Abstract
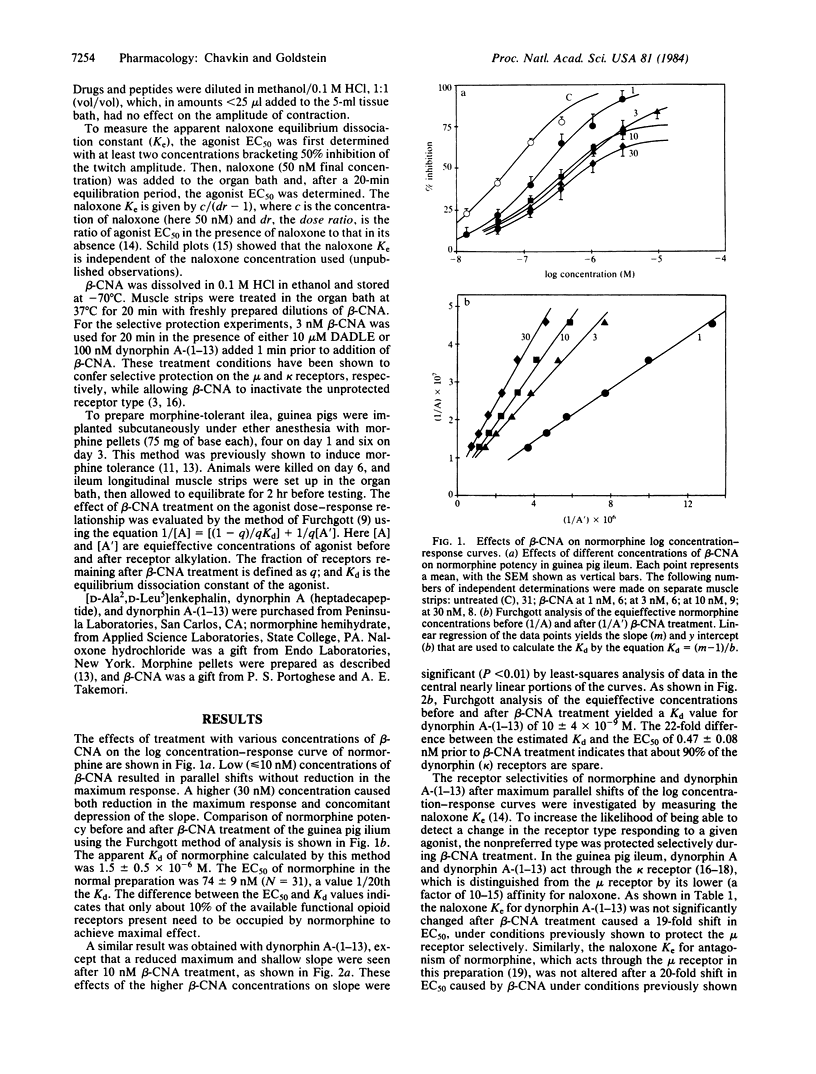
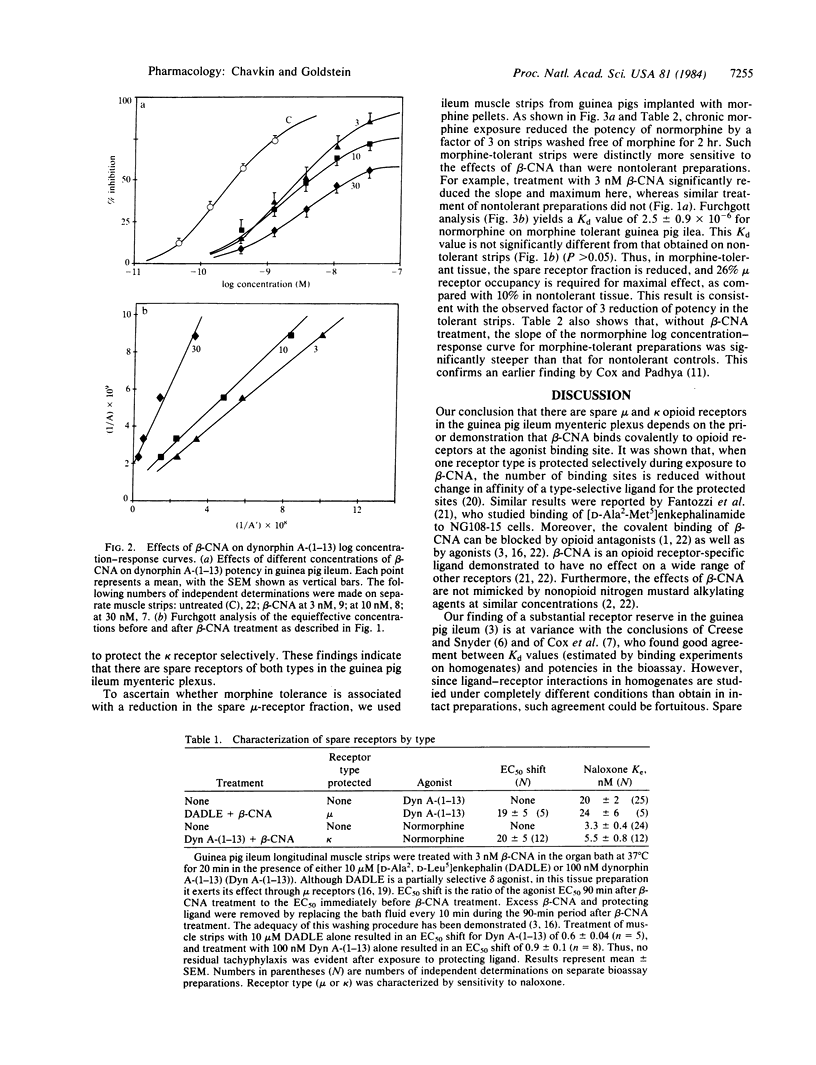
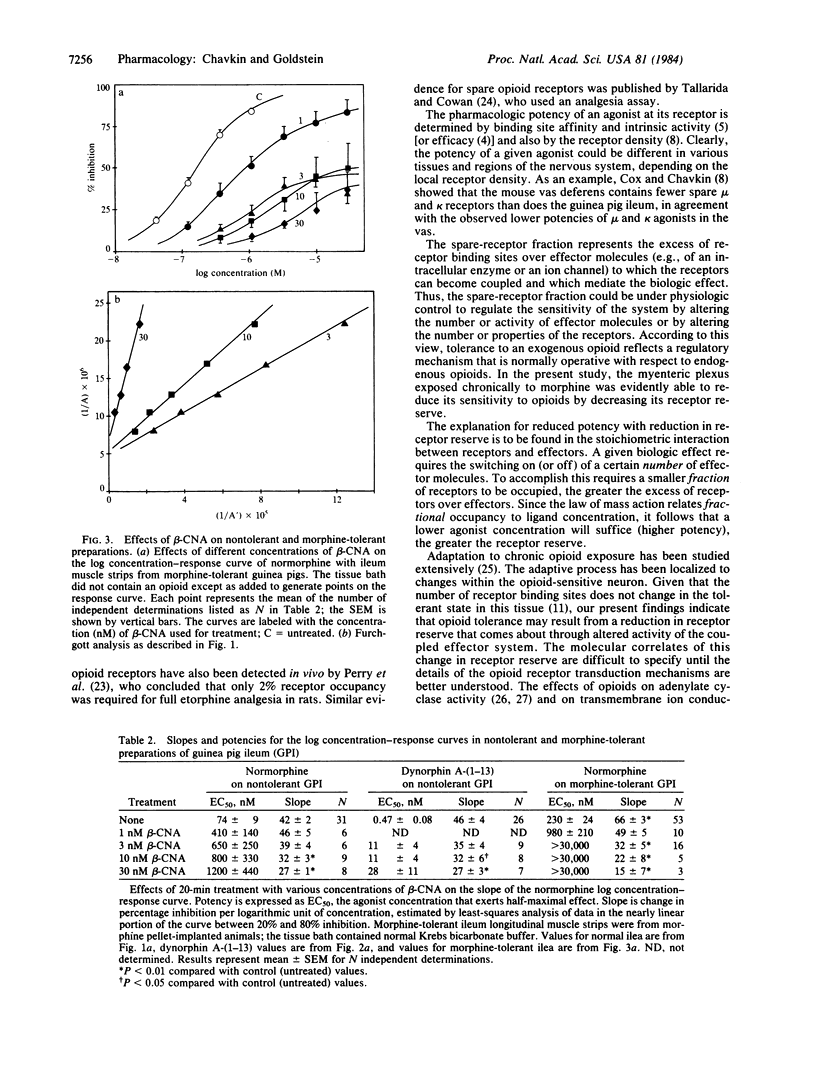
We have measured the opioid receptor reserve in the guinea pig ileum myenteric plexus by means of the site-directed alkylating agent, beta-chlornaltrexamine. Treatment of the tissue with low (less than 10 nM) concentrations of beta-chlornaltrexamine caused a parallel shift of the log concentration-response curves for both normorphine and dynorphin A-(1-13). Analysis of the resulting curves indicated that the Kd values were 1.5 +/- 0.5 X 10(-6) and 10 +/- 4 X 10(-9), respectively. Using the naloxone Ke to distinguish between the mu and kappa receptors in this tissue, we found that the receptor selectivities of normorphine and dynorphin A-(1-13) were unchanged after a maximum parallel shift, thus demonstrating that there are both spare mu and spare kappa receptors present. The spare-receptor fraction for both receptor types was about 90%. In morphine-tolerant preparations (chronic pellet implantation), there was an apparent reduction in the fraction of spare mu receptors without any change in the apparent affinity of normorphine. Reduction in the spare receptor fraction does not necessarily imply reduction in the number of binding sites. We suggest that this reduction in receptor reserve is the basis of opioid tolerance, since the agonist concentration needed to produce a given effect is expected to increase as the receptor reserve decreases.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARIENS E. J., van ROSSUM J., KOOPMAN P. C. Receptor reserve and threshold phenomena. I. Theory and experiments with autonomic drugs tested on isolated organs. Arch Int Pharmacodyn Ther. 1960 Sep 1;127:459–478. [PubMed] [Google Scholar]
- Caruso T. P., Larson D. L., Portoghese P. S., Takemori A. E. Pharmacological studies with an alkylating narcotic agonist, chloroxymorphamine, and antagonist, chlornaltrexamine. J Pharmacol Exp Ther. 1980 Jun;213(3):539–544. [PubMed] [Google Scholar]
- Caruso T. P., Takemori A. E., Larson D. L., Portoghese P. S. Chloroxymorphamine, and opioid receptor site-directed alkylating agent having narcotic agonist activity. Science. 1979 Apr 20;204(4390):316–318. doi: 10.1126/science.86208. [DOI] [PubMed] [Google Scholar]
- Chavkin C., Goldstein A. Demonstration of a specific dynorphin receptor in guinea pig ileum myenteric plexus. Nature. 1981 Jun 18;291(5816):591–593. doi: 10.1038/291591a0. [DOI] [PubMed] [Google Scholar]
- Chavkin C., Goldstein A. Reduction in opiate receptor reserve in morphine tolerant guinea pig ilea. Life Sci. 1982 Oct 18;31(16-17):1687–1690. doi: 10.1016/0024-3205(82)90186-2. [DOI] [PubMed] [Google Scholar]
- Chavkin C., James I. F., Goldstein A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science. 1982 Jan 22;215(4531):413–415. doi: 10.1126/science.6120570. [DOI] [PubMed] [Google Scholar]
- Collier H. O. Cellular site of opiate dependence. Nature. 1980 Feb 14;283(5748):625–629. doi: 10.1038/283625a0. [DOI] [PubMed] [Google Scholar]
- Collier H. O., Roy A. C. Morphine-like drugs inhibit the stimulation of E prostaglandins of cyclic AMP formation by rat brain homogenate. Nature. 1974 Mar 1;248(5443):24–27. doi: 10.1038/248024a0. [DOI] [PubMed] [Google Scholar]
- Cox B. M., Chavkin C. Comparison of dynorphin-selective Kappa receptors in mouse vas deferens and guinea pig ileum. Spare receptor fraction as a determinant of potency. Mol Pharmacol. 1983 Jan;23(1):36–43. [PubMed] [Google Scholar]
- Cox B. M., Padhya R. Opiate binding and effect in ileum preparations from normal and morphine pretreated guinea-pigs. Br J Pharmacol. 1977 Oct;61(2):271–278. doi: 10.1111/j.1476-5381.1977.tb08415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese I., Snyder S. H. Receptor binding and pharmacological activity of opiates in the guinea-pig intestine. J Pharmacol Exp Ther. 1975 Jul;194(1):205–219. [PubMed] [Google Scholar]
- Fantozzi R., Mullikin-Kilpatrick D., Blume A. J. Irreversible inactivation of the opiate receptors in the neuroblastoma x glioma hybrid NG108-15 by chlornaltrexamine. Mol Pharmacol. 1981 Jul;20(1):8–15. [PubMed] [Google Scholar]
- Goldstein A., Schulz R. Morphine-tolerant longitudinal muscle strip from guinea-pig ileum. Br J Pharmacol. 1973 Aug;48(4):655–666. doi: 10.1111/j.1476-5381.1973.tb08254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James I. F., Chavkin C., Goldstein A. Preparation of brain membranes containing a single type of opioid receptor highly selective for dynorphin. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7570–7574. doi: 10.1073/pnas.79.23.7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James I. F., Fischli W., Goldstein A. Opioid receptor selectivity of dynorphin gene products. J Pharmacol Exp Ther. 1984 Jan;228(1):88–93. [PubMed] [Google Scholar]
- Lord J. A., Waterfield A. A., Hughes J., Kosterlitz H. W. Endogenous opioid peptides: multiple agonists and receptors. Nature. 1977 Jun 9;267(5611):495–499. doi: 10.1038/267495a0. [DOI] [PubMed] [Google Scholar]
- North R. A., Williams J. T. Opiate activation of potassium conductance inhibits calcium action potentials in rat locus coeruleus neurones. Br J Pharmacol. 1983 Oct;80(2):225–228. doi: 10.1111/j.1476-5381.1983.tb10023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry D. C., Rosenbaum J. S., Kurowski M., Sadée W. [3H]Etorphine receptor binding in vivo. Small fractional occupancy elicits analgesia. Mol Pharmacol. 1982 Mar;21(2):272–279. [PubMed] [Google Scholar]
- Porreca F., Burks T. F. Affinity of normorphine for its pharmacologic receptor in the naive and morphine-tolerant guinea-pig isolated ileum. J Pharmacol Exp Ther. 1983 Jun;225(3):688–693. [PubMed] [Google Scholar]
- Portoghese P. S., Larson D. L., Jiang J. B., Caruso T. P., Takemori A. E. Synthesis and pharmacologic characterization of an alkylating analogue (chlornaltrexamine) of naltrexone with ultralong-lasting narcotic antagonist properties. J Med Chem. 1979 Feb;22(2):168–173. doi: 10.1021/jm00188a008. [DOI] [PubMed] [Google Scholar]
- STEPHENSON R. P. A modification of receptor theory. Br J Pharmacol Chemother. 1956 Dec;11(4):379–393. doi: 10.1111/j.1476-5381.1956.tb00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R., Wüster M., Krenss H., Herz A. Lack of cross-tolerance on multiple opiate receptors in the mouse vas deferens. Mol Pharmacol. 1980 Nov;18(3):395–401. [PubMed] [Google Scholar]
- Sharma S. K., Klee W. A., Nirenberg M. Dual regulation of adenylate cyclase accounts for narcotic dependence and tolerance. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3092–3096. doi: 10.1073/pnas.72.8.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallarida R. J., Cowan A. The affinity of morphine for its pharmacologic receptor in vivo. J Pharmacol Exp Ther. 1982 Jul;222(1):198–201. [PubMed] [Google Scholar]
- Werz M. A., MacDonald R. L. Opioid peptides selective for mu- and delta-opiate receptors reduce calcium-dependent action potential duration by increasing potassium conductance. Neurosci Lett. 1983 Dec 2;42(2):173–178. doi: 10.1016/0304-3940(83)90402-0. [DOI] [PubMed] [Google Scholar]
- Wüster M., Rubini P., Schulz R. The preference of putative pro-enkephalins for different types of opiate receptors. Life Sci. 1981 Sep 21;29(12):1219–1227. doi: 10.1016/0024-3205(81)90226-5. [DOI] [PubMed] [Google Scholar]


