Abstract
INTRODUCTION
Hashimoto’s thyroiditis is the most common form of acquired hypothyroidism. Fine needle aspiration cytology is one important tool in diagnosing Hashimoto’s thyroditis, along with clinical, biochemical, immunological and ultrasonographical modalities. The present study examines cytological aspects of Hashimoto’s thyroiditis along with their correlation with clinical, biochemical and immunological findings, whenever available.
MATERIALS AND METHODS
This is a retrospective study of 50 cases of Hashimoto’s thyroiditis. Cytological findings were reviewed and correlated with clinical, biochemical and immunological findings whenever available.
RESULTS
The majority of the patients were middle-aged females, with a female to male ratio of 6.14:1. Most patients presented with diffuse thyromegaly (68%) and/or hypothyroidism (56.09%).
The antibody profile was available in 22% of patients. Of these, anti-thyroid peroxidase antibodies were raised in 81.81% of patients and anti-thyroglobulin antibodies were raised in 63.63% of patients.
In the present study, high lymphoid to epithelial cell ratio was seen in 78% of cases, and 74% of cases showed Hurthle cell change. Follicular atypia was seen in 36% of cases. Lymphoid follicle formation was seen in seen in 54% of cases.
Follicular cell infiltration by lymphocytes, eosinophils and neutrophils was seen in 72%, 48% and 26% of cases, respectively. Plasma cells were seen in 18% of cases.
CONCLUSION
Thyroid function tests and immunological tests cannot diagnose all cases of Hashimoto’s thyroiditis. Fine needle aspiration cytology continues to be a diagnostic tool of significance in diagnosing Hashimoto’s thyroiditis. The presence of inflammatory cells, particularly lymphocytes and eosinophils, was detected in a significant proportion of cases.
Keywords: Hashimoto’s thyroiditis, cytological findings, thyroid function test, anti-thyroid peroxidase antibody, anti-thyroglobulin antibody
Introduction
Hashimoto’s thyroiditis (HT) was first described in 1912 and is the most common form of thyroiditis.1–2 This is an autoimmune disease that affects women more frequently than men and may be associated with hypothyroidism, euthyroidism or occasionally hyperthyroidism. However, most cases present with hypothyroidism. The most important antibody directed against the thyroid tissue is thyroid peroxidase.3–5
The value of fine needle aspiration cytology (FNAC) and its role in management of thyroid diseases is undisputed. 6 FNAC also helps in preventing unnecessary surgeries in case of thyroiditis.7 FNAC is considered a superior and more cost-effective tool in diagnosing HT than antibody screening.8
Thus the present study aims at studying cytomorphological findings in the patients of HT, and their comparison with other studies and correlation with thyroid function test and antibody profile whenever available.
Materials and Methods
We studied 50 patients, diagnosed as HT (unequivocally), on the basis of fine needle aspiration cytology (FNAC) and close clinical follow-up, between 1.10.2009 to 1.2.2012. All the patients gave written, informed consent to reproduce their information or photographs. The diagnostic criteria used to diagnose HT on FNAC included: lymphocytes and plasma cells infiltrating the thyroid follicles, increased number of lymphocytes in the background with or without lymphoid follicles, Hurthle cell change, multinucleated giant cells, epithelioid cell clusters, anisonucleosis.9 The Hurthle cell is a large (10–15 μ), polygonal cell with distinct cell borders, abundant eosinophilic finely granular cytoplasm, a large hyperchromatic round to oval nucleus, and a prominent nucleolus.10
Thyroid function tests were done using a Competitive Enzyme Immunoassay from Monobind Inc. The normal ranges of T3, T4 and TSH using this method were 0.52–1.85 ng/mL, 4.4–10.8 μg/dL and 0.39–6.16 μIU/mL respectively.
Anti-thyroid peroxidase antibodies and anti-thyroglobulin were determined by means of Microplate Enzyme Immunoassay using Accubind Elisa Microwells from Monobind Inc. Values in excess of 40 IU/mL and 125 IU/mL were considered to be positive for anti-thyroid peroxidase antibodies and anti-thyroglobulin respectively.
Clinical details including age, sex and biochemical findings were tabulated. FNAC smears stained with May—Grünwald—Giemsa (MGG) were reviewed and the following data were recorded: lymphoid:epithelial cell ratio (more than 1:1 was considered high), presence or absence of Hurthle cells, follicular atypia, lymphoid follicle. The percentages of cases showing follicular cell infiltration by lymphocytes, eosinophils, neutrophils and plasma cells were also calculated. Levels of thyroid function test, anti-thyroid peroxidase antibody and anti-thyroglobulin antibody, wherever available, were recorded.
Results
The age of patients who were diagnosed with HT varied from 23 yrs to 49 yrs. The female to male ratio was 6.14:1. The clinical and laboratory findings of HT are summarised in Table 1. The majority of the patients presented with diffuse thyromegaly (68%), and compared with only 32% with nodular presentation.
Table 1.
Clinical and laboratory findings in cases of Hashimoto’s thyroiditis.
| CLINICAL AND LABORATORY FINDINGS | PRESENT STUDY | JAYARAM ET AL 2007(11) | EKAMBARAM M ET AL 2010(12) | MARWAHA RK ET AL 2000(13) | |
|---|---|---|---|---|---|
| 1. | Female: male | 6.14:1 | Not recorded | Not recorded | Only young females were studied |
| 2. | Nodular presentation | 16 (32%) | 33% | Not recorded | Not recorded |
| 3. | Thyroid profile | Available in 41 patients (82%) | Available in 68 patients (77.27%) | Available in 50 patients (100%) | Available in all 43 patients (100%) |
| Hypothyroid | 23 (56.09%) | 27 (39.7%) | 42 (84%) | 20% | |
| Hyperthyroid | 03 (7.31%) | 8 (11.7%) | 3 (06%) | 0% | |
| Euthyroid | 15 (36.58%) | 33 (48.5%) | 5 (10%) | 80% | |
| 4. | Antibody profile | Available in 11 patients (22%) | Available in 29 patients (32.95%) | Available in 40 patients (80%) | Available in 43 patients (100%) |
| Raised thyroid peroxidise antibody | 9 (81.81%) | 27 (93%) | 26 (65%) | 29 (67.4%) | |
| Raised thyroglobulin antibody | 7 (63.63%) | 24 (82.7%) | 26 (65%) | 18 (41.8%) |
The thyroid function tests were available in 82% of patients. The majority of these patients (56.09%) were found to be hypothyroid.
The antibody profile was available in 22% of patients. Anti-thyroid peroxidase antibodies were raised in 81.81% of patients and anti-thyroglobulin were raised in 63.63% of patients (Table 1).
The cytological features of HT are summarised in Table 2 and Figures 1 to 8.
Table 2.
Cytological features of Hashimoto’s thyroiditis.
| CYTOLOGICAL FEATURES | PRESENT STUDY | JAYARAM ET AL 2007(11) | HANDA ET AL 2008(14) | JAYARAM ET AL 1987(15) | FREIDMAN ET AL 1981(16) | KINI ET AL 1981(17) | |
|---|---|---|---|---|---|---|---|
| Number of cases | (n = 50) | (n = 88) | (n = 119) | (n = 40) | (n = 40) | (n = 87) | |
| 1. | Lymphoid:Epithelial cell ratio | High in 39 cases (78%) | High in 38% cases | Not recorded | High | High | High |
| 2. | Hurthle cells | 37 cases (74%) | 56% | (40.3%) | Many | 98% | Variable |
| 3. | Follicular atypia | 18 cases (36%) | 44% | Not recorded | Mild-moderate | Present | Often |
| 4. | Lymphoid follicle | Seen in 27 cases (54%) | 67% | Not recorded | Not recorded | Present | Present |
| 5. | Follicular cell infiltration by lymphocytes | 36 cases (72%) | 69% | Not recorded | Present | Not recorded | Present |
| 6. | Follicular cell infiltration by eosinophils. | 24 cases (48%) | 17% | Not recorded | Not recorded | Not recorded | Not recorded |
| 7. | Follicular cell infiltration by neutrophils | 13 (26%) | 17% | Not recorded | Not recorded | Not recorded | Not recorded |
| 8. | Plasma cells | 9 cases (18%) | 40% | Not recorded | Not recorded | Present | Present |
| 9. | Fire flares | 2 cases (4%) | 23% | 3.4% | 25% | Not recorded | Not recorded |
| 10. | Granuloma | 6 cases (12%) | 16% | Not recorded | 8% | Not recorded | Not recorded |
| 11. | Giant cell | 3 cases (6%) | 39% | 8.4% | 33% | Infrequent | Rare |
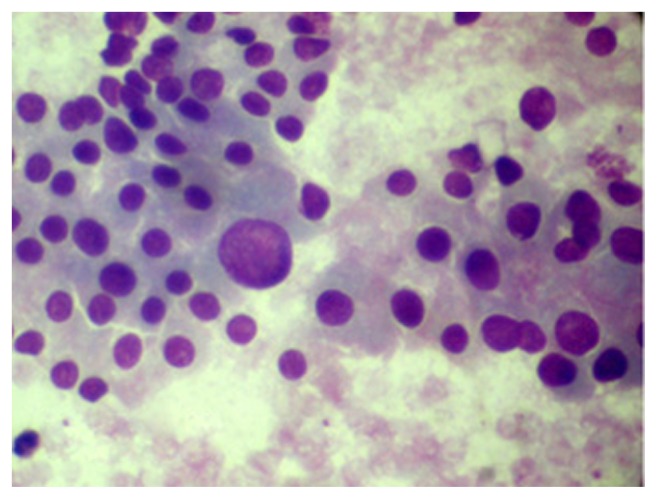
Figure 1.
High lymphoid:epithelial cell ratio (MGG 400X).
Figure 8.
Fire flares in Hashimoto’s thyroiditis (MGG 400X).
In the present study, high lymphoid:epithelial cell (L:E cell) ratio (>1) was seen in 39 patients (78%). Hurthle cell change was seen in 37 cases (74%). Follicular atypia was seen in 18 cases (36%). Lymphoid follicle formation was seen in 27 cases (54%).
Follicular cell infiltration by lymphocytes, eosinophils, neutrophils or plasma cells was seen in 36 cases (72%), 24 cases (48%), 13 (26%) and 9 cases (18%), respectively (Table 2).
Discussion and Conclusion
The present study shows that HT is more common in the female population. Eranga Himalee Siriweera et al also reported a female male ratio of 10.3:1 in cases of HT.18
The age group of patients who were diagnosed with HT varied from 23 yrs to 49 yrs.
Only 32% presented with nodular presentation. Jayaram et al 2007 also reported nodular presentation in 33% of patients with HT.11
Thyroid function tests were available in 41 patients (82%) and revealed that the majority of these patients were hypothyroid (56.09%). In the study done by Ekambaram M et al on 50 patients with HT,12 thyroid profile was available for all 50 patients. In their study, 84% of patients were hypothyroid. In our study also, a majority of the patients for whom thyroid function test results were available presented with hypothyroidism.
The antibody profile was available in 22% of patients. Thyroid peroxidase antibodies (TPO Ab) were raised in 81.81% of patients and thyroglobulin antibodies (TG Ab) were raised in 63.63% of patients. This is in concordance with the fact that TPO Ab are considered to be more specific than TG Ab. However, sero-negative cases of HT can be explained on the basis of localised antibody production by intrathyroidal lymphocytes.19–21 Jayaram et al 2007 and Marwaha RK et al 2000 also concluded that TPO Ab are more specific than TG Ab in diagnosing HT on cytology smears.11,13
The cytological findings of the current study are compared with other studies in Table 2.
High L:E cell ratio was seen in the present study in 78% of cases. Earlier studies done on HT also show the presence of high L:E cell ratio.11,15–17
Hurthle cell change was seen in 74%, which is higher than that seen in other studies. Friedman et al 1980 also reported a markedly high percentage of cases showing Hurthle cell change.16 Data in this study on follicular atypia and follicular cell infiltration by lymphocytes were comparable with the study of Jayaram et al 2007.11
Follicular cell infiltration by eosinophils was seen in 48% of cases. Ekambaram M et al studied 50 cases of HT and counted the average number of eosinophils in 10 high power fields (HPF).12 They found eosinophils in 42 cases (84%). They concluded that eosinophilic infiltration of the thyroid gland has higher association with HT.
Data here on follicular cell infiltration by neutrophils, seen in 13 cases (26%), are also concordant with Jayaram et al 2007.11 However, plasma cells, fire flares, granuloma and giant cells are seen here in very few cases when compared with Jayaram et al.11 One case with fire flares revealed hyperthyroidism on thyroid profile.
Conclusion
Thyroid function tests and immunological tests cannot diagnose all cases of Hashimoto’s thyroiditis. Fine needle aspiration cytology continues to be a tool of significance in diagnosing Hashimoto’s thyroiditis, especially in developing countries. However, in our study the presence of lymphocytes, neutrophils and eosinophils was found in a significant proportion of cases in comparison to previous studies. Emphasis should be placed on the cytological features of HT in cases of doubt, so that in future new aspects can be discovered to improve upon the efficacy of FNAC in diagnosing HT.
Figure 2.
Folliclular epithelial cells infiltrated by lymphocytes (MGG-400X).
Figure 3.
Hurthle cell change with sudden anisocytosis (MGG 400X).
Figure 4.
Granuloma in Hashimoto’s thyroiditis (MGG-400X).
Figure 5.
Giant cell in case of Hashimoto’s thyroiditis (MGG 400X).
Figure 6.
Eosinophils and neutrophils in Hashimoto’s thyroiditis (MGG 400X).
Figure 7.
Plasma cells in Hashimoto’s thyroiditis (MGG-400X).
Acknowledgements
We are thankful to Jayaram G, Iyengar K R, Sthaneshwar P, Hayati J N for their guidance. We are also thankful to Engineer AYUSH for his technical help.
Footnotes
COMPETING INTERESTS: Author(s) disclose no potential conflicts of interest.
Author Contributions
Conceived and designed the experiments: MR, FA, SKB, SA, AK, SD. Analysed the data: MR, FA, SKB, SA, AK, SD. Wrote the first draft of manuscript: MR. Contributed to the writing of the manuscript: MR, FA, SKB, SA, AK, SD. Agree with manuscript results and conclusions: MR, FA, SKB, SA, AK, SD. Jointly developed the structure and arguments for the paper: MR, FA, SKB, SA, AK, SD. Made critical revisions and approved the final version: MR, FA, SKB, SA, AK, SD. All the authors reviewed and approved of the final manuscript.
DISCLOSURES AND ETHICS
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests.
FUNDING: Author(s) disclose no funding sources.
REFERENCES
- 1.Kini SR. Thyroiditis. In: Kini SR, editor. Thyroid Cytopathology. first edition. Riverwoods, Illinois: Lippincott Williams and Wilkins; 2008. pp. 294–5. [Google Scholar]
- 2.Hashimoto H. Zur Kenntnis der Lymphomatosen Veranderungen der Schilddruse (Struma Lymphomatosa) Arch Klin Chir. 1912;97:219. [Google Scholar]
- 3.LiVolsi V. Surgical Pathology of Thyroid Major Problems in Pathology. Vol. 22. Philadelphia: WB Saunders; 1990. [Google Scholar]
- 4.Doniach I. The Thyroid Gland. In: Symmers WStC., editor. Systemic Pathology. Second edition. Vol. 4. Edinburgh: Churchill Livingstone; 1978. pp. 1975–2037. [Google Scholar]
- 5.Pearce EN, Farewell AP, Braveran E. Thyroiditis. N Engl J Med. 2003;348:2646. doi: 10.1056/NEJMra021194. [DOI] [PubMed] [Google Scholar]
- 6.Wong CK, Wheeler MH. Thyroid nodules: rational management. World J Surg. 2000;24:934–41. doi: 10.1007/s002680010175. [DOI] [PubMed] [Google Scholar]
- 7.Suen KC, Quenville NF. Fine needle aspiration biopsy of the thyroid gland: a study of 304 cases. J Clin Pathol. 1983;36:1036–45. doi: 10.1136/jcp.36.9.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poropatich, Marcus D, Oertel YC. Hashimoto’s Thyroiditis: Fine needle aspirations of 50 asymptomatic cases. Diagn Cytopathol. 1994;11:141–5. doi: 10.1002/dc.2840110207. [DOI] [PubMed] [Google Scholar]
- 9.Bhatia A, Rajwanshi A, Dash RJ, Mittal BR, Saxena AK. Lymphocytic thyroiditis—is cytological grading significant? A correlation of grades with clinical, biochemical, ultrasonographic and radionuclide parameters. Cytojournal. 2007;4:10. doi: 10.1186/1742-6413-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cannon J. The Significance of Hürthle Cells in Thyroid Disease. Oncologist. 2011 Oct;16:1380–7. doi: 10.1634/theoncologist.2010-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jayaram G, Iyengar KR, Sthaneshwar P, Hayati JN. Hashimoto’s thyroiditis—A Malaysian perspective. J Cytol. 2007;24:119–24. [Google Scholar]
- 12.Ekambaram M, Kumar B, Chowdhary N, Siddaraju N, Kumar S. Significance of eosinophils in diagnosing Hashimoto’s thyroiditis on fine-needle aspiration cytology. Indian J Pathol Microbiol. 2010;53:476–9. doi: 10.4103/0377-4929.68282. [DOI] [PubMed] [Google Scholar]
- 13.Marwaha RK, Tandon N, Karak AK, Gupta N, Verma K, Kochupillai N. Hashimoto’s thyroiditis: Countrywide screening of goitrous healthy young girls in postiodization phase in India. J Clin Endocrinol Metab. 2000;85:3798–802. doi: 10.1210/jcem.85.10.6924. [DOI] [PubMed] [Google Scholar]
- 14.Handa U, Garg S, Mohan H, Nagarkar N. Role of fine needle aspiration cytology in diagnosis and management of thyroid lesions: A study on 434 patients. J Cytol. 2008;25:13–7. [Google Scholar]
- 15.Jayaram G, Marwaha RK, Gupta RK, Sharma SK. Cytomorphologic aspects of thyroiditis—A study of 51 cases with functional, immunologic and ultrasonographic data. Acta Cytol. 1987;31:687–93. [PubMed] [Google Scholar]
- 16.Friedman M, Shimaoka K, Rao U, Tsukada Y, Gavigan M, Tamura K. Diagnosis of chronic lymphocytic thyroiditis (nodular presentation) by needle aspiration. Acta Cytol. 1981;25:513–22. [PubMed] [Google Scholar]
- 17.Kini SR, Miller JM, Hamburger JI. Problems in the cytologic diagnosis of the “cold” thyroid nodule in patients with lymphocytic thyroiditis. Acta Cytol. 1981;25:506–12. [PubMed] [Google Scholar]
- 18.Siriweera EH, Ratnatunga NV. Profile of Hashimoto’s Thyroiditis in Sri Lankans: Is There an Increased Risk of Ancillary Pathologies in Hashimoto’s Thyroiditis? Journal of Thyroid Research. 2010 doi: 10.4061/2010/124264. Article ID 124264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amino N. Thyroid directed antibodies. In: Ingbar SH, Braverman LE, editors. Werner’s the thyroid—a fundamental and clinical text. Philadelphia: J B Lippincott Co; 1986. pp. 546–59. [Google Scholar]
- 20.Fisher DA, Oddie TH, Johnson DE, Nelson JC. The diagnosis of Hashimoto’s Thyroiditis. J Clin Endocrinol Metab. 1975;40:795–801. doi: 10.1210/jcem-40-5-795. [DOI] [PubMed] [Google Scholar]
- 21.Baker JR, Jr, Saunders NB, Wartofsky L, Tseng YL, Burman KD. Seronegative Hashimoto’s thyroiditis with thyroid autoantibody production localized to the thyroid. Ann Intern Med. 1988;108:26–30. doi: 10.7326/0003-4819-108-1-26. [DOI] [PubMed] [Google Scholar]