Abstract
[3H]Cytochalasin B was used as a ligand to identify and characterize the glucose transporter in cerebral microvessels of the rat and the pig. Specific cytochalasin B binding, defined as that fraction of the total binding that is stereospecifically displaced by excess (500 mM) D-glucose, is saturable. Kinetic studies of this specific binding to cerebral microvessel preparations showed a dissociation constant (Kd) of 0.65-0.88 microM and a maximal binding (Bmax) of 60-80 pmol/mg of protein. In comparison, the Bmax of particulate fractions of the cerebral cortex was about one-tenth that of cerebral microvessels. The ability of various hexoses to displace specific cytochalasin B binding to cerebral microvessels in vitro correlated well with the capability of these hexoses to cross the blood-brain barrier in vivo. Irreversible photoaffinity labeling of the glucose transporter of cerebral microvessels with cytochalasin B followed by solubilization and polyacrylamide gel electrophoresis labeled a polypeptide(s) with a molecular weight of about 53,000. Antibodies prepared against the glucose transporter of human erythrocytes also reacted with a polypeptide(s) with a molecular weight of about 53,000 on electrophoresed preparations of cerebral microvessels. These results indicate that cerebral microvessels are richly endowed with a glucose transporter moiety of similar molecular weight and antigenic characteristics as the glucose transporter of human erythrocytes and other mammalian tissues.
Full text
PDF
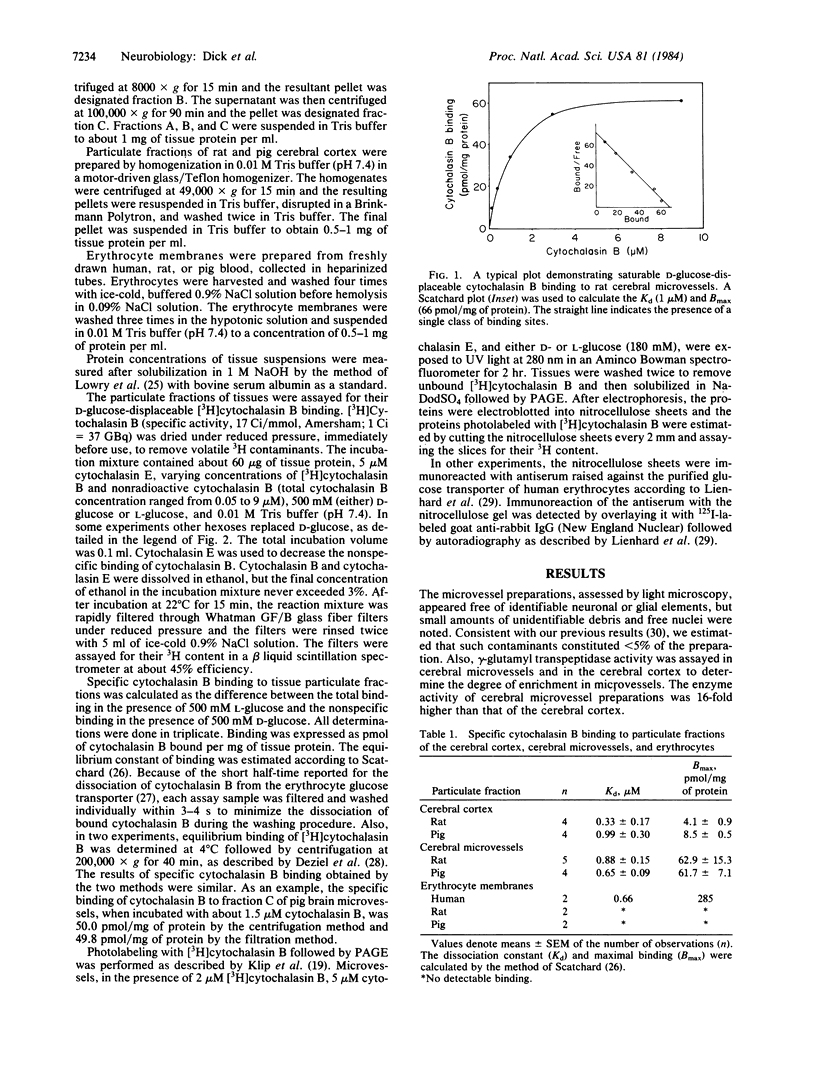
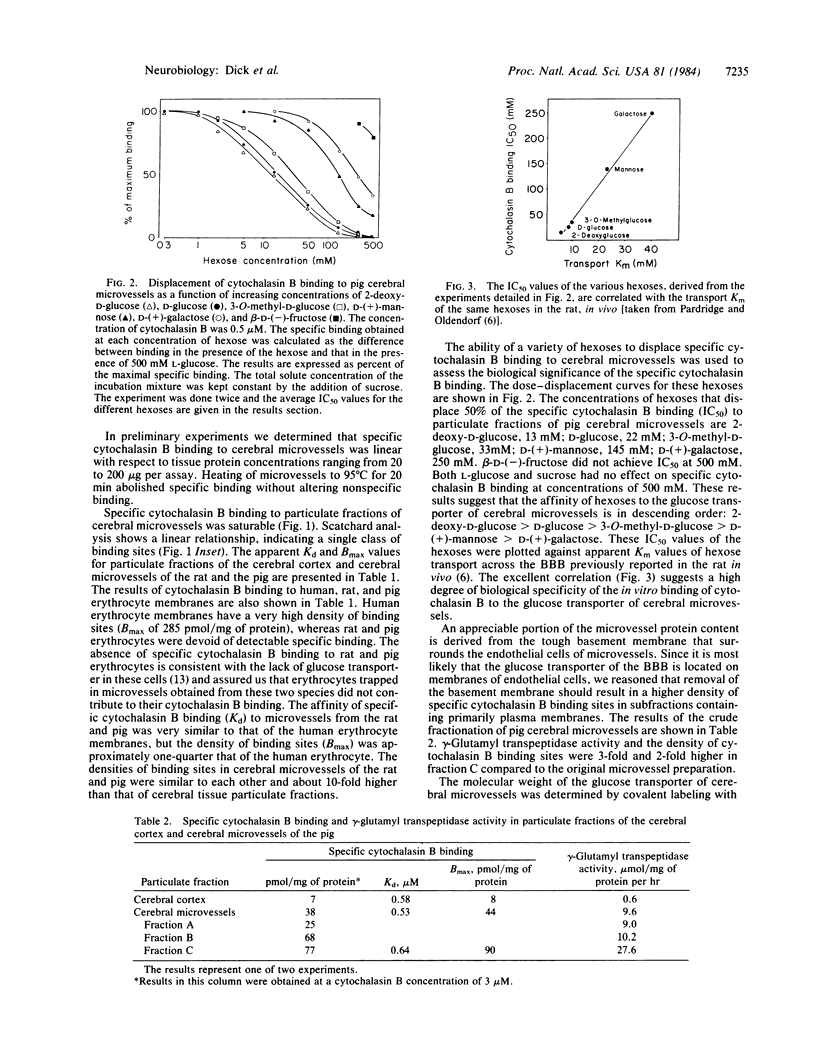
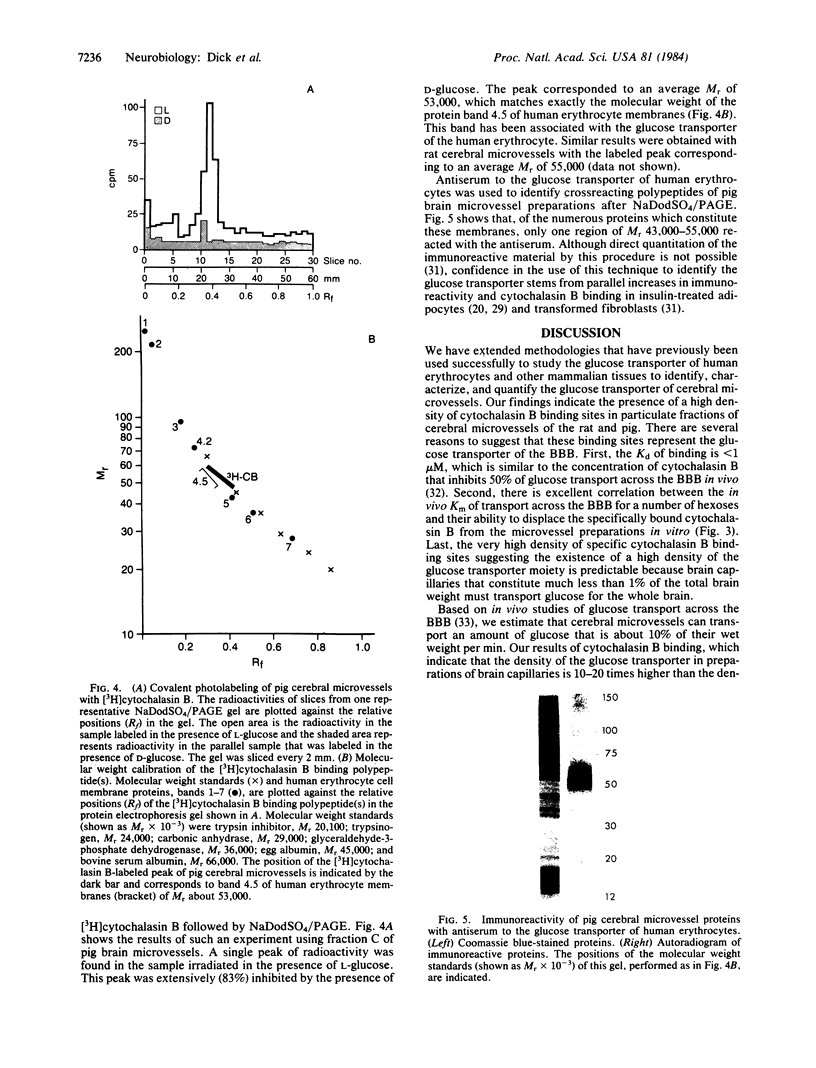

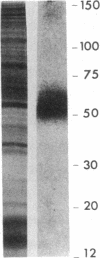
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert Z., Orlowski M., Rzucidlo Z., Orlowska J. Studies on gamma-glutamyl transpeptidase activity and its histochemical localization in the central nervous system of man and different animal species. Acta Histochem. 1966;25(5):312–320. [PubMed] [Google Scholar]
- Betz A. L., Csejtey J., Goldstein G. W. Hexose transport and phosphorylation by capillaries isolated from rat brain. Am J Physiol. 1979 Jan;236(1):C96–102. doi: 10.1152/ajpcell.1979.236.1.C96. [DOI] [PubMed] [Google Scholar]
- Betz A. L., Gilboe D. D., Yudilevich D. L., Drewes L. R. Kinetics of unidirectional glucose transport into the isolated dog brain. Am J Physiol. 1973 Sep;225(3):586–592. doi: 10.1152/ajplegacy.1973.225.3.586. [DOI] [PubMed] [Google Scholar]
- Betz A. L. Sodium transport in capillaries isolated from rat brain. J Neurochem. 1983 Oct;41(4):1150–1157. doi: 10.1111/j.1471-4159.1983.tb09065.x. [DOI] [PubMed] [Google Scholar]
- Brightman M. W. Morphology of blood-brain interfaces. Exp Eye Res. 1977;25 (Suppl):1–25. doi: 10.1016/s0014-4835(77)80008-0. [DOI] [PubMed] [Google Scholar]
- Crone C. Facilitated transfer of glucose from blood into brain tissue. J Physiol. 1965 Nov;181(1):103–113. doi: 10.1113/jphysiol.1965.sp007748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel P. M., Love E. R., Pratt O. E. Insulin and the way the brain handles glucose. J Neurochem. 1975 Oct;25(4):471–476. doi: 10.1111/j.1471-4159.1975.tb04352.x. [DOI] [PubMed] [Google Scholar]
- Deziel M., Pegg W., Mack E., Rothstein A., Klip A. Labelling of the human erythrocyte glucose transporter with 3H-labelled cytochalasin B occurs via protein photoactivation. Biochim Biophys Acta. 1984 May 30;772(3):403–406. doi: 10.1016/0005-2736(84)90157-3. [DOI] [PubMed] [Google Scholar]
- Drewes L. R., Horton R. W., Betz A. L., Gilboe D. D. Cytochalasin B inhibition of brain glucose transport and the influence of blood components on inhibitor concentration. Biochim Biophys Acta. 1977 Dec 15;471(3):477–486. doi: 10.1016/0005-2736(77)90051-7. [DOI] [PubMed] [Google Scholar]
- Gjedde A. Modulation of substrate transport to the brain. Acta Neurol Scand. 1983 Jan;67(1):3–25. doi: 10.1111/j.1600-0404.1983.tb04541.x. [DOI] [PubMed] [Google Scholar]
- Gjedde A. Rapid steady-state analysis of blood-brain glucose transfer in rat. Acta Physiol Scand. 1980 Apr;108(4):331–339. doi: 10.1111/j.1748-1716.1980.tb06541.x. [DOI] [PubMed] [Google Scholar]
- Gorga F. R., Lienhard G. E. Equilibria and kinetics of ligand binding to the human erythrocyte glucose transporter. Evidence for an alternating conformation model for transport. Biochemistry. 1981 Sep 1;20(18):5108–5113. doi: 10.1021/bi00521a003. [DOI] [PubMed] [Google Scholar]
- Harik S. I., Sharma V. K., Wetherbee J. R., Warren R. H., Banerjee S. P. Adrenergic and cholinergic receptors of cerebral microvessels. J Cereb Blood Flow Metab. 1981;1(3):329–338. doi: 10.1038/jcbfm.1981.36. [DOI] [PubMed] [Google Scholar]
- Johnson L. W., Smith C. H. Identification of the glucose transport protein of the microvillous membrane of human placenta by photoaffinity labelling. Biochem Biophys Res Commun. 1982 Nov 30;109(2):408–413. doi: 10.1016/0006-291x(82)91736-3. [DOI] [PubMed] [Google Scholar]
- Jones M. N., Nickson J. K. Monosaccharide transport proteins of the human erythrocyte membrane. Biochim Biophys Acta. 1981 Jun 16;650(1):1–20. doi: 10.1016/0304-4157(81)90006-x. [DOI] [PubMed] [Google Scholar]
- Karnieli E., Zarnowski M. J., Hissin P. J., Simpson I. A., Salans L. B., Cushman S. W. Insulin-stimulated translocation of glucose transport systems in the isolated rat adipose cell. Time course, reversal, insulin concentration dependency, and relationship to glucose transport activity. J Biol Chem. 1981 May 25;256(10):4772–4777. [PubMed] [Google Scholar]
- Klip A., Walker D., Ransome K. J., Schroer D. W., Lienhard G. E. Identification of the glucose transporter in rat skeletal muscle. Arch Biochem Biophys. 1983 Oct 1;226(1):198–205. doi: 10.1016/0003-9861(83)90285-0. [DOI] [PubMed] [Google Scholar]
- Klip A., Walker D. The glucose transport system of muscle plasma membranes: characterization by means of [3H]cytochalasin B binding. Arch Biochem Biophys. 1983 Feb 15;221(1):175–187. doi: 10.1016/0003-9861(83)90134-0. [DOI] [PubMed] [Google Scholar]
- Kolber A. R., Bagnell C. R., Krigman M. R., Hayward J., Morell P. Transport of sugars into microvessels isolated from rat brain: a model for the blood-brain barrier. J Neurochem. 1979 Aug;33(2):419–431. doi: 10.1111/j.1471-4159.1979.tb05171.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lidinsky W. A., Drewes L. R. Characterization of the blood-brain barrier: protein composition of the capillary endothelial cell membrane. J Neurochem. 1983 Nov;41(5):1341–1348. doi: 10.1111/j.1471-4159.1983.tb00831.x. [DOI] [PubMed] [Google Scholar]
- Lienhard G. E., Kim H. H., Ransome K. J., Gorga J. C. Immunological identification of an insulin-responsive glucose transporter. Biochem Biophys Res Commun. 1982 Apr 14;105(3):1150–1156. doi: 10.1016/0006-291x(82)91090-7. [DOI] [PubMed] [Google Scholar]
- Lund-Andersen H. Transport of glucose from blood to brain. Physiol Rev. 1979 Apr;59(2):305–352. doi: 10.1152/physrev.1979.59.2.305. [DOI] [PubMed] [Google Scholar]
- ORLOWSKI M., MEISTER A. ISOLATION OF GAMMA-GLUTAMYL TRANSPEPTIDASE FROM HOG KIDNEY. J Biol Chem. 1965 Jan;240:338–347. [PubMed] [Google Scholar]
- Pardridge W. M. Brain metabolism: a perspective from the blood-brain barrier. Physiol Rev. 1983 Oct;63(4):1481–1535. doi: 10.1152/physrev.1983.63.4.1481. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M., Oldendorf W. H. Kinetics of blood-brain transport of hexoses. Biochim Biophys Acta. 1975 Mar 25;382(3):377–392. doi: 10.1016/0005-2736(75)90279-5. [DOI] [PubMed] [Google Scholar]
- Pessin J. E., Tillotson L. G., Yamada K., Gitomer W., Carter-Su C., Mora R., Isselbacher K. J., Czech M. P. Identification of the stereospecific hexose transporter from starved and fed chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2286–2290. doi: 10.1073/pnas.79.7.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese T. S., Karnovsky M. J. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol. 1967 Jul;34(1):207–217. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter D. W., Baldwin S. A., Lienhard G. E., Weber M. J. Proteins antigenically related to the human erythrocyte glucose transporter in normal and Rous sarcoma virus-transformed chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1540–1544. doi: 10.1073/pnas.79.5.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan M. F. Cytochalasin B. A natural photoaffinity ligand for labeling the human erythrocyte glucose transporter. J Biol Chem. 1982 Jul 10;257(13):7290–7293. [PubMed] [Google Scholar]
- Shanahan M. F., Olson S. A., Weber M. J., Lienhard G. E., Gorga J. C. Photolabeling of glucose-sensitive cytochalasin B binding proteins in erythrocyte, fibroblast and adipocyte membranes. Biochem Biophys Res Commun. 1982 Jul 16;107(1):38–43. doi: 10.1016/0006-291x(82)91666-7. [DOI] [PubMed] [Google Scholar]
- Wardzala L. J., Cushman S. W., Salans L. B. Mechanism of insulin action on glucose transport in the isolated rat adipose cell. Enhancement of the number of functional transport systems. J Biol Chem. 1978 Nov 25;253(22):8002–8005. [PubMed] [Google Scholar]
- Wardzala L. J., Jeanrenaud B. Identification of the D-glucose-inhibitable cytochalasin B binding site as the glucose transporter in rat diaphragm plasma and microsomal membranes. Biochim Biophys Acta. 1983 Apr 21;730(1):49–56. doi: 10.1016/0005-2736(83)90315-2. [DOI] [PubMed] [Google Scholar]
- Wheeler T. J., Simpson I. A., Sogin D. C., Hinkle P. C., Cushman S. W. Detection of the rat adipose cell glucose transporter with antibody against the human red cell glucose transporter. Biochem Biophys Res Commun. 1982 Mar 15;105(1):89–95. doi: 10.1016/s0006-291x(82)80014-4. [DOI] [PubMed] [Google Scholar]
- Williams S. K., Gillis J. F., Matthews M. A., Wagner R. C., Bitensky M. W. Isolation and characterization of brain endothelial cells: morphology and enzyme activity. J Neurochem. 1980 Aug;35(2):374–381. doi: 10.1111/j.1471-4159.1980.tb06274.x. [DOI] [PubMed] [Google Scholar]