (myc-associated factor X) transcription factor acts for genes involved in cellular proliferation, differentiation, and apoptosis. In a study of 59 patients with either bilateral pheo or an age of onset of less than 30 years, the prevalence was 8.5%30. Another study showed that 1.1% of 1694 individuals with pheo or pgl had a mutation31. Even though the number of patients described is limited, there appears to be a tendency toward multiple tumours (8 of 12 patients had bilateral adrenal pheos) and malignancy (3 of 8 had malignant pheos)30. However, the association with malignancy was not confirmed in another study (2 of 23 patients)31. Because malignancy can be assessed only by the presence of metastasis, which can appear many years after the primary tumour, long-term follow-up will be necessary to approach a definitive conclusion. The MAX gene is the third gene with evidence of preferential paternal transmission, but the mechanism is unknown8.
Three other genes are thought to be associated with predisposition to pgl-pheo, but their role and clinical significance are still unclear. A germline mutation in the KIF1B gene has been reported in a single patient with pheo32. This gene could be a good candidate gene, given that it is frequently implicated in inherited and sporadic neural crest tumours such as neuroblastomas3,32,33; however, other studies are necessary to pinpoint its impact. Finally, in 1 patient with mutation in the EGLN1 gene (phd2 enzyme), recurrent pgl and erythrocytosis have been described34; however, no tumours were found in relatives with the same mutation. Still, EGLN1 is a potential pgl susceptibility gene given that it has a critical role in regulating hypoxia-inducible factor, although another study of 82 cases with hereditary pheo found no mutations in this gene35. Another patient with polycythemia and pheo–pgl was found to carry a germline HIF2A gene mutation. However, there was no loss of heterozygosity or additional somatic mutation of that gene in the tumour, indicating that HIF2A may be a lower-risk susceptibility gene rather than a directly causative factor in pheo–pgl36. In support of a role in pathogenesis, 2 patients with pheo–pgl and polycythemia have been reported to have somatic mutations in HIF2A37.
Finally, other genes have recently been associated with sporadic pheo–pgl tumours. Recurrent somatic mutations in the HRAS gene have been found in pheo and pgls38. However, those mutations are not inherited, being only found in the tumour and not in the germline.
2.4. Testing Protocol
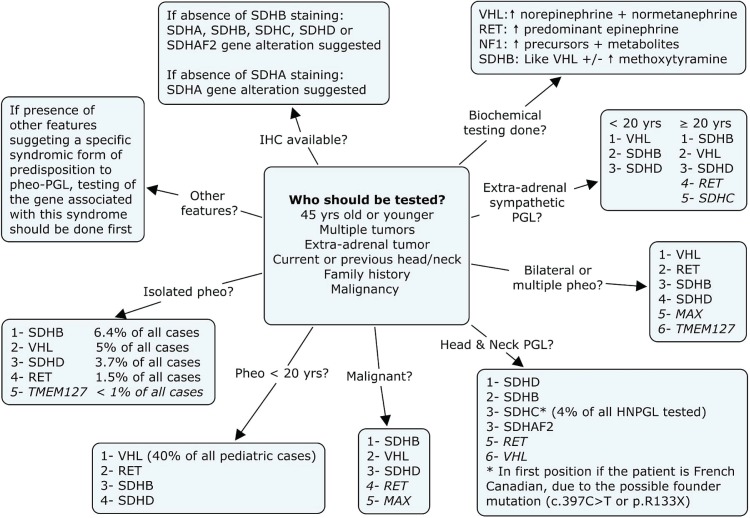
Because numerous genes are associated with the development of pheo–pgl, it is not surprising that some clinical laboratories are beginning to offer a panel for all of them; however, these panels are yet not widely used in the clinical setting because of their high price (more than $5,000). On the other hand, one-by-one testing for the 10 genes is time-consuming and would cost even more if all genes were to be tested. Thus, we believe that algorithms are still important to prioritize gene testing and to limit the need for either panel testing or repeated single-gene testing. To help with the decision about the order in which to offer sequential genetic testing, we present, in Figure 3, a testing algorithm for pheo–pgl, based on testing first for the gene most probably implicated. However, the first important question that the practicing clinician faces is whether a patient should undergo any genetic investigation. Figure 3 also provides important information about the pertinence of sending a specific patient to a genetic clinic6–8,14,15.
FIGURE 3.
Referral and testing protocol (based on Fishbein and Nathanson6, Jimerez et al.7, Gimenez–Roqueplo et al.14, Jafri and Maher8, and Erlic et al.15). The numbering system in the boxes refers to the likelihood of identifying mutations, with 1 being most likely. Criteria in the middle should trigger a referral to oncogenetics. Pheo = pheochromocytoma; Pgl = paraganglioma; ihc = immunohistochemistry.
2.4.1. Other Tools for Genetic Testing
Immunohistochemistry of the SDH subunits, which is offered by some European pathology laboratories, is another approach that can help to determine the genetic cause of a tumour. When any component of mitochondrial complex ii is completely inactivated, the entire complex becomes unstable, and the result is degradation of the SDHB subunit. Staining for SDHB is therefore absent whenever SDHA, SDHB, SDHC, SDHD, or SDHAF2 is completely inactivated. The precise gene involved cannot be determined by this test, only whether it is an SDHx gene39. Immunohistochemistry of SDHA has been also used for detecting the absence of SDHA18.
2.4.2. Screening and Follow-Up Recommendations for Carriers of a Predisposing Mutation
There are no official guidelines for the follow-up of carriers of a genetic predisposition to pheo–pgl. Many authors have published their own guidelines, which can differ significantly one from the other. Here, we propose our own screening recommendations, based on a review of the literature (Table iv). Clinical follow-up should be offered to
individuals who are known to have a hereditary pheo–pgl syndrome.
individuals who have known disease-causing mutations in a pheo–pgl predisposing gene.
relatives at risk (based on their position in the pedigree) who have not yet undergone gene testing.
TABLE IV.
Screening and follow-up recommendations for risk of pheochromocytoma or paraganglioma in mutation carriers
| Recommendation | Susceptibility gene | Syndrome | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| SDHB | SDHC | SDHD | vhl | men2 | nf1 | |
| Age to begin screening (years) | 5–10 | 5–10 | 5–10 | 5 | 8–20 | 5 |
| Physical exam and bp | Every 6–12 months | Every 6–12 months | Every 6–12 months | Annually | Annually | Annually |
| Urinary excretion of fractionated metanephrines and catecholamines in 24 hours | Annually | Annually | Annually | Annually after age 11 | Annually | If abnormal bp |
| mri–ct of abdomen, thorax, and pelvis | Every 6–24 months | Every 1–4 years | Every 1–4 years | If abnormal biochemistry | If abnormal biochemistry | If abnormal biochemistry |
| mri–ct of skull base and neck | Every 2–4 years | Every 6–36 months | Every 6–36 months | — | — | — |
| Periodic mibg scintigraphy | Every 2–4 years | Every 1–4 years | Every 1–4 years | — | — | — |
| Screening for renal cell carcinoma | Consider | — | — | Abdominal us or mri annually after age 16 | — | — |
bp = blood pressure; mri = magnetic resonance imaging; ct = computed tomography; mibg = metaiodobenzylguanidine; us = ultrasonography.
No clear consensus has been developed for the screening protocol, and given the rarity of cases, no clear recommendations have emerged for SDHA, SDHAF2, TMEM127, and MAX mutation carriers.
For SDHB, SDHC, and SDHD mutation carriers, screening should begin between the ages of 5 and 10 years3,6. Some authors recommend that the monitoring program should start in the first decade of life for SDHB carriers and in the second decade of life for SDHC and SDHD carriers2. However, it remains unclear whether imaging studies should be conducted as frequently in childhood as in adulthood.
Whatever the screening protocol, it should include lifelong annual biochemical and clinical surveillance:
Careful history, physical examination (including tympanic membrane), and measurement of blood pressure every 6 months to 1 year2,3,6,16
Evaluation of 24-hour urinary excretion of fractionated metanephrines and catecholamines (including chromogranin A and methoxytyramine, if available)2,3,6,16
Follow-up imaging (computed tomography, magnetic resonance imaging, positron-emission tomography, and so on) in addition to regular imaging surveillance, if the fractionated metanephrines and catecholamines become elevated2,3,6,16
Screening for gist in the presence of gastrointestinal symptoms, obstruction, or anemia3
Advice to all patients to avoid smoking and high altitudes3
The screening recommendations for syndromic forms of predisposition to pheo–pgl also include particular screening for other features of those conditions not discussed here. Patients with vhl syndrome should be followed annually, with measurement of blood pressure, beginning at 5 years of age6. Urinary excretion of fractionated metanephrines and catecholamines (24-hour collection) should also be evaluated every year after 11 years of age11.
All men2 carriers should undergo annual screening for pheo beginning at 8–20 years of age (depending on the exact mutation and codon implicated)40. The screening should include 24-hour urine studies for vanillylmandelic acid, metanephrines, and catecholamines, and annual serum measurements for catecholamines and chromogranin A, if available6. Imaging for routine surveillance is controversial, however. Magnetic resonance imaging (or computed tomography) should be used only if a biochemical test is abnormal11.
Finally, nf1 patients should have their blood pressure measured every year. Only those with high blood pressure should undergo tests specific for pheo6.
3. GENETIC TESTING: THE FUTURE
Given the rapid evolution in molecular genetic testing and the appearance of next-generation sequencing in clinical practice, it is likely that stepwise genetic testing will soon not be required, because many genes will be tested simultaneously, rapidly, and at lower cost. The result will be a higher mutation discovery rate, more complete genetic counselling to patients, and a better understanding of the clinical phenotypes of these predisposition syndromes41.
4. ACKNOWLEDGMENTS
We thank Dr. Mercedes Robledo [Centro Nacional de Investigaciones Oncológicas (Spanish National Cancer Research Center)] for identifying the mutation in case 2. We also acknowledge Drs. Marc Tischkowitz, Nancy Deslauriers, and Israel Fortin for their comments on earlier drafts of this manuscript, and the cancer genetics teams at the Montreal General Hospital and the Jewish General Hospital for clinical input.
5. CONFLICT OF INTEREST DISCLOSURES
The authors report that they have no financial or commercial conflicts of interest.
6. REFERENCES
- 1.Dumas N, Edmont M, Grunenwald S, et al. Characterisation of genetics aspects of paragangliomas and pheochromocytomas in the Quebec French-Canadian population. Presented at the 12th International Congress of Human Genetics; Montreal, QC. October 11–15, 2011; [Available online at: http://www.ichg2011.org/cgi-bin/showdetail.pl?absno=10669; cited June 17, 2013] [Google Scholar]
- 2.Pasini B, Stratakis CA. SDH mutations in tumorigenesis and inherited endocrine tumours: lesson from the phaeochromocytoma–paraganglioma syndromes. J Intern Med. 2009;266:19–42. doi: 10.1111/j.1365-2796.2009.02111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirmani S, Young WF. Hereditary paraganglioma–pheochromocytoma syndromes. In: Pagon RA, Bird TD, Dolan CR, et al., editors. GeneReviews. Seattle, WA: University of Washington; 1993. [Available online at: http://www.ncbi.nlm.nih.gov/books/NBK1548; cited August 30, 2012] [PubMed] [Google Scholar]
- 4.DeLellis RA, Lloyd RV, Heitz PU, Eng C, editors. WHO Classification of Tumours Pathology and Genetics Tumours of Endocrine Organs. Lyon, France: IARC Press; 2004. [Google Scholar]
- 5.Erlic Z, Neumann HPH. Familial pheochromocytoma. Hormones. 2009;8:29–38. doi: 10.14310/horm.2002.1219. [DOI] [PubMed] [Google Scholar]
- 6.Fishbein L, Nathanson KL. Pheochromocytoma and paraganglioma: understanding the complexities of the genetic background. Cancer Genet. 2012;205:1–11. doi: 10.1016/j.cancergen.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jimerez C, Cote G, Arnold A, Gagel RF. Review: should patients with apparently sporadic pheochromocytomas or paragangliomas be screened for hereditary syndromes? J Clin Endocrinol Metab. 2006;91:2851–8. doi: 10.1210/jc.2005-2178. [DOI] [PubMed] [Google Scholar]
- 8.Jafri M, Maher ER. The genetics of phaeochromocytoma: using clinical features to guide genetic testing. Eur J Endocrinol. 2012;166:151–8. doi: 10.1530/EJE-11-0497. [DOI] [PubMed] [Google Scholar]
- 9.Favier J, Gimenez–Roqueplo AP. Genetics of paragangliomas and pheochromocytomas [French] Med Sci (Paris) 2012;28:625–32. doi: 10.1051/medsci/2012286016. [DOI] [PubMed] [Google Scholar]
- 10.de Wailly P, Oragano L, Radé F, et al. Malignant pheochromocytoma: new malignancy criteria. Langenbecks Arch Surg. 2012;397:239–46. doi: 10.1007/s00423-011-0850-3. [DOI] [PubMed] [Google Scholar]
- 11.Hodgson SV, Foulkes WD, Eng C, Maher ER. A Practical Guide to Human Cancer Genetics. 3rd ed. Cambridge, UK: Cambridge University Press; 2007. [Google Scholar]
- 12.Neumann HP, Bausch B, McWhinney SR, et al. Germ-line mutations in nonsyndromic pheochromocytoma. N Engl J Med. 2002;346:1459–66. doi: 10.1056/NEJMoa020152. [DOI] [PubMed] [Google Scholar]
- 13.Cui Q, Wang W, Fu Z, et al. Integrated dna-based/biochemical screening for early diagnosis of multiple endocrine neoplasia type 2A (men2a) J Biomed Res. 2013;27:145–50. doi: 10.7555/JBR.27.20120121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gimenez–Roqueplo AP, Dahia PL, Robledo M. An update on the genetics of paraganglioma, pheochromocytoma, and associated hereditary syndromes. Horm Metab Res. 2012;44:328–33. doi: 10.1055/s-0031-1301302. [DOI] [PubMed] [Google Scholar]
- 15.Erlic Z, Rybicki L, Peczkowska M, et al. on behalf of the European–American Pheochromocytoma Study Group Clinical predictors and algorithm for the genetic diagnosis of pheochromocytoma patients. Clin Cancer Res. 2009;15:6378–85. doi: 10.1158/1078-0432.CCR-09-1237. [DOI] [PubMed] [Google Scholar]
- 16.Kantorovich V, King KS, Pacak K. SDH-related pheochromocytoma and paraganglioma. Best Pract Res Clin Endocrinol Metab. 2010;24:415–24. doi: 10.1016/j.beem.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burnichon N, Briere JJ, Libe R, et al. SDHA is a tumor suppressor gene causing paraganglioma. Hum Mol Genet. 2010;19:3011–20. doi: 10.1093/hmg/ddq206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korpershoek E, Favier J, Gaal J, et al. SDHA immunohistochemistry detects germline SDHA gene mutations in apparently sporadic paragangliomas and pheochromocytomas. J Clin Endocrinol Metab. 2011;96:E1472–6. doi: 10.1210/jc.2011-1043. [DOI] [PubMed] [Google Scholar]
- 19.Boedeker CC, Neumann HP, Offergeld C, et al. Clinical features of paraganglioma syndromes. Skull Base. 2009;19:17–24. doi: 10.1055/s-0028-1103123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ricketts CJ, Forman JR, Rattenberry E, et al. Tumor risks and genotype-phenotype-proteotype analysis in 358 patients with germline mutations in SDHB and SDHD. Hum Mutat. 2010;31:41–51. doi: 10.1002/humu.21136. [DOI] [PubMed] [Google Scholar]
- 21.Baysal BE. Mitochondrial complex ii and genomic imprinting in inheritance of paraganglioma tumors. Biochim Biophys Acta. 2013;1827:573–7. doi: 10.1016/j.bbabio.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Yeap PM, Tobias ES, Mavraki E, et al. Molecular analysis of pheochromocytoma after maternal transmission of SDHD mutation elucidates mechanism of parent-of-origin effect. J Clin Endocrinol Metab. 2011;96:E2009–13. doi: 10.1210/jc.2011-1244. [DOI] [PubMed] [Google Scholar]
- 23.Hao HX, Khalimonchuk O, Schraders M, et al. SDH5, a gene required for flavination of succinate dehydrogenase, is mutated in paraganglioma. Science. 2009;325:1139–42. doi: 10.1126/science.1175689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunst HP, Rutten MH, de Monnink JP, et al. SDHAF2 (PGL2–SDH5) and hereditary head and neck paraganglioma. Clin Cancer Res. 2011;17:247–54. doi: 10.1158/1078-0432.CCR-10-0420. [DOI] [PubMed] [Google Scholar]
- 25.Bayley JP, Kunst HP, Cascon A, et al. SDHAF2 mutations in familial and sporadic paraganglioma and phaeochromocytoma. Lancet Oncol. 2010;11:366–72. doi: 10.1016/S1470-2045(10)70007-3. [DOI] [PubMed] [Google Scholar]
- 26.Qin Y, Yao L, King EE, et al. Germline mutations in TMEM127 confer susceptibility to pheochromocytoma. Nat Genet. 2010;42:229–33. doi: 10.1038/ng.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burnichon N, Lepoutre–Lussey C, Laffaire J, et al. A novel TMEM127 mutation in a patient with familial bilateral pheochromocytoma. Eur J Endocrinol. 2011;164:141–5. doi: 10.1530/EJE-10-0758. [DOI] [PubMed] [Google Scholar]
- 28.Neumann HP, Sullivan M, Winter A, et al. Germline mutations of the TMEM127 gene in patients with paraganglioma of head and neck and extraadrenal abdominal sites. J Clin Endocrinol Metab. 2011;96:E1279–82. doi: 10.1210/jc.2011-0114. [DOI] [PubMed] [Google Scholar]
- 29.Yao L, Schiavi F, Cascon A, et al. Spectrum and prevalence of FP/TMEM127 gene mutations in pheochromocytomas and paragangliomas. JAMA. 2010;304:2611–19. doi: 10.1001/jama.2010.1830. [DOI] [PubMed] [Google Scholar]
- 30.Comino–Mendez I, Garcia–Aznarez FJ, Schiavi F, et al. Exome sequencing identifies MAX mutations as a cause of hereditary pheochromocytoma. Nat Genet. 2011;43:663–7. doi: 10.1038/ng.861. [DOI] [PubMed] [Google Scholar]
- 31.Burnichon N, Cascon A, Schiavi F, et al. MAX mutations cause hereditary and sporadic pheochromocytoma and paraganglioma. Clin Cancer Res. 2012;18:2828–37. doi: 10.1158/1078-0432.CCR-12-0160. [DOI] [PubMed] [Google Scholar]
- 32.Schlisio S, Kenchappa RS, Vredeveld LC, et al. The kinesin kif1bβ acts downstream from EglN3 to induce apoptosis and is a potential 1p36 tumor suppressor. Genes Dev. 2008;22:884–93. doi: 10.1101/gad.1648608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galan SR, Kann PH. Genetics and molecular pathogenesis of pheochromocytoma and paraganglioma. Clin Endocrinol (Oxf) 2013;78:165–75. doi: 10.1111/cen.12071. [DOI] [PubMed] [Google Scholar]
- 34.Ladroue C, Carcenac R, Leporrier M, et al. PHD2 mutation and congenital erythrocytosis with paraganglioma. N Engl J Med. 2008;359:2685–92. doi: 10.1056/NEJMoa0806277. [DOI] [PubMed] [Google Scholar]
- 35.Astuti D, Ricketts CJ, Chowdhury R, et al. Mutation analysis of HIF prolyl hydroxylases (PHD/EGLN) in individuals with features of phaeochromocytoma and renal cell carcinoma susceptibility. Endocr Relat Cancer. 2010;18:73–83. doi: 10.1677/ERC-10-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lorenzo F, Yang C, Ng Tang Fui M, et al. A novel EPAS1/HIF2A germline mutation in a congenital polycythemia with paraganglioma. J Mol Med (Berl) 2013;91:507–12. doi: 10.1007/s00109-012-0967-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhuang Z, Yang C, Lorenzo F, et al. Somatic HIF2A gain-of-function mutations in paraganglioma with polycythemia. N Engl J Med. 2012;367:922–30. doi: 10.1056/NEJMoa1205119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crona J, Delgado Verdugo A, Maharjan R, et al. Somatic mutations in H-RAS in sporadic pheochromocytoma and paraganglioma identified by exome sequencing. J Clin Endocrinol Metab. 2013;98:E1266–71. doi: 10.1210/jc.2012-4257. [DOI] [PubMed] [Google Scholar]
- 39.Gill AJ, Benn DE, Chou A, et al. Immunohistochemistry for SDHB triages genetic testing of SDHB, SDHC, and SDHD in paraganglioma–pheochromocytoma syndromes. Hum Pathol. 2010;41:805–14. doi: 10.1016/j.humpath.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Moline J, Eng C. Multiple endocrine neoplasia type 2. In: Pagon RA, Bird TD, Dolan CR, et al., editors. GeneReviews. Seattle, WA: University of Washington; 1993. [Available online at: http://www.ncbi.nlm.nih.gov/books/NBK1257; cited January 10, 2013] [Google Scholar]
- 41.Rattenberry E, Vialard L, Yeung A, et al. A comprehensive next generation sequencing based genetic testing strategy to improve diagnosis of inherited pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 2013;98:E1248–56. doi: 10.1210/jc.2013-1319. [DOI] [PubMed] [Google Scholar]