Abstract
The inflammatory response is integral to maintaining health, by functioning to resist microbial infection and repair tissue damage. Large numbers of neutrophils are recruited to inflammatory sites to neutralise invading bacteria through phagocytosis and the release of proteases and reactive oxygen species into the extracellular environment. Removal of the original inflammatory stimulus must be accompanied by resolution of the inflammatory response, including neutrophil clearance, to prevent inadvertent tissue damage. Neutrophil apoptosis and its temporary inhibition by survival signals provides a target for anti-inflammatory therapeutics, making it essential to better understand this process. GM-CSF, a neutrophil survival factor, causes a significant increase in mRNA levels for the known anti-apoptotic protein Serum and Glucocorticoid Regulated Kinase 1 (SGK1). We have characterised the expression patterns and regulation of SGK family members in human neutrophils, and shown that inhibition of SGK activity completely abrogates the anti-apoptotic effect of GM-CSF. Using a transgenic zebrafish model, we have disrupted sgk1 gene function and shown this specifically delays inflammation resolution, without altering neutrophil recruitment to inflammatory sites in vivo. These data suggest SGK1 plays a key role in regulating neutrophil survival signalling, and thus may prove a valuable therapeutic target for the treatment of inflammatory disease.
Introduction
The inflammatory response is triggered by tissue injury or infection and is essential to protect the body from invading pathogens and to return damaged tissues to homeostasis. Neutrophils are the first cells recruited to sites of inflammation (1) and are an essential component of the cellular inflammatory response. Their main role is the removal of microbes through phagocytosis and intracellular killing (2). In addition, neutrophils may release intracellular contents either as neutrophil extracellular traps (3), or by degranulation of their potentially harmful granule contents into the inflammatory environment (4). Thus, tight regulation of neutrophil function is essential to the maintenance of health. In a perfectly balanced inflammatory response, there is removal of the initiating stimulus combined with the avoidance of tissue damage by successful inflammation resolution. A number of prerequisites must be met for the resolution of inflammation; of which the safe removal of leukocytes from the inflammatory site is of critical importance (5). An important mechanism of clearance is the apoptosis of neutrophils and their removal by macrophage phagocytosis in situ (6). At sites of inflammation, many signals are produced which suppress neutrophil apoptosis leading to an extended lifespan. Host derived signals such as ATP (7) or GM-CSF (8) and pathogen associated molecular patterns (PAMPs) (9) combine with physical properties of the inflammatory environment, such as hypoxia (10), to suppress neutrophil apoptosis, leading to delay or failure of inflammation resolution. This in turn results in tissue damage, which is a further stimulus for neutrophil recruitment and survival via damage associated molecular patterns (DAMPs) and underlies many chronic inflammatory diseases (11, 12). Understanding how these survival signals act at a molecular level within the neutrophil should identify targets for novel drug therapies for inflammatory disease.
GM-CSF is an important host-derived signal influencing neutrophil lifespan, and upon binding to its receptor initiates well-characterised intracellular signalling events. The heterodimeric GM-CSF receptor complex activates at least 2 distinct signalling pathways: activation of class IA PI3Ks (13) and JAK/STAT signalling (14). PI3K activation leads to generation of the secondary messenger phosphatidylinositol 3,4,5-triphosphate (PIP3) and downstream phosphorylation of AKT (15, 16), which in turn phosphorylates a wide range of cellular substrates with diverse cellular effects (17). AKT is seen as a central player in the transduction of cell surface signals to the core apoptotic machinery and AKT inhibitors have been shown to delay neutrophil apoptosis (18). However, fMLP receptors also signal via PI3K activation and activate AKT, but without affecting neutrophil apoptosis (19). There is, therefore, a dissociation of apoptosis signalling and AKT activation, suggesting roles for other signalling molecules in addition to PI3K activation in apoptosis regulation.
Activation of the JAK/STAT pathway downstream of GM-CSF stimulation is thought to act predominantly via transcriptional effects. Traditionally, neutrophils were thought not to be subject to transcriptional regulation but there is evidence of considerable regulation of neutrophil mRNA repertoire by inflammatory stimuli (including GM-CSF) (20, 21), with potential further regulation by miRNAs (22). In one study, mRNA changes in neutrophils were detected by microarray following GM-CSF stimulation (21). One of the most up-regulated mRNAs was that of the known anti-apoptotic protein Serum and Glucocorticoid Regulated Kinase 1 (SGK1) which, with GM-CSF stimulation, was up-regulated 13.2 fold. This protein had previously been shown to be regulated by GM-CSF and also upon treatment with other neutrophil survival agents such as LPS and TNFα (23). SGK1 shares 54% amino acid homology with AKT (24) and is also regulated by phosphorylation downstream of PI3K (25). We hypothesised that SGK1 might be an important link between extracellular anti-apoptotic stimuli and the downstream apoptosis machinery in neutrophils.
Materials and Methods
Reagents
Reagents used were qVD-OPh (R&D Systems, Abingdon, UK), GM-CSF (PeproTech, London, UK), DMOG, LPS, ATPγs and the DMSO control (all from Sigma-Aldrich, Poole, UK). Three SGK inhibitors were used: GSK650394 (Tocris Bioscience, Bristol, UK), GSK1558634A and GSK398689A (Glaxo Smith Kline, Stevenage, UK). The sgk1 morpholino and standard control morpholino were from Genetools (Philomath, OR, USA).
Purification of peripheral blood neutrophils
Peripheral venous blood was taken from healthy volunteers in accordance with the specific approval of the South Sheffield Research Ethics Committee (reference number: STH13927), and neutrophils prepared as previously described (26). Negatively selected neutrophil preparations were >98% pure. Apoptotic neutrophils were counted by an observer blind to the experimental condition, from cytospins stained with Quick-Diff (Gentaur, Brussels, Belgium).
Protein detection by western blot analysis
Samples were prepared from approximately 5×106 neutrophils as previously described (26), or from 30 pooled 5 dpf zebrafish embryos, and Western blotting was performed according to standard protocols (27) using a Protean 2 gel assembly (Bio-Rad, Hemel Hempstead, UK) and transferred to Hybond™-P PVDF membrane (GE Healthcare Life Sciences, Bucks, UK) using a Trans-Blot® semi dry blotter (Bio-Rad, Hemel Hempstead, UK). Antibodies used were anti-SGK (#3272; Cell Signaling Technology, Hertfordshire, UK) at 1:1000, anti-Mcl-1 (sc-819; Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 1:1000, anti-phospho-NDRG-1 (#3217; Cell Signaling Technology, Hertfordshire, UK ) at 1:1000, anti-total-NDRG-1 (#5196; Cell Signaling Technology, Hertfordshire, UK ) at 1:1000, or anti-Actin (A2066; Sigma-Aldrich, Poole, UK) at 1:2000 and Polyclonal Goat anti-Rabbit Secondary Antibody (Dako, Cambridge, UK) at 1:2000 (1:5000 for anti-Actin). Densitometry was performed using ImageJ as previously described (26).
PCR analysis of SGK mRNA isoforms and morpholino knockdown
RNA was extracted from approximately 7×106 human neutrophils (>96% purity), or 10-15 zebrafish larvae, using TRIzol® Reagent (Invitrogen, Paisley, UK) according to manufacturer’s instructions. Reverse Transcriptase PCR used Superscript III RT Enzyme (Invitrogen, paisley, UK) and PCR used Finnzymes Phusion® (New England Biolabs, Hertfordshire, UK) and was carried out in a C1000 Thermal Cycler. Primers used are shown in Supplemental Table 1.
Zebrafish husbandry and assays
Adult fish were maintained on a 14:10-hour light/dark cycle at 28°C in UK Home Office approved facilities. Experiments were performed using the Tg(mpx:GFP)i114 line, which specifically labels neutrophils with GFP (28), or the Tg(lyz:PHAkt-EGFP) which detects PI3K activation in neutrophils by recruitment of the EGFP-tagged plekstrin homology domain of AKT to PIP3 in the membrane (29). Larvae were collected and maintained according to standard protocols (30). Assays of neutrophil recruitment to tailfin injury were performed as previously described (28). For analysis of total neutrophil number, larvae were mounted in 1% Agarose and imaged using the 2X objective on a Nikon Eclipse TE2000U Inverted Microscope; neutrophils were counted blind, using ImageJ and Volocity® (Perkin Elmer, Cambridge, UK). Neutrophil apoptosis rates were assessed in PFA fixed larvae followed by Tyramide Signal Amplification (TSA) staining (TSATM-Plus, Perkin Elmer, Cambridge, UK) and ApopTag® Red In Situ Apoptosis Detection Kit (TUNEL) (Millipore Corporation, Herts, UK) as previously described (31).
Agilent microarray expression analysis
Six adult fish (from an mpx:GFP background) were macerated using a scalpel and passed through a series of μm meshes to further dissociate the cells. Cells were sorted by FACS analysis into those cells showing appropriate SSC and FSC to be neutrophil-like and further sorted into GFP positive and negative sets. GFP negative cells were used as a control. RNA was extracted from sorted cells using the mirVana™ kit as per manufacturer’s instructions (Ambion, Huntingdon, UK). Microarray analysis was performed using a Zebrafish (V3) Gene Expression Microarray containing 43663 probes (Agilent Technologies, Amstelveen, The Netherlands) as previously described (32).
Mass spectrometric analysis of drug penetration of larvae
3 dpf zebrafish samples (20 fish per time point) were sonicated (Soniprep 150, MSE, Sussex, UK) as previously described (33). Homogenised zebrafish samples were extracted by protein precipitation with the addition of 750 μL acetonitrile containing a structural analogue internal standard. Samples were mixed and centrifuged at 2200 × g for 20 min. LC-MS/MS analysis was performed using an API 4000 triple quadrupole mass spectrometer (AB Sciex) with an electrospray ionisation source operating in positive ion mode. The UHPLC system incorporated a Jasco XLC dual pump system with a gradient elution method utilising acetonitrile +0.1% formic acid (v/v) and water +0.1% formic acid (v/v) mobile phase and a Phenomenex Kinetex analytical column (C18, 2.6 μm, 50 × 2.1 mm). Samples were analysed against calibration standards prepared in a control zebrafish homogenate matrix over a concentration range of 1 to 5000 ng/mL, with an assay LLQ of 1 ng/mL. We estimated the volume of a zebrafish larva to be 0.0375 μl.
Statistical analyses
Data were analysed (Prism 5.0; GraphPad Software) using either unpaired, 2-tailed Student’s t-tests for comparisons between 2 groups or 1-way ANOVA with Bonferroni post-test adjustment for other data, except where indicated in the figure legend.
Results
Multiple SGK isoforms are expressed in neutrophils and regulated by GM-CSF
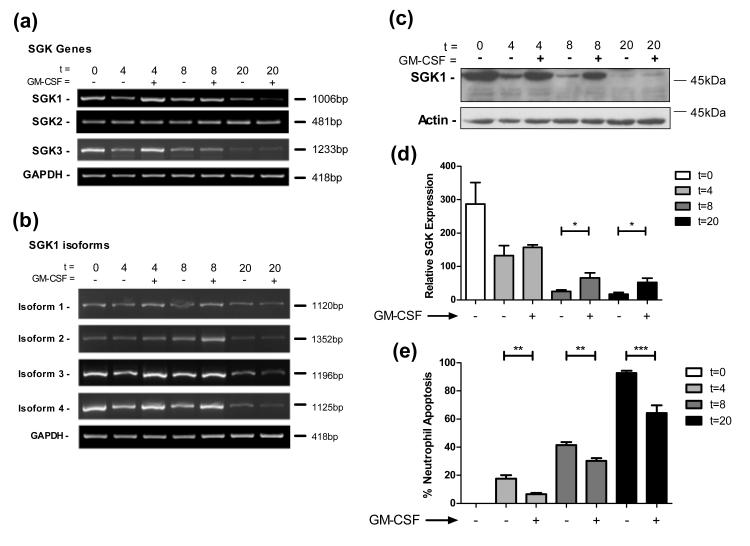
Kobayashi et al showed GM-CSF stimulation increased levels of SGK1 mRNA and prevented time dependent decreases in SGK1 protein levels (21). There are, however, three SGK genes present in humans: SGK1, 2 and 3, each sharing 80% homology at their catalytic domain (34). In addition there are four alternatively spliced isoforms of SGK1 and two for both SGK2 and 3 (Supplemental Figure 1a). We determined the expression profile of these different genes and isoforms in ultrapure human neutrophils (35). Using specific primer pairs in RT-PCR reactions we identified full-length isoforms of SGK1, SGK2 and SGK3 mRNA in human neutrophils (Figure 1a). SGK1 mRNA was detected at higher levels following GM-CSF stimulation at early time-points, whereas SGK2 expression was unaltered with GM-CSF stimulation. SGK3 mRNA levels, in contrast to previous reports (34), appeared to be increased initially but declined over time. Since GM-CSF regulation was largely confined to SGK1, we examined expression of the different isoforms of this gene. We aligned the sequences of the four SGK1 isoforms and identified the important domains and regulatory regions of the gene (Supplemental Figure 1b). We found that all isoforms varied only at their amino termini, where amino acids encoded by the first exons varied between isoforms both in length and sequence, with isoform 2 having additional exons. Functional domains within the protein were maintained between isoforms excepting the first 60 amino acids; these have been identified as playing a role in the ubiquitination and degradation of SGK1 (36). PCR analysis shows mRNAs for all SGK1 isoforms are regulated by GM-CSF (Figure 1b) at early time-points, though not at 20 hours.
Figure 1. Multiple SGK transcripts are present in human neutrophils, and are regulated by GM-CSF.
Human neutrophils were cultured for the times indicated ± GM-CSF and lysed for protein or mRNA analysis. a) RT-PCR analysis of mRNA levels show expression and regulation of multiple SGK genes in human neutrophils. b) Multiple isoforms of SGK1 are present and regulated in human neutrophils. c) Western blotting reveals the presence of SGK1 and its regulation by GM-CSF. The example shown is representative of 5 independent experiments. d) Densitometry and quantification of Western blots confirm statistically significant regulation of SGK1 expression (t-test, *p<0.05, n=5). e) GM-CSF causes a significant reduction in neutrophil apoptosis at 4, 8 and 20 hours in the samples used in (d) (**p<0.01, ***p<0.005, n=5).
To confirm changes in mRNA expression were paralleled by changes at the protein level, we analysed the change in SGK1 protein levels over time and following GM-CSF stimulation. We found, in agreement with Kobayashi et al, that GM-CSF prevented the reduction in SGK1 levels over time (Figure 1c, d) in parallel with increases in neutrophil survival (Figure 1e). Although the antibody used detects all members of the SGK protein family (SGK1, 2 and 3), no bands corresponding to full length SGK2 or SGK3 were identified. A band was routinely identified corresponding to full length SGK1 (isoform 1), which has a predicted size of 49kDa. To investigate whether the different levels of SGK1 detected were due to its decrease over time or merely due to the different numbers of viable neutrophils at the time points analysed, we used the caspase inhibitor, Q-VD-OPh, to prevent all neutrophil death (26). Following Q-VD-OPh treatment, SGK1 levels with or without GM-CSF stimulation were unchanged from DMSO treated control levels, despite the almost complete absence of apoptosis. This suggests that levels of SGK1 do not fall as a consequence of engagement of neutrophil apoptotic pathways (Supplemental Figure 2).
SGK1 inhibition blocks GM-CSF induced human neutrophil survival
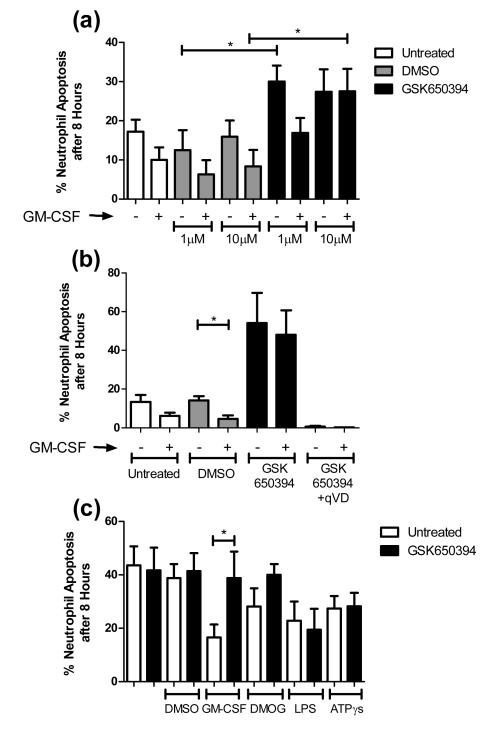
SGK1 is up-regulated at both the mRNA and protein level with GM-CSF treatment. We hypothesised this up-regulation was functionally important, and that the activity of SGK1 contributed to GM-CSF induced neutrophil survival. To test this hypothesis, we studied the effects on neutrophil apoptosis of the only commercially available inhibitor of SGK enzymes, GSK650394. GM-CSF induced neutrophil survival was clearly seen at 8 hours of culture, but with addition 10 μM GSK650394 this effect was totally abrogated (Figure 2a). GSK650394 also increased constitutive neutrophil apoptosis, suggesting that the compound was directly influencing neutrophil lifespan. To confirm the caspase dependence of the morphological changes of apoptosis observed in these experiments, the pan-caspase inhibitor, qVD-OPh was shown to prevent all cell death (Figure 2b).
Figure 2. Chemical inhibition of SGK1 abrogates neutrophil survival through an increase in apoptosis.
Human neutrophils were treated as indicated for 8 hours and percentage apoptosis scored by cytospin analysis. a) GSK650394 causes an increase in apoptosis rates and the 10 μM concentration significantly inhibits GM-CSF induced neutrophil survival (t-test, *p<0.05, n=5). b) Caspase inhibition prevents all neutrophil death (t-test, *p0.05, n=4). c) Neutrophil survival factors lower apoptosis rates at 8 hours, GSK650394 treatment abrogates survival caused by GM-CSF and DMOG but not that by LPS and ATPγs (*p<0.05, n=3).
To investigate whether the inhibition of neutrophil survival signalling by GSK650394 was specific to GM-CSF survival, we investigated its effect on a number of other neutrophil survival stimuli including activation of HIF signalling (mimicking hypoxia, induced here by the chemical DMOG) (31), LPS (37) and ATPγs (7). All survival factors reduced the amount of apoptosis compared to the control, although to a lesser degree than GM-CSF (Figure 2c). GSK650394 treatment abrogated the survival induced by GM-CSF and hypoxia, but did not affect that induced by LPS and ATPγs (Figure 2c), indicating that SGK1 is important to some, but not all, survival pathways.
To further probe the mechanism by which SGK1 inhibition might lead to neutrophil apoptosis, we tested whether SGK1 inhibition might cause decreases in the levels of Mcl-1, a key neutrophil survival protein. By western blotting, we found that Mcl-1 levels fell with GSK650394 treatment, suggesting a possible mechanism for enhanced apoptosis (Supplemental Figure 3).
Sgk1 inhibition enhances the resolution of inflammation in vivo, in part through an increase in neutrophil apoptosis
Acceleration of apoptosis in vitro may not always correspond to acceleration of inflammation resolution in vivo, particularly if anti-apoptotic factors predominate at the inflammatory site or large numbers of apoptotic neutrophils overwhelm clearance mechanisms. We therefore wished to study the effect of Sgk1 inhibition in a simple in vivo model of neutrophilic inflammation. Our preferred model for visualising individual inflammatory cell behaviours in a genetically and pharmacologically manipulable organism is the transgenic zebrafish model established in our laboratory (28). This model allows direct visualisation of vertebrate neutrophils during inflammation in vivo, and has proven invaluable in the identification of unique features of neutrophil behaviour (31, 38). By 3 dpf, zebrafish larvae have a fully functioning innate immune system and, importantly for these experiments, the zebrafish sgk1 gene is highly similar to the human gene in both amino acid sequence and conservation of key functional domains (Supplemental Figure 1c). By microarray analysis of FACS-sorted zebrafish neutrophils, sgk1 is present in zebrafish neutrophils at a level 7.84 times higher than in non-neutrophil cells, underlining its importance in this cell type.
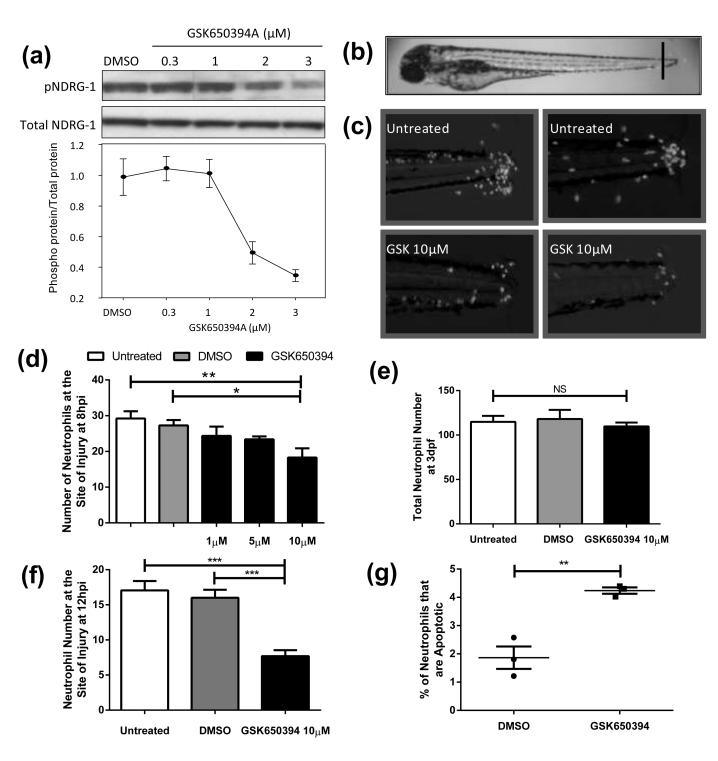
To investigate whether the chemical inhibitor of human SGK1, GSK650394, could penetrate zebrafish at this larval stage we incubated 3 dpf larvae with GSK650394 at 10 μM for an hour. We then subjected the larvae to mass spectrometric analysis as described in Materials and Methods. We measured 0.089 nmol of compound per larva, or 237 μM, confirming considerable concentration of compound within each larva. To further test whether the compound could successfully inhibit zebrafish Sgk1 we studied its phosphorylation activity in vivo. NDRG1 is known to be phosphorylated solely by SGK1 (39) and thus its phosphorylation can be used as a read out for SGK1 activity. In larvae treated with GSK650394 there was a dose dependent reduction in phosphorylated Ndrg1 without any effect on the total amount of Ndrg1 present (Figure 3a). This indicates that GSK650394 successfully inhibits Sgk1 activity in our assays.
Figure 3. Inhibition of Sgk1 enhances inflammation resolution in a model of tissue injury.
a) Protein extracts (30 μg) from 30 pooled 5 dpf zebrafish embryos exposed for 4 h to GSK650394 at the indicated concentrations were immunoblotted for p-NDRG1 and total NDRG1 showing inhibition of Sgk1 activity by GSK650394. b-d) 3 dpf mpx:GFP zebrafish larvae were subjected to tailfin transection at the site shown in (b) and treated as indicated at 4 hpi; neutrophil number was counted at 8 hpi. c) Representative micrographs of the larvae used for (d). d) GSK650394 significantly reduced the number of neutrophils at the site of injury in a dose dependent manner (*p<0.05, **p<0.01. n=18 performed as three independent experiments). e) Total neutrophil counts show no difference between GSK650394 treatment and the control. f g) 3 dpf mpx:GFP larvae were injured as above, treated at 6 hpi, and at 12 hpi neutrophil number was counted or larvae were fixed and subsequently stained with neutrophil markers (TSA) and apoptosis markers (TUNEL) to allow calculation of percentage apoptosis. f) GSK650394 causes a highly significant reduction in neutrophil number at the site of injury (***p<0.0001, n=18 performed as three independent experiments). g) GSK650394 treatment causes a significant increase in neutrophil apoptosis (**p<0.005, n=135 performed as three independent experiments).
Inflammation is induced in 3 dpf zebrafish larvae by transection of the caudal fin (Figure 3b), and the cellular component of the inflammatory response is quantified by counting the number of neutrophils present at the site of injury. In untreated larvae, recruitment of neutrophils to the site of injury occurs within the first 6 hours post injury (hpi) followed by spontaneous resolution within 24 hours. In this model, GSK65394 added during peak inflammation caused specific reduction in neutrophil numbers (Figure 3c, d), without altering numbers of neutrophils elsewhere in the animal (Figure 3e).
To see whether the effects on inflammation resolution were mediated by induction of neutrophil apoptosis in vivo, we dual stained for endogenous neutrophil peroxidase activity and for double stranded DNA breaks as a marker for apoptosis using TUNEL. These experiments were performed by adding the compound at peak neutrophil number (6 hpi) and measuring apoptosis at 12 hpi, during peak inflammation resolution. Neutrophil numbers were reduced significantly in these experiments compared to control (Figure 3f). Rates of apoptosis were comparable to previous studies (31) and were significantly higher for the GSK650394 group (Figure 3g). Interestingly, these effects were specific to inflammatory neutrophils, as rates of neutrophil apoptosis were not increased with GSK650394 treatment of uninjured larvae (data not shown).
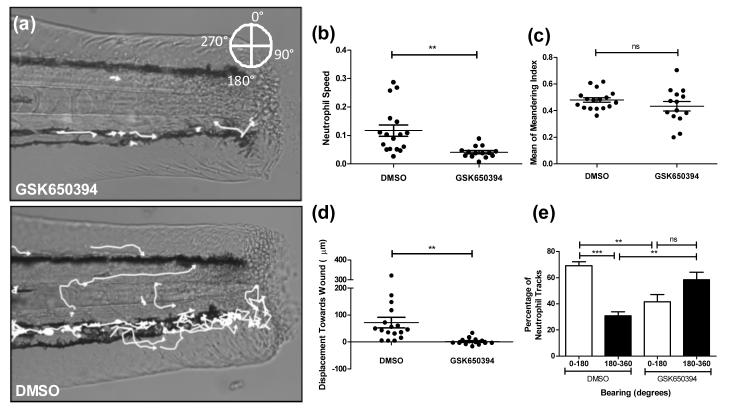
GSK650394 inhibits the recruitment of neutrophils to the site of injury through disruption of the direction and speed of neutrophil chemotaxis
To test whether the SGK1 inhibitor had any effect on other aspects of neutrophil function in vivo, we assessed the effects of GSK650394 on recruitment assays in the injured transgenic zebrafish model. Using Volocity® software, we tracked neutrophils as they moved towards the site of tailfin injury (Figure 4a) and saw a marked reduction in the movement of neutrophils treated with GSK650394. Further analysis revealed that this was due to a reduction in neutrophil speed (Figure 4b) whilst the meandering index (displacement divided by path length) of neutrophils was unaffected (Figure 4c). We noted that many neutrophils remained in one area, extending and retracting lamellipodia without making any directional movements. This was reflected in a reduction in the displacement of neutrophils towards the wound in GSK650394 treated larvae (Figure 4d). Additionally the bearing at which neutrophils moved is significantly different after GSK650394 treatment: control neutrophils preferentially migrate towards the wound, but GSK650394 treated larvae do not (Figure 4e).
Figure 4. GSK650394 inhibits neutrophil recruitment through disrupted chemotaxis.
3 dpf mpx:GFP larvae were pre-treated for two hours, injured and a timelapse image sequence taken for 1 hour. Neutrophil chemotaxis was analysed using Volocity® software. a) Representative images of treated larvae with neutrophil tracks superimposed. b) Neutrophil speed is significantly reduced with GSK650394 treatment (**p<0.005, n=14 performed as three independent experiments). c) Neutrophil meandering index is unaffected. d) GSK650394 treated neutrophils show a lack of movement towards the site of injury, as represented by positive values (**p<0.005, n=14 performed as three independent experiments). e) Analysis of the bearing at which neutrophils move demonstrates that directional movement of neutrophils towards the wound is removed with GSK650394 treatment (**p<0.005, ***p<0.001, n=14 performed as three independent experiments).
Genetic inhibition of sgk1 has a different anti-inflammatory phenotype to pharmacological inhibition of Sgk1
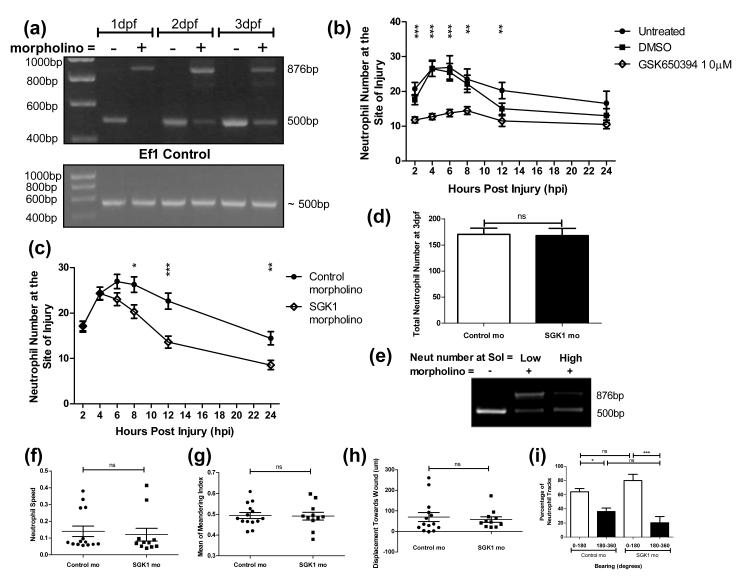
Pharmacological modulators of neutrophil function are prone to confounding results by off-target effects, and genetic confirmation of the phenotype is essential to confirm potential drug targets. Efficient knockdown of gene function in zebrafish can be achieved by injection of morpholino-modified antisense constructs into the fertilised egg (40). This can lead to altered expression of the target gene for many days. We therefore designed a “morpholino” to alter the pre-mRNA splicing of the sgk1 gene by targeting the splice site of intron 5 and causing intron inclusion, introducing a premature stop codon and leading to protein truncation. Effective gene knockdown was confirmed by RT-PCR analysis on RNA extracted from morphant larvae, with maintenance of the intron seen as a 385nt shift in the sgk1 band detected (Figure 5a).
Figure 5. sgk1 inhibition enhances inflammation resolution without affecting neutrophil recruitment.
3 dpf mpx:GFP larvae were injured, treated as indicated and neutrophil counts performed at the timepoints shown. (a, c-e) 1-2 nl of sgk1 morpholino was injected to larvae at the one cell stage. Knock-down disrupts the splicing of sgk1 pre-mRNA causing intron 5 to be retained; this can be detected as a 385 bp band shift by PCR. a) RT-PCR showing sgk1 knock down from 1-3 dpf with addition of the sgk1 morpholino. b) GSK650394 causes a highly significant inhibition of neutrophil recruitment to the site of injury (**p<0.01, ***p<0.005, n=18 performed as three independent experiments). c) The sgk1 morpholino increases resolution of the inflammatory response with a significant lowering of neutrophil number from 8 hpi (*p<0.05, **p<0.01, ***p<0.005, n=34 performed as three independent experiments). d) Total neutrophil counts show no difference between the sgk1 morpholino and the control. e) RT-PCR analysis of larvae separated after the morpholino timecourse into those with high and low neutrophil number at the site of injury show that larvae with fewer neutrophils show more complete morpholino knock down. f-i) mpx:GFP larvae were injected with 1-2 nl of 0.25 mM sgk1 morpholino at the one cell stage, injured at 3 dpf and a timelapse video taken for 1 hour. Neutrophil chemotaxis was analysed using Volocity®. Neutrophils from the sgk1 morphants did not show any significant difference from the controls in their speed (f), meandering (g) or displacement towards the wound (h) (n=11 performed as three independent experiments). i) There is no difference in the bearing at which neutrophils move, between the control and sgk1 morpholino injected larvae (*p<0.05, **p<0.005, n=11 performed as three independent experiments).
To isolate and identify the specific stage of the inflammatory response at which Sgk1 inhibition was having its effect, we analysed neutrophil number at the site of injury over the course of an entire inflammatory response by performing neutrophil counts at 2, 4, 6, 8, 12 and 24 hpi. In GSK650394 treated larvae there was a clear inhibition of neutrophil recruitment (Figure 5b).
To investigate whether reduction in neutrophil recruitment was due to specific inhibition of Sgk1 we repeated this timecourse using sgk1 morpholino injected larvae. We found that genetic knockdown of sgk1 caused an increase in the resolution of the inflammatory response (Figure 5c), without affecting recruitment or total neutrophil number (Figure 5d). Additional confirmation of the importance of Sgk1 levels on resolution of inflammation was obtained by separating larvae into groups showing the highest and lowest rates of inflammation resolution. The group having fewer neutrophils had better knockdown of sgk1 (Figure 5e), confirming its importance in regulating inflammation resolution.
GSK650394 inhibits neutrophil recruitment by off-target effects, in part through the inhibition of PI3K
Detailed analysis of neutrophil recruitment behaviour in sgk1 morphants revealed no difference in the morphant neutrophils’ speed of movement (Figure 5f) or their ability to follow the chemotactic gradient (Figure 5g, h, i) suggesting GSK650394 might be acting on a target other than Sgk1 to block chemotaxis. The profound effect on neutrophil recruitment, and differences in the shape of GSK650394 treated neutrophils (data not shown) led us to hypothesise that these effects might relate to off-target inhibition of the PI3K pathway. This was possible as GSK650394 was known to inhibit more than 30 enzymes, other than SGK1, with a pIC50 of >6 (data not shown). To further investigate this hypothesis, the effect of PI3K inhibition on neutrophil recruitment was studied in the zebrafish model. PI3K inhibition reduces the number of neutrophils recruited to a site of injury in a similar manner to GSK650394 (Figure 6a). A more detailed investigation of neutrophil chemotaxis shows that this reduction in recruitment is due to an inhibition of neutrophil speed (Figure 6b), similar to that seen with SGK inhibition. However, in contrast to findings with GSK650394 treated larvae, the bearing at which neutrophils moved was unaffected (Figure 6c). Furthermore, using a PHAkt-EGFP line (29) to indicate the subcellular localisation of phosophoinositides produced by PI3K activity, we investigated whether there were changes in PI3K activity in response to GSK650394 or Sgk1 knockdown. To quantify PI3K activity in individual cells in vivo, we used an assay we had previously developed to quantify PI3K activity in neutrophils in response to pharmacological treatments (29). This assay gives a numerical value of the cell polarisation as indicated by PHAkt-EGFP distribution within neutrophils. Using this system we were able to show that both PI3K inhibitors and GSK650394 reduced the polarity index (Figure 6d, e), whereas genetic manipulation of Sgk1 did not (Figure 6f).
Figure 6. PI3K inhibition attenuates neutrophil recruitment through a reduction in speed.
3 dpf mpx:GFP larvae were pre-treated for two hours, injured and a timelapse image sequence taken for 1 hour. Neutrophil chemotaxis was analysed using Volocity®. a) Both GSK650394 and LY294002 reduce the number of neutrophils recruited to a site of injury (***p<0.0001, n=12 performed as two independent experiments). b) Neutrophil speed is significantly reduced with LY294002 treatment (***p<0.0001, n=15 performed as two independent experiments). c) The bearing at which neutrophils migrate is not affected by PI3K inhibition (***p<0.0001, **p<0.05, n=15 performed as two independent experiments). d-f) 3 dpf PHAkt-EGFP larvae were pretreated for 2 hours as indicated and subsequently injured. Neutrophils were imaged and their polarity index calculated. d) Representative images of treated neutrophils and fluorescence intensity per pixel graphs. e) GSK650394 and LY294002 significantly reduce the polarity index of neutrophils (***p<0.005, n=2 performed on 3 separate occasions). f) Injection of the sgk1 morpholino has no effect upon the polarity index of neutrophils (n=26 performed on 3 separate occasions).
This phenotype demonstrates the complexity of understanding off-target pharmacological inhibition, with only part of the effect of pharmacological sgk inhibition likely to act via the PI3K pathway, while underscoring the importance of genetic confirmation for all inhibitor studies.
Highly specific SGK1 inhibitors block GM-CSF induced neutrophil survival and also lower neutrophil number at an in vivo site of injury
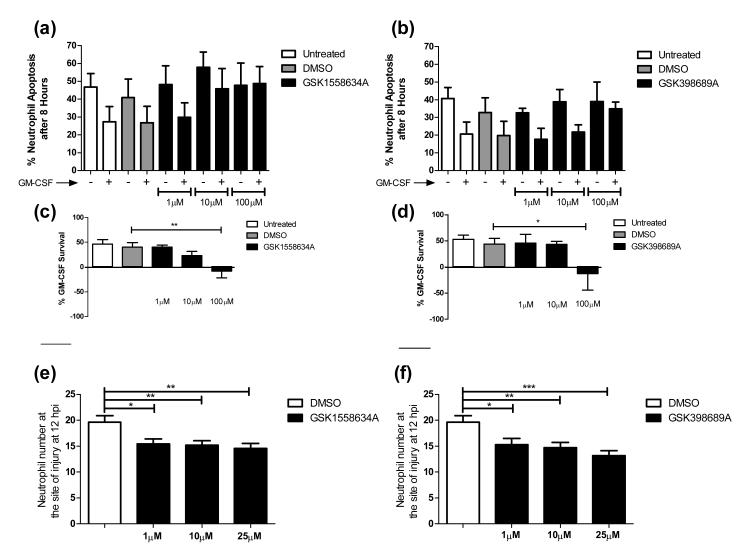
We have shown that sgk1 genetic inhibition removes neutrophils from a site of inflammation, however for this to be useful in a clinical situation SGK1 inhibition would have to be achieved using a chemical inhibitor. We have seen that GSK650394 has off-target effects and would not fulfil this role; we therefore used 2 potent SGK1 inhibitors from a chemical template distinct from that of GSK650394: GSK1558634A and GSK398689A. These compounds have a pIC50 of 8.2 and 8.8 respectively with limited off-target effects. GSK1558634A inhibits only 3 other enzymes with a pIC50 >6, these are AURA, AURB and 3,5-diarylpyr(id/az)inebenzimidazole, GSK398689A additionally inhibits YAK3 (GSK internal data). Treatment of human neutrophils with either of these new inhibitors removes the survival response to GM-CSF in a dose dependent manner, with total abrogation by 100 μM (Figure 7 a-d). Importantly, these compounds, had no effect upon constitutive apoptosis of human neutrophils.
Figure 7. Highly specific SGK1 inhibitors removes GM-CSF induced neutrophil survival.
a-d) Human neutrophils were treated as incubated for 8 hours and percentage apoptosis scored by cytospin analysis. a and c) GSK1558634A causes a dose dependent inhibition of GM-CSF induced neutrophil survival (**p<0.01, n=4). b and d) GSK398689A causes an inhibition of GM-CSF induced neutrophil survival (*p<0.05, n=5). e,f) 3 dpf mpx:GFP zebrafish larvae were subjected to tailfin transection and treated as indicated at 6 hpi; neutrophil number was counted at 12 hpi. Both GSK1558634A e) and GSK398689A f) significantly reduce the number of neutrophils present at the site of injury in a dose dependent manner (*p<0.05, **p<0.01, ***p<0.005, n=4).
Furthermore, zebrafish larvae treated with either GSK1558634A or GSK398689A after transection of the caudal fin, show a significant and dose dependent reduction in the number of neutrophils at the site of injury (Figure 7 e, f). The ability of two additional, more specific, inhibitors of SGK1 to replicate both the inhibition of GM-CSF induced neutrophil survival and the rapid resolution of the inflammatory response in vivo suggests that this occurs via inhibition of the SGK1 protein, not through off-target actions, adding support for a key role for SGK1 in the regulation of neutrophil lifespan during inflammation resolution.
Discussion
Previous work using human neutrophils has shown increased amounts of SGK1 mRNA and protein following GM-CSF stimulation (21); we have now produced genetic and pharmacological data showing the importance of SGK1 in controlling neutrophil lifespan in vitro and in vivo. We have not distinguished between new transcription and regulation of protein and mRNA stability, but we have shown by highly effective Caspase inhibition that the changes in SGK1 protein level are independent of levels of apoptosis. Use of the only commercially available SGK inhibitor, GSK650394, together with newer more selective compounds and a morpholino against sgk1, have shown it to play an important role in maintaining neutrophils at a site of injury. Sgk inhibition leads to rapid resolution of the inflammatory response without affecting neutrophil recruitment. SGK1 is therefore an important component of the pathways that link certain survival stimuli with the neutrophil apoptotic machinery. In other cell types, SGK1 is known to act downstream of PI3K and is activated by PDK1 through phosphorylation of Thr-256 and by PDK2 via Ser-422 (25, 41). We do not yet know completely how, once activated, SGK1 plays its anti-apoptotic role; in other cell types it regulates this process through inactivation of the FOXO3a transcription factor (42) and up-regulation of NFκB signalling (43), both of which are important in neutrophils (44, 45). Interestingly, SGK1 phosphorylates and inactivates Glycogen Synthase Kinase-3 (GSK-3) (25) which is known to phosphorylate the key neutrophil anti-apoptotic protein Mcl-1 leading to its destabilisation and degradation (46), a key upstream event in neutrophil apoptosis (26). We now show that inhibition of Mcl-1 turnover in human neutrophils is a likely final mechanism for the anti-apoptotic action of SGK1.
Chemical inhibition of Sgk enzymes with GSK650394 accelerates inflammation resolution, and reduces neutrophil recruitment. Genetic ablation of sgk1 alone has no effect on neutrophil recruitment. Studies of PI3K inhibition in vivo suggest that GSK650394, but not the genetic strategy, may act upstream of PI3K to modulate neutrophil recruitment, suggesting that inhibition of neutrophil recruitment is an off-target effect. It remains possible that the lack of effect on neutrophil recruitment with sgk1 morpholino could be due to incomplete knock-down of sgk1, as shown by the maintenance of a wild type sgk1 band in our PCRs (Figure 5a); the small amounts of wild type sgk1 present may be sufficient for neutrophil recruitment, but not their maintenance, at a site of injury. More probable, given the different effects of treatment with the more specific inhibitors, is that the recruitment phenotype is an off-target effect of GSK650394. Over 30 enzymes are known to be inhibited by GSK650394 with a pIC50 >6, including ALK5 (activin receptor-like kinase 5), AURB (aurora kinase B), CAMKK (calcium/calmodulin-dependent protein kinase kinase) 1 and CAMKK 2, CDK2 (cyclin dependent kinase 2), ITK (Il-2 inducible T-cell kinase), JNK1, JNK3, and the Class III PI3K, VPS34 (vacuolar protein sorting 34), and it is likely that additional kinases which have not been included in these kinase profiling studies are also inhibited by GSK650394 (Farrow, S and Zuercher, W unpublished data). Thus there is considerable scope for significant off-target effects at the dose used. To overcome this limitation of the pharmacological approach, we used new inhibitors, GSK1558634A and GSK398689A. These compounds have a pIC50 of 8.2 and 8.8 respectively, inhibiting only 3 and 4 other enzymes with a pIC50 >6. The fact that both compounds show the same pro-resolution phenotype as GSK650394 strongly supports the role of SGK1 in the regulation of neutrophil responses to survival signals. The effects on chemotaxis may relate to inefficient AKT2 recruitment following changes in PI3Kγ activation, as knock out of either gene causes frustrated chemotaxis (47, 48). Interestingly, the morphology of PI3Kγ knock out neutrophils is very similar to that seen in our time-lapse videos of GSK650394 treated neutrophils in the extending and retracting of lamellopodia.
The use of the zebrafish model of inflammation has allowed us to dissect the cellular consequences of SGK inhibition in ways that would not be possible in mammalian systems. The ability to dissociate effects of SGK inhibition on recruitment and neutrophil removal allows the pathway to be understood in detail. While murine models might allow the identification of reduced neutrophil numbers, dissociating therapeutic differences in recruitment and clearance is more challenging, but critically important. There is an important therapeutic difference between removing an unwanted neutrophil, and preventing recruitment of all neutrophils to sites of potential infection.
SGK1, like AKT, is activated downstream of PI3K by PDK1/2 at similar residues (15, 25, 41) and most protein targets of SGK1 are also phosphorylated by other protein kinases such as AKT (49). However, it has previously been noted that some stress stimuli which activated SGK1 did not result in AKT phosphorylation, showing separate rather than redundant roles for these two protein kinases (50). Although AKT activation is thought to delay neutrophil apoptosis (18), AKT can also be activated without influencing apoptosis, for example by fMLP (19). Thus, SGK1 might in some circumstances act to transduce apoptotic signals downstream of PI3K, in parallel to AKT.
The up-regulation of SGK1 by survival signals (21, 23) links to a clinically important increase in SGK1 expression seen in a number of inflammatory or fibrotic diseases during which the inflammatory response is involved, including Crohn’s disease (50) and glomerulonephritis (51). Prolonged neutrophil lifespan has been implicated in the extensive host tissue damage seen in these conditions, further underlining the therapeutic potential of SGK1 inhibition. Many current anti-inflammatory treatments block neutrophil recruitment to the site of injury but fail to remove neutrophils already there. We propose that SGK1 represents a possible target for anti-inflammatory therapeutics specifically targeting the resolution phase of inflammation while still allowing neutrophils to mount a response against infection, without the extended survival and degranulation associated with disease.
Supplementary Material
Acknowledgments
Grant support This work was supported by an MRC Senior Clinical Fellowship (G0701932; S.A.R.) and an MRC centre grant (G0700091). Microscopy studies were supported by a Wellcome Trust grant to the MBB/BMS Light Microscopy Facility (GR077544AIA).
Abbreviations
- SGK
serum and glucocorticoid regulated kinase
- PDK
phosphoinositide dependent kinase
- Mcl-1
myeloid cell leukaemia-1
References
- 1.Schleimer RP, Freeland HS, Peters SP, Brown KE, Derse CP. An assessment of the effects of glucocorticoids on degranulation, chemotaxis, binding to vascular endothelium and formation of leukotriene B4 by purified human neutrophils. J Pharmacol Exp Ther. 1989;250:598–605. [PubMed] [Google Scholar]
- 2.Liu H, Pope RM. Phagocytes: mechanisms of inflammation and tissue destruction. Rheum Dis Clin North Am. 2004;30:19–39. v. doi: 10.1016/S0889-857X(03)00107-8. [DOI] [PubMed] [Google Scholar]
- 3.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 4.Dahlgren C, Karlsson A. Respiratory burst in human neutrophils. J Immunol Methods. 1999;232:3–14. doi: 10.1016/s0022-1759(99)00146-5. [DOI] [PubMed] [Google Scholar]
- 5.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 6.Fox S, Leitch AE, Duffin R, Haslett C, Rossi AG. Neutrophil Apoptosis: Relevance to the Innate Immune Response and Inflammatory Disease. J Innate Immun. 2010;2:216–227. doi: 10.1159/000284367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaughan KR, Stokes L, Prince LR, Marriott HM, Meis S, Kassack MU, Bingle CD, Sabroe I, Surprenant A, Whyte MK. Inhibition of neutrophil apoptosis by ATP is mediated by the P2Y11 receptor. J Immunol. 2007;179:8544–8553. doi: 10.4049/jimmunol.179.12.8544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee A, Whyte MK, Haslett C. Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J Leukoc Biol. 1993;54:283–288. [PubMed] [Google Scholar]
- 9.Parker LC, Whyte MK, Dower SK, Sabroe I. The expression and roles of Toll-like receptors in the biology of the human neutrophil. J Leukoc Biol. 2005;77:886–892. doi: 10.1189/jlb.1104636. [DOI] [PubMed] [Google Scholar]
- 10.Hannah S, Mecklenburgh K, Rahman I, Bellingan GJ, Greening A, Haslett C, Chilvers ER. Hypoxia prolongs neutrophil survival in vitro. FEBS Lett. 1995;372:233–237. doi: 10.1016/0014-5793(95)00986-j. [DOI] [PubMed] [Google Scholar]
- 11.Brannigan AE, O’Connell PR, Hurley H, O’Neill A, Brady HR, Fitzpatrick JM, Watson RW. Neutrophil apoptosis is delayed in patients with inflammatory bowel disease. Shock. 2000;13:361–366. doi: 10.1097/00024382-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Matute-Bello G, Liles WC, Radella F, 2nd, Steinberg KP, Ruzinski JT, Jonas M, Chi EY, Hudson LD, Martin TR. Neutrophil apoptosis in the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1997;156:1969–1977. doi: 10.1164/ajrccm.156.6.96-12081. [DOI] [PubMed] [Google Scholar]
- 13.Klein JB, Rane MJ, Scherzer JA, Coxon PY, Kettritz R, Mathiesen JM, Buridi A, McLeish KR. Granulocyte-macrophage colony-stimulating factor delays neutrophil constitutive apoptosis through phosphoinositide 3-kinase and extracellular signal-regulated kinase pathways. J Immunol. 2000;164:4286–4291. doi: 10.4049/jimmunol.164.8.4286. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe S, Itoh T, Arai K. JAK2 is essential for activation of c-fos and c-myc promoters and cell proliferation through the human granulocyte-macrophage colony-stimulating factor receptor in BA/F3 cells. J Biol Chem. 1996;271:12681–12686. doi: 10.1074/jbc.271.21.12681. [DOI] [PubMed] [Google Scholar]
- 15.Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 2002;14:381–395. doi: 10.1016/s0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- 16.Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. Embo J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 17.Hannigan MO, Huang CK, Wu DQ. Roles of PI3K in neutrophil function. Curr Top Microbiol Immunol. 2004;282:165–175. doi: 10.1007/978-3-642-18805-3_6. [DOI] [PubMed] [Google Scholar]
- 18.Zhu D, Hattori H, Jo H, Jia Y, Subramanian KK, Loison F, You J, Le Y, Honczarenko M, Silberstein L, Luo HR. Deactivation of phosphatidylinositol 3,4,5-trisphosphate/Akt signaling mediates neutrophil spontaneous death. Proc Natl Acad Sci U S A. 2006;103:14836–14841. doi: 10.1073/pnas.0605722103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tilton B, Andjelkovic M, Didichenko SA, Hemmings BA, Thelen M. G-protein-coupled receptors and Fc gamma-receptors mediate activation of Akt protein kinase B in human phagocytes. J Biol Chem. 1997;272:28096–28101. doi: 10.1074/jbc.272.44.28096. [DOI] [PubMed] [Google Scholar]
- 20.Brach MA, deVos S, Gruss HJ, Herrmann F. Prolongation of survival of human polymorphonuclear neutrophils by granulocyte-macrophage colony-stimulating factor is caused by inhibition of programmed cell death. Blood. 1992;80:2920–2924. [PubMed] [Google Scholar]
- 21.Kobayashi SD, Voyich JM, Whitney AR, DeLeo FR. Spontaneous neutrophil apoptosis and regulation of cell survival by granulocyte macrophage-colony stimulating factor. J Leukoc Biol. 2005;78:1408–1418. doi: 10.1189/jlb.0605289. [DOI] [PubMed] [Google Scholar]
- 22.Ward JR, Heath PR, Catto JW, Whyte MKB, Milo M, Renshaw SA. Regulation of Neutrophil Senescence by MicroRNAs. PLoS One. 2011:6. doi: 10.1371/journal.pone.0015810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cowling RT, Birnboim HC. Expression of serum- and glucocorticoid-regulated kinase (sgk) mRNA is up-regulated by GM-CSF and other proinflammatory mediators in human granulocytes. J Leukoc Biol. 2000;67:240–248. doi: 10.1002/jlb.67.2.240. [DOI] [PubMed] [Google Scholar]
- 24.Mikosz CA, Brickley DR, Sharkey MS, Moran TW, Conzen SD. Glucocorticoid receptor-mediated protection from apoptosis is associated with induction of the serine/threonine survival kinase gene, sgk-1. J Biol Chem. 2001;276:16649–16654. doi: 10.1074/jbc.M010842200. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi T, Cohen P. Activation of serum- and glucocorticoid-regulated protein kinase by agonists that activate phosphatidylinositide 3-kinase is mediated by 3-phosphoinositide-dependent protein kinase-1 (PDK1) and PDK2. Biochem J. 1999;339(Pt 2):319–328. [PMC free article] [PubMed] [Google Scholar]
- 26.Wardle DJ, Burgon J, Sabroe I, Bingle CD, Whyte MK, Renshaw SA. Effective caspase inhibition blocks neutrophil apoptosis and reveals Mcl-1 as both a regulator and a target of neutrophil caspase activation. PLoS One. 2011;6:e15768. doi: 10.1371/journal.pone.0015768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning : a laboratory manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, N.Y: 1989. [Google Scholar]
- 28.Renshaw SA, Loynes CA, Trushell DM, Elworthy S, Ingham PW, Whyte MK. A transgenic zebrafish model of neutrophilic inflammation. Blood. 2006;108:3976–3978. doi: 10.1182/blood-2006-05-024075. [DOI] [PubMed] [Google Scholar]
- 29.Xingang Wang ALR, Li Jingyu, Chai Ruth Jinfen, Haishan Wang, Sadiku Pranvera, Ogryzko Nikolay V., Everett Martin, Yoganathan Kanagasundaram, Luo Hongbo Robert, Renshaw Stephen A., Ingham Philip W. Novel natural product inhibitors of neutrophil recruitment identified through a transgenic zebrafish screen. Disease Models and Mechanisms. 2013 doi: 10.1242/dmm.012047. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nu\sslein-Volhard C, Dahm R. Zebrafish : a practical approach. Oxford University Press; Oxford: 2002. [Google Scholar]
- 31.Elks PM, van Eeden FJ, Dixon G, Wang X, Reyes-Aldasoro CC, Ingham PW, Whyte MK, Walmsley SR, Renshaw SA. Activation of hypoxia-inducible factor-1alpha (Hif-1alpha) delays inflammation resolution by reducing neutrophil apoptosis and reverse migration in a zebrafish inflammation model. Blood. 2011;118:712–722. doi: 10.1182/blood-2010-12-324186. [DOI] [PubMed] [Google Scholar]
- 32.van Rooijen E, Voest EE, Logister I, Korving J, Schwerte T, Schulte-Merker S, Giles RH, van Eeden FJ. Zebrafish mutants in the von Hippel-Lindau tumor suppressor display a hypoxic response and recapitulate key aspects of Chuvash polycythemia. Blood. 2009;113:6449–6460. doi: 10.1182/blood-2008-07-167890. [DOI] [PubMed] [Google Scholar]
- 33.Berghmans S, Butler P, Goldsmith P, Waldron G, Gardner I, Golder Z, Richards FM, Kimber G, Roach A, Alderton W, Fleming A. Zebrafish based assays for the assessment of cardiac, visual and gut function--potential safety screens for early drug discovery. J Pharmacol Toxicol Methods. 2008;58:59–68. doi: 10.1016/j.vascn.2008.05.130. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi T, Deak M, Morrice N, Cohen P. Characterization of the structure and regulation of two novel isoforms of serum- and glucocorticoid-induced protein kinase. Biochem J. 1999;344(Pt 1):189–197. [PMC free article] [PubMed] [Google Scholar]
- 35.Prince LR, Allen L, Jones EC, Hellewell PG, Dower SK, Whyte MK, Sabroe I. The role of interleukin-1beta in direct and toll-like receptor 4-mediated neutrophil activation and survival. Am J Pathol. 2004;165:1819–1826. doi: 10.1016/s0002-9440(10)63437-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brickley DR, Mikosz CA, Hagan CR, Conzen SD. Ubiquitin modification of serum and glucocorticoid-induced protein kinase-1 (SGK-1) J Biol Chem. 2002;277:43064–43070. doi: 10.1074/jbc.M207604200. [DOI] [PubMed] [Google Scholar]
- 37.Sabroe I, Prince LR, Jones EC, Horsburgh MJ, Foster SJ, Vogel SN, Dower SK, Whyte MK. Selective roles for Toll-like receptor (TLR) 2 and TLR4 in the regulation of neutrophil activation and life span. The Journal of Immunology. 2003;170:5268–5275. doi: 10.4049/jimmunol.170.10.5268. [DOI] [PubMed] [Google Scholar]
- 38.Ellett F, Pase L, Hayman JW, Andrianopoulos A, Lieschke GJ. mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish. Blood. 2011;117:e49–56. doi: 10.1182/blood-2010-10-314120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murray JT, Cummings LA, Bloomberg GB, Cohen P. Identification of different specificity requirements between SGK1 and PKBalpha. FEBS Lett. 2005;579:991–994. doi: 10.1016/j.febslet.2004.12.069. [DOI] [PubMed] [Google Scholar]
- 40.Nasevicius A, Ekker SC. Effective targeted gene ‘knockdown’ in zebrafish. Nat Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- 41.Park J, Leong ML, Buse P, Maiyar AC, Firestone GL, Hemmings BA. Serum and glucocorticoid-inducible kinase (SGK) is a target of the PI 3-kinase-stimulated signaling pathway. EMBO J. 1999;18:3024–3033. doi: 10.1093/emboj/18.11.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.You H, Jang Y, You-Ten AI, Okada H, Liepa J, Wakeham A, Zaugg K, Mak TW. p53-dependent inhibition of FKHRL1 in response to DNA damage through protein kinase SGK1. Proc Natl Acad Sci U S A. 2004;101:14057–14062. doi: 10.1073/pnas.0406286101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang L, Cui R, Cheng X, Du J. Antiapoptotic effect of serum and glucocorticoid-inducible protein kinase is mediated by novel mechanism activating I{kappa}B kinase. Cancer Res. 2005;65:457–464. [PubMed] [Google Scholar]
- 44.Ward C, Walker A, Dransfield I, Haslett C, Rossi AG. Regulation of granulocyte apoptosis by NF-kappa B. Biochem Soc T. 2004;32:465–467. doi: 10.1042/BST0320465. [DOI] [PubMed] [Google Scholar]
- 45.Jonsson H, Allen P, Peng SL. Inflammatory arthritis requires Foxo3a to prevent Fas ligand-induced neutrophil apoptosis. Nat Med. 2005;11:666–671. doi: 10.1038/nm1248. [DOI] [PubMed] [Google Scholar]
- 46.Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21:749–760. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 47.Chen J, Tang H, Hay N, Xu J, Ye RD. Akt isoforms differentially regulate neutrophil functions. Blood. 2010;115:4237–4246. doi: 10.1182/blood-2009-11-255323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hannigan M, Zhan L, Li Z, Ai Y, Wu D, Huang CK. Neutrophils lacking phosphoinositide 3-kinase gamma show loss of directionality during N-formyl-Met-Leu-Phe-induced chemotaxis. Proc Natl Acad Sci U S A. 2002;99:3603–3608. doi: 10.1073/pnas.052010699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lang F, Bohmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V. Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev. 2006;86:1151–1178. doi: 10.1152/physrev.00050.2005. [DOI] [PubMed] [Google Scholar]
- 50.Waldegger S, Klingel K, Barth P, Sauter M, Lanzendorfer M, Kandolf R, Lang F. h-sgk serine-threonine protein kinase gene as transcriptional target of transforming growth factor beta in human intestine. Gastroenterology. 1999;116:1081–1088. doi: 10.1016/s0016-5085(99)70011-9. [DOI] [PubMed] [Google Scholar]
- 51.Friedrich B, Warntges S, Klingel K, Sauter M, Kandolf R, Risler T, Muller GA, Witzgall R, Kriz W, Grone HJ, Lang F. Up-regulation of the human serum and glucocorticoid-dependent kinase 1 in glomerulonephritis. Kidney Blood Press Res. 2002;25:303–307. doi: 10.1159/000066794. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.