Abstract
Thiamine deficiency has been documented to be prevalent in patients with diabetes mellitus, and correction of thiamine deficiency in this population may provide beneficial effects in several cardiometabolic parameters, including prevention of impending complications secondary to chronic hyperglycemia. In this interventional study, we aim to determine whether thiamine supplementation is associated with cardiometabolic improvements in patients with diabetes mellitus type 2 (DMT2). A total of 86 subjects (60 DMT2 and 26 age- and BMI-matched controls) were included and were given thiamine supplements (100 mg/day) for six months. Anthropometrics and metabolic profiles were measured routinely. Serum thiamine and its derivatives were measured using high performance liquid chromatography. In all groups, there was a significant decrease in total cholesterol after three months (p = 0.03) as well as in HDL cholesterol after six months of thiamine supplementation (p = 0.009). Significant improvements were also observed in the mean serum levels of creatinine (p = 0.001), as well as thiamine and its derivatives in both serum and urinary levels across follow-up visits (p-values 0.002 and <0.001, respectively). In the DMT2 group, improvements were observed in lipid profile (mean serum LDL and total cholesterol with p-values 0.008 and 0.006, respectively), serum thiamine (p < 0.001), TMP (p < 0.001), TDP (p < 0.001), urinary thiamine (p < 0.001) and serum creatinine (p < 0.001). Thiamine supplementation is a promising adjuvant therapy for patients with DMT2. Longer clinical trials are needed to determine its protective effect in DMT2 complications.
Keywords: thiamine, diabetes mellitus type 2, Saudi, supplements
Introduction
As of 2011, the overall prevalence of diabetes mellitus type 2 (DMT2) in the kingdom of Saudi Arabia (KSA) is 31.6%.1 In terms of complications, the prevalence of diabetic retinopathy in KSA is 31% as of 1998.2 Microvascular diseases (nephropathy, retinopathy and neuropathy) develop in most diabetic patients over time and manifest within a period of 10–15 years. These are common and debilitating complications of DM with no effective therapy. Diabetic nephropathy is characterized by the development of proteinuria, culminating in end-stage renal disease (ESRD) with a particularly high risk of cardiovascular morbidity and mortality. The initial stage of development of nephropathy, incipient nephropathy, is characterized by the onset of persistent microalbuminuria and hyperfiltration.3 Over the years the mechanisms of hyperglycemia leading to diabetes complications, specifically nephropathy, have been deciphered, but not totally. Nevertheless, current knowledge suggests that certain processes initiate and sustain cellular dysfunction in microvascular complications: hyperfiltration, renal mesangial cell expansion, basement membrane thickening, nephrosclerosis and podocyte loss in diabetic nephropathy, loss of pericytes, vessel weakening and endothelial dysfunction in diabetic retinopathy, and endothelial dysfunction and hypoxia in endoneural microvessels in diabetic neuropathy.4 A strategy to reverse the underlying hyperglycemia could alleviate multiple pathways of biochemical dysfunction. Whilst there are several biomarkers related to diabetes and its complications, targeting thiamine levels through high dose thiamine therapy may be the way to achieve this goal. We have recently documented that Saudi patients with both DM type 1 and type 2 have low levels of circulating thiamine, and the presence of such diseases is related to increased thiamine clearance.5 In this follow-up study, we aim to determine whether oral thiamine supplementation for up to 6 months can increase thiamine levels and improve cardiometabolic parameters.
Materials and Methods
Study design and settings
This is a comparative six-month prospective study in which Saudi patients with DMT2, aged 1–65 years old, have been selected randomly from subjects who participated in the ongoing “Biomarker Screening” study of the Research Center for Biomarkers Research Laboratory in King Saud University, Riyadh, Saudi Arabia. Ethical approval was obtained from the College of Medicine and Research Center (CMRC) in King Khalid University Hospital, King Saud University Hospital, Riyadh, Saudi Arabia. The study was also conducted in accordance with the guidelines set by the International Committee of Medical Journal Editors (ICMJE) for intervention studies.
Subjects
A total of 60 patients with DMT2, plus 26 age- and BMI-matched controls, (total N = 86) were randomly selected from the master database of the Biomarkers Research Program (BRP). They have been recruited previously from a cross-sectional study assessing circulating levels of thiamine by high performance liquid chromatography (HPLC).5 Subjects were given Thiavit® (Thiamine hydrochloride) (Julphar Industries, UAE) 100 mg daily for six months and were monitored regularly for compliance.
Inclusion criteria
Study groups matched for age (1–65 years), sex, diabetes duration (five or more years), HbA1c (<10%) and body mass index (19–40 kg/m2). All subjects reported no change in medications during the entire study.
Exclusion criteria
Patients with end stage renal disease (creatinine clearance <10 mL/min), with significant co-morbidities, known allergy or intolerance to thiamine, patients who have been taking thiamine supplements or goldenseal (herbal supplements), patients participating in an interventional study within 30 days, patients with renal and/or pancreatic transplant, women who are pregnant or breast feeding and women of child-bearing potential not using adequate contraceptive precautions were excluded.
Blood analysis
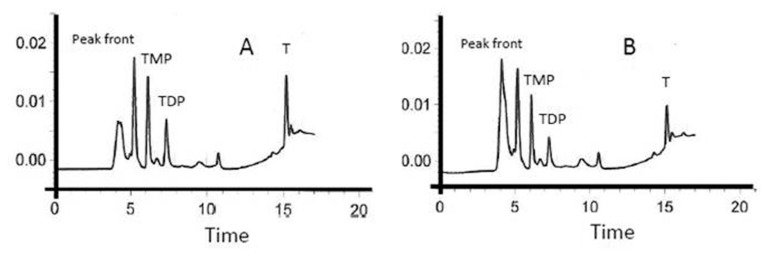
A detailed methodology has been described previously.5 In brief, fasting blood samples were collected and anthropometrics measured. Fasting blood, lipid and renal profile were determined routinely. LDL-cholesterol was calculated using the Friedwald equation. Blood thiamine concentration, its phosphate esters and urine thiamine were quantified using HPLC.5 Recovery studies were conducted after fortification to 0.002 and 0.01 mg L−1. All spiked samples gave satisfactory recovery rates for the target analytes (see Fig. 1). The recovery values ranged from 91% to 112% for urine and from 95% to 115% for blood, respectively. The measurements were repeated after the third and sixth months of supplementation.
Figure 1.
Thiamine (T), Thiamine monophosphate (TMP), and Thiamine diphosphate (TDP chromatograms obtained from A) Blood samples fortified with T, TMP and TDP, and B) Subjects’ blood samples (5).
Data analysis
Data was analyzed using SPSS version 16.5 (SPSS, Chicago, IL, USA). Continuous variables were presented as mean ± standard deviation. The generalized linear model (GLM) was used to compare differences of variables over time. Statistically significant follow-up differences or effects were determined using the Bonferroni multiple comparison test. Level of significance was set at p < 0.05.
Results
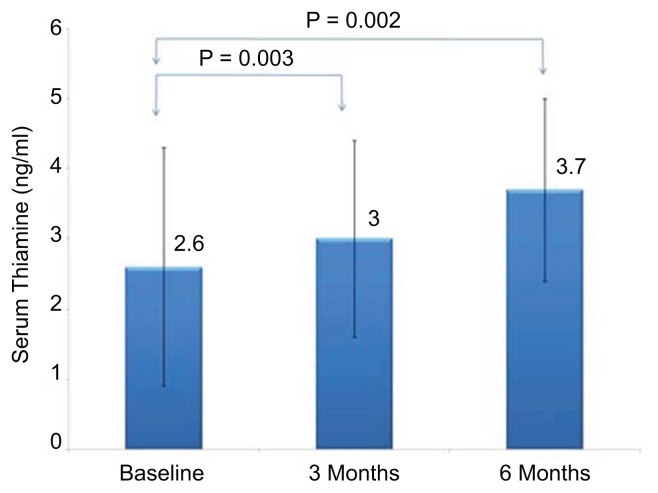
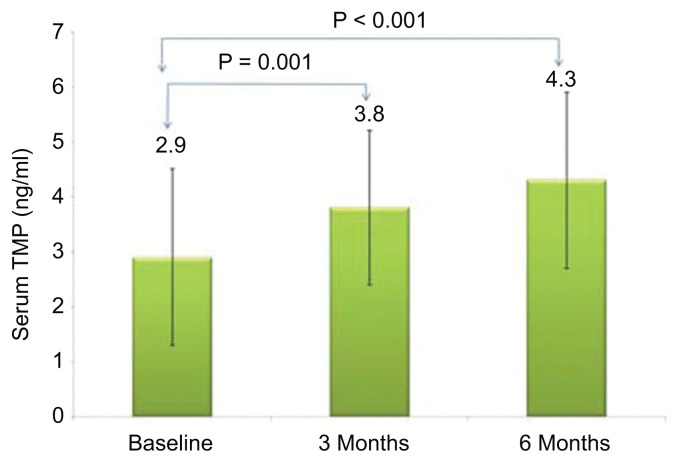
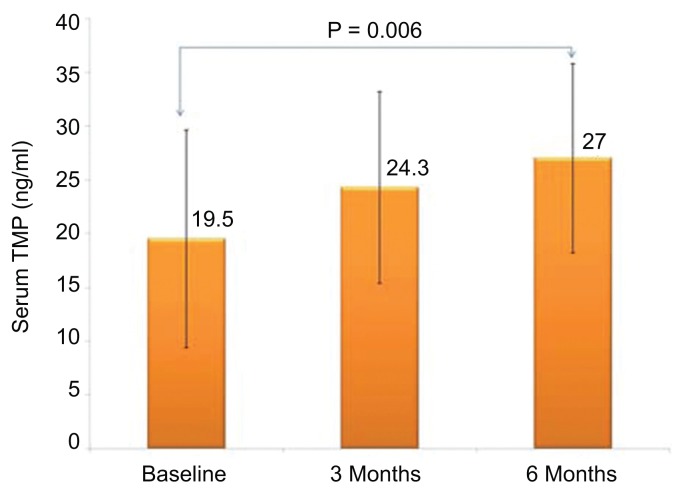
Figure 1 shows the chromatogram of thiamine and its derivatives in both fortified and subjects’ samples. Table 1 shows the clinical profile of all subjects according to follow up. Comparisons showed significant decrease in total cholesterol after three months (p = 0.03) as well as HDL-cholesterol after six months of thiamine supplementation (p = 0.009). Significant improvements were also observed in the mean serum levels of creatinine (p = 0.001). With regards to thiamine and its derivatives, there was a significant increase in both serum and urinary thiamine levels across follow-up visits (p-values 0.002 and <0.001, respectively; see Fig. 2), and this was in parallel with the increased mean levels of serum TDP and TMP (p-values 0.009 and <0.001, respectively; see Figs. 3 and 4). No significant changes were observed among the anthropometric indices, and borderline significant variations were noted in mean serum levels of albumin and calcium (p-values 0.07 and 0.09, respectively; see Table 1). We then divided the subjects according to presence of DMT2. Mean serum thiamine was not significantly different between control and DMT2 groups (not shown in table). Table 2 shows the clinical and metabolic parameters of control subjects according to various time points. Significant improvement was seen in the mean serum creatinine levels (p < 0.001) during the 1st and 2nd follow-up as compared to baseline and this was in parallel with the significantly increased urinary excretion of thiamine (p < 0.001). The rest of the parameters were not significantly different from one another. Table 3 shows the comparisons among the DMT2 subjects. As opposed to the control subjects, more improvements were observed in terms of lipid profile (mean serum LDL- and total cholesterol, with p-values 0.008 and 0.006, respectively), serum thiamine (p < 0.001), TMP (p < 0.001), TDP (p < 0.001), urinary thiamine (p < 0.001) and serum creatinine (p < 0.001). Other parameters demonstrated no significant changes over time.
Table 1.
General characteristics of all subjects.
| BASELINE | 3 MONTHS | 6 MONTHS | P VALUE | |
|---|---|---|---|---|
| N | 86 | |||
| Age (years) | 51.8 ± 10.9 | |||
| M/F | 54/33 | |||
| BMI (kg/m2) | 31.0 ± 5.7 | 31.3 ± 6.1 | 30.1 ± 5.3 | 0.38 |
| Systolic BP (mmHg) | 123.4 ± 16.1 | 126.6 ± 17.8 | 127.7 ± 18.2 | 0.24 |
| Diastolic BP (mmHg) | 76.7 ± 12.7 | 77.3 ± 13.0 | 78.1 ± 13.8 | 0.78 |
| Waist (cm) | 95.5 ± 18.6 | 93.8 ± 17.5 | 94.6 ± 18.0 | 0.91 |
| Hips (cm) | 109.5 ± 15.0 | 107.8 ± 14.9 | 107.3 ± 14.5 | 0.75 |
| Glucose (mmol/l) | 8.3 ± 3.9 | 7.1 ± 2.9 | 8.3 ± 4.1 | 0.34 |
| Triglycerides (mmol/l) | 1.7 ± 0.72 | 1.6 ± 0.62 | 1.5 ± 0.67 | 0.43 |
| Total cholesterol (mmol/l) | 5.3 ± 1.0 | 4.6 ± 1.2a | 4.9 ± 1.0 | 0.03 |
| LDL-cholesterol (mmol/l) | 3.9 ± 1.0 | 3.2 ± 0.96a | 3.8 ± 1.1b | 0.006 |
| HDL-cholesterol (mmol/l) | 1.1 ± 0.27 | 1.2 ± 0.38 | 0.81 ± 0.58a,b | 0.009 |
| TDP (ng/ml) | 19.5 ± 10.1 | 24.3 ± 8.9 | 27.0 ± 8.8a | 0.009 |
| TMP (ng/ml) | 2.9 ± 1.6 | 3.8 ± 1.4a | 4.3 ± 1.6a | <0.001 |
| Serum thiamine (ng/ml) | 2.6 ± 1.7 | 3.0 ± 1.4 | 3.7 ± 1.3a,b | 0.002 |
| Serum albumin (g/l) | 35.5 ± 4.8 | 37.0 ± 5.7 | 32.4 ± 6.8 | 0.07 |
| Serum creatinine (umol/l) | 120.0 ± 26.6 | 96.3 ± 24.0a | 88.0 ± 18.6a | 0.001 |
| Thiamine urine (ng/ml) | 924.8 ± 95.6 | 1097.9 ± 274.3a | 2401.5 ± 323.6a,b | <0.001 |
Notes: Data represented by Mean ± standard deviation;
denotes significance as compared to baseline;
denotes significance as compared to 3 months; Level of significance is given at p ≤ 0.05.
Figure 2.
Serum thiamine according to follow-up visits (all groups).
Figure 3.
Serum thiamine monophosphate according to follow-up visits (all groups).
Figure 4.
Serum thiamine diphosphate according to follow-up visits (all groups).
Table 2.
Characteristics of control subjects according to visit.
| CONTROL | BASELINE | 3 MONTHS | 6 MONTHS | P VALUE |
|---|---|---|---|---|
| N | 26 | 26 | 26 | |
| Age (years) | 50.7 ± 12.4 | |||
| M/F | 16/10 | |||
| BMI (kg/m2) | 30.2 ± 5.6 | 32.6 ± 5.4 | 29.8 ± 4.3 | 0.14 |
| Systolic BP (mmHg) | 120.6 ± 14.3 | 121.3 ± 13.9 | 123.6 ± 15.3 | 0.73 |
| Diastolic BP (mmHg) | 75.1 ± 11.0 | 76.8 ± 13.2 | 76.8 ± 13.5 | 0.85 |
| Waist (cm) | 93.6 ± 15.2 | 93.2 ± 16.3 | 94.0 ± 17.7 | 0.98 |
| Hips (cm) | 106.8 ± 13.2 | 106.9 ± 13.8 | 107.3 ± 14.0 | 0.97 |
| Glucose (mmol/l) | 5.3 ± 0.95 | 5.2 ± 1.9 | 5.3 ± 0.68 | 0.98 |
| Triglycerides (mmol/l) | 1.3 ± 0.54 | 1.4 ± 0.69 | 1.4 ± 0.56 | 0.79 |
| Total cholesterol (mmol/l) | 5.2 ± 0.67 | 4.7 ± 1.5 | 5.4 ± 1.0 | 0.37 |
| LDL-cholesterol (mmol/l) | 3.5 ± 0.88 | 3.0 ± 1.5 | 3.7 ± 1.1 | 0.31 |
| HDL-cholesterol (mmol/l) | 1.2 ± 0.47 | 1.3 ± 0.71 | 1.4 ± 0.52 | 0.68 |
| TDP (ng/ml) | 23.8 ± 13.4 | 23.0 ± 9.0 | 25.9 ± 8.5 | 0.72 |
| TMP (ng/ml) | 3.0 ± 1.4 | 3.6 ± 0.95 | 3.6 ± 1.1 | 0.19 |
| Serum thiamine (ng/ml) | 2.5 ± 0.92 | 2.8 ± 0.88 | 3.0 ± 1.3 | 0.36 |
| Serum albumin (g/l) | 36.2 ± 3.0 | 33.5 ± 4.3 | 34.8 ± 6.3 | 0.39 |
| Serum creatinine (umol/l) | 116.1 ± 25.3 | 92.8 ± 24.6 | 87.2 ± 23.6a,b | <0.001 |
| Thiamine urine (ng/ml) | 968.8 ± 268.7 | 1678.6 ± 298.8a | 2269.7 ± 312.9a,b | <0.001 |
Notes: Data represented by Mean ± standard deviation;
denotes significance as compared to baseline;
denotes significance as compared to 3 months; Level of significance is given at p ≤ 0.05.
Table 3.
Characteristics of DMT2 subjects according to visit.
| BASELINE | 3 MONTHS | 6 MONTHS | P VALUE | |
|---|---|---|---|---|
| N | 60 | 60 | 60 | |
| Age (years) | 52.1 ± 10.6 | |||
| M/F | 39/21 | |||
| BMI (kg/m2) | 31.1 ± 5.8 | 31.1 ± 6.4 | 30.5 ± 5.5 | 0.81 |
| Systolic BP (mmHg) | 126.4 ± 17.6 | 129.9 ± 18.6 | 130.2 ± 18.7 | 0.45 |
| Diastolic BP (mmHg) | 77.6 ± 12.9 | 78.6 ± 14.6 | 79.6 ± 15.0 | 0.74 |
| Waist (cm) | 96.6 ± 18.7 | 95.6 ± 17.8 | 95.0 ± 18.1 | 0.90 |
| Hips (cm) | 110.2 ± 16.3 | 109.8 ± 16.4 | 108.6 ± 16.1 | 0.85 |
| Glucose (mmol/l) | 8.8 ± 3.9 | 8.3 ± 3.6 | 8.8 ± 3.6 | 0.68 |
| Triglycerides (mmol/l) | 2.0 ± 0.97 | 1.7 ± 0.89 | 2.0 ± 1.2 | 0.41 |
| Total cholesterol (mmol/l) | 5.4 ± 1.2 | 4.9 ± 1.2 | 4.8 ± 0.89a | 0.006 |
| LDL-cholesterol (mmol/l) | 4.0 ± 1.2 | 3.7 ± 1.1 | 3.4 ± 0.81a | 0.008 |
| HDL-cholesterol (mmol/l) | 1.0 ± 0.21 | 1.1 ± 0.28 | 1.1 ± 0.25 | 0.18 |
| TDP (ng/ml) | 16.7 ± 8.4 | 22.1 ± 8.2a | 23.8 ± 8.0a | <0.001 |
| TMP (ng/ml) | 2.3 ± 1.0 | 3.2 ± 1.4a | 3.9 ± 1.6a | <0.001 |
| Serum thiamine (ng/ml) | 2.3 ± 1.5 | 2.7 ± 1.3 | 3.4 ± 1.2a | <0.001 |
| Serum albumin (g/l) | 37.8 ± 5.7 | 37.1 ± 5.6 | 36.9 ± 6.7 | 0.81 |
| Serum creatinine (umol/l) | 122.3 ± 26.3 | 97.8 ± 25.9 | 92.5 ± 24.7a,b | <0.001 |
| Thiamine urine (ng/ml) | 810.6 ± 78.1 | 998.7 ± 268.7a | 2319.9 ± 268.7a,b | <0.001 |
Notes: Data represented by Mean ± standard deviation;
denotes significance as compared to baseline;
denotes significance as compared to 3 months; Level of significance is given at p ≤ 0.05.
Discussion
Early studies on the relationship between thiamine status and diabetes-associated co-morbidities have raised the possibility of thiamine correction to reverse potential complications among the DMT2 community.6,7 In this short follow-up interventional study, we were able to observe remarkable improvements in the metabolic profile of DMT2 subjects secondary to oral thiamine supplementation, particularly in raising serum circulating thiamine levels and its derivatives, lipid profile and creatinine levels. No adverse effects were observed during the entire supplementation period and supplementation was well tolerated by the subjects. The association of circulating thiamine with lipid profile has been observed previously among elderly people.8 Furthermore, the improved lipid profile, particularly LDL- and total cholesterol, has been previously demonstrated in animal models,9,10 but not in humans,11 with the latter study’s intervention duration being comparatively shorter than the present study. It has been suggested that short duration, high-dose thiamine therapy is required to achieve normalization of lipids particularly among patients with diabetes, but such clinical trials are yet to be conducted.12
The improved levels of thiamine and its derivatives secondary to oral supplementation have been documented previously in small sample-sized, short- and long-duration clinical trials with no subsequent improvement in renal function.13,14 In the present study, while we were not able to measure various markers of renal function, we observed a significant decrease in serum creatinine over time, which can be considered a good indicator of renal function. The difference in the results of Alkhalaf et al,13 and the present study can be attributed to sample size, supplement dose and supplement duration. Preservation of renal function through thiamine treatment, however, has already been observed in animal models.15
The authors acknowledge several limitations and therefore results should be interpreted with caution. Dietary carbohydrate intake, which is known to influence thiamine status,16,17 and medication history were not taken into consideration in this study and could have been a confounding variable, which can explain why improvements in certain parameters, such as LDL-cholesterol, were not observed. Furthermore, since the DM patients had stable glycemic control, no complications were noted at baseline and therefore there was little space for a meaningful degree of improvement in the reversal of diabetic complications, if any. Lastly, there is no placebo group, and comparisons were done only for those with and without diabetes mellitus. The study, however, deserves merit in that the sample size was considerably larger as compared to previous intervention studies and a moderate duration of supplementation was implemented, providing new insights as to the beneficial effects of 100 mg thiamine supplementation among patients with DMT2.
Conclusion
In conclusion, 100 mg thiamine supplementation for six months may be beneficial for patients with DMT2 in terms of thiamine status correction and improvement in lipid and creatinine levels. Further interventional studies are needed to determine whether thiamine correction can reverse diabetic complications.
Footnotes
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Author Contributions
Conceived and designed the experiments: OA and NA. Analyzed the data: SA and BV. Wrote the first draft of the manuscript: SS. Contributed to the writing of the manuscript: OA, NA, MA, SA, BV, SS. Agree with manuscript results and conclusions: OA, NA, MA, SA, BV, SS. All authors reviewed and approved of the final manuscript.
DISCLOSURES AND ETHICS
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests.
FUNDING: This study has been graciously funded by the Center of Excellence in Biotechnology Research, King Saud University, Riyadh, Saudi Arabia.
REFERENCES
- 1.Al-Daghri NM, Al-Attas OS, Alokail MS, et al. Diabetes Mellitus type 2 and other chronic non-communicable diseases in the central region, Saudi Arabia (Riyadh cohort 2): a decade of an epidemic. BMC Med. 2011;9:76. doi: 10.1186/1741-7015-9-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Asrar AM, Al-Rubeaan KA, Al-Amro SA, et al. Risk factors for diabetic retinopathy among Saudi Diabetics. Int Ophthalmolol. 1998;22:155–161. doi: 10.1023/a:1006240928938. [DOI] [PubMed] [Google Scholar]
- 3.Parving HH, Harrington JT, Lewis J, et al. Diabetic nephropathy: prevention and treatment. Kidney Internat. 2001;60:2041–2055. doi: 10.1046/j.1523-1755.2001.00020.x. [DOI] [PubMed] [Google Scholar]
- 4.Thornalley PJ. Cell activation by glycated proteins, AGE receptors, receptor recognition factors and functional classification of AGEs. Cell Mol Biol. 1998;44:1013–1023. [PubMed] [Google Scholar]
- 5.Al-Attas OS, Al-Daghri NM, Alfadda A, et al. Blood thiamine and derivatives as measured by high performance liquid chromatography: levels and associations in DM patients with varying degrees of microalbuminuria. J Endocr Invest. 2012;35:951–956. doi: 10.3275/8126. [DOI] [PubMed] [Google Scholar]
- 6.Thornalley PJ. The potential role of thiamine (vitamin B1) in diabetic complications. Curr Diabetes Rev. 2005;1:287–298. doi: 10.2174/157339905774574383. [DOI] [PubMed] [Google Scholar]
- 7.Rabbani N, Thornalley PJ. Emerging role of thiamine therapy for prevention and treatment of early-stage diabetic nephropathy. Diabetes Obes Metab. 2001;13:577–583. doi: 10.1111/j.1463-1326.2011.01384.x. [DOI] [PubMed] [Google Scholar]
- 8.Hooper PL, Hooper EM, Hunt WC, et al. Vitamins, lipids and lipoproteins in a healthy elderly population. Int J Vitamin Nutr Res. 1983;53:412–419. [PubMed] [Google Scholar]
- 9.Naveed AK, Qamar T, Ahmad I, et al. Effect of thiamine on lipid profile in diabetic rats. J Coll Physicians Surg Pak. 2009;19:165–168. [PubMed] [Google Scholar]
- 10.Karachalias N, Babaei-Jadidi R, Kupich C, et al. High-dose thiamine therapy counters dyslipidemia and advanced glycation of plasma protein in streptozotocin- induced diabetic rats. Ann N Y Acad Sci. 2005;1043:777–783. doi: 10.1196/annals.1333.090. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Ortiz M, Martinez-Abundis E, Robles-Cervantes JA, et al. Effect of thiamine administration on metabolic profile, cytokines and inflammatory markers in drug-naïve patients with type 2 diabetes. Eur J Nutr. 2011;50:145–149. doi: 10.1007/s00394-010-0123-x. [DOI] [PubMed] [Google Scholar]
- 12.Babaei-Jadidi R, Karachalias N, Kupich C, et al. High-dose thiamine therapy counters dyslipidemia in streptozotocin-induced diabetic rats. Diabetologia. 2004;47:2235–2246. doi: 10.1007/s00125-004-1582-5. [DOI] [PubMed] [Google Scholar]
- 13.Alkhalaf A, Klooster A, van Oeveren W, et al. A double-blind, randomized, placebo-controlled clinical trial on benfotiamine treatment in patients with diabetic nephropathy. Diabetes Care. 2010;33:1598–1601. doi: 10.2337/dc09-2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fraser DA, Diep LM, Hovden IA, et al. The effects of long-term oral benfotiamine supplementation on peripheral nerve function and inflammatory markers in patients with type 1 diabetes: a 24-month, double-blind, randomized, placebo-controlled trial. Diabetes Care. 2010;35:1095–1097. doi: 10.2337/dc11-1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka T, Kono T, Terasaki F, et al. Thiamine prevents obesity and obesity-associated metabolic disorders in OLETF rats. J Nutr Sci Vitaminol (Tokyo) 2010;56:335–346. doi: 10.3177/jnsv.56.335. [DOI] [PubMed] [Google Scholar]
- 16.Bakker SJ, Hoogeveen EK, Nijpels G, et al. The association of dietary fibers with glucose tolerance is partly explained by concomitant intake of thiamine: the Hoorn Study. Diabetologia. 1998;41:1168–1175. doi: 10.1007/s001250051047. [DOI] [PubMed] [Google Scholar]
- 17.Vindedzis SA, Stanton KG, Sherriff JL, et al. Thiamine deficiency in diabetes—is diet relevant? Diab Vasc Dis Res. 2008;5:215. doi: 10.3132/dvdr.2008.035. [DOI] [PubMed] [Google Scholar]