Abstract
IMPORTANCE
Soy consumption has been suggested to reduce risk or recurrence of prostate cancer, but this has not been tested in a randomized trial with prostate cancer as the end point.
OBJECTIVE
To determine whether daily consumption of a soy protein isolate supplement for 2 years reduces the rate of biochemical recurrence of prostate cancer after radical prostatectomy or delays such recurrence.
DESIGN, SETTING, AND PARTICIPANTS
Randomized, double-blind trial conducted from July 1997 to May 2010 at 7 US centers comparing daily consumption of a soy protein supplement vs placebo in 177 men at high risk of recurrence after radical prostatectomy for prostate cancer. Supplement intervention was started within 4 months after surgery and continued for up to 2 years, with prostate-specific antigen (PSA) measurements made at 2-month intervals in the first year and every 3 months thereafter.
INTERVENTION
Participants were randomized to receive a daily serving of a beverage powder containing 20 g of protein in the form of either soy protein isolate (n=87)or, as placebo, calcium caseinate (n=90).
MAIN OUTCOMES AND MEASURES
Biochemical recurrence rate of prostate cancer (defined as development of a PSA level of ≥0.07 ng/mL) over the first 2 years following randomization and time to recurrence.
RESULTS
The trial was stopped early for lack of treatment effects at a planned interim analysis with 81 evaluable participants in the intervention group and 78 in the placebo group. Overall, 28.3% of participants developed biochemical recurrence within 2 years of entering the trial (close to the a priori predicted recurrence rate of 30%). Among these, 22 (27.2%) occurred in the intervention group and 23 (29.5%) in the placebo group. The resulting hazard ratio for active treatment was 0.96 (95% CI, 0.53–1.72; log-rank P = .89). Adherence was greater than 90% and there were no apparent adverse events related to supplementation.
CONCLUSION AND RELEVANCE
Daily consumption of a beverage powder supplement containing soy protein isolate for 2 years following radical prostatectomy did not reduce biochemical recurrence of prostate cancer in men at high risk of PSA failure.
Prostate cancer is the most frequently diagnosed malignancy and the second most frequent cause of male cancer death in the United States and other Western countries1 but is far less frequent in Asian countries.2 Prostate cancer risk has been inversely associated with intake of soy and soy foods in observational studies,3,4 which may explain this geographic variation because soy consumption is low in the United States and high in Asian countries.5,6 Although it has been repeatedly proposed that soy may prevent prostate cancer development,5,7,8 this hypothesis has not been tested in randomized studies with cancer as the end point. A substantive fraction (48%–55%) of men diagnosed as having prostate cancer use dietary supplements including soy products, although the exact proportion is not known.9–11 However, no evidence exists that soy supplementation has any prostate cancer–related benefits for these men. Soy contains several constituents, including isoflavones, which possess anticancer activities in laboratory studies.12,13 Several animal studies also provide support for the hypothesis that soy consumption may protect against prostate cancer.14
The majority of prostate cancers detected by prostate-specific antigen (PSA) screening are indolent; only those that have potential to progress to an aggressive, fatal phenotype are clinically (or biologically) significant.15 Thus, prevention approaches focusing on biologically significant cancers have the greatest potential to reduce prostate cancer–specific mortality. We investigated the effect of soy supplementation on biologically significant prostate cancer in a randomized trial in men who were at high risk of recurrence after radical prostatectomy. The objective was to determine whether daily consumption of a soy protein–based supplement for 2 years reduced the rate of recurrence or delayed recurrence after radical prostatectomy using PSA failure as the intermediate end point. To our knowledge, there have been no randomized clinical trials testing this hypothesis.
Methods
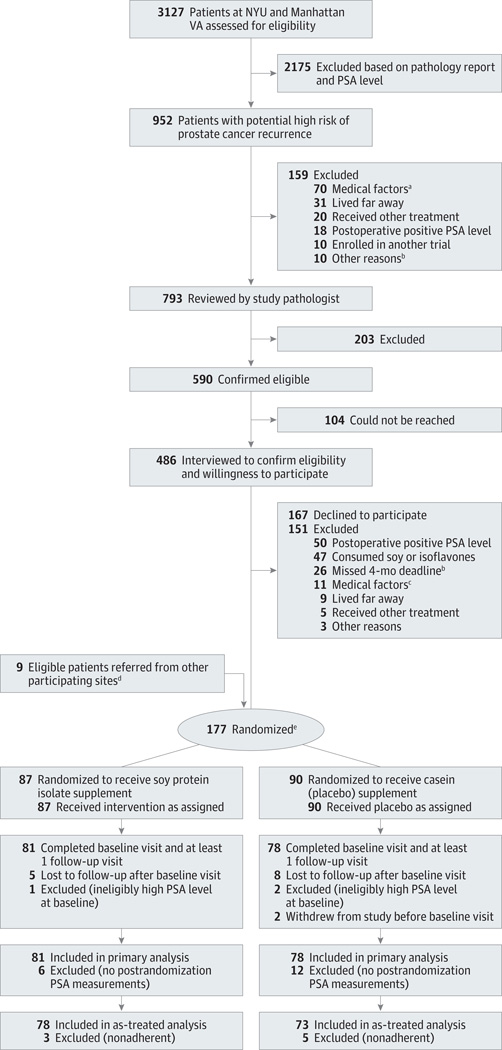
This study and its consent process were approved by the institutional review boards of all participating institutions. Flow of study participant screening, enrollment, and treatment is shown in Figure 1.
Figure 1. Participant Flow.
NYU indicates New York University; PSA, prostate-specific antigen.
aExclusionary medical factors included recent or current history of anemia, iron deficiency problems or subclinical iron deficiency at baseline, diabetes or insulin resistance requiring use of medication, thyroid disease, significant renal impairment, need for a sodium-restricted diet, substantive tendency to be constipated (grade ≥2 experienced regularly), a medical problem precluding the consumption of soy or casein such as allergies to soy or milk protein, and postoperative PSA of 0.07 ng/mL or higher. Fifty-four patients had diabetes, 8 had thyroid disease, 4 had anemia or low iron status, 2 had other malignancies, 1 had renal disease, and 1 had mental disease.
bThe 4-month postsurgery deadline had passed for these patients before pathology review could occur.
cPreviously undetected exclusionary medical factors included 4 patients who had diabetes, 1 who had thyroid disease, 1 who had anemia, 2 who had protein allergies, 1 who had recurrent constipation, 1 who had a restricted diet, and 1 who had mental disease.
dEight eligible participants were referred to the study by participating clinical sites other than NYU and the Manhattan VA; 1 eligible participant was referred to the study by a urologist in private practice. All 9 participants were confirmed to be at high risk by pathology review.
eThe median time between surgery and randomization was identical in both groups (14 weeks; 95% CI, 13.1–14.1; range, 7 or 8 to 18 weeks).
Eligibility
Patients were eligible if they had undergone radical prostatectomy for clinically localized (T1c or T2) prostate cancer less than 4 months before randomization, had a postsurgery PSA value of less than 0.07 ng/mL confirmed by the assay used in this study, and fulfilled 1 or more of the following criteria for high risk: preoperative PSA of greater than 20.0 ng/mL, final Gleason score of 8 or greater, established positive surgical margins (but not apical margins16), established extracapsular extension (but not in the bladder neck17), seminal vesicle invasion, or micrometastases in any removed pelvic lymph nodes. To confirm eligibility, prostatectomy slides of each prospective participant were centrally reviewed by 1 of the 4 participating pathologists (J.M., M.X.K., V.M., and A.K.-B.) who collectively had established standardized diagnostic criteria as members of the Cooperative Prostate Cancer Tissue Resource.18
Recruitment
Participants were enrolled at the New York University School of Medicine (NYU) (88%) and the Manhattan VA Medical Center (VA) (7%), both in New York City, as well as 5 other medical centers (5%) (Duke University, Durham, North Carolina; Moffitt Cancer Center, Tampa, Florida; Long Beach VA Medical Center, Long Beach, California; University of Chicago, Chicago, Illinois; and University of Illinois at Chicago); one private practitioner referred a single eligible patient. The pathology reports of all radical prostatectomy patients at the VA and NYU sites between July 1997 and November 2005 (VA) or May 2009 (NYU) were systematically screened. At the other sites, no systematic procedure to identify eligible patients was implemented, and enrollment relied on individual urologists. Recruitment at the University of Chicago and the University of Illinois at Chicago was continued until May 2010. Patients whose potential eligibility was confirmed by a study pathologist were first approached by participating urologists (by letter at NYU) asking them to contact study staff. If they agreed to participate and no exclusionary factors (Figure 1) were identified during an in-person or telephone interview, a baseline visit was conducted by a study coordinator to obtain written informed consent, demographic information (including self-identified race/ethnicity using categories listed in Table 1), and a blood sample to confirm postoperative PSA level. A significant baseline intake of soy (more than once per week) was also an exclusionary criterion; this was assessed using a standardized questionnaire.19
Table 1.
Baseline Characteristics of Evaluable Study Participants
| Characteristics |
Intervention (n=81) |
Placebo (n=78) |
P Value for Difference |
|---|---|---|---|
| Age at randomization, mean (SD), y | 61.3 (7.2) | 60.7 (6.6) | .56a |
| Race/ethnicity, No. (%) | |||
| White | 71 (88) | 70 (90) | .82b,c |
| African American | 6 (7) | 5 (7) | .91b,d |
| Hispanic | 1 (1) | 1 (1) | |
| Asian | 1 (1) | 1 (1) | |
| Other (including Pacific Islander) | 2 (3) | 1 (1) | |
| Weight, mean (SD), kg | 87.1 (13.4) | 88.9 (14.8) | .36a |
| Height, mean (SD), cm | 177.4 (7.1) | 178.6 (8.4) | .31a |
| BMIe | |||
| Mean (SD) | 27.6 (3.6) | 27.9 (4.2) | .64a |
| Median (IQR) | 27.4 (3.90) | 27.4 (5.58) | .91b |
| Serum total cholesterol level, mean (SD), mg/dLf | 183.4 (29.8) | 187.4 (36.9) | .48a |
| High-risk characteristics | |||
| Preoperative PSA level, ng/mL | |||
| Mean (SD) | 7.13 (3.87) | 7.71 (4.20) | .36a |
| Median (IQR) | 5.90 (3.20) | 6.62 (3.98) | .21g |
| Gleason score, mean (SD) | 7.00 (0.82) | 7.01 (0.80) | .98g |
| 5 | 1 (1) | 0 | .78b,h |
| 6 | 19 (23) | 18 (23) | |
| 7 | 45 (56) | 47 (60) | |
| 8 | 11 (14) | 7 (9) | |
| 9 | 5 (6) | 6 (8) | |
| Positive surgical margins, No. (%) | 38 (47) | 27 (35) | .15i |
| Extracapsular extension, No. (%) | 54 (67) | 61 (78) | .11i |
| Positive seminal vesicle(s), No. (%) | 15 (19) | 12 (15) | .68i |
| Lymph node metastases, No./examined | 0/36 | 2/31 | .50i |
| High-risk characteristics, mean (SD), No. | 1.54 (0.78) | 1.50 (0.75) | .72a |
| 1 | 48 (59) | 8 (62) | .87bj |
| ≥2 | 33 (41) | 30 (38) |
Abbreviations: BMI, body mass index, calculated as weight in kilograms divided by height in meters squared; IQR, interquartile range; PSA, prostate-specific antigen.
SI conversion: To convert total cholesterol to mmol/L, multiply by 0.0259.
By t test.
By χ2 test.
White vs all others.
White vs African American vs all others.
Calculated in 81 intervention participants and 77 placebo participants.
Calculated in 74 intervention participants and 76 placebo participants.
By Mann-Whitney test.
Score of 5 or 6 vs 7 vs 8 vs 9.
By Fisher exact test.
None vs 1 vs 2.
Randomization and Blinding
After eligibility was confirmed, participants were randomized to the intervention or placebo groups (1:1) using the dynamic intervention allocation procedure of Begg and Iglewicz,20 stratified by (1) hospital/clinical site (NYU vs VA vs other sites); (2) number of high-risk characteristics (1 vs >1); and (3) self-defined race/ethnicity (African American vs non– African American). The randomization procedure was programmed using SAS software (SAS Institute Inc) and carried out by study staff. All study investigators and staff as well as all study participants remained blinded to the assigned treatments until after the interim analysis was conducted, the trial was stopped, and the intention-to-treat analysis had been completed.
Intervention
The intervention agent was soy protein isolate based and the placebo was a caseinate-based product, each incorporated into a beverage powder developed and manufactured for this clinical trial by Solae LLC. A daily 47-g serving of beverage powder contained protein in the form of either soy protein isolate (19.2 g as analyzed) or calcium caseinate (19.8 g). The soy protein dose represents 37% to 40% of the daily reference protein intake in the United States.21,22 The soy protein beverage powder contained per 1 g of protein 3.67mg of all forms of isoflavones (aglycones, glycosides, and glycoside esters) or 2.13 mg as aglycone equivalents, amounting to (in aglycone equivalents) 1.24 mg of genistein, 0.78 mg of daidzein, and 0.11 mg of glycitein. The beverage powders were sweetened with a mixture of sucrose and fructose to improve palatability. Artificial strawberry flavoring was added to mask the taste difference between the 2 powders, which were packaged identically and differed nutritionally only in the type of protein and the presence of isoflavones and other soy-specific constituents. The nutrient composition of the powders is listed in Table 2. Participants were instructed to consume one 47-g packet of beverage powder mixed in approximately 10 oz of water or fruit juice each day.
Table 2.
Supplement Nutritional Information
| Nutrient/Dietary Factor |
Calculated Amount Per 47-g Daily Serving |
Daily Value, %a | |
|---|---|---|---|
| IOM Nutrition Board | FDA | ||
| Protein, g | 20b | 36 | 40 |
| Total carbohydrate, g | 21 | 16 | 7 |
| Sugars, g | 20 | NA | NA |
| Total fat, g | 1b | NA | 1.5 |
| Energy, kcal | 175 | ∼9c | ∼9c |
| Sodium, mg | 200b | 15 | 8 |
| Calcium, mg | 700b | 70 | 70 |
| Phosphorus, mg | 500 | 71 | 50 |
| Magnesium, mg | 40 | 9.5 | 10 |
| Vitamin A, IU | 500 | 17 | 10 |
| Vitamin C, mg | 2.4 | 2.7 | 4 |
| Vitamin D, IU | 100 | 17 | 25 |
| Vitamin B6, mg | 0.12 | 7 | 6 |
| Vitamin B12, µg | 0.9 | 38 | 15 |
| Thiamin, mg | 0.09 | 7.5 | 6 |
| Folate, µg | 60 | 15 | 15 |
| Riboflavin, mg | 0.4 | 31 | 24 |
| Pantothenic acid, mg | 0.8 | 16 | 8 |
| Zinc, mg | 0.9 | 8 | 6 |
| Iron, mg | 3.6 | 45 | 20 |
| Total isoflavones, mg | 41d | NA | NA |
| Genistein, mg | 24d | NA | NA |
Abbreviations: NA, not applicable.
Percentage of dietary reference intake; the first number is based on the current Recommended Daily Allowances and Adequate Intakes based on the dietary reference intakes of the Nutrition Board of the Institute of Medicine (IOM)21 and the second number is based on the current food labeling regulations of the US Food and Drug Administration (FDA) based on a 2000-kcal/d diet with 50 g protein.22 Amounts of saturated fats, cholesterol, and dietary fiber were negligible.
The actual measured amounts were 19.2 and 19.8 g protein, 1.12 and 0.68 g total fat, 196 and 184 mg sodium, and 707 and 707 mg calcium for soy and placebo products, respectively.
Percentage of a 2000-kcal diet.
Isoflavones (as aglycone equivalents) from soy not present in the placebo product.
Participant Follow-up
Participants were counseled by a study coordinator on avoiding intake of soy-based food and drink and limiting excessive dairy products while in the study. If postsurgical serum PSA level was confirmed to be less than 0.07 ng/mL, participants started the intervention within 1 or 2 weeks following randomization for 2 years and returned for follow-up visits at 2-month intervals during the first year and at 3-month intervals thereafter. At each follow-up visit, a blood sample was obtained for PSA measurement and occurrence of adverse events was assessed using the National Cancer Institute’s Common Toxicity Criteria version 4.0. For some participants, a number of follow-up visits were conducted by telephone and blood samples were mailed overnight by express delivery to the trial office; this has been shown to be feasible without affecting PSA levels,23 which we confirmed. The intervention was stopped when biochemical recurrence or serious adverse effects developed.
Adherence
Adherence was assessed by measuring serum isoflavone levels at baseline and at least 2 time points while in the study by high-performance liquid chromatography with electrochemical detection using the procedure of Gamache and Acworth,24 with slight modifications as described by Franke et al.25,26 Self-reported adherence was also assessed using a daily calendar, and soy intake during the previous 2 to 3 months was evaluated at each follow-up visit using the aforementioned questionnaire.
End Points
The primary end points of this trial were the 2-year rate of biochemical cancer recurrence and time to recurrence for those in whom cancer recurred. Biochemical recurrence was defined as development of a serum PSA level of 0.07 ng/mL or higher confirmed by 2 subsequent PSA values of 0.07 ng/mL or higher at least 1 month apart. Serum PSA levels were measured with an ultrasensitive automated immunoenzymometric assay (Tosoh Bioscience)using the 2 standard Tandem Hybritech monoclonal antibodies.27 We determined that the limit of quantitation of this assay was 0.03 ng/mL and the intraassay and interassay variation were 4.0% to 6.2% and 8.4% to 13.9%, respectively.
Statistical Analysis
The low detection limit of the PSA assay allowed us to define biochemical recurrence as a sustained increase in PSA level above 0.07 ng/mL approximately 1 year earlier than if we had used a higher cutoff (≥0.1 or 0.2 ng/mL) as commonly used.27 On the basis of a literature review and an analysis of data from NYU, as detailed in the eTable in the Supplement, the projected 2-year biochemical recurrence rate in the placebo group was estimated to be 30%. With 252 evaluable participants (126 per group) the study had 80% power to detect a 50% reduction in biochemical recurrence rate with a 2-sided significance level of .05 and 1 planned interim analysis after observation of 45 recurrences using the O’Brien-Fleming spending function.28 The 50% reduction in recurrence was chosen because (1) the prostate cancer risk reduction by soy in animal studies has been in this range; (2) the prostate cancer rate differential between populations who habitually consume soy and those who do not is more than 2-fold; and (3) a risk reduction of this magnitude would be needed to have important public health effects.
Baseline participant characteristics and adverse events were compared between the 2 groups using the t test or the Mann-Whitney test for continuous variables and the χ2 test or Fisher exact test for categorical variables. Time to recurrence was calculated as the time (in weeks) between randomization and first occurrence of a sustained PSA level of 0.07 ng/mL or higher. Participants who ceased study participation and had a PSA value of less than 0.07 ng/mL at their last visit were censored at the time of their last PSA measurement. Because PSA measurements during follow-up were necessary for the assessment of the main study outcome (ie, PSA recurrence), participants who were found ineligible after randomization, with-drew before their baseline visit, or never returned after baseline were excluded from the main intention-to-treat analysis, which was therefore a modified intention-to-treat analysis. The Cox proportional hazards model was used to calculate the hazard ratios and 95% confidence intervals comparing the 2 treatment groups and the log-rank test to assess statistical significance. The proportional hazard assumption was checked by inclusion of the cross-product of log (follow-up time) by treatment. SAS software, versions 9.2 and 9.1.3, and GraphPad Prism software, version 4.0, were used for statistical analysis.
Results
A total of 177 eligible participants were randomized between July 1997 and May 2010. As planned, a blinded interim analysis was conducted once 45 participants had developed confirmed biochemical recurrence, which was reviewed by the study’s data safety and monitoring committee. At the recommendation of this committee, the trial was stopped at that time because of lack of evidence of treatment effect. The conditional power—ie, the probability of observing a significant result at the end of the trial if enrollment had continued until the target sample size had been reached and all participants had been followed-up for two years—was extremely low at 0.0012, given the data observed at the interim analysis.
Of the 177 randomized participants, 18 (10.2%) did not contribute data and were thus not evaluable because they had ineligibly high baseline PSA (n = 3), withdrew before their baseline visit (n = 2), or never returned after baseline (n = 13) (Figure 1). These participants were not evaluable because they had no postrandomization PSA measurements. A total of 159 participants (81 in the intervention group and 78 in the placebo group) completed the baseline visit and at least 1 follow-up visit and were therefore evaluable for biochemical recurrence. There were no significant differences between the 2 treatment groups in any of the baseline characteristics of these evaluable participants (Table 1). Thirteen of the evaluable participants (8.2%) withdrew before recurrence or completion of 2 years of treatment. They were included in the intention-to-treat analysis with censoring at the time of withdrawal; 6 of these were in the intervention group (median number of weeks in study, 15 [range, 8–69 weeks]) and 7 were in the placebo group (median number of weeks in study, 29 [range, 10–94 weeks]) (P = .88 for difference in dropout incidence and P = .07 for difference in number of weeks). Most evaluable participants (n = 146 [92%]) completed 2 years of intervention or experienced recurrence earlier. Less than 2% of all PSA follow-up data were not obtained and in no case did this impair detection of biochemical recurrence. Three participants were classified as having recurrence based on only 1 PSA measurement of 0.07 ng/mL or higher at their last study visit because there was evidence from chart review that they subsequently had more than 1 PSA value higher than 0.1 ng/mL and/or hormone ablation or radiation treatment.
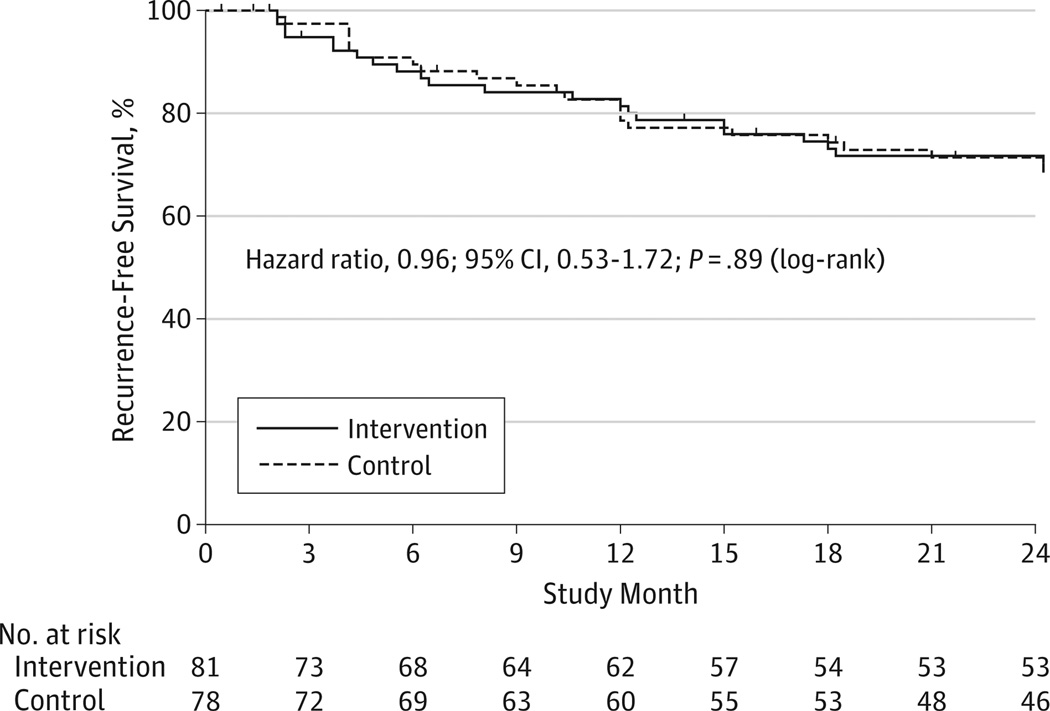
Twenty two (27.2%)of the 81 participants in the intervention group developed confirmed biochemical recurrence, whereas 23 (29.5%) of the 78 participants receiving placebo developed recurrence. The modified intention-to-treat analysis of the evaluable participants revealed no evidence of treatment effect with the 2 survival curves closely overlapping (Figure 2). The hazard ratio for the difference between the 2 groups was 0.96 (95% CI, 0.53–1.72; log-rank P = .89). Among participants who developed recurrence, the median time to recurrence was somewhat shorter in the intervention group (31.5 weeks) than in the placebo group (44 weeks), but this difference was not statistically significant (P = .62 by Mann-Whitney test).
Figure 2.
Biochemical Recurrence-Free Survival
Self-reported adherence was excellent, with 152 participants (96%) reporting having consumed more than 90% of the packets supplied. Only 7 evaluable participants reported serious nonadherence (consuming <50% of the packets), 3 in the intervention group and 4 in the placebo group. Analysis of serum genistein levels identified 1 additional case of nonadherence in the placebo group in a participant who consistently had genistein levels above 150 ng/mL. These 8 participants were considered definitively nonadherent. An additional 13 participants (7 in the intervention group and 6 in the placebo group) had serum genistein levels that were potentially inconsistent with their treatment assignment (>20 ng/mL in the placebo group and <20 ng/mL in the intervention group); these participants were considered possibly nonadherent. Median serum genistein levels were 0.0 ng/mL (interquartile range, 0.0–3.3 ng/mL), 94.7 ng/mL (interquartile range, 36.7–201.7 ng/mL), and 129.8 ng/mL (interquartile range, 64.6–208.6 ng/mL) at baseline and after 6 and 12 months, respectively, in the 33 of 69 fully adherent participants taking soy for whom we had complete data at these time points.
Eleven evaluable participants who were considered fully adherent stopped treatment at some point during the study but continued follow-up without treatment; they were included in the intention-to-treat analysis up to the point of last follow-up. Analysis censoring these participants at the time they ceased taking their assigned treatment and excluding participants who were considered definitively nonadherent (n = 8) did not change the results (hazard ratio, 0.89; 95% CI: 0.49–1.62; log-rank P = .70). Further eliminating the 13 participants who were possibly nonadherent from the analysis also did not change the outcome (hazard ratio, 0.97; 95% CI, 0.50–1.73; log-rank P = .81).
There were no differences in adverse events between the 2 groups (Table 3). The major reason for withdrawal from the study was difficulty with palatability or sweetness of the products. The second most frequent reason was lack of time or frequent traveling interfering with participation. Three participants (2 in the placebo group and 1 in the intervention group) stopped treatment because of symptoms that proved to be not related to treatment and 1 participant in the intervention group stopped because of recurrent grade 2 constipation considered treatment related; all 4 participants continued follow-up. No deaths occurred during the study.
Table 3.
Most Frequent Adverse Events by Treatment Group
| Adverse Events |
Intervention (n = 81) |
Placebo (n = 78) |
P Value for Difference |
|---|---|---|---|
| No. of adverse events per participant, mean (SD) | 1.74 (1.84) | 1.42 (1.53) | .40a |
| Participants with event, No. (%) | |||
| Any adverse event | 50 (61.7) | 50 (64.1) | .87b |
| 1 Adverse event | 26 (32.1) | 28 (35.9) | .88c |
| >1 Adverse event | 33 (40.7) | 30 (38.4) | |
| Gastrointestinal issues, all | 13 (16.1) | 8 (10.3) | .35b |
| Potentially treatment relatedd | 6 (7.4) | 6 (7.7) | .99b |
| Not treatment related | 7 (8.6) | 2 (2.6) | .19b |
| Urinary tract issues (surgery related) | 13 (16.1) | 8 (10.2) | .35b |
| Initiation of high cholesterol treatment | 9 (11.1) | 10 (12.8) | .81b |
| Initiation of hypertension treatment | 9 (11.1) | 7 (9.0) | .79b |
| Musculoskeletal pain, not treatment related | 11 (13.6) | 4 (5.1) | .10b |
By Mann-Whitney test.
By Fisher exact test.
By χ2 test comparing no adverse events with 1 or more than 1.
Gastrointestinal problems that could be treatment related included constipation, bloating, irregular bowel movements, diverticulosis, diverticulitis, and heartburn.
Discussion
This randomized clinical trial was stopped early because it was found that development of biochemical recurrence of prostate cancer after radical prostatectomy was not reduced or delayed by daily consumption of a 20-g soy protein isolate supplement. Intention-to-treat survival analysis yielded overlapping curves and a hazard ratio close to 1. An analysis limited to confirmed adherent participants did not change the results. To our knowledge, this is the first randomized clinical trial to test the efficacy of soy protein in reducing biochemical recurrence of prostate cancer. The soy treatment duration in this study was one of the longest reported to date, and this study demonstrated that soy protein isolate supplementation for 2 years is well tolerated and safe in men.
The only other interventional studies with soy relevant to prostate cancer used serum PSA levels as an end point and had mixed results. Soy protein or isoflavone supplementation did not significantly affect serum PSA levels in randomized trials with healthy men of different ages,29–32 men with prostate cancer under active surveillance,33 men with high-grade prostatic intraepithelial neoplasia but no cancer on biopsy, and men with untreated prostate cancer.34–36 Consumption of isoflavones or soy as supplements to the diet slowed down progression in subsets of men with increasing PSA after surgical intervention or radiation in small studies with short intervention periods.37–42
The lack of protective activity of soy against prostate cancer recurrence observed in this study was limited to men at above-average risk of recurrence within the first 2 years after surgery and to the soy protein dose tested. The findings of this study may therefore not be generalizable to prostate cancer patients at average risk of recurrence. It is also possible that the dose or duration of treatment used in this trial were not sufficient to affect aggressive prostate cancer. It is unlikely that the use of a casein-based supplement as placebo prevented detection of a protective effect of soy as, to our knowledge, no published evidence exists that milk or milk protein reduces risk of prostate cancer or improves clinical outcome. The modest representation of minority participants (self-identified non-white) limits the generalizability of the results to white men. The accrual to this study was slow and the possibility of accrual bias thus cannot be excluded but is unlikely because accrual by calendar year was evenly distributed between the 2 groups.
The design of the present study represents one possible approach to clinical studies for the assessment of the efficacy of chemoprevention agents for prostate cancer. Prostate cancer chemoprevention treatments are likely to be initiated in middle-aged men when as many as 50% already have small prostate cancers, of which only a small fraction have aggressive potential.15,43 Thus, focusing on prostate cancer patients at high risk of postsurgical recurring cancers, which are aggressive and biologically significant, has potential to identify treatments that reduce prostate cancer mortality. In addition, the study design is simple, does not interfere with clinical practice, and requires a much smaller sample size than prevention trials with healthy men at high or average risk of prostate cancer. Also, men with prostate cancer may be eager to participate in such trials (50% in this study), but effective recruitment requires strong support from participating urologists.
The findings of this study provide another example that associations in observational epidemiologic studies between purported preventive agents and clinical outcomes need confirmation in randomized clinical trials. Not only were these findings at variance with the epidemiologic evidence on soy consumption and prostate cancer risk, they were also not consistent with results from experiments with animal models of prostate carcinogenesis, which also suggest reduced risk.14,44–47 One possible explanation for these discrepant results is that in both epidemiologic studies and animal experiments, soy exposure typically occurred for most or all of the life span of the study participants or animals; there are no reports of such studies in which soy exposure started later in life. Thus, it is conceivable that soy is protective against prostate cancer when consumption begins early in life but not later or when prostate cancer is already present. If this is the case, chemoprevention of prostate cancer with soy is unlikely to be effective if started later in life, given the high prevalence of undetected prostate cancer in middle-aged men.43
Conclusion
This randomized clinical trial demonstrated that development of biochemical recurrence of prostate cancer after radical prostatectomy was not reduced or delayed by daily consumption of a 20-g soy protein isolate supplement in men at high risk of recurrence, but the intervention appeared safe and was well tolerated.
Supplementary Material
Acknowledgments
Funding/Support: This work was supported in part by National Institute of Health grants U01 CA072290 and R01 CA166195 as well as National Institute of Health grants P50 CA16087 and UL1 TR000050, with minor support from the Prevent Cancer Foundation and the United Soybean Board. Solae LLC provided the intervention materials.
Role of the Sponsor: The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; or decision to submit the manuscript for publication. Solae LLC developed and provided the intervention materials and reviewed parts of the manuscript for accuracy.
Additional Contributions: We thank all study participants for their dedicated contributions and we gratefully acknowledge the contributions of Nikola Baumann, PhD (University of Illinois at Chicago), for help with the validation of the PSA assay and Pablo Torre, MD (Manhattan VA Medical Center), for his help in recruiting participants. We also thank David M. Albala, MD (Duke University), Nagi Kumar, PhD (Moffitt Cancer Center), Anne R. Simoneau, MD (Long Beach VA), Gregory P. Zagaya, MD (University of Chicago), and Michael L. Howard, MD (Rotolo Howard and Leitner Associates), for enrolling study participants, as well as all members of the various incarnations of the data safety and monitoring board of this study. Drs Albala, Kumar, and Zagaya received compensation on a per-participant basis via a subcontract to their institution; the others did not receive compensation. This article is dedicated to the memory of Ernst L. Wynder, MD, who was the first to propose that a prostate cancer prevention study with a soy intervention be conducted.
Footnotes
Author Contributions: Dr Bosland had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Bosland, Kato, Zeleniuch-Jacquotte.
Acquisition of data: Bosland, Kato, Zeleniuch-Jacquotte, Schmoll, Enk Rueter, Melamed, Kong, Macias, Kajdacsy-Balla, Lumey, Walden, Lepor, Taneja, Randolph, Schlicht, Meserve-Watanabe, Davies.
Analysis and interpretation of data: Bosland, Zeleniuch-Jacquotte, Enk Rueter, Xie, Gao, Deaton.
Drafting of the manuscript: Bosland, Kato, Zeleniuch-Jacquotte, Lumey.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Bosland, Xie, Gao.
Obtained funding: Bosland.
Administrative, technical, and/or material support: Bosland, Kato, Zeleniuch-Jacquotte, Schmoll, Enk Rueter, Lumey, Walden, Lepor, Taneja, Randolph, Schlicht, Meserve-Watanabe, Deaton, Davies.
Study supervision: Bosland, Kato, Zeleniuch-Jacquotte, Melamed, Kajdacsy-Balla, Walden, Lepor.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Taneja reports that he has served as consultant for Eigen, Gtx, and Bayer and as a speaker for Janssen, receives royalties from Elsevier, and is a clinical trial investigator for Steba. No other disclosures were reported.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Yan L, Spitznagel EL. Soy consumption and prostate cancer risk in men: a revisit of a meta-analysis. Am J Clin Nutr. 2009;89(4):1155–1163. doi: 10.3945/ajcn.2008.27029. [DOI] [PubMed] [Google Scholar]
- 4.Hwang YW, Kim SY, Jee SH, Kim YN, Nam CM. Soy food consumption and risk of prostate cancer: a meta-analysis of observational studies. Nutr Cancer. 2009;61(5):598–606. doi: 10.1080/01635580902825639. [DOI] [PubMed] [Google Scholar]
- 5.Wynder EL, Rose DP, Cohen LA. Nutrition and prostate cancer: a proposal for dietary intervention. Nutr Cancer. 1994;22(1):1–10. doi: 10.1080/01635589409514327. [DOI] [PubMed] [Google Scholar]
- 6.Nomura AM, Kolonel LN. Prostate cancer: a current perspective. Epidemiol Rev. 1991;13:200–227. doi: 10.1093/oxfordjournals.epirev.a036069. [DOI] [PubMed] [Google Scholar]
- 7.Steele VE, Pereira MA, Sigman CC, Kelloff GJ. Cancer chemoprevention agent development strategies for genistein. J Nutr. 1995;125(3) suppl:713S–716S. doi: 10.1093/jn/125.3_Suppl.713S. [DOI] [PubMed] [Google Scholar]
- 8.Barnes S, Peterson TG, Coward L. Rationale for the use of genistein-containing soy matrices in chemoprevention trials for breast and prostate cancer. J Cell Biochem Suppl. 1995;22:181–187. doi: 10.1002/jcb.240590823. [DOI] [PubMed] [Google Scholar]
- 9.Greenlee H, White E, Patterson RE, Kristal AR. Vitamins and Lifestyle Study Cohort. Supplement use among cancer survivors in the Vitamins and Lifestyle (VITAL) study cohort. J Altern Complement Med. 2004;10(4):660–666. doi: 10.1089/acm.2004.10.660. [DOI] [PubMed] [Google Scholar]
- 10.Uzzo RG, Brown JG, Horwitz EM, et al. Prevalence and patterns of self-initiated nutritional supplementation in men at high risk of prostate cancer. BJU Int. 2004;93(7):955–960. doi: 10.1111/j.1464-410X.2004.04759.x. [DOI] [PubMed] [Google Scholar]
- 11.Westerlund A, Steineck G, Bälter K, Stattin P, Grönberg H, Hedelin M. Dietary supplement use patterns in men with prostate cancer: the Cancer Prostate Sweden study. Ann Oncol. 2011;22(4):967–972. doi: 10.1093/annonc/mdq456. [DOI] [PubMed] [Google Scholar]
- 12.Bektic J, Guggenberger R, Eder IE, et al. Molecular effects of the isoflavonoid genistein in prostate cancer. Clin Prostate Cancer. 2005;4(2):124–129. doi: 10.3816/cgc.2005.n.021. [DOI] [PubMed] [Google Scholar]
- 13.Steiner C, Arnould S, Scalbert A, Manach C. Isoflavones and the prevention of breast and prostate cancer: new perspectives opened by nutrigenomics. Br J Nutr. 2008;99E(suppl 1):S78–S108. doi: 10.1017/S0007114508965788. [DOI] [PubMed] [Google Scholar]
- 14.Ozten-Kandaş N, Bosland MC. Chemoprevention of prostate cancer: natural compounds, antiandrogens, and antioxidants—in vivo evidence. J Carcinog. 2011;10:27. doi: 10.4103/1477-3163.90438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ploussard G, Epstein JI, Montironi R, et al. The contemporary concept of significant vs insignificant prostate cancer. Eur Urol. 2011;60(2):291–303. doi: 10.1016/j.eururo.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Shah O, Melamed J, Lepor H. Analysis of apical soft tissue margins during radical retropubic prostatectomy. J Urol. 2001;165(6 pt 1):1943–1948. doi: 10.1097/00005392-200106000-00023. [DOI] [PubMed] [Google Scholar]
- 17.Yossepowitch O, Sircar K, Scardino PT, et al. Bladder neck involvement in pathological stage pT4 radical prostatectomy specimens is not an independent prognostic factor. J Urol. 2002;168(5):2011–2015. doi: 10.1016/S0022-5347(05)64284-X. [DOI] [PubMed] [Google Scholar]
- 18.Melamed J, Datta MW, Becich MJ, et al. The cooperative prostate cancer tissue resource: a specimen and data resource for cancer researchers. Clin Cancer Res. 2004;10(14):4614–4621. doi: 10.1158/1078-0432.CCR-04-0240. [DOI] [PubMed] [Google Scholar]
- 19.Williams AE, Maskarinec G, Hebshi S, Oshiro C, Murphy S, Franke AA. Validation of a soy questionnaire with repeated dietary recalls and urinary isoflavone assessments over 1 year. Nutr Cancer. 2003;47(2):118–125. doi: 10.1207/s15327914nc4702_2. [DOI] [PubMed] [Google Scholar]
- 20.Begg CB, Iglewicz B. A treatment allocation procedure for sequential clinical trials. Biometrics. 1980;36(1):81–90. [PubMed] [Google Scholar]
- 21.Dietary Reference Intakes: the Essential Guide to Nutrient Requirements. Washington, DC: National Academy Press; 2006. [Google Scholar]
- 22.Nutrition labeling of food. 21 CFR §101.9.Revised April 1, 2012. [Google Scholar]
- 23.Arcangeli CG, Smith DS, Ratliff TL, Catalona WJ. Stability of serum total and free prostate specific antigen under varying storage intervals and temperatures. J Urol. 1997;158(6):2182–2187. doi: 10.1016/s0022-5347(01)68191-6. [DOI] [PubMed] [Google Scholar]
- 24.Gamache PH, Acworth IN. Analysis of phytoestrogens and polyphenols in plasma, tissue, and urine using HPLC with coulometric array detection. Proc Soc Exp Biol Med. 1998;217(3):274–280. doi: 10.3181/00379727-217-44232. [DOI] [PubMed] [Google Scholar]
- 25.Franke AA, Custer LJ, Cerna CM, Narala K. Rapid HPLC analysis of dietary phytoestrogens from legumes and from human urine. Proc Soc Exp Biol Med. 1995;208(1):18–26. doi: 10.3181/00379727-208-43826. [DOI] [PubMed] [Google Scholar]
- 26.Franke AA, Custer LJ, Wang W, Shi CY. HPLC analysis of isoflavonoids and other phenolic agents from foods and from human fluids. Proc Soc Exp Biol Med. 1998;217(3):263–273. doi: 10.3181/00379727-217-44231. [DOI] [PubMed] [Google Scholar]
- 27.Prestigiacomo AF, Stamey TA. A comparison of 4 ultrasensitive prostate specific antigen assays for early detection of residual cancer after radical prostatectomy. J Urol. 1994;152(5 pt 1):1515–1519. doi: 10.1016/s0022-5347(17)32459-x. [DOI] [PubMed] [Google Scholar]
- 28.O'Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35(3):549–556. [PubMed] [Google Scholar]
- 29.Urban D, Irwin W, Kirk M, et al. The effect of isolated soy protein on plasma biomarkers in elderly men with elevated serum prostate specific antigen. J Urol. 2001;165(1):294–300. doi: 10.1097/00005392-200101000-00082. [DOI] [PubMed] [Google Scholar]
- 30.Jenkins DJ, Kendall CW, D'Costa MA, et al. Soy consumption and phytoestrogens: effect on serum prostate specific antigen when blood lipids and oxidized low-density lipoprotein are reduced in hyperlipidemic men. J Urol. 2003;169(2):507–511. doi: 10.1097/01.ju.0000046060.59113.57. [DOI] [PubMed] [Google Scholar]
- 31.Adams KF, Chen C, Newton KM, Potter JD, Lampe JW. Soy isoflavones do not modulate prostate-specific antigen concentrations in older men in a randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2004;13(4):644–648. [PubMed] [Google Scholar]
- 32.Maskarinec G, Morimoto Y, Hebshi S, Sharma S, Franke AA, Stanczyk FZ. Serum prostate-specific antigen but not testosterone levels decrease in a randomized soy intervention among men. Eur J Clin Nutr. 2006;60(12):1423–1429. doi: 10.1038/sj.ejcn.1602473. [DOI] [PubMed] [Google Scholar]
- 33.deVere White RW, Tsodikov A, Stapp EC, Soares SE, Fujii H, Hackman RM. Effects of a high dose, aglycone-rich soy extract on prostate-specific antigen and serum isoflavone concentrations in men with localized prostate cancer. Nutr Cancer. 2010;62(8):1036–1043. doi: 10.1080/01635581.2010.492085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamilton-Reeves JM, Rebello SA, Thomas W, Kurzer MS, Slaton JW. Effects of soy protein isolate consumption on prostate cancer biomarkers in men with HGPIN, ASAP, and low-grade prostate cancer. Nutr Cancer. 2008;60(1):7–13. doi: 10.1080/01635580701586770. [DOI] [PubMed] [Google Scholar]
- 35.Spentzos D, Mantzoros C, Regan MM, et al. Minimal effect of a low-fat/high soy diet for asymptomatic, hormonally naive prostate cancer patients. Clin Cancer Res. 2003;9(9):3282–3287. [PubMed] [Google Scholar]
- 36.Lazarevic B, Boezelijn G, Diep LM, et al. Efficacy and safety of short-term genistein intervention in patients with localized prostate cancer prior to radical prostatectomy: a randomized, placebo-controlled, double-blind phase 2 clinical trial. Nutr Cancer. 2011;63(6):889–898. doi: 10.1080/01635581.2011.582221. [DOI] [PubMed] [Google Scholar]
- 37.Pendleton JM, Tan WW, Anai S, et al. Phase II trial of isoflavone in prostate-specific antigen recurrent prostate cancer after previous local therapy. BMC Cancer. 2008;8:132. doi: 10.1186/1471-2407-8-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwan W, Duncan G, Van Patten C, Liu M, Lim J. A phase II trial of a soy beverage for subjects without clinical disease with rising prostate-specific antigen after radical radiation for prostate cancer. Nutr Cancer. 2010;62(2):198–207. doi: 10.1080/01635580903305318. [DOI] [PubMed] [Google Scholar]
- 39.deVere White RW, Hackman RM, Soares SE, Beckett LA, Li Y, Sun B. Effects of a genistein-rich extract on PSA levels in men with a history of prostate cancer. Urology. 2004;63(2):259–263. doi: 10.1016/j.urology.2003.09.061. [DOI] [PubMed] [Google Scholar]
- 40.van Veldhuizen PJ, Thrasher JB, Ray G, et al. Dose effect of soy supplementation in prostate cancer: a pilot study. Oncol Rep. 2006;16(6):1221–1224. [PubMed] [Google Scholar]
- 41.Hussain M, Banerjee M, Sarkar FH, et al. Soy isoflavones in the treatment of prostate cancer. Nutr Cancer. 2003;47(2):111–117. doi: 10.1207/s15327914nc4702_1. [DOI] [PubMed] [Google Scholar]
- 42.Kumar NB, Cantor A, Allen K, et al. The specific role of isoflavones in reducing prostate cancer risk. Prostate. 2004;59(2):141–147. doi: 10.1002/pros.10362. [DOI] [PubMed] [Google Scholar]
- 43.Sakr WA, Haas GP, Cassin BF, Pontes JE, Crissman JD. The frequency of carcinoma and intraepithelial neoplasia of the prostate in young male patients. J Urol. 1993;150(2 pt 1):379–385. doi: 10.1016/s0022-5347(17)35487-3. [DOI] [PubMed] [Google Scholar]
- 44.McCormick DL, Johnson WD, Bosland MC, Lubet RA, Steele VE. Chemoprevention of rat prostate carcinogenesis by soy isoflavones and by Bowman-Birk inhibitor. Nutr Cancer. 2007;57(2):184–193. doi: 10.1080/01635580701277478. [DOI] [PubMed] [Google Scholar]
- 45.Pollard M, Wolter W, Sun L. Prevention of induced prostate-related cancer by soy protein isolate/isoflavone-supplemented diet in Lobund-Wistar rats. In Vivo. 2000;14(3):389–392. [PubMed] [Google Scholar]
- 46.Wang J, Eltoum IE, Lamartiniere CA. Genistein chemoprevention of prostate cancer in TRAMP mice. J Carcinog. 2007;6:3. doi: 10.1186/1477-3163-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harper CE, Cook LM, Patel BB, et al. Genistein and resveratrol, alone and in combination, suppress prostate cancer in SV-40 tag rats. Prostate. 2009;69(15):1668–1682. doi: 10.1002/pros.21017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.