Abstract
Purpose
The main goal of treating ductal carcinoma in situ (dcis) is to prevent the development of invasive breast cancer. Most women are treated with breast-conserving surgery (bcs) and radiotherapy. Age at diagnosis may be a risk factor for recurrence, leading to concerns that additional treatment may be necessary for younger women. We report a population-based study of women with dcis treated with bcs and radiotherapy and an evaluation of the effect of age on local recurrence (lr).
Methods
All women diagnosed with dcis in Ontario from 1994 to 2003 were identified. Treatments and outcomes were collected through administrative databases and validated by chart review. Women treated with bcs and radiotherapy were included. Survival analyses were performed to evaluate the effect of age on outcomes.
Results
We identified 5752 cases of dcis; 1607 women received bcs and radiotherapy. The median follow-up was 10.0 years. The 10-year cumulative lr rate was 27% for women younger than 45 years, 14% for women 45–50 years, and 11% for women more than 50 years of age (p < 0.0001). The 10-year cumulative invasive lr rate was 22% for women younger than 45 years, 10% for women 45–50 years, and 7% for women more than 50 years of age (p < 0.0001). On multivariate analyses, young age (<45 years) was significantly associated with lr and invasive lr [hazard ratio (hr) for lr: 2.6; 95% confidence interval (ci): 1.9 to 3.7; p < 0.0001; hr for invasive lr: 3.0; 95% ci: 2.0 to 4.4; p < 0.0001]. An age of 45–50 years was also significantly associated with invasive lr (hr: 1.6; 95% ci: 1.0 to 2.4; p = 0.04).
Conclusions
Age at diagnosis is a strong predictor of lr in women with dcis after treatment with bcs and radiotherapy.
Keywords: Ductal carcinoma in situ, age, recurrence, young patients, radiation
1. BACKGROUND
Ductal carcinoma in situ (dcis) is a noninvasive form of breast cancer that is most often diagnosed during mammographic screening. Although dcis is not life-threatening, up to 20% of women with dcis can develop invasive breast cancer at 10 years, which is associated with an increased risk of breast cancer mortality1–6. The goals of treating dcis are to maximize breast conservation, optimize cosmesis, and prevent the development of invasive breast cancer. Most women with dcis are treated with breast-conserving surgery (bcs), followed by whole-breast irradiation. Radiotherapy after bcs has been proved to reduce the rate of local recurrence (both in situ and invasive)7–12. Randomized and nonrandomized clinical trials report that after bcs and radiation, approximately 12%–15% of women will experience a local recurrence within 10 years, and 5%–10% will develop an invasive local recurrence7–12.
It is important to identify factors associated with the development of local recurrence and subsequent invasive breast cancer, because women with such factors may be candidates for more extensive therapy. It has been suggested that younger age at diagnosis is a risk factor for local recurrence after treatment for dcis7,12–14. Multivariable analyses from randomized clinical trials suggest that the efficacy of radiation in preventing local recurrence after bcs may be less in younger women, but data on long-term outcomes (rates of dcis recurrence and invasive breast cancer) in younger women with dcis treated with bcs and radiation are limited5,7,12,15.
Past estimates of outcomes in younger women with dcis treated with bcs and radiation were derived from subgroup analyses of randomized clinical trials or institutional case series, which may not be representative of outcomes in young women with dcis from the population at large. No population-based studies of young women treated for dcis have been published. The long-term rates of recurrence and invasive breast cancer in young women with dcis treated with bcs and radiation and the independent effect of age at diagnosis therefore remain unclear.
We report the results of a population-based analysis of women with dcis treated with bcs and radiation with long-term follow-up. The objective of our study was to evaluate long-term outcomes in a population of young women with dcis treated with bcs and radiation. We describe the rates of local recurrence and of invasive breast cancer and evaluate the independent effect of age at diagnosis as a predictor of subsequent invasive breast cancer.
2. METHODS
2.1. Study Cohort
To identify the study population, all breast pathology reports held by the Ontario Cancer Registry (ocr) for tissue removed between January 1, 1994, and December 31, 2003, including reports with codes 174 (invasive breast cancer) or 233 (dcis) from the International Classification of Diseases, 9th revision (icd-9) or with benign diagnoses were obtained (n = 129,140). The ocr is a population-based registry for the province of Ontario, the largest province in Canada. The ocr collects information on every newly diagnosed case of cancer in the province using multiple data sources. All hospitals and laboratories in Ontario forward copies of all cancer-associated pathology reports to the ocr16.
We initially reviewed and abstracted all pathology reports held at the ocr and used automated text extraction to identify cases involving a diagnosis of dcis. We excluded cases with a final diagnosis of invasive breast cancer or benign breast disease (n = 118,905) or dcis with microinvasion (n = 1447). We found 7282 cases with a final diagnosis of dcis, and 2953 reports with dcis and microinvasion. We subsequently linked cases diagnosed as pure dcis or dcis and microinvasion with the ocr database to exclude cases with a prior history of any invasive cancer. We excluded 4483 such cases. The provincial cohort included 5752 cases of pure dcis.
Age at initial diagnosis was determined for each case. Information on the type of presentation (screen-detected vs. clinical symptoms) was not available. Follow-up for each patient was obtained using the Registered Persons Database, which includes demographic information on all residents in Ontario (place of residence, date of death, and date of last medical contact). The study was approved by the Research Ethics Board at Sunnybrook Hospital and the Ontario Cancer Research Ethics Board.
2.2. Treatment
To identify surgical treatment (mastectomy vs. bcs), we used deterministic linkage between the study cohort database and the Canadian Institute for Health Information (cihi) database. The cihi database is an administrative database containing information about every patient discharged from an Ontario hospital. It includes patient demographics (age, sex, and postal code), major diagnoses, and procedures (including same-day surgery). The date of definitive diagnosis was the date of histologic confirmation of dcis. We reviewed all surgical procedures performed within 6 months of the date of definitive diagnosis to determine if the final surgical treatment included bcs or mastectomy. Cases treated with mastectomy and bcs alone were excluded from the study cohort because our objective was to evaluate outcomes in women treated with bcs and radiation. To identify patients treated with breast radiation, we linked the study cohort database with the Ontario Health Insurance Program physician billings database to identify patients seen in consultation by a radiation oncologist within 12 months of definitive diagnosis (thus allowing for the possibilities of multiple surgical procedures and of an extended waiting period before radiotherapy consultation).
All radiation data were obtained by primary chart abstraction. For each case, we abstracted the radiation scheme used (total dose, number of fractions), beam energy (megavolts), and whether additional radiation treatment was delivered to the tumour cavity (“boost” radiation). In 89 cases, the administration of boost radiation was not indicated in the chart, and we therefore performed multivariate analyses categorizing cases with unreported boost both on their own and combined with “no boost” cases. There being no difference in the results, “unreported boost” and “no boost” cases were combined for presentation in this report. All surgical and radiation treatments were validated through primary review of hospital charts17.
2.3. Pathology
When original slides were available, a centralized pathology review of all diagnostic slides was performed by an expert breast pathologist. Data from the pathology review were included in all analyses involving those cases. In the remaining cases, we abstracted data from the original pathology report using an electronic data mining algorithm18.
We initially validated the accuracy of the data mining algorithm in a subset of 1000 cases of dcis by comparing the pathology data extracted electronically to the data obtained by manual data abstraction. The data algorithm achieved more than 95% accuracy for the following features: architectural subtype, nuclear grade, margin status, presence of comedonecrosis, and presence of multifocality. Tumour size and width of the resection margin were not consistently reported during the time interval of this study and were missing from many pathology reports. Those variables were therefore not abstracted. The data elements abstracted from the original pathology report were nuclear grade (low, intermediate, high, unreported), presence of comedonecrosis (present, absent, unreported), multifocality (present, absent, unreported), and margin status (positive, negative, unreported). Margin status was defined as “positive” if tumour cells were identified at the inked resection margin.
2.4. Outcomes
“Any local recurrence” was defined as detection of dcis or invasive breast cancer that developed in the same breast 6 months or more after the initial diagnosis of dcis. To determine any local recurrence, we identified all surgical procedures performed on the same breast (ipsilateral) as the index dcis lesion 6 months or more after the date of diagnosis through the cihi database. We abstracted the associated diagnosis (invasive, dcis, or benign) from the cihi database. We found that a cihi diagnosis of benign breast lesion was 98% accurate and a diagnosis coded as either invasive or dcis was more than 90% accurate. Therefore, to validate local recurrences that were invasive breast cancer, we linked each surgical procedure date to the ocr. Cases with a corresponding icd diagnostic code of 174 (invasive breast cancer) were accepted as an invasive local recurrence, because the accuracy of ascertainment of invasive cancers by the ocr is more than 95%16. Cases in which the corresponding hardcopy pathology report confirmed invasive breast cancer were coded as invasive breast cancer. The remaining cases were coded as dcis recurrence. Contralateral cancer is defined as dcis or invasive carcinoma developing in the contralateral breast after a diagnosis of dcis.
Overall survival was determined using the Registered Persons Database to determine the date of death from any cause. The date of last follow-up of the cohort was March 31, 2010.
2.5. Statistical Analysis
We conducted descriptive analyses for any local recurrence (in situ, invasive), invasive local recurrence, breast-cancer specific survival, and overall survival. We examined the effect of age as a continuous variable and as a categorical variable using cut-points previously reported in the literature: less than 45 years, 45–50 years, and more than 50 years of age8,11,13,14,19. Actuarial results for any local recurrence, invasive local recurrence, local recurrence-free survival, invasive local recurrence-free survival, and overall survival were calculated by the Kaplan–Meier method. The statistical significance of differences between actuarial curves was tested using the log-rank test. Proportional differences for categorical variables were tested using chi-square tests, and mean differences for continuous variables, by t-tests. A p value of 0.05 or less was considered statistically significant. To evaluate the independent effect of age on the outcomes of interest (any local recurrence, invasive local recurrence, and in situ local recurrence), univariate and multivariate survival analyses were performed using Cox proportional hazards models once it had been confirmed that the assumptions of the proportional hazards model were not violated.
3. RESULTS
3.1. Population Cohort
Using data from the ocr, we located 1949 patients diagnosed with dcis without microinvasion from 1994 to 2003 who were treated with bcs and radiation. A pathology review was performed in 1363 of the cases (70%). After the pathology review, 314 cases (16%) were excluded because the diagnosis changed to invasive cancer, lobular carcinoma in situ, benign disease, or dcis with microinvasion. Another 10 cases were excluded because the treatment was mastectomy and not bcs, and 18 cases were excluded because the invasive recurrence was found less than 6 months after the initial diagnosis, leaving 1607 patients in the study cohort. In that group, median age at diagnosis was 56 years (range: 20–85 years), with 195 of the women (12%) being less than 45 years of age at diagnosis, 281 (17%) being 45–50 years, and 1131 (70%) being more than 50 years. Most of the women received a conventional dose–fractionation radiation scheme of 50 Gy in 25 fractions delivered over 5 weeks (n = 969, 60%). The remaining 638 women (40%) received a hypofractionated regimen (40–44 Gy in 16 fractions). Boost radiation was given to 30% of the women (n = 488, Table i).
TABLE I.
Patient and tumour characteristics by age of the patient at diagnosis
| Variable |
Patient group [n (%)]
|
p Value | |||
|---|---|---|---|---|---|
| Whole cohort | <45 Years | 45–50 Years | >50 Years | ||
| Patients | 1607 | 195 | 281 | 1131 | — |
| Nuclear grade | |||||
| High | 591 (36.8) | 74 (37.9) | 112 (39.9) | 405 (35.8) | 0.31 |
| Moderate | 671 (41.8) | 87 (44.6) | 114 (40.6) | 470 (41.6) | |
| Low | 95 (5.9) | 9 (4.6) | 10 (3.6) | 76 (6.7) | |
| Unreported | 250 (15.6) | 25 (12.8) | 45 (16.0) | 180 (15.9) | |
| Necrosis | |||||
| Present | 948 (59.0) | 114 (58.5) | 175 (62.3) | 659 (58.3) | 0.45 |
| Absent | 374 (23.3) | 52 (26.7) | 58 (20.6) | 264 (23.3) | |
| Unreported | 285 (17.7) | 29 (14.9) | 48 (17.1) | 208 (18.4) | |
| Multifocality | |||||
| Present | 343 (21.3) | 33 (16.9) | 69 (24.6) | 241 (21.3) | 0.12 |
| Absent | 981 (61.0) | 117 (60.0) | 165 (58.7) | 699 (61.8) | |
| Unreported | 283 (17.6) | 45 (23.1) | 47 (16.7) | 191 (16.9) | |
| Subtype | |||||
| Solid | 1,073 (66.8) | 137 (70.3) | 184 (65.5) | 752 (66.5) | 0.94 |
| Cribriform | 332 (20.7) | 36 (18.5) | 65 (23.1) | 231 (20.4) | |
| Micropapillary | 21 (1.3) | 2 (1.0) | 3 (1.1) | 16 (1.4) | |
| Other | 50 (3.1) | 6 (3.1) | 9 (3.2) | 35 (3.1) | |
| Unreported | 131 (8.2) | 14 (7.2) | 20 (7.1) | 97 (8.6) | |
| Margin status | |||||
| Negative | 1,001 (62.3) | 121 (62.1) | 170 (60.5) | 710 (62.8) | 0.56 |
| Positive | 256 (15.9) | 27 (13.8) | 53 (18.9) | 176 (15.6) | |
| Unreported | 350 (21.8) | 47 (24.1) | 58 (20.6) | 245 (21.7) | |
| Radiation scheme | |||||
| Conventional | 827 (51.5) | 107 (54.9) | 150 (53.4) | 570 (50.4) | 0.01 |
| Conventional+boost | 142 (8.8) | 15 (7.7) | 31 (11.0) | 96 (8.5) | |
| Hypofractionation | 292 (18.2) | 23 (11.8) | 36 (12.8) | 233 (20.6) | |
| Hypofractionation+boost | 346 (21.5) | 50 (25.6) | 64 (22.8) | 232 (20.5) | |
| Boost radiation | |||||
| Yes | 488 (30.4) | 65 (33.3) | 95 (33.8) | 328 (29.0) | 0.18 |
| No | 1,119 (69.6) | 130 (66.7) | 186 (66.2) | 803 (71.0) | |
| Year of diagnosis | |||||
| 1994–1996 | 38 (19.5) | 59 (21.0) | 248 (21.9) | 345 (21.5) | 0.36 |
| 1997–1999 | 78 (40.0) | 106 (37.7) | 378 (33.4) | 562 (35.0) | |
| 2000–2003 | 79 (40.5) | 116 (41.3) | 505 (44.7) | 700 (43.6) | |
3.2. Outcomes
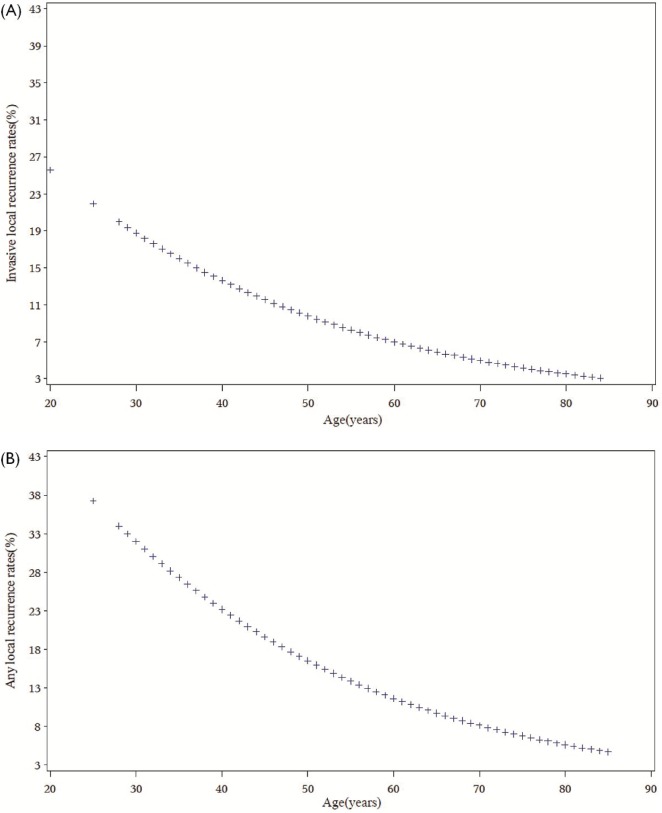
After a median follow-up of 10.0 years, 209 women treated with bcs and radiotherapy (13%) experienced a local recurrence (in situ or invasive). The risk of local recurrence increased with lower age at diagnosis. The effect of age on the risk of local recurrence and invasive recurrence was continuous. For each year of increase in age, local recurrence decreased 4% [hazard ratio (hr): 0.96; 95% confidence interval (ci): 0.95 to 0.98; p < 0.0001], and invasive local recurrence decreased 5% (hr: 0.95; 95% ci: 0.94 to 0.97; p < 0.0001; Figure 1). The 10-year cumulative rates of any local recurrence were 27% among women less than 45 years of age at diagnosis, 14% among women 45–50 years, and 11% among women more than 50 years (log-rank p < 0.0001, Table ii).
FIGURE 1.
Effect of age as continuous variable on (A) invasive local recurrence and (B) local recurrence of any kind.
TABLE II.
Outcomes in women with ductal carcinoma in situ (dcis) treated with breast-conserving surgery and radiation, by age at diagnosis
| Variable |
Patient group
|
p Value | |||
|---|---|---|---|---|---|
| Whole cohort (n=1607) | <45 Years (n=195) | 45–50 Years (n=281) | >50 Years (n=1131) | ||
| Local recurrence [n (%)] | |||||
| Any | 209 (13) | 48 (25) | 42 (15) | 119 (11) | <0.0001 |
| Invasive | 148 (9) | 36 (18) | 31 (11) | 81 (7) | <0.0001 |
| dcis | 61 (4) | 12 (6) | 11 (4) | 38 (3) | 0.17 |
| Local recurrence-free survival (%) | |||||
| Actuarial | |||||
| 5-Year | 91 | 84 | 90 | 93 | <0.0001 |
| 10-Year | 87 | 73 | 86 | 89 | |
| Invasive | |||||
| 5-Year | 95 | 88 | 94 | 96 | <0.0001 |
| 10-Year | 90 | 78 | 90 | 93 | |
| dcis | |||||
| 5-Year | 96 | 95 | 96 | 97 | 0.13 |
| 10-Year | 96 | 94 | 96 | 97 | |
| Contralateral breast cancer–free survival (%) | |||||
| 5-Year | 97 | 98 | 99 | 97 | 0.82 |
| 10-Year | 95 | 94 | 95 | 94 | |
| Overall survival (%) | |||||
| 5-Year | 97 | 98 | 97 | 97 | 0.002 |
| 10-Year | 91 | 91 | 95 | 89 | |
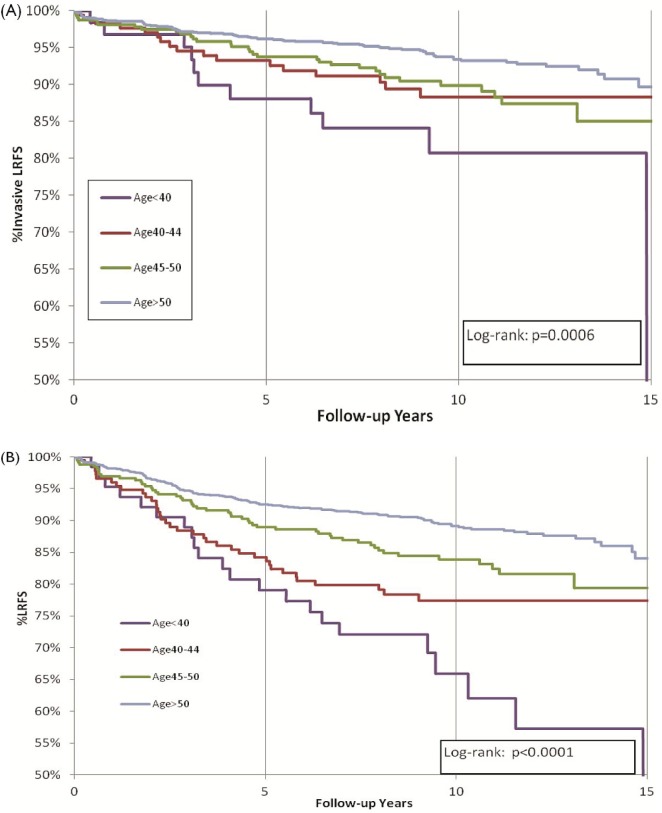
The rate of invasive local recurrence decreased progressively with increasing age: 22% for the group less than 45 years of age at diagnosis, 10% for those 45–50 years, and 7% for those more than 50 years (p < 0.0001, Figure 2). The cumulative incidence rates for dcis recurrence were 6% among women less than 45 years of age, 4% among those 45–50 years, and 3% among those more than 50 years (log-rank p = 0.17). We observed no significant difference in the 10-year rates of contralateral breast cancer by age, which ranged between 5% and 6% (Table ii).
FIGURE 2.
Local recurrence-free survival (lrfs) by age at diagnosis. (A) Invasive local recurrence. (B) Local recurrence of any kind.
The effect of age on the development of local recurrence remained significant among women treated with boost radiation. Among those who received boost radiation (n = 488), the cumulative local recurrence rate was 30% for those less than 45 years of age at diagnosis, 12% for those 45–50 years, and 9% for those more than 50 years (log-rank p = 0.0001). Among individuals who did not receive boost radiation, the local recurrence rate was 25% among those less than 45 years of age, 15% among those 45–50 years, and 11% among those more than 50 years (log-rank p = 0.0003; Figure 3).
FIGURE 3.
Local recurrence-free survival (lrfs) by age at diagnosis in women who (A) received and (B) did not receive boost radiation.
We performed multivariate analyses to evaluate the effect of age at diagnosis and other independent prognostic variables on the development of any local recurrence, invasive local recurrence, and dcis local recurrence after bcs and radiotherapy. On multivariate analyses, age less than 45 years (hr: 2.6; 95% CI: 1.9 to 3.7; p < 0.0001) and positive or unreported margin status (hr: 1.5; 95% CI: 1.1 to 2.0; p = 0.01) were significantly associated with the development of local recurrence (Table iii).
TABLE III.
Factors associated with any local recurrence: univariate and multivariate analysis, adjusted for year of diagnosis
| Variable |
Multivariate analysis
|
p Value | |
|---|---|---|---|
| hr | 95% cl | ||
| Age | |||
| <45 Years | 2.6 | 1.9, 3.7 | <0.0001 |
| 45–50 Years | 1.4 | 0.98, 2.0 | 0.06 |
| >50 Years | Reference | ||
| Multifocality | |||
| Present | 1.4 | 0.99, 1.9 | 0.06 |
| Absent | Reference | ||
| Unreported | 1.2 | 0.84, 1.7 | 0.32 |
| Margin status | |||
| Positive or unreported | 1.5 | 1.1, 2.0 | 0.01 |
| Negative | Reference | ||
| Nuclear grade | |||
| High | 1.2 | 0.9, 1.7 | 0.14 |
| Moderate or low | Reference | ||
| Unreported | 0.7 | 0.5, 1.1 | 0.15 |
| Boost | |||
| Yes | 0.8 | 0.6, 1.1 | 0.12 |
| No | Reference | ||
hr = hazard ratio; cl = confidence limits.
Age less than 45 years (hr: 3.0; 95% ci: 2.0 to 4.4; p < 0.0001) and 45–50 years at diagnosis (hr: 1.6; 5% ci: 1.0 to 2.4; p = 0.04), positive or unreported margin status (hr: 1.6; 95% ci: 1.1 to 2.2; p = 0.01), and unreported nuclear grade (hr: 0.6; 95% ci: 0.3 to 0.95; p = 0.04) were associated with the development of invasive local recurrence after treatment with bcs and radiotherapy (Table iv). On multivariate analysis, age less than 45 years (hr: 1.9; 95% ci: 1.0 to 3.7; p = 0.05) and 45–50 years at diagnosis (hr: 1.1; 95% ci: 0.6 to 2.1; p = 0.79) and high nuclear grade (hr: 2.8; 95% ci: 1.6 to 5.0; p = 0.0005) were associated with the development of dcis recurrence (Table v).
TABLE IV.
Factors associated with invasive local recurrence: multivariate analysis adjusted for year of diagnosis
| Variable |
Multivariate analysis
|
p Value | |
|---|---|---|---|
| hr | 95% cl | ||
| Age | |||
| <45 Years | 3.0 | 2.0, 4.4 | <0.0001 |
| 45–50 Years | 2.4 | 1.6, 1.0 | 0.04 |
| >50 Years | Reference | ||
| Multifocality | |||
| Present | 1.3 | 0.9, 2.0 | 0.16 |
| Absent | Reference | ||
| Unreported | 1.1 | 0.7, 1.7 | 0.77 |
| Margin status | |||
| Positive or unreported | 1.6 | 1.1, 2.2 | 0.01 |
| Negative | Reference | ||
| Nuclear grade | |||
| High | 0.9 | 0.6, 1.3 | 0.56 |
| Moderate or low | Reference | ||
| Unreported | 0.6 | 0.3, 0.95 | 0.04 |
| Boost | |||
| Yes | 0.7 | 0.5, 1.1 | 0.11 |
| No | Reference | ||
hr = hazard ratio; cl = confidence limits.
TABLE V.
Factors associated with ductal carcinoma in situ local recurrence: multivariate analysis adjusted for year of diagnosis
| Variable |
Multivariate analysis
|
p Value | |
|---|---|---|---|
| hr | 95% cl | ||
| Age | |||
| <45 Years | 1.9 | 1.0, 3.7 | 0.05 |
| 45–50 Years | 1.1 | 0.6, 2.1 | 0.79 |
| >50 Years | Reference | ||
| Multifocality | |||
| Present | 1.5 | 0.8, 2.8 | 0.18 |
| Absent | Reference | ||
| Unreported | 1.6 | 0.8, 3.1 | 0.16 |
| Margin status | |||
| Positive or unreported | 1.2 | 0.7, 2.1 | 0.47 |
| Negative | Reference | ||
| Nuclear grade | |||
| High | 2.8 | 1.6, 5.0 | 0.0005 |
| Moderate or low | Reference | ||
| Unreported | 1.5 | 0.6, 3.7 | 0.39 |
| Boost | |||
| Yes | 0.9 | 0.5, 1.6 | 0.75 |
| No | Reference | ||
hr = hazard ratio; cl = confidence limits.
4. DISCUSSION
We report long-term outcomes in a large, diverse population of women diagnosed with dcis and treated with bcs and radiation. Among those women, we found that young age at diagnosis is the strongest predictor of local recurrence, invasive local recurrence, and in situ local recurrence. Our results demonstrate that the effect of age on recurrence and invasive local recurrence is continuous. For women under 45 years of age at diagnosis, the 10-year local recurrence-free survival and invasive local recurrence-free survival after bcs and radiation were 73% and 78% respectively.
Our study corroborates a recent analysis of two large randomized trials, the National Surgical Adjuvant Breast and Bowel Project B-17 and B-24 trials, which demonstrated that women less than 45 years of age experienced higher invasive local recurrence even after controlling for other factors including tumour size, presence of comedonecrosis, and clinical presentation5. Half the women enrolled in B-17 did not receive radiotherapy. The strength of that study is that it included a large and diverse population of more than 1600 women with dcis treated with bcs and radiotherapy in the province of Ontario.
Young age at diagnosis and positive or unreported resection margins were significantly associated with an increased risk of local recurrence and invasive local recurrence after treatment with bcs and radiotherapy for dcis. The risk of local recurrence increased progressively with decreasing age at diagnosis after adjusting for the effect of nuclear grade and boost radiation. At 10 years, the rates of local recurrence after bcs and radiotherapy were 11% for women more than 50 years of age at diagnosis, 15% for those 45–50 years, and 25% for those less than 45 years.
We found that the increased risk of local recurrence associated with younger age was present regardless of the use of boost radiation to the tumour cavity. Excluding women with positive resection margins, the 10-year local recurrence-free survival rates among women who did and did not receive boost radiation were, respectively, 70% and 75% for women less than 45 years of age at diagnosis, 88% and 85% for those 45–50 years, and 91% and 89% for those more than 50 years. Although our study included 1607 women diagnosed over a 10-year period, only 65 of the women less than 45 years of age were treated with boost radiation because boost radiation was not commonly used during the period of our study. In a previous study, we had found no significant effect of boost radiation, but our ability to evaluate the effect of boost radiation on the outcomes of young women with dcis is limited18. The benefit of boost radiation in young women (<45 years of age) was reported in the Rare Cancer Network study19. In that report, the 10-year local relapse-free survival was 86% in women treated with boost radiotherapy and 72% in women treated with breast radiotherapy without boost. The results of our study and the Rare Cancer Network study demonstrate that the 10-year rate of local recurrence in young women with dcis treated with bcs and radiotherapy ranges from 14% to 27%. We could not evaluate the effect of tamoxifen because, during the study period, few women in the cohort received tamoxifen. Further research is needed to determine the optimal dose–fractionation regimen and the effect of tamoxifen in further reducing the risk of local recurrence in young women.
The reasons why younger women have higher rates of local recurrence and invasive recurrence are unknown. We did not identify any differences in the pathologic features of dcis (such as high grade, presence of necrosis, or resection margin status) in younger women. A previous study noted that, compared with women diagnosed between 40 and 70 years of age, younger women with dcis (<40 years) were more likely to be diagnosed with multicentric disease (29.3% vs. 17.7%)20. It is also possible that younger women are more likely to present with clinical symptoms (for example, a palpable lump) or more likely to have dense breasts or a family history of breast cancer, which have been shown to be associated with an increased risk of recurrence21–25. It has also been hypothesized that the efficacy of radiotherapy may be less in younger women11. A study by Holmberg et al.15 reported that radiotherapy had a small benefit with respect to cancer recurrence in women less than 50 years of age, but that a large degree of protection was seen in women more than 60 years. The differential benefit could not be explained by differences in lesion size, completeness of excision, multifocality, or mode of detection, a finding which suggests that the age effect may be to some extent attributable to responsivity to radiotherapy and not solely to an intrinsically higher recurrence risk in young women. That suggestion of differential response might be resolved if studies were to be undertaken in cohorts of women who did and did not receive radiotherapy; however, the latter studies are few in number. Further research is needed to determine the reasons that younger women have a higher risk of recurrence. In this regard, several studies of gene expression profiling are underway.
5. CONCLUSIONS
Young age at diagnosis and positive surgical resection margins are significantly associated with an increased risk of local recurrence after bcs and radiotherapy for dcis. The results of our study do not suggest that breast-conserving therapy is contraindicated in younger women for dcis; rather, the findings can be used to guide clinicians in their treatment decisions for women with dcis—such as ensuring complete clearance of the disease with negative resection margins. Further research is needed to understand the underlying biologic reasons that younger women have higher rates of recurrence and to explore ways to improve outcomes of women with dcis.
6. ACKNOWLEDGMENTS
The authors thank Ms. Barb Zurowski for assistance with manuscript preparation and Mr. Bruce Haan for assistance with computer programming.
7. CONFLICT OF INTEREST DISCLOSURES
The work reported here was supported by a grant from the Canadian Cancer Society Research Institute. IK’s fellowship was funded by the Canadian Breast Cancer Research Foundation, Ontario Chapter. The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or manuscript preparation. The corresponding author had full access to all the data in the study and took final responsibility for the decision to submit for publication.
8. REFERENCES
- 1.Page DL, Dupont WD, Rogers LW, Landenberger M. Intraductal carcinoma of the breast: follow-up after biopsy only. Cancer. 1982;49:751–8. doi: 10.1002/1097-0142(19820215)49:4<751::AID-CNCR2820490426>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 2.Lee LA, Silverstein MJ, Chung CT, et al. Breast cancer–specific mortality after invasive local recurrence in patients with ductal carcinoma-in-situ of the breast. Am J Surg. 2006;192:416–19. doi: 10.1016/j.amjsurg.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Romero L, Klein L, Ye W, et al. Outcome after invasive recurrence in patients with ductal carcinoma in situ of the breast. Am J Surg. 2004;188:371–6. doi: 10.1016/j.amjsurg.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 4.Silverstein MJ, Lagios MD, Martino S, et al. Outcome after invasive local recurrence in patients with ductal carcinoma in situ of the breast. J Clin Oncol. 1998;16:1367–73. doi: 10.1200/JCO.1998.16.4.1367. [DOI] [PubMed] [Google Scholar]
- 5.Wapnir IL, Dignam JJ, Fisher B, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in nsabp B-17 and B-24 randomized clinical trials for dcis. J Natl Cancer Inst. 2011;103:478–88. doi: 10.1093/jnci/djr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Page DL, Dupont WD, Rogers LW, Jensen RA, Schuyler PA. Continued local recurrence of carcinoma 15–25 years after a diagnosis of low grade ductal carcinoma in situ of the breast treated only by biopsy. Cancer. 1995;76:1197–200. doi: 10.1002/1097-0142(19951001)76:7<1197::AID-CNCR2820760715>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 7.Correa C, McGale P, Taylor C, et al. on behalf of the Early Breast Cancer Trialists’ Collaborative Group (ebctcg) Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Inst Monogr. 2010;2010:162–77. doi: 10.1093/jncimonographs/lgq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher B, Dignam J, Wolmark N, et al. Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-17. J Clin Oncol. 1998;16:441–52. doi: 10.1200/JCO.1998.16.2.441. [DOI] [PubMed] [Google Scholar]
- 9.Fisher B, Land S, Mamounas E, Dignam J, Fisher ER, Wolmark N. Prevention of invasive breast cancer in women with ductal carcinoma in situ: an update of the National Surgical Adjuvant Breast and Bowel Project experience. Semin Oncol. 2001;28:400–18. doi: 10.1016/S0093-7754(01)90133-2. [DOI] [PubMed] [Google Scholar]
- 10.Houghton J, George WD, Cuzick J, et al. Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia, and New Zealand: randomised controlled trial. Lancet. 2003;362:95–102. doi: 10.1016/S0140-6736(03)13859-7. [DOI] [PubMed] [Google Scholar]
- 11.Julien JP, Bijker N, Fentiman IS, et al. Radiotherapy in breast-conserving treatment for ductal carcinoma in situ: first results of the eortc randomised phase iii trial 10853. eortc Breast Cancer Cooperative Group and eortc Radiotherapy Group. Lancet. 2000;355:528–33. doi: 10.1016/S0140-6736(99)06341-2. [DOI] [PubMed] [Google Scholar]
- 12.Bijker N, Meijnen P, Peterse JL, et al. on behalf of the eortc Breast Cancer Cooperative Group Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in-situ: ten-year results of European Organisation for Research and Treatment of Cancer randomized phase iii trial 10853—a study by the eortc Breast Cancer Cooperative Group and eortc Radiotherapy Group. J Clin Oncol. 2006;24:3381–7. doi: 10.1200/JCO.2006.06.1366. [DOI] [PubMed] [Google Scholar]
- 13.Vicini FA, Kestin LL, Goldstein NS, et al. Impact of young age on outcome in patients with ductal carcinoma-in-situ treated with breast-conserving therapy. J Clin Oncol. 2000;18:296–306. doi: 10.1200/JCO.2000.18.2.296. [DOI] [PubMed] [Google Scholar]
- 14.Vicini FA, Recht A. Age at diagnosis and outcome for women with ductal carcinoma-in-situ of the breast: a critical review of the literature. J Clin Oncol. 2002;20:2736–44. doi: 10.1200/JCO.2002.07.137. [DOI] [PubMed] [Google Scholar]
- 15.Holmberg L, Garmo H, Granstrand B, et al. Absolute risk reductions for local recurrence after postoperative radiotherapy after sector resection for ductal carcinoma in situ of the breast. J Clin Oncol. 2008;26:1247–52. doi: 10.1200/JCO.2007.12.7969. [DOI] [PubMed] [Google Scholar]
- 16.Clarke EA, Marrett LD, Kreiger N. Cancer registration in Ontario: a computer approach. IARC Sci Publ. 1991:246–57. [PubMed] [Google Scholar]
- 17.Rakovitch E, Narod SA, Nofech–Moses S, et al. Impact of boost radiation in the treatment of ductal carcinoma in situ: a population-based analysis. Int J Radiat Oncol Biol Phys. 2013;86:491–7. doi: 10.1016/j.ijrobp.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 18.Currie AM, Fricke T, Gawne A, Johnston R, Liu J, Stein B. Automated extraction of free-text from pathology reports. AMIA Annu Symp Proc. 2006:899. [PMC free article] [PubMed] [Google Scholar]
- 19.Omlin A, Amichetti M, Azria D, et al. Boost radiotherapy in young women with ductal carcinoma in situ: a multicentre, retrospective study of the Rare Cancer Network. Lancet Oncol. 2006;7:652–6. doi: 10.1016/S1470-2045(06)70765-3. [DOI] [PubMed] [Google Scholar]
- 20.Alvarado R, Lari SA, Roses RE, et al. Biology, treatment, and outcome in very young and older women with dcis. Ann Surg Oncol. 2012;19:3777–84. doi: 10.1245/s10434-012-2413-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bijker N, Peterse JL, Duchateau L, et al. Risk factors for recurrence and metastasis after breast-conserving therapy for ductal carcinoma-in-situ: analysis of European Organization for Research and Treatment of Cancer Trial 10853. J Clin Oncol. 2001;19:2263–71. doi: 10.1200/JCO.2001.19.8.2263. [DOI] [PubMed] [Google Scholar]
- 22.Habel LA, Dignam JJ, Land SR, Salane M, Capra AM, Julian TB. Mammographic density and breast cancer after ductal carcinoma in situ. J Natl Cancer Inst. 2004;96:1467–72. doi: 10.1093/jnci/djh260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Habel LA, Daling JR, Newcomb PA, et al. Risk of recurrence after ductal carcinoma in situ of the breast. Cancer Epidemiol Biomarkers Prev. 1998;7:689–96. [PubMed] [Google Scholar]
- 24.Kerlikowske K, Molinaro A, Cha I, et al. Characteristics associated with recurrence among women with ductal carcinoma in situ treated by lumpectomy. J Natl Cancer Inst. 2003;95:1692–702. doi: 10.1093/jnci/djg097. [DOI] [PubMed] [Google Scholar]
- 25.Virnig BA, Tuttle TM, Shamliyan T, Kane RL. Ductal carcinoma in situ of the breast: a systematic review of incidence, treatment, and outcomes. J Natl Cancer Inst. 2010;102:170–8. doi: 10.1093/jnci/djp482. [DOI] [PubMed] [Google Scholar]