Abstract
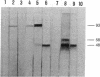
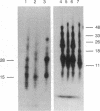
The glycine receptor of rat spinal cord is an oligomeric membrane glycoprotein of molecular mass 250,000 daltons that contains three polypeptides of 48,000, 58,000, and 93,000 daltons. Monoclonal antibodies (mAbs) were prepared against the affinity-purified glycine receptor protein by using 125I-labeled receptor preparations for the detection of positive hybrids. From nine monoclonal antibodies obtained, six recognized denatured receptor polypeptides blotted to nitrocellulose paper. Two of these antibodies bound to more than one glycine receptor polypeptide: mAb GlyR 4a stained the 48,000- and 58,000-dalton polypeptides, and mAb GlyR 7a stained the 48,000- and 93,000-dalton polypeptides. Common antigenic determinants thus are shared by the different subunits of the glycine receptor. Complementary results were obtained by peptide mapping of 125I-labeled glycine receptor polypeptides with various proteases. A set of peptide fragments of the same apparent molecular mass was produced from the different glycine receptor polypeptides by using V8 protease, chymotrypsin, and elastase. These data suggest that the subunits of the glycine receptor have significant homology within their primary structure and may have evolved from a common ancestor receptor polypeptide.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betz H., Graham D., Rehm H. Identification of polypeptides associated with a putative neuronal nicotinic acetylcholine receptor. J Biol Chem. 1982 Oct 10;257(19):11390–11394. [PubMed] [Google Scholar]
- Betz H. Neues von der Nervenzellmembran. Fortschritte bei der Strukturanalyse transmembranärer Ionenkanäle. Naturwissenschaften. 1984 Jul;71(7):363–368. doi: 10.1007/BF00410741. [DOI] [PubMed] [Google Scholar]
- Betz H., Pfeiffer F. Monoclonal antibodies against the alpha-bungarotoxin-binding protein of chick optic lobe. J Neurosci. 1984 Aug;4(8):2095–2105. doi: 10.1523/JNEUROSCI.04-08-02095.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Curtis D. R., Hösli L., Johnston G. A., Johnston I. H. The hyperpolarization of spinal motoneurones by glycine and related amino acids. Exp Brain Res. 1968;5(3):235–258. doi: 10.1007/BF00238666. [DOI] [PubMed] [Google Scholar]
- Ellisman M. H., Miller J. A., Agnew W. S. Molecular morphology of the tetrodotoxin-binding sodium channel protein from Electrophorus electricus in solubilized and reconstituted preparations. J Cell Biol. 1983 Dec;97(6):1834–1840. doi: 10.1083/jcb.97.6.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Graham D., Pfeiffer F., Betz H. Photoaffinity-labelling of the glycine receptor of rat spinal cord. Eur J Biochem. 1983 Apr 5;131(3):519–525. doi: 10.1111/j.1432-1033.1983.tb07292.x. [DOI] [PubMed] [Google Scholar]
- Graham D., Pfeiffer F., Betz H. UV light-induced cross-linking of strychnine to the glycine receptor of rat spinal cord membranes. Biochem Biophys Res Commun. 1981 Oct 30;102(4):1330–1335. doi: 10.1016/s0006-291x(81)80157-x. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Gullick W. J., Tzartos S., Lindstrom J. Monoclonal antibodies as probes of acetylcholine receptor structure. 1. Peptide mapping. Biochemistry. 1981 Apr 14;20(8):2173–2180. doi: 10.1021/bi00511a016. [DOI] [PubMed] [Google Scholar]
- Kistler J., Stroud R. M., Klymkowsky M. W., Lalancette R. A., Fairclough R. H. Structure and function of an acetylcholine receptor. Biophys J. 1982 Jan;37(1):371–383. doi: 10.1016/S0006-3495(82)84685-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Takahashi H., Tanabe T., Toyosato M., Kikyotani S., Furutani Y., Hirose T., Takashima H., Inayama S., Miyata T. Structural homology of Torpedo californica acetylcholine receptor subunits. Nature. 1983 Apr 7;302(5908):528–532. doi: 10.1038/302528a0. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Pfeiffer F., Betz H. Solubilization of the glycine receptor from rat spinal cord. Brain Res. 1981 Dec 7;226(1-2):273–279. doi: 10.1016/0006-8993(81)91099-4. [DOI] [PubMed] [Google Scholar]
- Pfeiffer F., Graham D., Betz H. Purification by affinity chromatography of the glycine receptor of rat spinal cord. J Biol Chem. 1982 Aug 25;257(16):9389–9393. [PubMed] [Google Scholar]
- Raftery M. A., Hunkapiller M. W., Strader C. D., Hood L. E. Acetylcholine receptor: complex of homologous subunits. Science. 1980 Jun 27;208(4451):1454–1456. doi: 10.1126/science.7384786. [DOI] [PubMed] [Google Scholar]
- Snyder S. H., Bennett J. P., Jr Neurotransmitter receptors in the brain: biochemical identification. Annu Rev Physiol. 1976;38:153–175. doi: 10.1146/annurev.ph.38.030176.001101. [DOI] [PubMed] [Google Scholar]
- Sutter A., Riopelle R. J., Harris-Warrick R. M., Shooter E. M. Nerve growth factor receptors. Characterization of two distinct classes of binding sites on chick embryo sensory ganglia cells. J Biol Chem. 1979 Jul 10;254(13):5972–5982. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzartos S. J., Lindstrom J. M. Monoclonal antibodies used to probe acetylcholine receptor structure: localization of the main immunogenic region and detection of similarities between subunits. Proc Natl Acad Sci U S A. 1980 Feb;77(2):755–759. doi: 10.1073/pnas.77.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werman R., Davidoff R. A., Aprison M. H. Inhibition of motoneurones by iontophoresis of glycine. Nature. 1967 May 13;214(5089):681–683. doi: 10.1038/214681a0. [DOI] [PubMed] [Google Scholar]
- Young A. B., Snyder S. H. Strychnine binding associated with glycine receptors of the central nervous system. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2832–2836. doi: 10.1073/pnas.70.10.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]