Abstract
Background
The existence of Tc17 cells was recently shown in several types of infectious and autoimmune diseases, but their distribution and functions in uterine cervical cancer (UCC) have not been fully elucidated.
Methods
The frequency of Tc17 cells in peripheral blood samples obtained from UCC patients, cervical intraepithelial neoplasia (CIN) patients and healthy controls was determined by flow cytometry. Besides, the prevalence of Tc17 cells and their relationships to Th17 cells and Foxp3-expressing T cells as well as microvessels in tissue samples of the patients were assessed by immunohistochemistry staining.
Results
Compared to controls, patients with UCC or CIN had a higher proportion of Tc17 cells in both peripheral blood and cervical tissues, but the level of Tc17 cells in UCC tissues was significantly higher than that in CIN tissues. Besides, the increased level of Tc17 in UCC patients was associated with the status of pelvic lymph node metastases and increased microvessel density. Finally, significant correlations of infiltration between Tc17 cells and Th17 cells or Foxp3-expressing T cells were observed in UCC and CIN tissues.
Conclusions
This study indicates that Tc17 cell infiltration in cervical cancers is associated with cancer progression accompanied by increased infiltrations of Th17 cells and regulatory T cells as well as promoted tumor vasculogenesis.
Introduction
Uterine cervical cancer (UCC), the second most prevalent malignancy in women worldwide [1], is considered to be an important immunogenic tumor, as human papillomavirus (HPV) high-risk subtypes cause multistep carcinogenesis from cervical intraepithelial neoplasia (CIN) through carcinoma in situ to invasive cancer and metastatic cancer. Meanwhile, the responses of the host immune systems, especially the cellular immune response, play an important role in the control of both HPV infections and HPV-associated neoplastic formation [2], [3]. Although the cellular adaptive immunity is an important component in the tumor immune surveillance, the mechanisms underlying tumor immunity is not fully understood [4].
The primary cells responsible for the cellular immune response are a set of T subsets, including helper T cells (Th), cytotoxic T cells (Tc), and suppressor T cells (Ts). A recently described Th subset, CD4+ T cells with IL-17 production (Th17 cells), has been shown to play an important role in the conditions of inflammation, autoimmunity and allergic reactions [5]–[7]. In a recent study, we observed that Th17 cells were highly enriched in peripheral blood of human UCC patients, and their levels were positively correlated with the status of lymph node metastases and vasoinvasion [8]. However, the subsets of IL-17+CD8+ T cells (Tc17 cells), recently found in several conditions of infection and autoimmune diseases [9]–[12], have not been fully studied and their biological functions are still lacking.
CD8+ cytotoxic T cells (Tc cells) play a crucial role in the host immune response to intracellular pathogens and cancer. Due to the redundant expression of T-box transcription factor Eomes and T-bet, Tc cells are fated to develop into cytolytic effector cells that produce IFN-γ and express granzyme B and perforin to kill the target cells [13], [14]. However, studies of the effects of Tc17 cells on immune responses are scarce. In contrast to classic CD8+ Tc cells, Tc17 cells are negative for granzyme B as well as perforin and lacking cytolytic activity in vitro [9], [12], [15], and the absence of Eomes in tumor infiltrating lymphocytes is correlated with enhanced lymph node metastasis in colorectal cancers [16]. Nevertheless, it is interesting that after adoptive transfer of highly purified, Ag-specific, IL-17-secreting CD8+ T cells to Ag-expressing hosts, the transferred Tc17 cells could convert to IFN-γ-producing effector cells lacking IL-17 expression, which could be accumulated remarkably and cause significant pulmonary pathologic alterations [12] or the regression of large established tumors [15], [17]. These findings may be explained by the existence of the plasticity of Tc17 cells. However, the exact effects of naturally existed Tc17 cells in vivo such as in the lung, in the digestive mucosa [18], and in the tumor-bearing mice [19] are still largely unknown. Tc17 cells were recently detected in human hepatocellular carcinoma [20], but data concerning their biological function as well as regulatory mechanisms are still lacking. Here, we aimed to investigate the levels as well as the possible biologic functions of Tc17 cells in UCC, which is known to be a type of highly immunogenic cancer initiated by the persistent infection of high-risk HPV.
In this study, we sought to determine the distribution of Tc17 cells in bothperipheral blood and cervical tissues from healthy controls, CIN and UCC patients. Moreover, to determine the potential roles of Tc17 cells, relationship between Tc17 cells and clinical features of UCC as well as microvessel density in cervical tissues were investigated. Furthermore, combined with our previously report [8], [25], the correlations of the levels of Tc17 cells with Th17 cells or Foxp3-expressing T cells were also determined.
Design and Methods
Ethics statement
Enrollment took place between May 2009 and April 2012 in Qilu Hospital, Shandong University. Our research was approved by the Medical Ethical Committee of Qilu Hospital, Shandong University. A written informed consent document was obtained from each participant. Informed consent declared that remnants of the patient's peripheral blood and cervical tissues excised during surgery would be used for the examination of T subsets. These samples were used to identify Tc17 cells in UCC and CIN, and peripheral blood was also drawn from healthy volunteers for this research.
Patient and control specimens
Tumor or peripheral blood samples were obtained from 88 untreated UCC patients (age range 34–70 years, median 44 years) and 53 untreated CIN patients (28–60 years, median 42 years) with pathologically confirmed active disease at Qilu Hospital, Shandong University. Samples from 46 UCC patients who underwent curative resection, and 28 CIN patients who underwent cervical conization were used for immunohistochemistry. Blood samples from 49 UCC patients and 25 CIN patients were used for flow cytometric analyses. Individuals with concurrent autoimmune disease, active or chronic infection, cardiovascular diseases, connective tissue diseases or a history of malignant tumors were excluded. None of the patients previously received immunosuppressive, radiotherapy or chemotherapy. The characteristics of the studied population are presented in Tables 1 and 2. Blood specimens (n = 28; 26–67 years, median 42 years) and histological specimens of cervices (n = 18; 31–60 years, median 46 years) from healthy women who underwent hysterectomies for benign uterine diseases with no cervical abnormalities were collected. These histological specimens were obtained as Paraffin-embedded blocks from the Department of Pathology of Qilu Hospital, Shandong University.
Table 1. Clinical Characteristics of UCC Patients.
| Characteristic | Category | N = 88 (%) |
| FIGO stage | IA | 14 (16) |
| IB | 46 (52) | |
| IIA | 20 (23) | |
| IIB | 8 (9) | |
| Histology type | SCC | 77 (88) |
| ADC/ADSC | 11 (12) | |
| Tumor differentiation | Well | 14 (16) |
| Moderate | 33 (37) | |
| Poor | 41 (47) | |
| Lymph node metastases | Positive | 26 (30) |
| Negative | 62 (70) | |
| Tumor size (cm) | <4 | 48 (54) |
| ≥4 | 40 (46) | |
| Infiltration depth (mm) | <15 | 39 (44) |
| ≥15 | 49 (56) | |
| Vasoinvasion | Yes | 28 (32) |
| No | 51 (58) | |
| Unknown | 9 (10) |
Abbreviation: FIGO, International Federation of Gynecologists and Obstetricians; SCC, squamous cell carcinoma; ADC, adenocarcinoma; ADSC, adenosquamous carcinoma.
Table 2. Clinical Characteristics of CIN Patients.
| Category | Subcategory | Result |
| No. patients | 53 | |
| Age (y) | Median | 42 |
| Range | 28–60 | |
| Clinical stage | CINII | 23 |
| CINIII | 30 |
Flow cytometric analysis of Tc17 cells
To analyze the prevalence of Tc17 cells, IL-17-producing CD8(+) lymphocytes were evaluated by flow cytometry. In brief, heparinized peripheral whole blood (200 µl) with an equal volume of RPMI-1640 medium was incubated for 4 h at 37°C in 5% CO2 in the presence of 25 ng/ml of phorbol myristate acetate (PMA), 1 µg/ml of ionomycin, and 1.7 µg/ml monensin (all from Alexis Biochemicals, San Diego, CA). Then, the cells were incubated with PE-Cy5-conjugated anti-human CD3 and FITC-conjugated anti-human CD8 monoclonal antibodies (eBioscience, San Diego, CA) at room temperature in the dark for 15 min to stain the surface. After fixation and permeabilization, according to the manufacturer's instructions, the cells were stained with a PE-conjugated anti-IL-17 monoclonal antibody for 15 min. Isotype controls were used to enable correct compensation and confirm antibody specificity. Stained cells were analyzed by flow cytometric analysis using a FACScan cytometer equipped with CellQuest software (BD Bioscience PharMingen).
Immunohistochemistry detection of Tc17 cells and microvessel density (MVD)
Paraffin-embedded tissue sections were deparaffinized and rehydrated, followed by microwave antigen retrieval in EDTA (pH 9.1). For immunohistochemical double staining of Tc17 cells, the sections were processed with the Doublestain system (kit-9999), and the following antibodies were used:rabbit anti-human IL-17 antibody (1∶700 v/v; Abcam, USA) and mouse anti-human CD8 antibody (1∶30 v/v; Abcam, USA). For immunohistochemical analysis of microvessel density, the sections were blocked with blocking solution (serum albumin) diluted in phosphate buffer saline (PBS) 3 times for 5 min. Then, the sections were incubated with primary antibody in a moist chamber at 4°C overnight. The CD34 monoclonal antibody (Abcam, USA) was used in this study. After washing 3 times with PBS for 5 min, a horseradish peroxidase (HRP) polymer-linked secondary antibody was added and incubated for 15 min at 37°C. The sections were then visualized with diaminobenzadine (DAB) and counterstained with hematoxylin. Negative control staining was performed with an isotype control and PBS instead of the primary antibody.
Statistical analysis
Values were expressed as the mean ± SD. The data were assessed by an ANOVA. Comparisons between two groups were assessed by student's t test. Correlations between variables were determined using linear regression analysis. All tests were performed using the SPSS 13.0 system. P<0.05 was considered statistically significant.
Results
Elevated circulating Tc17 cells in untreated UCC and CIN patients
We analyzed the expression of IL-17 on CD8+ T cells (Tc17 cells) in peripheral blood using flow cytometry. The expression of typical Tc17 cells in representative patients and controls was shown in Fig. 1. Besides, the percentage of Tc17 cells was significantly higher in UCC patients (0.49±0.37%, P = 0.0076) and CIN patients (0.51±0.39%, P = 0.0149), compared to that in healthy controls (0.27±0.27%) (Fig. 2a). No significant difference was found between the level of Tc17 cells in CIN and that in UCC patients (P>0.05). To investigate the possible role of the increased Tc17 cells, we next sought to determine whether this Tc17 skewing was associated with clinical features of the disease. Notably, we observed a significant increase in the frequency of Tc17 cells in UCC patients with lymph node metastasis (0.77±0.43%; P = 0.0026), when compared to that in patients without lymph node metastases (0.36±0.26%) (Fig. 2b). No association was found between the levels of Tc17 cells and other clinical characteristics such as clinical stage, infiltration depth, vasoinvasion, histological tumor type, or primary tumor size in UCC patients (P>0.05).
Figure 1. The levels of circulation Tc17 cells in representative controls, CIN patients and UCC patients.
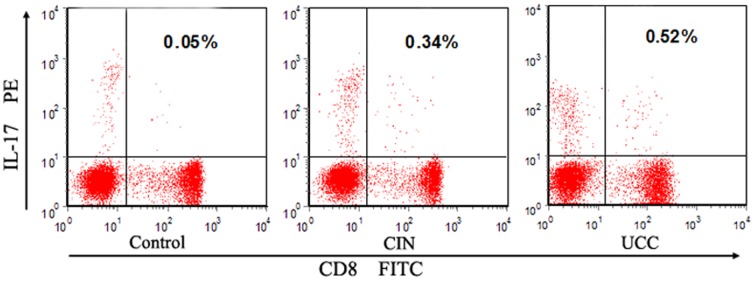
Upper right quadrants are the domains of Tc17 (CD8+ IL-17+) cells and the percentages of them were shown in each panel.
Figure 2. The frequency of Tc17 cells by flow cytometry.
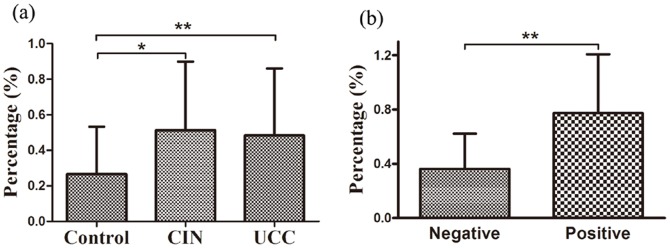
(a) Tc17 frequencies in the three groups. Significantly increased Tc17 cells were found in untreated UCC patients (P = 0.0076, n = 49) and CIN patients (P = 0.0149, n = 25) compared to healthy controls (n = 28). (b) Tc17 frequency in positive or negative lymph node metastases in UCC patients. Compared with patients without lymph node metastases (n = 15), significantly increased Tc17 frequency (P = 0.0026) was found in lymph node metastases patients (n = 35). Student's t test was used and bars represent SD, *P<0.05, **P<0.01.
Distribution of Tc17 cells and the microvessel density (MVD) in tissue samples of UCC and CIN patients
We previously found that IL-17+ cells were enriched in human UCC, and most of the circulating IL-17+ cells were CD4+ cell population (Th17 cells) [8]. However, in tumor tissues, a more abundance of the cells were CD8+ T cells (Tc17 cells). To study the distribution of Tc17 cells on a local scale, we examined the prevalence of Tc17 cells in tumor tissues from 46 UCC patients and cervical tissues from 28 CIN patients using immunohistochemical double staining (Fig. 3). As shown in Fig. 4a, the level of Tc17 cells significantly increased in tissues of UCC patients (142.50±28.24 cells/HPF) and CIN patients (23.42±7.65 cells/HPF), when compared to that in tissues of healthy controls (5.52±2.25 cells/HPF; P UCC = 0.0007, P CIN = 0.026). A significant difference was also found between CIN and UCC patients (P = 0.0086). Again, the level of Tc17 cells in tissues of UCC patients was positively correlated with the status of lymph node metastases, since significantly higher level of Tc17 cells was observed in tissues from UCC patients with lymph node metastases (178.20±35.47 cells/HPF) compared to that in UCC patients without lymph node metastases (116.59±25.57 cells/HPF, P = 0.035) (Fig. 4b). No correlation was found between the level of Tc17 cells with the other clinical characteristics such as clinical stage, infiltration depth, vasoinvasion, tumor size and histological tumor type of UCC patients (P>0.05).
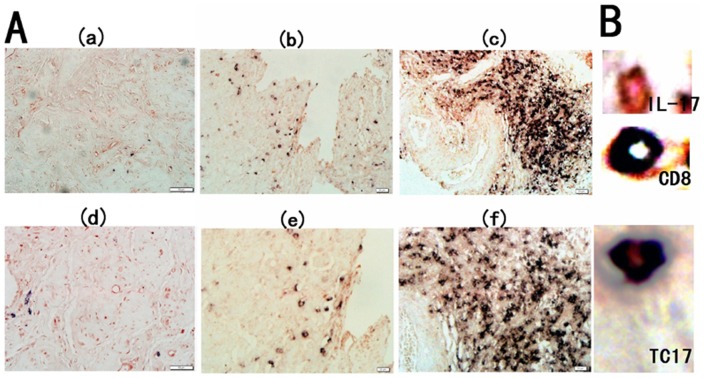
Figure 3. Expression of Tc17 cells in cervical tissues of the three groups.
(A) Immunohistochemical double staining for Tc17 cells in the control group (a and d), CIN group (b and e) and UCC group (c and f). Representative sites with low (200×, upper panels) and high (400×, lower panels) magnification were shown. (B) IL-17-producing cells were stained red (in the cytoplasm) and CD8+ cells were stained black (in the membrane). The co-expression of CD8 and IL-17 confirmed that a proportion of Tc17 cells.
Figure 4. The frequency of Tc17 cells by immunohistochemical staining.
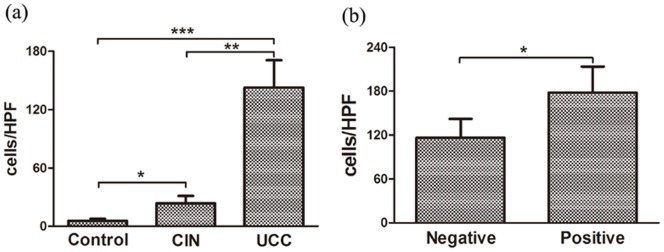
(a) Compared to healthy controls (n = 30), significantly increased numbers of Tc17 cells were found in both tissues of UCC patients (P = 0.0007, n = 46) and CIN patients (P = 0.026, n = 28). Significant difference was also found between CIN and UCC tissues (P = 0.0086). (b) In tumor region, those UCC patients with lymph node metastases (n = 30) were detected significantly statistical higher Tc17 cells frequency than those patients without lymph node metastases (P = 0.035, n = 16). *P<0.05, **P<0.01, ****P<0.001.
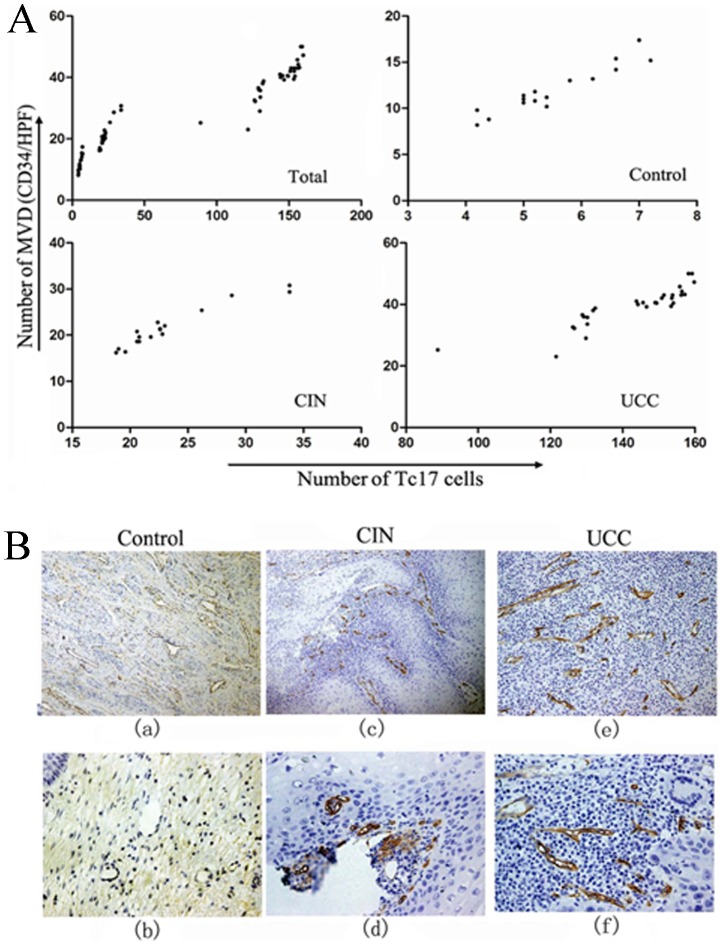
Next, the density of microvessels in cervical tissues was determined by immunohistochemistry staining with antibody against angiogenic marker- CD34. As shown in Fig. 5b, MVD was more frequently observed in UCC (38.37±7.04/HPF, P<0.0001) and CIN tissues (23.36±5.34/HPF, P<0.0001) than that in the control tissues (11.81±2.47/HPF). Also, significantly higher level of MVD was found in UCC group when compared with CIN group (P<0.0001). Since IL-17 is known to be able to promote tumor progression through fostering angiogenesis [21], [22], we analyzed the relationship between the level of Tc17 cells and the amount of microvessels. As shown in Fig. 5a, the levels of Tc17 cells were found to correlate positively with the microvessel density in cervical CIN and UCC tissues (r = 0.923,P<0.001 in CIN; and r = 0.938, P<0.0001 in UCC, respectively).
Figure 5. The correlations between Tc17 cells and microvessel density (MVD).
(A) Linear regression analysis between the levels of Tc17 cells and MVD (Total: r = 0.987, P<0.0001, n = 65; control: r = 0.814, P<0.001, n = 18, CIN: r = 0.923, P<0.001, n = 17; UCC: r = 0.938, P<0.0001, n = 30). MVD from each sample was plotted against Tc17 cells level from the same person. (B) Representative immunohistochemical staining of MVD in cervical tissues of three groups. Representative sites with low (100×, upper panels) and high (400×, lower panels) magnification were shown.
Correlations between the levels of Tc17 cells and Th17 cells as well as Foxp3-expression T cells
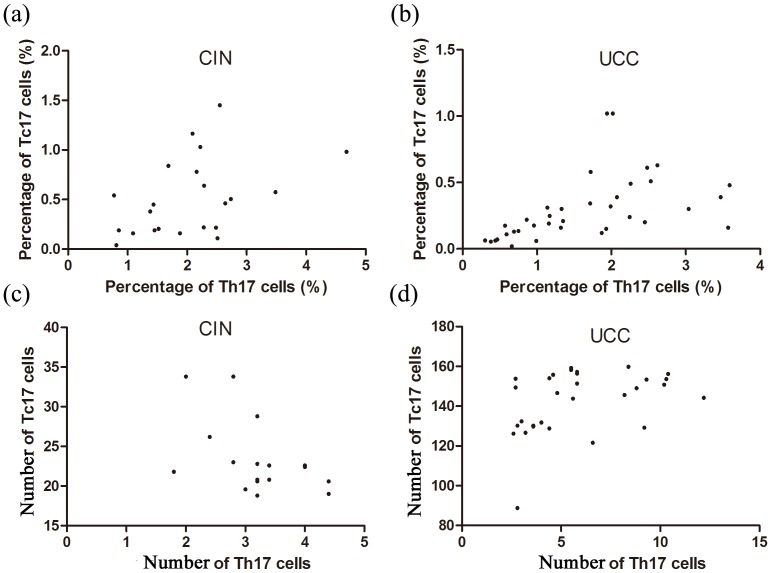
Since Tc17 cells are similar to Th17 cells in the respect of developmental pathway and possible effects [23], [24], it is reasonable to investigate the relation between them. The primary data and the methods for Th17 cells detection in the same three groups (UCC, CIN and the control groups) were shown in our two previous published papers [8], [25]. The results demonstrated that the levels of Th17 cells and Tc17 cells were positively correlated in the CIN and UCC groups in both peripheral blood (rCIN = 0.435, P = 0.042; rUCC = 0.403, P = 0.016) (Fig. 6 a and b) and cervical tissues (rCIN = 0.441, P = 0.039; rUCC = 0.693, P = 0.026) (Fig. 6 c and d).
Figure 6. The correlations between Tc17 cells and Th17 cells.
(a, b) Linear regression analysis between frequencies of Tc17 cell and Th17 cells in the blood (rCIN = 0.435, P = 0.042, n = 22; rUCC = 0.403, P = 0.016, n = 36). (c, d) Linear regression analysis between the levels of Tc17 cells and Th17 cells in the cervical tissues (rCIN = 0.441, P = 0.039, n = 17; rUCC = 0.693, P = 0.026, n = 30).
Besides, since Foxp3-expressing T cells are well known to be immune-suppressive for the T cell responses [25], [26], the relationship between the levels of Tc17 and Foxp3-expressing T cells in the patient's (CIN and UCC) cervical tissues were also analyzed. The primary data and the methods of Foxp3-expressing T cells detection were in the reference 38. As shown in Fig. 7, the levels of Tc17 cells and Foxp3-expressing T cells in the patient's cervical tissues were positively correlated (r = 0.841, P<0.001).
Figure 7. The correlation between Tc17 cells and Foxp3-expressing T cells in the patients of CIN and UCC tissues.
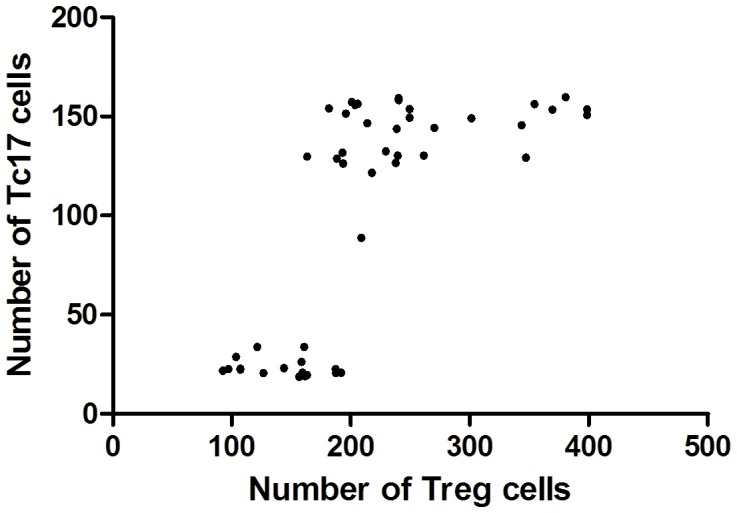
(r = 0.713, P<0.001, n = 47).
Discussion
The immune system plays a complex role in tumorigenesis. It was previously believed that CD8+ T mainly comprise CD8+ cytotoxic T cells, Tc cells, which play crucial role in host immune responses to intracellular pathogens and tumor formation by means of the expression of IFN-γ, granzyme B and perforin [13], [14]. However, a subset of CD8+ T cells with immunosuppressive properties have been described [27], [28]. Roberts et al. [29] found that CD8+ T cells could also promote the cutaneous carcinogenesis using a two-step chemical initiation/promotion protocol (DMBA and TPA). A DMBA/TPA carcinogenic regimen could up-regulate the expressions of TGF-β and IL-6, resulting in Tc17 cell skewing [30], [31]. Besides, they also found that a subset of tumor-promoting IL-17+CD8+ cell population might exist in this model.
Tc17 cells are recently defined as an IL-17-producing CD8+ T subset that is distinct from Tc1 and Tc2 cells. Although some advances have been made in the understanding of the differentiation and function of Tc17 cells in some types of human diseases and in the homeostatic regulations [9], [10], [32], little is known about the biological function and mechanism underlying the regulation of Tc17 cells in human tumors. Our study demonstrated that the levels of Tc17 cells and Th17 cells prominently increased in both peripheral blood and tumor tissues of UCC patients. Besides, we found that the frequency of Tc17 cells was positively correlated with that of Th17 cells in both peripheral blood and cervical tissues in patients of CIN and UCC. Another study has also shown an abundance of Tc17 cells in tumor-bearing mice, similar to that in inflammatory condition [18]. Also, Kuang et al. [20] found that IL-17+ cells (including Th17 cells and Tc17 cells) accumulated in human hepatocellular carcinomas(HCC) and Tc17 cells were enriched in the invading edge of HCCs. Thus, Tc17 cells enriched in UCC patients may play an important role in the development of this type of cancer.
It was reported that Tc17 cells and Th17 cells shared the similar developmental pathways with regard to the differentiation process [10]. For example, TGF-β, IL-6 and IL-21 could stimulate the differentiation of both Tc17 and Th17 cells [10], [19], [24]. Besides, studies have shown that Tc17 cells may display suppressed cytotoxic function similarly to the function of Th17 cells in autoimmune diseases [23], [33], infection [9], [23] and antitumor immunity [15]. Furthermore, IL-17 is a pro-inflammatory cytokine, which is produced by both Th17 cells and Tc17 cells. IL-17 producing T cells are the effectors involved in inducing inflammation and autoimmune tissue injury [34]. Thus, we speculate that the production of IL-17 may mediate the potential roles of Tc17 cells in cancer progression. However, the forced ectopic overexpression of IL-17 in tumor cells demonstrated conflicting effects on tumor growth: tumor inhibiting [35], [36] versus tumor promoting [21], [37]. Notably, Nam et al. [31] reported that IL-17 suppressed apoptosis in several tumor cell lines in vitro, and knockdown of the IL-17 receptor in 4T1 mouse mammary cancer cells enhanced apoptosis and decreased tumor growth in vivo. Also, we observed the elevated levels of TGF-β, IL-6, IL-17 and IL-21 in cervical cancers (unpublished observations). These observations suggest that UCC may actively secrete large amounts of TGF-βwhich may induce the differentiation of IL-17+ T cells, and the latter may in turn promote the development and survival of cancers in an IL-17-dependent manner [31], although further studies are still needed.
Recently, it has been shown that both Th17 and Tc17 cells are present in the tumor microenvironments of several types of human and mouse tumors [19], suggesting that this may be a relatively common phenomenon in tumorigenesis. Besides, we previously reported that Th17 cells might cause malignant progression and poor survival in UCC patients [8]. In this study, we also observed that the positive correlation between Tc17 cells and Th17 cells increased gradually with the progression from CIN to UCC. Furthermore, the prevalence of Tc17 cells was also correlated with lymph node metastases of UCC. Therefore, it is possible that the enrichment of Tc17 cells promotes the progression of UCC in concert with Th17 cells.
Despite the fact that Tc17 cells are effector cells, they could not kill target cells in the way that classical CD8+ T cells do [24]. Epidemiological studies have established that inflammation is associated with tumor progression [38]. Inflammation leads to the production of growth factors, angiogenic factors, and matrix-degrading proteases by leukocytes, which play an important role in tumor promotion and progression [4], [38]. Kuang et al. [20] found that Tc17 cells were predominantly enriched in the invading edge of human hepatocellular carcinoma (HCC). These infiltrating Tc17 cells expressed high levels of proinflammatory cytokines, which promote tumor extension via the inflammatory reaction. The neovascularization is another important hallmark of cancer progression. Proinflammatory cytokines such as IL-17 could also promote tumor growth by fostering angiogenesis [21], [22]. In our study, we also found a positive correlation between the frequency of Tc17 cell and microvessel density, supporting the proinflammatory property of IL-17–producing cells. Moreover, we also observed the correlation of Tc17 cells with Foxp3-expressing T cells in cervical cancer tissues,Foxp3-expressing T cells mediate strong immune-suppressive activity on T cell responses [39], [40]. These results indicate that Tc17 cells may promote tumor progression, but the exact function of Tc17 cells in UCC needs further investigation.
In summary, we showed for the first time that the frequency of Tc17 cells increased obviously in CIN and UCC patients, and the increased prevalence of Tc17 cells was associated with lymph node metastases and microvessel density, indicating an important role for Tc17 cells in the initiation and progression of UCC. A better understanding of the underlying mechanism of IL-17 regulating antitumor immune responses may lead to the development of novel therapeutic strategy for UCC patients.
Funding Statement
This study was supported by grants from the National Natural Science Foundation of China (Nos. 81172486, 81170515, and 81072122) and the Shandong Technological Development Project (No. 2006BS03060). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Parkin DM, Bray F (2006) Chapter 2: The burden of HPV-related cancers. Vaccine 24: 11–25. [DOI] [PubMed] [Google Scholar]
- 2. Delgado FG, Martínez E, Céspedes MA, Bravo MM, Navas MC, et al. (2009) Increase of human papillomavirus-16 E7-specific T helper type 1 response in peripheral blood of cervical cancer patients after radiotherapy. Immunology 126: 523–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sheu BC, Chang WC, Lin HH, Chow SN, Huang SC (2007) Immune concept of human papillomaviruses and related antigens in local cancer milieu of human cervical neoplasia. J Obstet Gynaecol Res 33: 103–113. [DOI] [PubMed] [Google Scholar]
- 4. Zhu Y, Ju S, Chen E, Dai S, Li C, et al. (2010) T-bet and eomesodermin are required for T cell-mediated antitumor immune responses. J Immunol 185: 3174–3183. [DOI] [PubMed] [Google Scholar]
- 5. Xie JJ, Wang J, Tang TT, Ghen J, Gao XL, et al. (2010) The Th17/Treg functional imbalance during atherogenesis in ApoE(−/−) mice. Cytokine 49: 185–193. [DOI] [PubMed] [Google Scholar]
- 6. Zhu X, Ma D, Zhang J, Peng J, Qu X, et al. (2010) Elevated interleukin-21 correlated to Th17 and Th1 cells in patients with immune thrombocytopenia. J Clin Immunol 30: 253–259. [DOI] [PubMed] [Google Scholar]
- 7. Bettelli E, Oukka M, Kuchroo VK (2007) T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol 8: 345–350. [DOI] [PubMed] [Google Scholar]
- 8. Zhang Y, Ma D, Zhang Y, Tian Y, Wang Y, et al. (2011) The imbalance of Th17/Treg in patients with uterine cervical cancer. Clin Chim Acta 412: 894–900. [DOI] [PubMed] [Google Scholar]
- 9. Hamada H, Garcia-Hernandez Mde L, Reome JB, Misra SK, Strutt TM, et al. (2009) Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J Immunol 182: 3469–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ciric B, El-behi M, Cabrera R, Zhang GX, Rostami A (2009) IL-23 drives pathogenic IL-17-producing CD8+ T cells. J Immunol 182: 5296–5305. [DOI] [PubMed] [Google Scholar]
- 11. Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, et al. (2008) Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol 172: 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yen HR, Harris TJ, Wada S, Grosso JF, Getnet D, et al. (2009) Tc17 CD8 T cells: functional plasticity and subset diversity. J Immunol 183: 7161–7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Intlekofer AM, Banerjee A, Takemoto N, Gordon SM, Dejong CS, et al. (2008) Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science 321: 408–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, et al. (2003) Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science 302: 1041–1043. [DOI] [PubMed] [Google Scholar]
- 15. Hinrichs CS, Kaiser A, Paulos CM, Cassard L, Sanchez-Perez L, et al. (2009) Type 17 CD8+ T cells display enhanced antitumor immunity. Blood 114: 596–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Atreya I, Schimanski CC, Becker C, Wirtz S, Dornhoff H, et al. (2007) The T-box transcription factor eomesodermin controls CD8 T cell activity and lymph node metastasis in human colorectal cancer. Gut 56: 1572–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yu Y, Cho HI, Wang D, Kaosaard K, Anasetti C, et al. (2013) Adoptive transfer of Tc1 or Tc17 cells elicits antitumor immunity against established melanoma through distinct mechanisms. J Immunol 190: 1873–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kryczek I, Wei S, Vatan L, Escara-Wilke J, Szeliga W, et al. (2007) Cutting edge: opposite effects of IL-1 and IL-2 on the regulation of IL-17+ T cell pool IL-1 subverts IL-2-mediated suppression. J Immunol 179: 1423–1426. [DOI] [PubMed] [Google Scholar]
- 19. Kryczek I, Wei S, Zou L, Altuwaijri S, Szeliga W, et al. (2007) Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J Immunol 178: 6730–6733. [DOI] [PubMed] [Google Scholar]
- 20. Kuang DM, Peng C, Zhao Q, Wu Y, Zhu LY, et al. (2010) Tumor-activated monocytes promote expansion of IL-17-producing CD8+ T cells in hepatocellular carcinoma patients. J Immunol 185: 1544–1549. [DOI] [PubMed] [Google Scholar]
- 21. Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, et al. (2003) Interleukin-17 promotes angiogenesis and tumor growth. Blood 101: 2620–2627. [DOI] [PubMed] [Google Scholar]
- 22. Semela D, Dufour JF (2004) Angiogenesis and hepatocellular carcinoma. J Hepatol 41: 864–880. [DOI] [PubMed] [Google Scholar]
- 23. Huber M, Heink S, Grothe H, Guralnik A, Reinhard K, et al. (2009) A Th17-like developmental process leads to CD8(+) Tc17 cells with reduced cytotoxic activity. Eur J Immunol 39: 1716–1725. [DOI] [PubMed] [Google Scholar]
- 24. Kondo T, Takata H, Matsuki F, Takiquchi M (2009) Cutting edge: Phenotypic characterization and differentiation of human CD8+ T cells producing IL-17. J Immunol 182: 1794–1798. [DOI] [PubMed] [Google Scholar]
- 25. Hou F, Li Z, Ma D, Zhang WJ, Zhang YZ, et al. (2012) Distribution of Th17 cells and Foxp3-expressing T cells in tumor-infiltrating lymphocytes in patients with uterine cervical cancer. Clin Chim Acta 413: 1848–1854. [DOI] [PubMed] [Google Scholar]
- 26. Zhang B, Rong G, Wei H, Zhang M, Bi J, et al. (2008) The prevalence of Th17 cells in patients with gastric cancer. Biochem Biophys Res Commun 374: 533–537. [DOI] [PubMed] [Google Scholar]
- 27. Wei S, Kryczek I, Zou L, Daniel B, Cheng P, et al. (2005) Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma. Cancer Res 65: 5020–5026. [DOI] [PubMed] [Google Scholar]
- 28. Xystrakis E, Dejean AS, Bernard I, Druet P, Liblau R, et al. (2004) Identification of a novel natural regulatory CD8 T-cell subset and analysis of its mechanism of regulation. Blood 104: 3294–3301. [DOI] [PubMed] [Google Scholar]
- 29. Roberts SJ, Ng BY, Filler RB, Lewis J, Glusac EJ, et al. (2007) Characterizing tumor-promoting T cells in chemically induced cutaneous carcinogenesis. Proc Natl Acad Sci U S A 104: 6770–6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dwivedi VP, Tousif S, Bhattacharya D, Prasad DV, Van Kaer L, et al. (2012) Transforming growth factor-beta protein inversely regulates in vivo differentiation of interleukin-17 (IL-17)-producing CD4+ and CD8+ T cells. J Biol Chem 287: 2943–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nam JS, Terabe M, Kang MJ, Chae H, Voong N, et al. (2008) Transforming growth factor beta subverts the immune system into directly promoting tumor growth through interleukin-17. Cancer Res 68: 3915–3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Henriques A, Inês L, Couto M, Pedreiro S, Santos C, et al. (2010) Frequency and functional activity of Th17, Tc17 and other T-cell subsets in Systemic Lupus Erythematosus. Cell Immunol 264: 97–103. [DOI] [PubMed] [Google Scholar]
- 33. Ortega C, Fernández-A S, Carrillo JM, Romero P, Molina IJ, et al. (2009) IL-17-producing CD8+ T lymphocytes from psoriasis skin plaques are cytotoxic effector cells that secrete Th17-related cytokines. J Leukoc Biol 86: 435–443. [DOI] [PubMed] [Google Scholar]
- 34. Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, et al. (2003) Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med 198: 1951–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Benchetrit F, Ciree A, Vives V, Warnier G, Gey A, et al. (2002) Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood 99: 2114–2121. [DOI] [PubMed] [Google Scholar]
- 36. Hirahara N, Nio Y, Sasaki S, Minari Y, Takamura M, et al. (2001) Inoculation of human interleukin-17 gene-transfected Meth-A fibrosarcoma cells induces T cell-dependent tumor-specific immunity in mice. Oncology 61: 79–89. [DOI] [PubMed] [Google Scholar]
- 37. Numasaki M, Watanabe M, Suzuki T, Takahashi H, Nakamura A, et al. (2005) IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. J Immunol 175: 6177–6189. [DOI] [PubMed] [Google Scholar]
- 38. Karin M (2006) Nuclear factor-kappaB in cancer development and progression. Nature 441: 431–436. [DOI] [PubMed] [Google Scholar]
- 39. Qinfeng S, Depu W, Xiaofeng Y, Shah W, Hongwei C, et al. (2013) In situ observation of the effects of local irradiation on cytotoxic and regulatory T lymphocytes in cervical cancer tissue. Radiat Res 179: 584–589. [DOI] [PubMed] [Google Scholar]
- 40. Chen ZF, Xu Q, Ding JB, Zhang Y, Du R, et al. (2012) CD4+CD25+Foxp3+ Treg and TGF-beta play important roles in pathogenesis of Uygur cervical carcinoma. Eur J Gynaecol Oncol 33: 502–507. [PubMed] [Google Scholar]