Summary
Sleep is universal, tightly regulated, and its loss impairs cognition. But why does the brain need to disconnect from the environment for hours every day? The synaptic homeostasis hypothesis (SHY) proposes that sleep is the price the brain pays for plasticity. During a waking episode, learning statistical regularities about the current environment requires strengthening connections throughout the brain. This increases cellular needs for energy and supplies, decreases signal-to-noise ratios, and saturates learning. During sleep, spontaneous activity renormalizes net synaptic strength and restores cellular homeostasis. Activity-dependent down-selection of synapses can also explain the benefits of sleep on memory acquisition, consolidation, and integration. This happens through the off-line, comprehensive sampling of statistical regularities incorporated in neuronal circuits over a lifetime. This review considers the rationale and evidence for SHY and points to open issues related to sleep and plasticity.
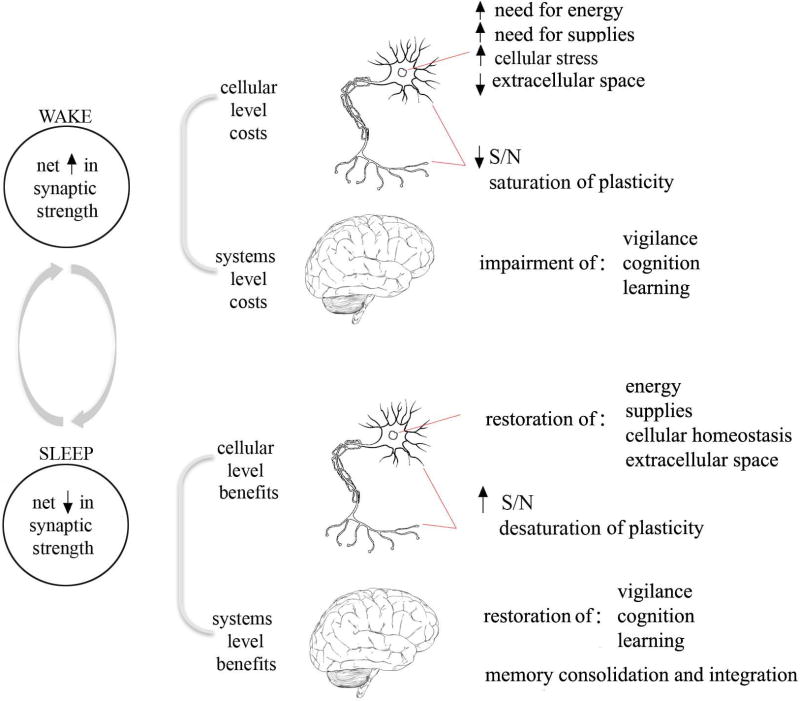
Why we need to sleep seems clear: without sleep, we become tired, irritable, and our brain functions less well. After a good night of sleep, brain and body feel refreshed and we are restored to normal function. However, what exactly is being restored by sleep has proven harder to explain. Sleep occupies a large fraction of the day, it occurs from early development to old age, and it is present in all species carefully studied so far, from fruit flies to humans. Its hallmark is a reversible disconnection from the environment, usually accompanied by immobility. The risks inherent in forgoing vigilance, and the opportunity costs of not engaging in more productive behaviors, suggest that allowing the brain to go periodically ‘off-line’ must serve some important function. Here we review a proposal concerning what this function might be - the synaptic homeostasis hypothesis or SHY (Tononi and Cirelli, 2003, 2006). SHY proposes that the fundamental function of sleep is the restoration of synaptic homeostasis, which is challenged by synaptic strengthening triggered by learning during wake and by synaptogenesis during development (Fig. 1). In other words, sleep is “the price we pay for plasticity.” Increased synaptic strength has various costs at the cellular and systems level including higher energy consumption, greater demand for the delivery of cellular supplies to synapses leading to cellular stress, and associated changes in support cells such as glia. Increased synaptic strength also reduces the selectivity of neuronal responses and saturates the ability to learn. By renormalizing synaptic strength, sleep reduces the burden of plasticity on neurons and other cells while restoring neuronal selectivity and the ability to learn, and in doing so enhances signal-to-noise ratios (S/N), leading to the consolidation and integration of memories.
Figure 1. The Synaptic Homeostasis Hypothesis (SHY).
Synaptic homeostasis and sleep function
Neurobiological and informational constraints
SHY was initially motivated by considering neurobiological and informational constraints faced by neurons in the wake state, as outlined in the following section.
Neurons should fire sparsely and selectively
Energetically, a neuron is faced with a major constraint: firing is more expensive than not firing and firing strongly (bursting) is especially expensive (Attwell and Gibb, 2005). Informationally, a neuron is a tight bottleneck: it can receive a very large number of different input patterns over thousands of synapses, but through its single axon it produces only a few different outputs. Simplifying a bit, a neuron's dilemma is “to fire or not to fire” or “to burst or not to burst.” Together, these energetic and informational constraints force neurons to fire sparsely and selectively: bursting only in response to a small subset of inputs while remaining silent or only firing sporadic spikes in response to a majority of other inputs (Balduzzi and Tononi, 2013). In line with this requirement and with theoretical predictions (Barlow, 1985), firing rates are very low under natural conditions (Haider et al., 2013) and responses to stimuli are sparse, especially in the cerebral cortex (Barth and Poulet, 2012).
Neurons should detected and communicate suspicious coincidences
Since a neuron must fire sparsely, it should choose well when to do so. A classic idea is that a neuron should fire for “suspicious coincidences” - when inputs occur together more frequently than would be expected by chance (Barlow, 1985). Suspicious coincidences suggest regularities in the input and ultimately in the environment, such as the presence and persistence in time of objects, which a neuron should learn to predict. Importantly, due to sparse firing, excess coincidences of firing are easier to detect than coincidences of silence (Hashmi et al., 2013). Thus, a neuron should integrate across its many inputs to best detect suspicious coincidences of firing. Moreover, it should communicate their detection by firing in response, assuming that other neurons will also pay attention to firing. A good strategy to reliably communicate to other neurons would therefore be to fire most (burst) for the most suspicious coincidences, less so for less suspicious ones, and not at all for all other inputs. Finally, in order to fire when it detects suspicious coincidences, a neuron should make sure that the synapses carrying them are strong.
Neurons should strengthen synapses in wake, when interacting with the environment
A neuron cannot allocate high synaptic strength to input lines carrying suspicious coincidences once and for all: neurons must remain plastic and appropriately increase synaptic strength to become selective for novel suspicious coincidences and ensure that they can percolate through the brain. Clearly, this should happen in wake, and especially when organsims explore their environment and interact with it, encounter novel situations and pay attention to salient events. There are a variety of plasticity mechanisms that can promote some form of synaptic potentiation during wake, and which are known to occur during exploration (Clem and Barth, 2006), association learning (Gruart et al., 2006), contextual memory formation (Hu et al., 2007), fear conditioning (Matsuo et al., 2008; Rumpel et al., 2005), visual perceptual learning (Sale et al., 2011), cue-reward learning (Tye et al., 2008), and avoidance learning (Whitlock et al., 2006). While there are also forms of learning “by depression”, including reversal learning in the hippocampus (e.g. (Dong et al., 2013)), some aspects of fear extinction in the amygdala (reviewed in (Quirk et al., 2010)), and familiarity recognition in perirhinal cortex (e.g. (Cho et al., 2000)), enduring synaptic depression is associated more with forgetting what was previously known, than with acquiring new knowledge (Collingridge et al., 2010).
While potentiating synapses in wake when the organism is interacting with the environment is essential, doing so in sleep, when neural activity is disconnected from the environment and the brain is exposed to its own “fantasies”, may instead be maladaptive. For example, more than half of nocturnal awakenings reveal the occurrence of imaginary scenes or full-fledged dreams, so it could be dangerous if they gave rise to new declarative memories (Nir and Tononi, 2010). Similarly, non-declarative skills are acquired and refined with environmental feedback in wake, but if new learning occurred during sleep without such feedback, these skills could easily become corrupted. Indeed, the strengthening of fantasies is a known problem in neural networks that learn based on a wake-sleep algorithm in which feed-forward (“recognition”) connections that match feed-back (“generative”) connections are potentiated in the sleep phase (Hinton et al., 1995).
Neurons should renormalize synapses in sleep, when they can sample memories comprehensively
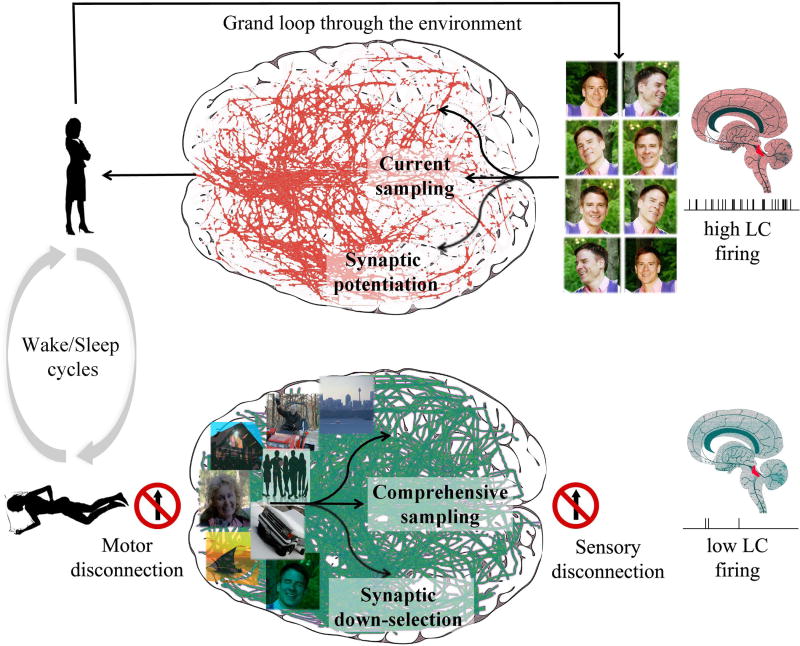
While neurons should learn primarily by potentiating synapses in wake, synaptic strength is a costly resource. One set of reasons is cell-biological: stronger synapses consume more energy, require extra supplies, and lead to cellular stress (see below). Another reason is informational, and can be termed the plasticity-selectivity dilemma: when a neuron strengthens additional input lines, a broader distribution of its input patterns can make it burst, reducing its ability to capture suspicious coincidences because it will also begin to fire for chance, spurious coincidences (Balduzzi and Tononi, 2013; Hashmi et al., 2013). Clearly, as recognized in many models of learning, neurons must eventually renormalize total synaptic strength in order to restore cellular functions as well as selectivity. SHY proposes that renormalization through synaptic depression should happen during sleep. This is because, when the brain goes off-line in sleep, the continuously changing patterns of spontaneous activity allows neurons to obtain a “comprehensive” sampling of the brain's overall knowledge of the environment (Fig. 2, bottom) – one acquired over evolution, development, and a lifetime of learning (Tononi et al., 1996). During a period of wake, instead, an organism is faced with the “current” sampling of the environment that is necessarily limited and biased. For example, consider spending a day with a new acquaintance (Fig. 2, top). By the evening, neurons in various brain areas will have learned to recognize the person's face, voice, posture, clothes, and many other aspects by strengthening incoming synapses. But it would not be a good idea if, to renormalize total synaptic strength, synapses underutilized during that particular waking day were to be weakened and possibly eliminated - otherwise one would remember the new acquaintance and forget old friends, a problem known as the plasticity-stability dilemma (Abraham and Robins, 2005; Grossberg, 1987).
Figure 2. SHY, wake-sleep cycles, and the plasticity-stability dilemma.
Top: during wake the brain interacts with the environment (grand loop) and samples a limited number of inputs dictated by current events (current sampling, here represented by a new acquaintance). High levels of neuromodulators, such as noradrenaline released by the locus coeruleus (LC), ensure that suspicious coincidences related to the current sampling percolate through the brain and lead to synaptic potentiation. Bottom: during sleep, when the brain is disconnected from the environment on both the sensory and motor sides, spontaneous activity permits a comprehensive sampling of the brain's knowledge of the environment, including old memories about people, places, etc. Low levels of neuromodulators, combined with the synchronous, ON and OFF firing pattern of many neurons during NREM sleep events such as slow waves, spindles, and sharp-wave ripples, are conducive to synaptic down-selection: synapses belonging to the fittest circuits, those that were strengthened repeatedly during wake and/or are better integrated with older memories are protected and survive. By contrast, synapses belonging to circuits that were only rarely activated during wake and/or fit less well with old memories, are progressively depressed and eventually eliminated over many wake/sleep cycles. The green lines in the sleeping brain (right), taken from (Murphy et al., 2009), illustrate the propagation of slow waves during NREM sleep, as established using high-density EEG and source modeling.
In summary, SHY claims that neurons should achieve some basic goals with respect to plasticity: i) New learning should happen primarily by synaptic potentiation. In this way firing that signals suspicious coincidences can percolate throughout the brain; ii) Synaptic potentiation should occur primarily in wake, when the organism interacts with its environment, not in sleep when it is disconnected. In this way what the organism learns is controlled by reality and not by fantasy; iii) Renormalization of synaptic strength should happen primarily during sleep, when the brain is spontaneously active off-line, not in wake when a neuron's inputs are biased by a particular situation. In this way neurons can sample comprehensively the brain's overall statistical knowledge of its environment.
Heuristic rules for neuronal plasticity in wake and sleep
Learning by potentiation in wake
The actual plasticity mechanisms employed by specific neuronal populations are bound to be complex, variable, and adaptable to local conditions and firing patterns (Feldman, 2009). However, learning and communicating down-stream important events that occur during wake can in principle be achieved using a few heuristic rules (Nere et al., 2012). First, a neuron should pay attention to inputs that fire strongly, because they could signal the detection of suspicious coincidences by upstream neurons. Furthermore, a strong input that persists over time could signal the presence of something (like an object) that remains present longer than expected by chance. Positive correlations between pre- and postsynaptic spikes, whether over pairs of spikes (spike-timing-dependent plasticity, STDP) or as an average, signal that the neuron must have detected enough suspicious coincidences, by integrating over its dendritic tree, to make it fire strongly within a restricted time frame (tens to hundreds of milliseconds), so they should be rewarded by increasing synaptic strength. Suspicious coincidences in input firing that occur over a restricted dendritic domain may be especially important (Legenstein and Maass, 2011; Winnubst and Lohmann, 2012), particularly if they involve both feed-forward and feed-back inputs. Such coincidences suggest the closure of a loop between input and output in which the neuron may have played a causal role (Hashmi et al., 2013). They also indicate that the feed-forward suspicious coincidences the neuron has captured, presumably originating in the environment, can be matched internally, within the same dendritic domain, by feed-back coincidences generated higher-up in the brain, indicating that bottom-up data fit at least in part with top-down expectations. This is a sign that the brain can model internally what it captures externally and vice-versa - a good recipe for increasing the matching between its causal structure and that of the environment (Hinton et al., 1995; Tononi, 2012). Finally, in this scheme a neuron should enable the strengthening of connections only when it is awake and engaged in situations worth remembering. This can be signaled globally by neuromodulatory systems that gate plasticity and are active during wake, especially during salient, unexpected, or rewarding circumstances.
Renormalization by down-selection in sleep
Various synaptic rules enforcing activity-dependent depression during sleep are compatible with the renormalization process predicted by SHY. In all cases, the end result is a competitive “down-selection” whereby after sleep some synapses become less effective than others. Computer implementations of down-selection include: a downscaling rule where all synapses decrease in strength proportionally, but those that end up below a minimal threshold become virtually ineffective (Hill et al., 2008); a modified STDP rule by which stronger synapses are depressed less than weaker ones (Olcese et al., 2010); and a “protection from depression” rule (Hashmi et al., 2013; Nere et al., 2013). In this last implementation of down-selection, when a neuron detects many suspicious coincidences during sleep (thus fires strongly), rather than potentiating the associated synapses as in the awake state, it protects them from depression (Fig. 2). This competitive down-selection mechanism has the advantage that synapses activated strongly and consistently during sleep survive mostly unchanged and may actually consolidate, in the classic sense of becoming more resistant to interference and decay. By contrast, synapses that are comparatively less activated are depressed, resulting in the consolidated synapses being stronger in relative terms. Thus, down-selection ensures the survival of those circuits that are “fittest,” because they were strengthened repeatedly during wake or better integrated with older memories, whereas synapses that were only occasionally strengthened during wake, or fit less well with old memories, are depressed and eventually eliminated. The simulations also show that down-selection during sleep increases S/N and promotes memory consolidation, gist extraction, and the integration of new memories with established knowledge, while ensuring that no new memories are formed in the absence of reality checks (Nere et al., 2013). Finally, it should be noted that in the special case of a neuron that received all its inputs from the same source (or from strongly correlated sources), down-selection would be ineffective because it could not enforce any competition among synapses. Neurons “taken over” by a particular source might be relevant for memories that are extremely stable, such as traumatic ones.
A few cellular mechanisms could explain why during sleep strongly activated synapses could depress less, or not at all. For instance, high calcium levels can partially or totally block calcineurin, a phosphatase that promotes synaptic depression and whose expression is upregulated in sleep (Cirelli et al., 2004). Another potential mechanism involves the endogenous inhibitor of CamKII (CamKIIN), which decreases synaptic strength by directly impairing the binding of CaMKII to the NMDA receptor (Sanhueza and Lisman, 2013). The alpha isoform of CaMKIIN is upregulated during sleep (Cirelli et al., 2004), and its inhibitory function is reduced by high calcium levels (Gouet et al., 2012). Alternatively Arc/Arg3.1, an activity-induced immediate early gene that enters spines and mediates receptor internalization (Bramham et al., 2010; Okuno et al., 2012) may be excluded from the spines that need to be protected, while synapses that are activated in isolation are not protected and depress progressively in the course of sleep. In sleep, the switch to a mode of plasticity where synaptic potentiation is prevented and synapses can at most be protected or depressed in an activity-dependent manner may be signaled globally by a drop in the level of neuromodulators, such as noradrenaline, histamine, and serotonin, that are high in wake and low in sleep. Indeed, the radically altered balance of neuromodulators and trophins such as brain-derived neurotrophic factor (BDNF) during sleep can reverse the sign of plastic changes compared to wake, blocking potentiation and promoting depression (Aicardi et al., 2004; Harley, 1991; Seol et al., 2007).
The schematic scenario described above is indicative of the general principles that would allow neurons to learn suspicious coincidences during wake and renormalize synaptic strength during sleep. Nevertheless, given the variety and complexity of plasticity mechanisms, the specific synaptic rules followed by neurons in order to learn during wake and to renormalize synapses during sleep are likely to differ in different species, brain structures, neuronal types, and developmental times (Tononi and Cirelli, 2012). For instance, it is unclear whether inhibitory connections also need to be renormalized after wake. It is also unknown whether invertebrates, such as the fruit fly, or ancient brain structures, such as the brainstem, use the same mechanisms of renormalization as the vertebrate cortex, or may not even require activity and oscillations in membrane potentials. Moreover, while SHY unambiguously predicts that wake should result in a net increase in synaptic strength and sleep in a net decrease, it does not rule out that some degree of synaptic depression may also occur in wake and some potentiation in sleep.
Sleep and synaptic homeostasis: the evidence
In view of the multiplicity of mechanisms of synaptic potentiation, depression, metaplasticity, homeostatic plasticity, and intrinsic plasticity, it is natural to assume that neurons have many ways to keep overall synaptic strength balanced (Kubota et al., 2009). However, for the reasons outlined above, SHY claims that such a balance is best achieved through an alternation of net synaptic potentiation in wake and net depression in sleep. Over the past few years, the core claim of SHY has been investigated using molecular, electrophysiological, and structural approaches (Fig. 3) that will be discussed in the following section.
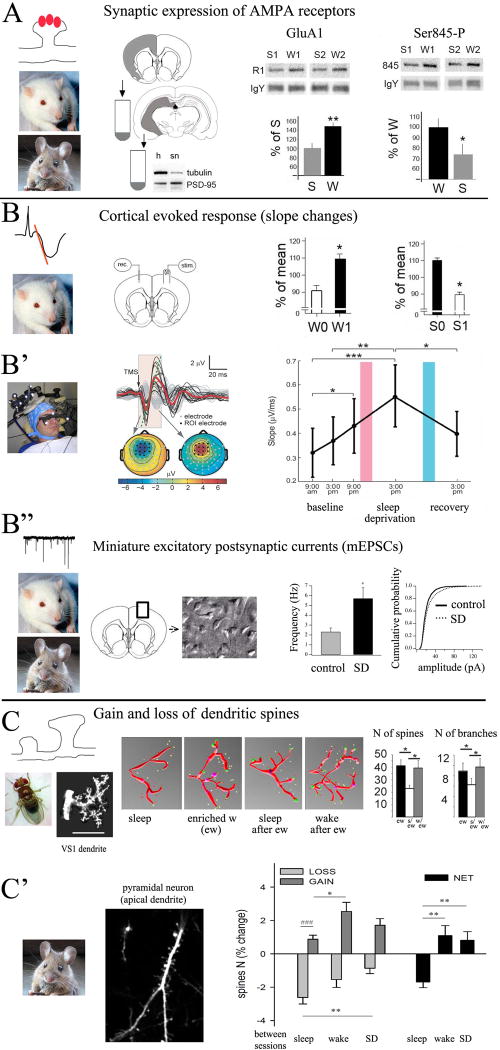
Figure 3. Evidence supporting SHY.
A, experiments in rats and mice show that the number and phosphorylation levels of GluA1-AMPARs increase after wake (data from rats (Vyazovskiy et al., 2008)). B, B′, electrophysiological analysis of cortical evoked responses using electrical stimulation (in rats; from (Vyazovskiy et al., 2008)) and TMS (in humans, from (Huber et al., 2012)) shows increased slope after wake and decreased slope after sleep. In B, W0 and W1 indicate onset and end of ∼ 4h of wake; S0 and S1 indicate onset and end of ∼ 4h of sleep, including at least 2h of NREM sleep. In B′, pink and blue bars indicate a night of sleep deprivation and a night of recovery sleep, respectively. B″, in vitro analysis of mEPSCs in rats and mice shows increased frequency and amplitude of mEPSCs after wake and sleep deprivation (SD) relative to sleep (control). Data from rats (Liu et al., 2010). C, in flies, the number of spines and dendritic branches in the visual neuron VS1 increase after enriched wake (ew) and decrease only if flies are allowed to sleep (from (Bushey et al., 2011). C′, structural studies in adolescent mice show a net increase in cortical spine density after wake and sleep deprivation (SD) and a net decrease after sleep (from (Maret et al., 2011).
Molecular evidence
The trafficking of GluA1-containing AMPARs in and out of the synaptic membrane is considered a primary mechanism for the occurrence of synaptic potentiation and depression, respectively (Kessels and Malinow, 2009). GluA1-containing AMPARs are permeable to calcium and their expression shows a supralinear relationship with the area of the post-synaptic density (Shinohara and Hirase, 2009) making them especially powerful in affecting synaptic strength. Levels of GluA1-containing AMPARs are 30-40% higher after wakefulness than after sleep in rats (Vyazovskiy et al., 2008) and phosphorylation changes of AMPARs, and of the enzymes CamKII and GSK3β, are also consistent with net synaptic potentiation during wake and depression during sleep (Vyazovskiy et al., 2008) (Fig. 3A). Similar sleep/wake changes in AMPARs expression have been found in other studies, for example the insertion of GluA1-containing AMPAR during wake (Qin et al., 2005) and their removal during sleep (Lante et al., 2011); as well as increases and decreases in a molecular hallmark of synaptic depression, dephosphorylation of GluA1-containing AMPARs at Ser845 (Kessels and Malinow, 2009), with time spent in sleep and wake respectively (Hinard et al., 2012).
Electrophysiological evidence
The slope of the early (monosynaptic) response evoked by electrical stimulation delivered in vivo is a classical measure of synaptic strength. In rat frontal cortex, the first negative component of the response evoked by transcallosal stimulation increases with time spent awake and decreases with time spent asleep, and the sleep-related decline correlates with the extent of the decline in slow wave activity (Vyazovskiy et al., 2008) (Fig. 3B). The slope of the response evoked in the rat hippocampal CA3 region by electrical stimulation of the fimbria also declines in the sleep period following a wake episode (Lubenov and Siapas, 2008). Similarly, in humans, the slope of the early response evoked in frontal cortex by transcranial magnetic stimulation (TMS) increases progressively in the course of 18 hours of continuous wake and returns to baseline levels after one night of recovery sleep (Huber et al., 2012)(Fig. 3B′). These changes in the slope of evoked responses occurred after several hours of sleep or wake with the subjects fully awake when post-sleep responses were recorded. By contrast, a recent study in head-restrained cats saw an increase in the cortical response evoked by medial lemniscus stimulation after sleep (Chauvette et al., 2012). Notably, the effect was observed after as little as 10 min of sleep, and saturated after two short sleep episodes. While species-specific differences may exist, EEG and intracellular recordings in the report suggest that the membrane potential in the “awake” condition immediately post-sleep was hyperpolarized, implying that the enhanced responses were most likely due to sleep inertia.
Other experiments measure amplitude and frequency of miniature excitatory postsynaptic currents (mEPSCs) from slices of frontal cortex (Fig. 3B″). Changes in mEPSCs frequency reflect modifications of the presynaptic component of synaptic transmission, while amplitude changes indicate alterations in the postsynaptic component. In the cerebral cortex of mice and rats, both parameters are lower after a few hours of sleep, higher after a few hours of wake, and decline during recovery sleep following sleep deprivation (Liu et al., 2010). This suggests that synaptic efficacy varies between sleep and wake because of changes at the postsynaptic level, as already indicated by changes in AMPARs expression (Vyazovskiy et al., 2008), as well as at the presynaptic level. Consistent with these findings, the mean firing rates of cortical neurons increase after prolonged wake (Vladyslav et al., 2009), and levels of glutamate in the rat cortical extrasynaptic space rise progressively during wake and decrease during NREM sleep (Dash et al., 2009). A study that tested excitatory synapses on hypocretin/orexin neurons of the hypothalamus also found an increase in both frequency and amplitude of mEPSCs after sleep deprivation (Rao et al., 2007), suggesting that changes in synaptic efficacy due to sleep/wake may not be restricted to cortical areas.
Structural evidence
Structural correlates of synaptic strength also support SHY. In Drosophila, protein levels of pre- and postsynaptic components are high after wake and decline in the course of sleep (Gilestro et al., 2009). Moreover, the number or size of synapses in four different neural circuits increase after a few hours of wake and decrease only if flies are allowed to sleep (Bushey et al., 2011; Donlea et al., 2009; Donlea et al., 2011). For instance, in the first giant tangential neuron in the visual system, the number of dendritic spines increases after 12 hours of wake spent in an enriched environment, and returns to pre-enrichment levels only if the flies are allowed to sleep (Bushey et al., 2011) (Fig. 3C). In mammals, structural synaptic changes due to sleep and wake have been studied by repeated two-photon microscopy in transgenic YFP-H mice. With only a few apical dendrites of layer V pyramidal neurons expressing yellow fluorescent protein, spines were counted twice within ∼12-16 hours, after a period spent mostly asleep or mostly awake (Maret et al., 2011)(Fig. 3C′). In adolescent 1-month old mice, spines form and disappear at all times, but spine gain prevails during wake, resulting in a net increase in spine density, while spine loss is larger during sleep, resulting in a net spine decrease (Maret et al., 2011). Another study using younger YFP-H mice (3-week old) also found greater formation of spines and filopodia (possible precursors of mature spines) during the dark period, when mice are mostly awake, and more elimination of these protrusions during the light period, when mice are mostly asleep (Yang and Gan, 2012). These findings confirm that, in young mice, a few hours of sleep and wake can affect the density of cortical synapses. By contrast, spine turnover is limited and is not impacted by sleep and wake in adult mice (Maret et al., 2011), suggesting that after adolescence synaptic homeostasis may be mediated primarily by changes in synaptic strength rather than number.
While the cellular, electrophysiological and structural evidence discussed above largely support SHY, it is important to bear in mind the limitations of these markers. Changes in evoked responses or firing rates may also be explained by fast changes in neuronal excitability due to neuromodulators such as norepinephrine, although synaptic strength and neuronal excitability are usually co-regulated in the same direction (Cohen-Matsliah et al., 2010; Kim and Linden, 2007). Moreover, in vitro changes in mEPSCs may not reflect what happens in vivo, structural changes of synapses do not always reflect changes in efficacy, and changes in the number and/or phosphorylation levels of AMPARs may not fully capture their functional status. Thus, more refined approaches, such as Cre-dependent tagging of activated circuits, will be needed to establish precisely which synapses strengthen and weaken during and after a specific learning task, and whether they mostly do so in wake and in sleep, respectively. Finally, in most of the studies highlighted, increases in synaptic strength after wake and their renormalization after sleep occurred in the absence of specific training paradigms, merely requiring that the experimental subjects stay awake. Regardless, it should be kept in mind that, even without any explicit instruction to learn, at the end of a typical waking day we can recollect an extraordinary amount of events, facts, and scenes, including many irrelevant details (Brady et al., 2008; Standing, 1973).
Synaptic homeostasis and slow wave activity
In mammals and birds, a reliable marker of sleep need is the amount of slow wave activity (SWA, 0.5-4.5 Hz) in the EEG of NREM sleep. As shown by many experimental and modeling studies, SWA is highest at sleep onset, decreases with the time spent asleep, increases further if one stays awake longer, and is reduced by naps (Fig. 4A). SWA occurs when, due to changes in neuromodulation in NREM sleep, cortical neurons become bistable and undergo a slow oscillation (<1 Hz) in membrane potential (Steriade et al., 2001). This consists of a depolarized up state, when neurons show sustained firing, and a hyperpolarized down state, characterized by neuronal silence, which corresponds to the negative down-stroke of EEG slow waves. Computer simulations show that, for a given level of neuromodulatory and inhibitory tone, the amplitude and slope of EEG slow waves are related to the number of neurons that enter an up state or a down state near-synchronously. In turn, synchrony is directly related to the number, strength, and distribution of synaptic connections among them (Esser et al., 2007; Olcese et al., 2010).
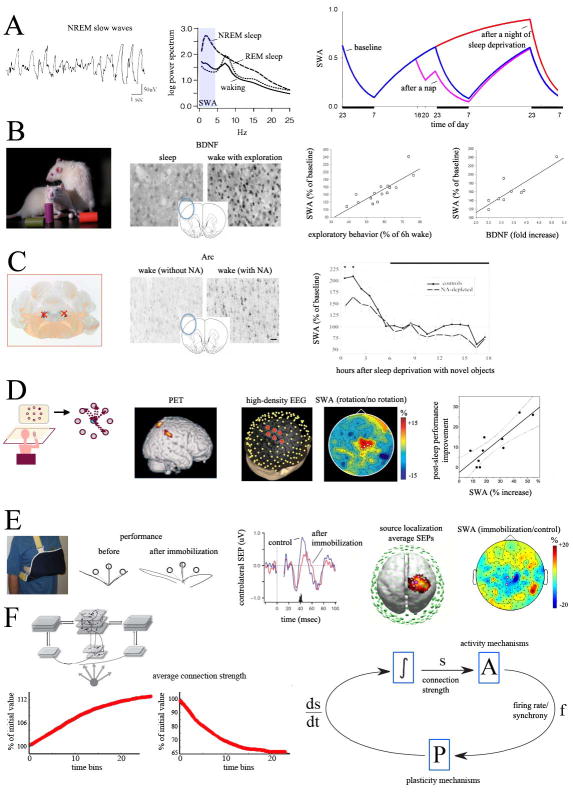
Figure 4. SHY and slow wave activity (SWA).
A, SWA, a quantitative measure of the number and amplitude of slow waves (left), is high in NREM sleep and low in REM sleep and wake (middle). SWA increases with time spent awake and decreases during sleep, thus reflecting sleep pressure (right). B, in rats kept awake for 6 hours by exposure to novel objects, longer times spent exploring result in greater cortical induction of BDNF during wake, as well as in larger subsequent increases in SWA at sleep onset (from (Huber et al., 2007b). C, after bilateral lesions of the LC expression of plasticity-related genes during wake is low; during subsequent sleep, SWA is lower than in non-lesioned controls (from (Cirelli et al., 1996; Cirelli and Tononi, 2000). D, during wake, subjects learn to adapt to systematic rotations imposed on the perceived cursor trajectory, a task which activates right parietal areas (Ghilardi et al., 2000); during subsequent NREM sleep, SWA in the same areas shows a local increase, which correlates with post-sleep improvements in performance (from (Huber et al., 2004)). E, after a subject's arm is immobilized during the day, motor performance in a reaching task deteriorates, and the P45 cortical component of the response evoked by stimulation of the median nerve (SEP) decreases in contralateral sensorimotor cortex. In sleep post-immobilization, the same area shows a local decrease in SWA (from (Huber et al., 2006). F, Control loop for the homeostatic regulation of connection strength and firing rate/synchrony, based on the results of computer simulations of slow wave sleep (Olcese et al., 2010). Here connection strength (s) affects firing rates and synchrony (f) via activity mechanisms (A). During slow wave sleep, plasticity mechanisms (P) lead to a depression of synaptic strength (ds/dt) that is proportional to f. The resulting integrated value of connection strength (∫), in turn, determines the new value of firing rates and synchrony (f). As an example, strong average connection strength will lead to high firing rates and synchrony which, in turn, will strongly depress synapses, to bring the system back to baseline values of connection strength. Conversely, when connections are renormalized, activity levels will not be able to induce significant plastic changes and the system will reach a self-limiting equilibrium point.
SWA as an index of synaptic homeostasis
An important corollary of SHY is that, to the extent that SWA reflect the homeostatic regulation of sleep need, it should reflect changes in synaptic strength. Work in humans and rodents is consistent with these predictions. For example, the increase in the slope of cortical evoked field potentials (an electrophysiological sign of increased synaptic strength) after a period of wakefulness correlates with SWA values at the onset of the following sleep period (Vyazovskiy et al., 2008). Furthermore, rats exposed to an enriched environment experience a diffuse induction of BDNF (a marker of synaptic potentiation) and show a widespread increase in SWA during subsequent sleep, which is positively correlated with the amount of the time spent exploring and with the cortical induction of BDNF (Huber et al., 2007b) (Fig. 4B). By contrast, the increase in sleep SWA after wake is dampened following noradrenergic lesions, which reduce levels of BDNF, Arc, and other markers of plasticity (Cirelli et al., 1996; Cirelli and Tononi, 2000) (Fig. 4C).
The link between plasticity, SWA and sleep is also seen locally. In rats, SWA increases locally both after learning a task involving motor cortex (Hanlon et al., 2009), and after locally infusing BDNF to induce synaptic potentiation (Faraguna et al., 2008). In humans, learning tasks that involve particular regions of cortex, i.e. right parietal cortex (Perfetti et al., 2011), leads to a local increase in sleep SWA and correlates with post-sleep performance improvement (Fig. 4D; (Huber et al., 2004); see also (Kattler et al., 1994; Landsness et al., 2009)). Similarly, visual perceptual learning, which depends on a restricted population of orientation-selective neurons in lateral occipital cortex, increases the number of slow waves initiated in these areas (Mascetti et al., 2013b). High-frequency TMS over motor cortex also leads to a local increase in the amplitude of EEG responses, indicative of potentiation of premotor circuits. The magnitude of this potentiation in wake predicts the local increase in SWA during the subsequent sleep episode (Huber et al., 2007a). By contrast, arm immobilization leads to motor performance deterioration with a decrease in somatosensory and motor evoked responses over contralateral sensorimotor cortex (indicative of local synaptic depression), and a decrease in sleep SWA over the same cortical area (Huber et al., 2006) (Fig. 4E). Sustained increases or decreases of cortical excitability induced by a paired associative stimulation protocol also result in local SWA increases and decreases, respectively (Huber et al., 2008), although some studies employing slightly different protocols failed to detect local changes in SWA (for details see (Hanlon et al., 2011). Overall, these results support the idea that sleep may be regulated locally (Krueger and Tononi, 2011).
SWA as a contributor to synaptic homeostasis
SHY also suggests that SWA may not simply reflect changes in synaptic strength, but that the underlying slow oscillations may contribute directly to synaptic renormalization. One scenario is that burst firing, which is common in slow wave sleep during transitions between intracellular up and down states, may lead to a long-lasting depression of excitatory postsynaptic potentials (Czarnecki et al., 2007), mainly via postsynaptic mechanisms. Indeed, repetitive burst firing without synaptic stimulation, or paired with synaptic stimulation in a way that mimics in vivo conditions, leads to long-term depression and removal of AMPARs via serine/threonine phosphatases and protein kinase C signaling (Lante et al., 2011). Another possible mode for SWA to enforce synaptic renormalization is by decoupling through synchrony (Lubenov and Siapas, 2008). In recurrent networks with conduction delays, synchronous bursts of activity typical of slow wave sleep would lead to net synaptic depression through STDP mechanisms. For example, if neurons A and B fire simultaneously and neuron A projects to neuron B then, due to conduction delays, the presynaptic spike will arrive after the postsynaptic spike has occurred, leading to synaptic depression.
A control loop for synaptic strength
If stronger synapses increase SWA and SWA contributes to the decrease of synaptic strength during sleep, the conditions are in place for implementing a control loop in which synaptic strength is the regulated variable (Olcese et al., 2010). In this loop (Fig. 4F), synapses are potentiated due to learning in wake, leading to higher neuronal firing rates and synchrony and thus to high SWA when entering the sleep mode. On the other hand, strong, synchronous firing during NREM sleep leads to synaptic depression. In turn, the progressive weakening of synapses reduces firing rates and synchrony, slowing the process of activity-dependent renormalization. Finally, the network reaches an equilibrium point where synaptic strength is sufficiently low that firing rates and synchrony are too low to further weaken connections. Altogether, this control loop ensures that the decline in synaptic strength and SWA during sleep is exponential and self-limiting (Olcese et al., 2010), in agreement with experimental data in mammals and birds. Consistent with the existence of a control loop, the suppression of SWA during the first 3 hours of sleep prevents the homeostatic decline of SWA (Dijk et al., 1987), suggesting that SWA is both a sensor and an effector in a homeostatic process occurring during sleep.
SWA and the specificity of cortical connections
In addition to the total amount of synaptic strength, the specificity of connections is another factor that may influence neuronal synchronization and SWA. Computer simulations show that, for the same total number and strength of synapses, synchronization is higher if the connectivity among cortical neurons is homogenous or random (all neurons tend to receive similar inputs) and lower if the connectivity reflects functional specialization (different groups of neurons receive different inputs) (Tononi et al., 1998; Tononi et al., 1994). As mentioned earlier, learning in wake can reduce selectivity of firing as neurons start responding to a broader distribution of inputs (Balduzzi and Tononi, 2013), which in turn leads to a reduction of specificity, with more neurons firing in response to the same inputs. Thus, a reduction of selectivity and specificity may also contribute to increased synchronization and increased SWA after prolonged wake. By the same token, the restoration of selectivity and specificity after sleep-dependent renormalization should decrease SWA by lessening synchronization.
The relationship between connection specificity and neural synchronization may be especially important during neural development, including adolescence, when SWA shows a remarkable decline (Campbell and Feinberg, 2009). In various periods of development, after the overall anatomical wiring patterns have been established, a process of synaptic refinement, often activity-dependent, leads to an increase in the specificity of connections not only through synaptic pruning, but also through synaptic redistribution (Sanes and Yamagata, 2009). Moreover, synapses may be rearranged within distinct dendritic domains of a single neuron, whereby synapses from correlated sources become clustered together and those from uncorrelated sources are eliminated from one dendritic domain and redirected to another one (Sanes and Yamagata, 2009; Winnubst and Lohmann, 2012). Characteristically, target cells are initially innervated by several axons from multiple neurons, then lose many inputs and become innervated more specifically by fewer sources (Ko et al., 2013). Electrophysiological evidence indicates that after developmental refinement, if two cortical neurons are connected by one synapse, they are more likely than average to be connected by further synapses (Ko et al., 2013; Markram et al., 1997). Thus, the decrease in SWA during adolescence may reflect not only a decline in cortical synaptic density, but also changes in the specificity of neuronal connections and, by extension, of cognitive maturation (Buchmann et al., 2011; de Vivo et al., 2013).
While we highlight SWA in this review, other mechanisms of synaptic renormalization may also play a role in synaptic homeostasis in sleep and should be kept in mind as well. For example, sharp-wave ripples in slow wave sleep or rest in CA1 hippocampal neurons could lead to a rescaling of synaptic strength via antidromic spikes that requires L-type calcium channel activation and functional gap junctions (Bukalo et al., 2013). Other sleep events grouped by the onset of the ON period of the slow oscillation, such as spindles and bursts of gamma activity, may also be involved in the overall effects of NREM sleep on plasticity (Rasch and Born, 2013). In general, the switch from a mode of net synaptic potentiation to one of net synaptic depression is likely mediated by the drop in the level of many neuromodulators, such as acetylcholine, norepinephrine, serotonin, histamine, and hypocretin during NREM sleep. Neuromodulators can powerfully affect plasticity, including STDP polarity (Pawlak et al., 2010). Specifically, changes in cholinergic and noradrenergic modulation during sleep can shift the STDP curve to favor depression (Isaac et al., 2009; Seol et al., 2007) and could in turn promote synaptic renormalization in sleep.
Synaptic homeostasis and the cellular benefits of sleep
If sleep does in fact enforce the renormalization of synaptic strength, what are the benefits? As mentioned earlier, if learning during wake produces a net increase in synaptic strength, there are consequences both at the cellular and at the systems level. For an average neuron this means higher energy consumption, larger synapses, greater need for the delivery of cellular supplies to thousands of synapses, and cellular stress (Fig. 1).
Energy
The human brain accounts for 2% of body mass but uses up to 25% of the whole body glucose consumption (Sokoloff, 1960). The average metabolic cost per neuron is not only high, but also fixed, as suggested by the fact that the total glucose use by the brain is a linear function of the number of its neurons (Herculano-Houzel, 2011). Synaptic activity as a whole accounts for most of the brain's energy use (Attwell and Gibb, 2005) due to the energetically expensive processes of synaptic signaling, including the release of neurotransmitter vesicles and their recycling, action potential initiation and propagation, spiking, and restoration of Na+ and K+ gradients via the Na+/K+ ATPase pump. Thus, a net increase in synaptic strength necessarily comes at the expense of an increase in energy consumption even for the same level of neural activity.
Moreover, despite the various mechanisms that ensure a tight balance between excitation and inhibition (Haider et al., 2006) and regulate excitability through intrinsic conductances (van Welie et al., 2004) and synaptic scaling (Turrigiano, 2012), synaptic potentiation can lead to increased probability of firing in the hippocampus (Buzsaki et al., 2002). Moreover, sustained wake leads to increased firing rates (Kostin et al., 2010; Vyazovskiy et al., 2009), while during the course of sleep firing decreases in cortex (Vyazovskiy et al., 2009) and hippocampus (Grosmark et al., 2012). Thus, if synaptic strengths and firing rates were to grow without check as a result of wake plasticity, they could eventually become energetically too expensive. It is well established that the brain's energy consumption is “state-dependent”, being higher in wake than in sleep, especially slow wave sleep (Kennedy et al., 1982; Madsen and Vorstrup, 1991). During this stage, the second-by-second occurrence of hyperpolarized down states is poised to reduce the energy consumption associated with synaptic activity and make more energy available for other cellular processes (Cirelli et al., 2004; Mackiewicz et al., 2007; Mongrain et al., 2010; Vyazovskiy and Harris, 2013). However, few studies have assessed whether the brain's energy consumption is also “history-dependent”, i.e. whether it increases in the course of wake and/or decreases in the course of sleep. The available evidence suggests that this may be the case, but only when wake is forced beyond its physiological duration (Braun et al., 1997; Buysse et al., 2004; Shannon et al., 2013; Vyazovskiy et al., 2004).
Cellular supplies
Synapses also require many cellular constituents, from mitochondria to synaptic vesicles to various proteins and lipids synthesized and often delivered over great lengths (Kleim et al., 2003; McCann et al., 2008). These needs grow acutely when synaptic strength increases. Indeed, one of the genes most consistently upregulated in the brain during wake is the endoplasmic reticulum (ER) chaperone BiP (Hspa5) (Cirelli, 2009). BiP assists in the folding of newly synthesized proteins, including those produced after learning (Kuhl et al., 1992; Vandenberghe et al., 2005). BiP also assists in the folding of misfolded proteins as part of the unfolded protein response (UPR), a global ER stress response whose corrective actions aim at preserving ER functions. For reasons that remain unclear, a few hours of sleep deprivation are sufficient to trigger the UPR, whose end result is an overall decrease in protein synthesis (Naidoo et al., 2005). Thus the induction of plastic changes during wake increases the need for protein synthesis, but when wake is extended beyond its physiological duration, protein synthesis becomes impaired. With the reduced consumption of energy by synaptic transmission during hyperpolarized down states, slow wave sleep may represent an elective time for brain cells to carry out many housekeeping functions, including protein translation, the replenishment of calcium in presynaptic stores, the replenishment of glutamate vesicles, the recycling of membranes, the resting of mitochondria (Cirelli et al., 2004; Mackiewicz et al., 2007; Mongrain et al., 2010; Vyazovskiy and Harris, 2013), and the clearance of the extracellular space (Xie et al., 2013).
White matter and glia
Finally, imaging studies in humans show that, as a result of learning, changes in grey and white matter can occur within a few hours or days even in the adult brain (Zatorre et al., 2012). Although the underlying cellular mechanisms are poorly characterized, changes in synaptic strength, synaptogenesis, and dendritic or axonal sprouting are often accompanied by astrocytic growth, proliferation of oligodendrocyte precursor cells, and possibly micro-vascular modifications. Whether sleep plays a specific role in the glial response to learning is unclear but should be explored in future studies, as many brain transcripts upregulated during sleep are involved in the synthesis and maintenance of membranes in general and of myelin in particular, and the proliferation of oligodendrocyte precursor cells is facilitated by sleep (Bellesi et al., 2013).
Synaptic homeostasis and the memory benefits of sleep
In this section, we consider how a process of activity-dependent synaptic down-selection can also be beneficial for neuronal communication and memory management (Fig. 1), thus accounting for many of the positive effects of sleep on memory. We then contrast down-selection with “instructive” models of memory consolidation, according to which sleep benefits memory by potentiating recent memory traces.
Synaptic renormalization by down-selection and memory
As illustrated by different computer models, SHY provides a parsimonious explanation for several of the positive consequences of sleep on memory processes including acquisition, consolidation, gist extraction, integration, and smart forgetting.
Acquisition
Restoration of the capacity to acquire new memories is one of the most evident benefits of sleep. For example, episodic memory retention is substantially impaired if the training session follows sleep deprivation, despite no change in reaction time at training, suggesting a decrease in encoding ability due to sleep loss (Yoo et al., 2007). Similarly, the encoding of novel images is impaired after a night of mild sleep disruption, which decreases SWA without reducing total sleep time (Van Der Werf et al., 2009). Conversely, a nap in which slow oscillations were enhanced by transcranial stimulation, relative to sham stimulation, enhanced the encoding of pictures, word pairs, and word lists (Antonenko et al., 2013). Synaptic renormalization provides a straightforward account of these beneficial effects of sleep, since the desaturation of synaptic weights (Olcese et al., 2010), the improvement in energy availability, and the reduction in cellular stress all lead to an improved ability to learn.
Consolidation
Activity-dependent down-selection of synapses can also explain various aspects of memory consolidation. At first it may seem implausible that synaptic weakening could enhance memory, until one considers that synapses supporting new memories may depress less than synapses supporting memories that are weak or less integrated with previous memories (Fig. 1). For example, a sequence-learning paradigm representative of non-declarative tasks that benefit from sleep was implemented in a large-scale model of the corticothalamic system equipped with a STDP-like down-selection rule (Olcese et al., 2010). When the model learned a sequence of activations during wake, the learned sequence was preferentially reactivated during sleep, and reactivation declined over time, in line with experimental results (Ji and Wilson, 2007; Kudrimoti et al., 1999). The simulations showed that, by biasing the STDP-like plasticity rule towards depression during sleep, weaker synapses were depressed more than stronger ones, with the result that S/N increased and learned sequences were better recalled by the model, in agreement with experimental results. Similar results were obtained with a downscaling rule under a threshold of minimal efficacy (Hill et al., 2008) and with a down-selection rule that protected the synapses that were most activated (Nere et al., 2013). In summary, different down-selection rules implemented in different models consistently yielded an increase in S/N and performance, potentially accounting for the consolidation of procedural memories. It remains to be determined whether specific down-selection mechanisms may be engaged in different species, brain circuits, and developmental periods, and whether different rules may offer specific advantages.
Activity-dependent down-selection during sleep also accounted for memory consolidation in a model of paired-associate (‘declarative’) learning (Nere et al., 2013). The simulations found that enhancing activation of a particular memory in the down-selection phase results in a selective enhancement of that memory, in line with experimental results showing the benefits of cuing during sleep (Antony et al., 2012; Bendor and Wilson, 2012; Diekelmann et al., 2011; Rasch et al., 2007; Rudoy et al., 2009). These simulations also examined the effects of further synaptic potentiation in wake and of potentiation during ‘reactivation’ in the sleep mode, followed by downscaling of connections (Lewis and Durrant, 2011). In both cases, S/N, performance, and recall showed a decrease rather than the increase observed with down-selection during sleep. This implies that further potentiation in wake or sleep may result in “overtraining” and saturation of relevant neural circuits, since both “signal” and “noise” synapses are potentiated. Similar conclusions have been reached from perceptual learning experiments in humans using the visual texture discrimination task, one of the best-characterized examples of sleep-dependent memory consolidation (Karni and Sagi, 1993; Karni et al., 1994). In this task, perceptual learning is assumed to occur through synaptic potentiation (Cooke and Bear, 2012) within the neural circuits specific for the trained background orientation (Karni and Sagi, 1991). However, performance in wake declines with overtraining and eventually does not recover even after sleep, consistent with saturation of both signal and noise synapses and in line with the idea that the benefits provided by sleep may be due to desaturation (Censor and Sagi, 2008, 2009).
Gist extraction
Simulations of hierarchically organized networks indicate that down-selection can also account for gist extraction – a prominent feature of memory that appears to be facilitated by sleep (Inostroza and Born, 2013; Lewis and Durrant, 2011; Rasch and Born, 2013; Stickgold and Walker, 2013). Gist extraction is related to the brain's penchant for forming more enduring memories of high-level invariants, such as faces, places, or even maps, than of low-level details and instances of a particular encounter with the environment. In the simulations, a hierarchically organized network was trained in the wake mode with stimuli that shared some invariant features but differed in specific details (Nere et al., 2013). Learning during wake led to the strengthening of many connections, most of all those of neurons in higher cortical areas relating to the invariant concepts. During sleep, connections in higher areas were protected by strong and frequent reactivations, while synaptic depression predominantly weakened synapses associated with details learned by lower cortical areas, in line with the more frequent origin of sleep slow waves in anterior rather than posterior cortices (Massimini et al., 2004; Murphy et al., 2009). A bias for preferential top-down activation during sleep can be predicted based on multiple factors: i) the inherent reversal of the flow of signals from bottom-up to top-down, due to the lack of driving input from low areas associated with the sensory disconnection of sleep; ii) the large number and long time constant of feed-back connections (due to a higher percentage of NMDARs (Self et al., 2012)); iii) the high likelihood that activation of higher areas can produce meaningful activation patterns that percolate top-down through diverging back-connections, in line with the evidence suggesting that cognitive activity during sleep is more akin to imagination than to perception (Nir and Tononi, 2010); iv) the low likelihood that random activations of neurons in lower areas may selectively activate neurons in higher areas through their specialized convergent connectivity. Therefore top-down spontaneous activation during sleep would have a competitive advantage over bottom-up, random activation of lower areas, which would resemble meaningless “TV noise” and thus would fail to percolate bottom up through feed-forward connections. Conceptually, the process of preserving the gist and removing the chaff resembles the increase in S/N through which sleep appears to benefit non-declarative memories. The benefits of sleep for gaining insight of a hidden rule, enhancing the extraction of second-order inferences, and helping abstraction in language-learning children – all tasks that are conceptually related to gist extraction (Stickgold and Walker, 2013) – may also be achieved through similar mechanisms.
Integration
Another prominent feature of memory is that new material is better remembered if it fits with previously learned schemas (Bartlett, 1932), that is, if the new memories are integrated or incorporated with an organized body of old memories (McClelland et al., 1995). Once again, sleep seems to facilitate this process (Inostroza and Born, 2013; Lewis and Durrant, 2011; Rasch and Born, 2013; Stickgold and Walker, 2013). Computer simulations confirm that memory integration can be obtained through down-selection (Nere et al., 2013) whereby new and old memories that fit well together are co-activated strongly and repeatedly during sleep and thus are comparatively protected, while new memories that fit less well with previous knowledge are less activated and are competitively down-selected.
Protection from interference
Sleep can also benefit declarative memories by sheltering them from interference (Alger et al., 2012; Ellenbogen et al., 2006; Korman et al., 2007; Sheth et al., 2012). A simple mechanism by which NREM sleep, like quiet wake, alcohol, and several drugs, can reduce interference is by blocking LTP-like potentiation and thus new learning (Mednick et al., 2011; Wixted, 2004). Another mechanism may involve the molecular or structural “stabilization” of synapses tagged during wake, although direct evidence that sleep may do so is missing. In this context, an interesting possibility is that learning in wake would promote the early / induction phase of synaptic potentiation, while sleep would promote the late / maintenance phase. GluA2-containing AMPARs are strongly involved in constitutive receptor cycling and synaptic depression, while GluA1-containing AMPARs are linked to synaptic potentiation (Kessels and Malinow, 2009). According to current models, the maintenance of synaptic potentiation requires that a constant amount of GluA2-containing AMPARs is preserved at the synaptic membrane, perhaps through the formation of CamKII-NMDA complexes acting as seeds to keep them anchored to the plasma membrane (Sanhueza and Lisman, 2013). Because these complexes are large and are made of many, partly redundant proteins with different lifespan, the turnover of each protein is unlikely to imperil the existence of the complex and thus of the memory (Sanhueza and Lisman, 2013). Thus, if sleep were to actively maintain previously induced synaptic potentiation rather than inducing it de novo, it would likely do so by preventing the removal of synaptic GluA2-containing AMPARs, rather than by promoting the new insertion of GluA1-containing AMPARs. The available evidence, however, suggests that synaptic expression of GluA2-containing AMPARs goes in the same direction as that of GluA1-containing AMPARs, i.e. it is higher in wake than in sleep, although the changes do not reach significance (Vyazovskiy et al., 2008).
Forgetting
Forgetting has been recognized as an important mechanism for dealing efficiently with the inevitable accumulation of unimportant details (Wixted, 2004). According to a recent view, forgetting relies heavily on active decay, and could involve the internalization of GluA2-containing AMPARs during sleep (Hardt et al., 2013). Indeed, computer simulations show that active forgetting, if performed off-line so as to weaken preferentially memory traces that represent details and are less integrated with the overall structure of knowledge, is likely to constitute a major benefit of sleep on memory (Hashmi et al., 2013), offering a potential solution to the plasticity-stability dilemma of learning new associations without wiping out previously learned ones (Abraham and Robins, 2005; Grossberg, 1987). The plasticity-stability dilemma is evident in artificial neural networks, where increasing connection strengths to store new associations can lead to “catastrophic interference” (French, 1999). The brain, despite its large memory capacity, is probably not immune to such problems, and the potential for sleep to help with this issue has been recognized before (Crick and Mitchison, 1995; Robins and McCallum, 1999). Down-selection during sleep provides an efficient and smart means for enforcing an overall renormalization of synaptic strength, thereby avoiding runaway potentiation and catastrophic interference (Hashmi et al., 2013).
Matching
Another benefit of down-selection becomes apparent when considering the systematic alternation between net synaptic potentiation during wake and depression during sleep (Hashmi et al., 2013). Most neurons in the brain only communicate with other neurons and not directly with sensory inputs and motor outputs. However, high levels of neuromodulators during wake alert neurons that they are connected in a ‘grand loop’ with the environment and learning should be enabled. Conversely in sleep, low levels of neuromodulators signal disconnection from the environment, leaving only internal loops operative, and enforce a bias towards smart, selective forgetting (Fig. 2). Over time, the systematic alternation between “connected” potentiation and “disconnected” depression should favor the acquisition of activity patterns related to statistical regularities in the environment that are presumably adaptive, at the expense of activity patterns that are unrelated to the environment and are potentially maladaptive. In this way, sleep can increase the “matching” between the causal structure of the brain and that of the environment to which it is adapted. In principle, matching can be assessed by measuring how much the brain states triggered when interacting with the environment differ from those triggered when it is exposed to uncorrelated noise. In a simple model in which changes in matching could be measured rigorously (Hashmi et al., 2013), the learning rules for potentiation in wake and down-selection in sleep led to a progressive increase in matching over repeated sleep-wake cycles. By contrast, matching decreased if down-selection occurred in wake or if synaptic potentiation occurred during sleep, due to the frequent strengthening of spurious coincidences not sampled from the environment. This result highlights a potential problem with the idea that sleep may help memory through “pseudorehearsal” - the systematic “relearning” of both new and old memories by random reactivation and synaptic potentiation (Robins and McCallum, 1999). By contrast, activity-dependent down-selection can lead to the transfer, transformation, and integration of memories, and to the stimulation of unused circuits, without the pitfalls of spurious potentations.
An alternative view of sleep-dependent memory consolidation: Replay-transfer-potentiation and active system consolidation
An alternative model suggests that sleep benefits memory consolidation by selectively strengthening certain synaptic traces. The original replay-transfer-potentiation model (Born et al., 2006) was inspired by three main sets of observations. First, in line with the standard system consolidation framework (McClelland et al., 1995; Squire et al., 2004), the hippocampus – a fast learner - stores memories for a short time before they are transferred to the cerebral cortex – a slow learner - for long-term storage. Second, firing patterns established during learning in wake are replayed in sleep, especially as accelerated sequences during sharp-wave ripples in NREM sleep, and impressed upon neocortical circuits. This evidence is often tied together with work suggesting that the dialogue between hippocampus and cortex may reverse in direction between wake and sleep (Buzsaki, 1998; Chrobak and Buzsaki, 1994), that the neuromodulatory milieu of sleep may favor outflow from hippocampus in some stages of sleep (Hasselmo, 1999), and that intense activity in the hippocampus during sleep may impinge upon cortex and modify the firing of cortical neurons (Logothetis et al., 2012; Siapas and Wilson, 1998). Third, there is strong evidence that sleep benefits declarative memory consolidation (Born et al., 2006; Diekelmann and Born, 2010; Stickgold and Walker, 2013; Wilhelm et al., 2012). Based on these premises, it is natural to consider the possibility that hippocampal replay during sleep may “transfer” memory representations from a short-term store in the hippocampus to long-term stores in the cortex. Similarly, it is plausible to infer that the activation of hippocampal circuits during sharp-wave ripples, followed by spindles and slow waves in the cortex, may be responsible for memory enhancements after sleep and “system consolidation” (Born et al., 2006). Finally, one can hypothesize that replay during sleep leads to an enhancement of memories through synaptic potentiation in the relevant neural circuits, in a process of “synaptic consolidation.”
While the replay-transfer-potentiation model is straightforward and elegant, some of its assumptions are problematic. Thus, the original idea that memories are transferred from short-term storage in the hippocampus to long-term storage in the cortex has lost support, in favor of the notion that an episodic memory trace is always a hippocampal-neocortical ensemble, where the role of the hippocampal formation is to index and bind together sparse cortical representations (Winocur and Moscovitch, 2011). Over time, memories are likely to be reactivated in multiple contexts, forming multiple related traces that slowly become integrated into a large body of semantic knowledge and lose their episodic character (Winocur and Moscovitch, 2011). There are also indications that neocortical circuits may not be ‘slow learners’ after all, but may rapidly achieve system level consolidation as long as a new memory can be easily assimilated into a body of related knowledge (Tse et al., 2011). It should also be noted that in the down-selection model the very uniqueness of the hippocampal, episodic component of memories would make them unsuitable to gist extraction and more liable to interference from the superposition of new memories, leading to an advantage of the new at the expense of the old in hippocampal circuits; whereas the cortical, semantic component of such memories would benefit from superposition and gist extraction, as is the case with non-declarative memories, leading to an advantage for the signal at the expense of the noise in cortical circuits.
Moreover, most of the evidence indicates that, during NREM sleep, synchronous volleys associated with slow waves percolate from cortex to hippocampus, rather than the other way around. Recent studies in animals and humans show that cortical slow waves typically begin in cortex and only later reach medial temporal lobe structures and the hippocampus (Isomura et al., 2006; Molle et al., 2006; Nir et al., 2011). Thus, most likely during sleep the interactions between cortex and hippocampus are bidirectional (Buhry et al., 2011; Diekelmann and Born, 2010; Ji and Wilson, 2007; Tononi et al., 2006) with up states in the cortex activating the hippocampus in a feed-forward manner, prompting the hippocampus itself to feedback on the cortex with sharp-wave ripple complexes.
More recent accounts of how sleep can benefit memory can be grouped under the general heading of “active system consolidation” models, which have modified and elaborated the standard replay-transfer-potentiation model in several important ways (Diekelmann and Born, 2010; Inostroza and Born, 2013; Lewis and Durrant, 2011; Rasch and Born, 2013; Stickgold and Walker, 2013). First, such models propose that sleep leads to a system-level transformation of memory representations and not just to a straightforward transfer from hippocampus to cortex. Moreover, some aspects of the renormalization model, including the claim that overall synaptic strength decreases during sleep, have been incorporated in the process of active system consolidation. For example, it has been proposed that synapses subject to replay during sleep may first be selectively potentiated and then globally downscaled (Lewis and Durrant, 2011), or may first be “tagged” for potentiation during NREM sleep replays, and then potentiated during subsequent REM sleep (Rasch and Born, 2013). Active system consolidation models can account for many experimental data and have inspired numerous experiments (Mascetti et al., 2013a; Rasch and Born, 2013). However, even in their latest incarnations, such models still differ from the down-selection model on a fundamental issue: whether memory consolidation and integration during sleep are achieved primarily by “instruction” or by “selection.”
Instruction or selection?
In both the active system consolidation model and the down-selection model, spontaneous activity during sleep, especially slow oscillations, spindle oscillations, and sharp-wave ripples, trigger plastic processes that ultimately account for the memory benefits of sleep. There is now evidence that promoting such oscillations can enhance memory consolidation and disrupting them can impair it (reviewed in (Rasch and Born, 2013)). What remains controversial is the direction of synaptic changes (potentiation or depression) during sleep and their synaptic and systems level consequences. In the active system consolidation model, “replays” of recent waking activity patterns in NREM sleep “instruct” learning, determining which connections should be strengthened selectively or “tagged” for subsequent strengthening in REM sleep (Diekelmann and Born, 2010; Rasch and Born, 2013). Such strengthening would explain why sleep not only enhances declarative memories, but also changes their quality, enabling the integration of newly learned material into preexistent schema, the emergence of insight, and even the formation of false memories. By contrast, in the down-selection model, spontaneous activity during sleep samples comprehensively the brain's knowledge basis in a neuromodulatory milieu that promotes depression. In doing so, spontaneous activity “selects” among preexisting memory traces those that are stronger and fit better with the overall organization of memory, protecting them preferentially and leading to the “survival of the fittest,” without requiring new learning. Of note, according to the down-selection model, spontaneous ensemble activation of cortico-hippocampal circuits during sleep does not need to be random, but may be highly structured, as long as it is comprehensive. For example, slow waves are more global early in the night, then become more local (Nir et al., 2011), suggesting that consolidation and integration of memory traces may first be achieved on a larger-scale and then, progressively, in more restricted circuits. Moreover, slow waves not only have varying sources of origin and propagation (Massimini et al., 2004; Murphy et al., 2009; Nir et al., 2011), but typically only involve a subset of brain areas (Nir et al., 2011). It could be that certain slow waves may be triggered preferentially by synapses that were recently strengthened during wake, thus priming certain circuits for preferential consolidation. Similarly, instructions to remember certain material, administered after learning but before sleep, may prime certain pathways for more frequent sleep-dependent consolidation. Which of these two frameworks – instruction and selection – fits better with the available data?
Replay to reinforce or play to select?
Active system consolidation models were initially galvanized by the demonstration of so-called “replays” or reactivations: patterns of neuronal firing during sleep that bear some resemblance to patterns of activity during preceding wake. Replays are especially evident during hippocampal sharp-wave ripples, but they can be demonstrated also during “ON” periods in cortex, corresponding to the up state of the slow oscillation. However, we now know that reactivations occur outside of sleep, i.e. in quiet wakefulness (Davidson et al., 2009; Diba and Buzsaki, 2007; Foster and Wilson, 2006; Karlsson and Frank, 2009; Kudrimoti et al., 1999), during initial learning (Singer et al., 2013), and during many states of cortical activation (Bermudez Contreras et al., 2013). This makes it difficult to understand why, if replays are important for rehearsing memories, animals should risk being asleep if they can do so when awake and monitoring the environment.
An equally serious issue is that replays during sleep are comparatively infrequent, are not very faithful to the original, are usually played several times faster, and decline rapidly during the first hour of sleep (Ji and Wilson, 2007; Kudrimoti et al., 1999; Nadasdy et al., 1999). Rare, noisy reactivations would not seem ideal for enhancing memories. Moreover, if replays strengthen the associated memory traces, why should replays themselves fade rapidly? Above all, what should one make of the overwhelming proportion of spontaneous activity that does not constitute replays? Even during quiet wake, firing sequences of CA1 hippocampal cells do not only replay previous events, but may instead anticipate (“preplay”) those that will be triggered by future interactions with the environment, suggesting that spontaneous firing patterns can be recruited to make plans and encode new memories (Dragoi and Tonegawa, 2011; Pfeiffer and Foster, 2013). In fact, since the brain is spontaneously active, it can be expected by default to “replay,” “preplay,” and just plain “play” many different combinations from its vast repertoire of memories (as suggested by dreams), whether old or recently modified, in wake or in sleep (Gupta et al., 2010). What is not easy to explain in an instructive model, then, is how the sleeping brain, disconnected from the environment, could distinguish the “right replays” from the “wrong” ones and make sure that only the former are potentiated, thus avoiding the formation of spurious memories. Of note, while enhanced hippocampal replay could explain why sensory cuing during sleep enhances memory, the beneficial effects of sensory cuing during sleep, as well as of pre-cuing through instructions to remember before sleep, can be explained just as well by increased down-selection triggered by increased activations (Nere et al., 2013).
Does synaptic potentiation occur during sleep?
As we have seen, structural, molecular, and electrophysiological studies consistently indicate that sleep is accompanied by a net depression of synaptic strength, although this evidence so far does not rule out the selective potentiation of a subset of synapses. The case for synaptic potentiation in sleep rests on several grounds. One is based on the assumption that phasic events such as hippocampal sharp-wave ripples and correlated cortical spindles (Siapas and Wilson, 1998; Sirota et al., 2003) may provide conditions conducive to long-term potentiation (e.g. (Buzsaki, 1989; Louie and Wilson, 2001; Pennartz et al., 2004)), because they may result in a large influx of calcium inside dendrites (Sejnowski and Destexhe, 2000; Steriade and Timofeev, 2003). However, it was recently shown that antidromic spikes produced during sharp-wave ripples produce an overall downscaling of synaptic strength through L-type calcium channel activation (Bukalo et al., 2013). In vitro and in vivo studies show that electrical stimulation near 10 Hz, which spans the spindle range (7-14 Hz), can result in either synaptic potentiation or depression, depending on the intensity of the stimulation and the pattern of cortical activity (Rosanova and Ulrich, 2005; Werk and Chapman, 2003; Werk et al., 2006). Moreover, high frequency stimulation in hippocampus consistently induces synaptic potentiation during wake and REM sleep, but rarely during NREM sleep (Bramham and Srebro, 1989; Leonard et al., 1987). Finally, the most ubiquitous and frequent pattern of activity during NREM sleep is burst-pause activity at around 0.8 Hz, corresponding to the up and down states of the slow oscillation, which leads to synaptic depression ((Lante et al., 2011), see also (Czarnecki et al., 2007)).
Another rationale is provided by imaging studies indicating that the relative activation of several brain areas increases during post-sleep retest but not at encoding (Mascetti et al., 2013a). These results are interpreted as evidence for the selective potentiation of connections during sleep. Yet, relative changes in fMRI responses after sleep could also result from a down-selection process, whereby certain memory traces are protected more than others from depression, changing the “synaptic landscape” of the brain.
Then there are some molecular studies in rats indicating that induction of electrical LTP or novel experience increase cortical expression of the immediate early genes zif-268 and Arc during REM sleep, though not during NREM sleep (Ribeiro et al., 2002; Ribeiro et al., 2007). Reactivation during NREM sleep may set the stage for the induction of synaptic potentiation during a subsequent REM sleep episode (Diekelmann and Born, 2010; Rasch and Born, 2013). However, the link between zif-268 and synaptic potentiation remains indirect (Davis et al., 2003; Knapska and Kaczmarek, 2004). Moreover, recent studies show that after early induction in response to neuronal activation, Arc enters weakly stimulated synapses and promote their depression via endocytosis of AMPARs (Okuno et al., 2012), and/or enters the nucleus to mediate cell-wide synaptic downscaling by repressing the transcription of the same receptors (Korb et al., 2013). Other experiments found that active avoidance learning increases the density of ponto-geniculo-occipital (PGO) waves during post-learning REM sleep, and this increase is correlated with the subsequent consolidation of the task (Datta, 2000). The expression of Arc, P-CREB, BDNF and zif-268 also increases in several brain areas 1-6 hours after avoidance learning (Ulloor and Datta, 2005), but whether the induction of these activity-dependent genes occurs specifically during REM sleep after training, and whether it is causally linked to the consolidation of the avoidance task, remains unclear. Altogether, how REM sleep may contribute to memory consolidation remains an open issue (see below).
A final rationale comes from developmental studies using monocular deprivation, which triggers first a decrease in the response of the deprived eye due to synaptic depression, followed by an increase in the response of the open eye due to homosynaptic and/or heterosynaptic potentiation (Smith et al., 2009). In kittens, an increase in the open eye response occurs during the 6 hours of sleep following eye closing (Frank et al., 2001). This result and the identification of a narrow window of 1-2 hours during which the phosphorylation of CamKII and other molecular markers of synaptic potentiation increases during sleep (Aton et al., 2009), poses a challenge to the down-selection model (Frank, 2012). However, while acute monocular deprivation is a powerful paradigm for investigating the occurrence and mechanisms of plasticity, it is also non-physiological. Under such conditions wake is accompanied not by the usual net potentiation, but by massive synaptic depression, and it is followed by a 40% decrease in slow waves during subsequent sleep (Miyamoto et al., 2003). What these experiments show, then, is that under special conditions synaptic potentiation can be induced during sleep, consistent with previous evidence that LTP induction during NREM sleep is difficult but not impossible (Bramham and Srebro, 1989; Leonard et al., 1987). However, these findings say less about the role played by sleep under physiological conditions.
Are new associations formed during sleep?
If replays and, more generally, neural activity during sleep were able to potentiate synapses and transform memories, they should also be able, under the right conditions, to promote the learning of new associations. However, a consistent feature of declarative memory consolidation is that, after acquisition, declarative memories do not improve in absolute terms, but always deteriorate. Thus, the positive effect of sleep on retention is that it slows down forgetting of certain memories. This observation is clearly more in line with relative down-selection rather than with absolute potentiation. Procedural memories do get better in absolute terms, due either to a net increase in speed of performance, as in visual discrimination learning, or in accuracy, as in visuomotor learning, both of which depend on slow wave sleep (Aeschbach et al., 2008; Landsness et al., 2009). Yet even in these cases, computer simulations show that an absolute increase in S/N can be obtained through down-selection (Hill et al., 2008; Nere et al., 2013; Olcese et al., 2010).
Three other cases suggesting that sleep may positively strengthen memory traces and create new associations are insight, false memories, and sleep-learning. The emergence of insight after sleep was shown using a modified version of the number reduction task, which subjects can solve slowly, by applying the instructions they are given at the onset of training, or quickly, if they realize that there is a hidden rule to reach the final solution. Subjects who slept were twice more likely to gain insight of the hidden rule than whose who stayed awake (Wagner et al., 2004), suggesting that sleep may have created new associations that may have led to insight. However, fMRI data indicates that insight solutions activate a specific brain region, the anterior superior temporal gyrus, more than non-insight solutions, and that the same area is already activated during the initial solving efforts (Jung-Beeman et al., 2004). Thus it may be that sleep, rather than creating an insight solution from scratch, simply lets it emerge more clearly after removing the “noise” around it, as suggested by the simulations of gist extraction discussed earlier in this review.
False recall is classically tested using the Deese-Roediger-McDermott paradigm, in which memorizing a list of related words (e.g. bed, rest, tired, dream, snooze, nap), elicits high recall of a “lure,” a word that is semantically associated to the list of studied words, but is never presented at training (e.g. sleep). False memories have been shown to increase after sleep relative to wake in some studies (Darsaud et al., 2011; Payne et al., 2009), but not in others (Diekelmann et al., 2008; Fenn et al., 2009). Even when sleep increased false recall, however, the false memories were already present at the end of training. Once again, it seems that sleep did not create new false memories from scratch, but at most slowed down their forgetting (Fenn et al., 2009; Payne et al., 2009).
As for sleep-learning, well-controlled studies have failed to show the transfer of any learning for declarative material from sleep to the following wake (Roth et al., 1988; Wyatt et al., 1994). If the EEG is monitored carefully, there is virtually no recall of verbal material presented during sleep (Simon and Emmons, 1956) unless subjects are awakened (Koukkou and Lehmann, 1968; Portnoff et al., 1966). On the other hand, some forms of non-declarative memory may be acquired during sleep. For example, subjects could learn a simple conditioned response (an EEG K complex triggered by a tone associated with a shock) and the learned response could be transferred to waking (Beh and Barratt, 1965). Also, in a recent study in which the sleep EEG was carefully monitored (Arzi et al., 2012), subjects did learn to inhale larger volumes when conditioned with tones paired with pleasant odors. However, simple conditioning is quite different from declarative learning (Stickgold, 2012) or even skill learning. Moreover, we know now that during EEG-defined sleep individual brain regions can be in a wake-like state (Nir et al., 2011). Thus, it is possible that the conditioning procedures may have induced a local wake-like activation that could not be detected with traditional EEG.
In summary, while a definitive assessment is still premature, there is no clear evidence that sleep can normally lead to new learning, that it can promote synaptic potentiation, and that during sleep the brain can distinguish between “replays” that should be potentiated and “plays” that should not.
Some open issues
In the future, systematic optogenetic stimulation of multiple inputs to a given neuron, simultaneously with calcium imaging in vivo, may establish more directly what happens to individual synapses during wake and sleep and adjudicate between the active system consolidation and down-selection models. Moreover, other important features of sleep will have to be addressed, including the role of REM sleep, the effects of sleep deprivation, and the function of sleep during development, as will be briefly discussed below.
REM sleep
While some of the evidence that wake leads to an increase in synaptic strength and sleep to its homeostatic renormalization points to a specific role for NREM sleep, many of the findings suggestive of renormalization were obtained in relation to total sleep, not just NREM sleep. Moreover, synaptic homeostasis occurs in invertebrates such as flies, where there is so far no indication for a distinction between different sleep stages. It is thus natural to ask how REM sleep may or may not fit with the hypothesized core function of sleep, and whether the regular alternation of NREM/REM periods in most mammals and birds may serve a particular function (Giuditta et al., 1995). These are classic questions that have implications for both the ontogeny and the phylogeny of REM sleep (Jouvet, 1998).
It has been argued that REM sleep may not serve an essential function, at least with respect to memory, since it can be largely suppressed for months by antidepressant treatment, or even permanently by brainstem lesions, without obvious ill effects (Siegel, 2001). On the other hand, REM sleep can have memory benefits (Graves et al., 2001; Karni et al., 1994), and the PGO waves, phasic events that are triggered by burst firing in the pons but extend to the forebrain, could be one of the underlying mechanisms (Datta, 2000). In rodents, REM sleep is also characterized by the occurrence of regular theta oscillations in the hippocampus. Theta oscillations also occur during active exploration in wake, travel across the hippocampal formation (Lubenov and Siapas, 2009), and can modulate plasticity in complex ways (Holscher et al., 1997; Poe et al., 2010). An intriguing possibility is that REM sleep may promote the insertion of AMPARs in the synaptic sites that are still effective after renormalization during NREM sleep, thus favoring their consolidation (Tononi and Cirelli, 2003). Similarly, it may potentiate synapses that were “tagged” by replays during NREM sleep (Rasch and Born, 2013). It may also stimulate unused synapses, another possible function that has been repeatedly attributed to sleep in general (Kavanau, 1997; Krueger and Obal, 1993, 2003), or prompt the formation of new synaptic contacts to refresh the repertoire of circuits available for the acquisition and selection of new memories.
An alternative possibility is suggested by the observation that intense spontaneous activity can lead to the cleansing of uncorrelated synapses and to the relative consolidation of correlated ones (Cohen-Cory, 2002; Zhou et al., 2003). In this view, REM sleep could lead, with different means and perhaps for different brain structures, to results similar to the ones postulated here for NREM sleep in the cerebral cortex of mammals and birds (Tononi and Cirelli, 2003). In a recent study (Grosmark et al., 2012), firing rates of hippocampal CA1 neurons decreased across sleep, as they do in cortex (Vyazovskiy et al., 2009), but did so in REM sleep as a function of REM theta power, and not as a function of SWA in NREM sleep, as they do in the cortex (Vyazovskiy et al., 2009). If these progressive changes in firing rate are indicative of changes of neuronal excitability and net synaptic strength, they would suggest that synaptic renormalization is brought about by SWA during NREM in the cortex, and by theta activity during REM sleep in the hippocampus. In this regard, it is notable that hippocampal cells lack the slow oscillation. Unlike cortical cells, hippocampal granule cells and CA3 and CA1 pyramidal cells do not show OFF states and are not bistable (Isomura et al., 2006). Considering the likely role of the slow oscillation in promoting synaptic depression during sleep, it may be that synaptic renormalization in the hippocampus uses different mechanisms, tied to theta/gamma activities, which are its dominant oscillatory modes. Indeed, there are several indications that the phase of theta activity can influence the direction of plasticity (Holscher et al., 1997; Poe et al., 2010), and the dynamics of theta-gamma coordination are different in REM sleep and wake (Montgomery et al., 2008). Finally, as in the cortex, the levels of neuromodulators such as noradrenaline, serotonin, and histamine are high in wake and low in REM sleep, potentially affecting the direction of plastic changes. In summary, while REM sleep could contribute to memory in many intriguing ways, we still do not know for sure if it is even necessary, and if so how it would perform its functions.
Sleep deprivation and local sleep in wake
Acutely extending wake or chronically curtailing sleep impairs many cognitive functions. The underlying cellular mechanisms remain unclear, but the recent identification of “local sleep” during wake offers some clues (Vyazovskiy et al., 2011). Multiarray recordings in rats show that, the longer an animal stays awake, the more its cortical neurons show brief periods of silence that are essentially indistinguishable from the OFF periods associated with the slow oscillations of sleep. These OFF periods are local in that they occur at different times in different brain regions, and are associated with a local EEG slow/theta wave (2-6 Hz). Since local OFF periods in wake are remarkably similar to sleep OFF periods, they may also result from increased neuronal bistability caused by an increased drive toward hyperpolarization. In turn, hyperpolarization could be a local consequence of synaptic overload caused by intense wake plasticity leading to an imbalance between energy supply and demand, possibly signaled by a local increase in extracellular adenosine (Brambilla et al., 2005). At present, it is unknown whether local OFF periods can carry out some of the restorative functions of sleep, including synaptic homeostasis. However, the occurrence of local OFF periods raises interesting new questions. For example, if local sleep in wake occurred in hypothalamic and brainstem neurons that exert a central control on arousal, it could help explain the increased sleepiness and global deficits in arousal and attention after sleep deprivation, especially for simple, boring tasks (Killgore, 2010; Lim and Dinges, 2010). Intriguingly, overall performance in sleep-deprived subjects is highly unstable, oscillating back and forth from normal levels to catastrophic mistakes (Doran et al., 2001; Zhou et al., 2011), just as would be expected given the stochastic, all-or-none occurrence of local sleep. In addition, the occurrence of local sleep at times in subcortical, arousal-promoting systems, and at other times in specific cortical areas, could explain the occasional dissociation between overall vigilance and specific cognitive functions under conditions of sleep deprivation (Blatter et al., 2005; Sagaspe et al., 2007; Sandberg et al., 2011).
Development
If sleep serves synaptic homeostasis, then it should do so even more prominently during development (Roffwarg et al., 1966). Childhood and adolescence are times of concentrated learning, which in itself would make sleep particularly important. Moreover, development is characterized by intense synaptic remodeling, with massive synaptogenesis accompanied by massive synaptic pruning. The increase in the number of synapses during early development is explosive and is likely to pose a risk of synaptic overload and associated cellular burdens for both neurons and glia. Thus, sleep may be essential for maintaining homeostasis not just in the strength but also in the number and distribution of synapses. For the usual reasons, such rebalancing is best achieved off-line, when neurons can sample most of their inputs in a comprehensive manner. It is worth noting that, during the initial, experience-independent phases of synaptic formation and refinement (Sanes and Yamagata, 2009), spontaneous activity during sleep might serve to restore synaptic homeostasis also in the positive direction, to avoid the risk that a neuron may end up connected just to a few sources.
As was mentioned above, in adolescent mice wake is associated with a net increase in the number of synapses and sleep with a net decrease, although the total number of synapses does not change appreciably over 24 hours (Maret et al., 2011; Yang and Gan, 2012). Even without changes in number, the addition and survival of some synapses, and the elimination of others, can enforce an activity-dependent process of synaptic rearrangement that increases the specificity of connections. For example, shared inputs may be redistributed to segregated neurons or to distinct dendritic domains within a neuron (Ko et al., 2013; Sanes and Yamagata, 2009; Winnubst and Lohmann, 2012). Cycles of synaptogenesis or synaptic strengthening in wake, followed by down-selection in sleep, may play a role in this process.
By the same token, if down-selection were impaired during developmental critical periods when a progressive weakening of short-range connections occurs in association with the strengthening of long-range connections (Uddin et al., 2010), there could be irreversible consequences on the wiring and function of neural circuits. For example, if circuits involving nearby neurons were activated more frequently than those involving neurons in a distant cortical area, the former would be consolidated, while the latter would be lost permanently. Increases in short-distance connections at the expense of long-distance ones have been reported in autism (Zikopoulos and Barbas, 2010), a developmental disorder with prominent sleep abnormalities (Reynolds and Malow, 2011), though the cause-effect relationships are not known. In the future, by mapping the axonal projections of specific cell types, it should be possible to determine if sleep deprivation or restriction in critical periods during development may alter the refinement of cortical and other connections. If this were the case, sleep loss early in life would lead not only to impaired performance, but also to a permanent mis-wiring of brain circuits.
Conclusions and caveats
So far, direct experimental evidence from structural, molecular, and electrophysiological studies in a variety of species is broadly consistent with the core idea behind SHY – that normal sleep allows the brain to reestablish synaptic and cellular homeostasis challenged by plastic changes occurring during normal wake. The wealth of data about how sleep benefits learning and memory are also compatible with SHY, though other interpretations are certainly possible. Finally, SHY offers a parsimonious rationale for why the brain needs sleep: to renormalize synaptic strength based on a comprehensive sampling of its overall knowledge of the environment, rather than being biased by the particular inputs of a particular waking day.
While we have discussed the strengths of SHY, there are many ways in which SHY may turn out to be wholly or at least partly wrong. One way is if synaptic homeostasis can be accomplished sufficiently in wake. For example, it could be that at a given time only a small subset of brain circuits are engaged by behavior, and all other circuits are effectively off-line even in wake. Conceivably, such off-line circuits could renormalize synaptic strength even while the organism is behaving. What is less easily conceivable is how the brain could determine and control which circuits are being engaged by behavior, and thus expected to potentiate, and which would be off-line, and thus expected to depress. Even so, it is important to emphasize that SHY is a hypothesis about sleep and plasticity under natural conditions, not about which plastic changes may be induced under non-physiological conditions. SHY does not endorse a specific mechanism for potentiation during wake and depression during sleep. Taken as a whole, SHY provides a possible and testable explanation for why sleep, a pervasive behavioral state of environmental disconnection and opportunity cost, may be universally needed to address the plasticity-selectivity and plasticity-stability dilemmas faced by the brain.
Acknowledgments
This work was supported by NIMH (1R01MH091326 and 1R01MH099231 to GT and CC). We thank present and past members of the laboratory and many colleagues for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abraham WC, Robins A. Memory retention--the synaptic stability versus plasticity dilemma. Trends Neurosci. 2005;28:73–78. doi: 10.1016/j.tins.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Aeschbach D, Cutler AJ, Ronda JM. A role for non-rapid-eye-movement sleep homeostasis in perceptual learning. J Neurosci. 2008;28:2766–2772. doi: 10.1523/JNEUROSCI.5548-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aicardi G, Argilli E, Cappello S, Santi S, Riccio M, Thoenen H, Canossa M. Induction of long-term potentiation and depression is reflected by corresponding changes in secretion of endogenous brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 2004;101:15788–15792. doi: 10.1073/pnas.0406960101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alger SE, Lau H, Fishbein W. Slow wave sleep during a daytime nap is necessary for protection from subsequent interference and long-term retention. Neurobiol Learn Mem. 2012;98:188–196. doi: 10.1016/j.nlm.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Antonenko D, Diekelmann S, Olsen C, Born J, Molle M. Napping to renew learning capacity: enhanced encoding after stimulation of sleep slow oscillations. Eur J Neurosci. 2013;37:1142–1151. doi: 10.1111/ejn.12118. [DOI] [PubMed] [Google Scholar]
- 6.Antony JW, Gobel EW, O'Hare JK, Reber PJ, Paller KA. Cued memory reactivation during sleep influences skill learning. Nat Neurosci. 2012;15:1114–1116. doi: 10.1038/nn.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arzi A, Shedlesky L, Ben-Shaul M, Nasser K, Oksenberg A, Hairston IS, Sobel N. Humans can learn new information during sleep. Nat Neurosci. 2012;15:1460–1465. doi: 10.1038/nn.3193. [DOI] [PubMed] [Google Scholar]
- 8.Aton SJ, Seibt J, Dumoulin M, Jha SK, Steinmetz N, Coleman T, Naidoo N, Frank MG. Mechanisms of sleep-dependent consolidation of cortical plasticity. Neuron. 2009;61:454–466. doi: 10.1016/j.neuron.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Attwell D, Gibb A. Neuroenergetics and the kinetic design of excitatory synapses. Nat Rev Neurosci. 2005;6:841–849. doi: 10.1038/nrn1784. [DOI] [PubMed] [Google Scholar]
- 10.Balduzzi D, Tononi G. What can neurons do for their brain? Communicate selectivity with bursts. Theory Biosci. 2013;132:27–39. doi: 10.1007/s12064-012-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barlow HB. The twelfth Bartlett memorial lecture: the role of single neurons in the psychology of perception. Q J Exp Psychol A. 1985;37:121–145. doi: 10.1080/14640748508400927. [DOI] [PubMed] [Google Scholar]
- 12.Barth AL, Poulet JF. Experimental evidence for sparse firing in the neocortex. Trends Neurosci. 2012;35:345–355. doi: 10.1016/j.tins.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Bartlett FC. Remembering: A Study in Experimental and Social Psychology. Cambridge, UK: Cambridge University Press; 1932. [Google Scholar]
- 14.Beh HC, Barratt PE. Discrimination and Conditioning During Sleep as Indicated by the Electroencephalogram. Science. 1965;147:1470–1471. doi: 10.1126/science.147.3664.1470. [DOI] [PubMed] [Google Scholar]
- 15.Bellesi M, Pfister-Genskow M, Maret S, Keles S, Tononi G, Cirelli C. Effects of sleep and wake on oligodendrocytes and their precursors. J Neurosci. 2013;33:14288–14300. doi: 10.1523/JNEUROSCI.5102-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bendor D, Wilson MA. Biasing the content of hippocampal replay during sleep. Nat Neurosci. 2012;15:1439–1444. doi: 10.1038/nn.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bermudez Contreras EJ, Schjetnan AG, Muhammad A, Bartho P, McNaughton BL, Kolb B, Gruber AJ, Luczak A. Formation and Reverberation of Sequential Neural Activity Patterns Evoked by Sensory Stimulation Are Enhanced during Cortical Desynchronization. Neuron. 2013;79:555–566. doi: 10.1016/j.neuron.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 18.Blatter K, Opwis K, Munch M, Wirz-Justice A, Cajochen C. Sleep loss-related decrements in planning performance in healthy elderly depend on task difficulty. J Sleep Res. 2005;14:409–417. doi: 10.1111/j.1365-2869.2005.00484.x. [DOI] [PubMed] [Google Scholar]
- 19.Born J, Rasch B, Gais S. Sleep to remember. Neuroscientist. 2006;12:410–424. doi: 10.1177/1073858406292647. [DOI] [PubMed] [Google Scholar]
- 20.Brady TF, Konkle T, Alvarez GA, Oliva A. Visual long-term memory has a massive storage capacity for object details. Proc Natl Acad Sci U S A. 2008;105:14325–14329. doi: 10.1073/pnas.0803390105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brambilla D, Chapman D, Greene R. Adenosine mediation of presynaptic feedback inhibition of glutamate release. Neuron. 2005;46:275–283. doi: 10.1016/j.neuron.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 22.Bramham CR, Alme MN, Bittins M, Kuipers SD, Nair RR, Pai B, Panja D, Schubert M, Soule J, Tiron A, Wibrand K. The Arc of synaptic memory. Exp Brain Res. 2010;200:125–140. doi: 10.1007/s00221-009-1959-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bramham CR, Srebro B. Synaptic plasticity in the hippocampus is modulated by behavioral state. brain research. 1989;493:74–86. doi: 10.1016/0006-8993(89)91001-9. [DOI] [PubMed] [Google Scholar]
- 24.Braun AR, Balkin TJ, Wesenten NJ, Carson RE, Varga M, Baldwin P, Selbie S, Belenky G, Herscovitch P. Regional cerebral blood flow throughout the sleep-wake cycle. An H2(15)O PET study. Brain. 1997;120:1173–1197. doi: 10.1093/brain/120.7.1173. [DOI] [PubMed] [Google Scholar]
- 25.Buchmann A, Ringli M, Kurth S, Schaerer M, Geiger A, Jenni OG, Huber R. EEG sleep slow-wave activity as a mirror of cortical maturation. Cereb Cortex. 2011;21:607–615. doi: 10.1093/cercor/bhq129. [DOI] [PubMed] [Google Scholar]
- 26.Buhry L, Azizi AH, Cheng S. Reactivation, replay, and preplay: how it might all fit together. Neural Plast. 2011;2011:203462. doi: 10.1155/2011/203462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bukalo O, Campanac E, Hoffman DA, Fields RD. Synaptic plasticity by antidromic firing during hippocampal network oscillations. Proc Natl Acad Sci U S A. 2013;110:5175–5180. doi: 10.1073/pnas.1210735110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bushey D, Tononi G, Cirelli C. Sleep and synaptic homeostasis: structural evidence in Drosophila. Science. 2011;332:1576–1581. doi: 10.1126/science.1202839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buysse DJ, Nofzinger EA, Germain A, Meltzer CC, Wood A, Ombao H, Kupfer DJ, Moore RY. Regional brain glucose metabolism during morning and evening wakefulness in humans: preliminary findings. Sleep. 2004;27:1245–1254. doi: 10.1093/sleep/27.7.1245. [DOI] [PubMed] [Google Scholar]
- 30.Buzsaki G. Two-stage model of memory trace formation: a role for “noisy” brain states. Neuroscience. 1989;31:551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- 31.Buzsaki G. Memory consolidation during sleep: a neurophysiological perspective. J Sleep Res. 1998;7(Suppl 1):17–23. doi: 10.1046/j.1365-2869.7.s1.3.x. [DOI] [PubMed] [Google Scholar]
- 32.Buzsaki G, Csicsvari J, Dragoi G, Harris K, Henze D, Hirase H. Homeostatic maintenance of neuronal excitability by burst discharges in vivo. Cereb Cortex. 2002;12:893–899. doi: 10.1093/cercor/12.9.893. [DOI] [PubMed] [Google Scholar]
- 33.Campbell IG, Feinberg I. Longitudinal trajectories of non-rapid eye movement delta and theta EEG as indicators of adolescent brain maturation. Proc Natl Acad Sci U S A. 2009;106:5177–5180. doi: 10.1073/pnas.0812947106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Censor N, Sagi D. Benefits of efficient consolidation: short training enables long-term resistance to perceptual adaptation induced by intensive testing. Vision Res. 2008;48:970–977. doi: 10.1016/j.visres.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 35.Censor N, Sagi D. Global resistance to local perceptual adaptation in texture discrimination. Vision Res. 2009;49:2550–2556. doi: 10.1016/j.visres.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 36.Chauvette S, Seigneur J, Timofeev I. Sleep oscillations in the thalamocortical system induce long-term neuronal plasticity. Neuron. 2012;75:1105–1113. doi: 10.1016/j.neuron.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho K, Kemp N, Noel J, Aggleton JP, Brown MW, Bashir ZI. A new form of long-term depression in the perirhinal cortex. Nat Neurosci. 2000;3:150–156. doi: 10.1038/72093. [DOI] [PubMed] [Google Scholar]
- 38.Chrobak JJ, Buzsaki G. Selective activation of deep layer (V-VI) retrohippocampal cortical neurons during hippocampal sharp waves in the behaving rat. J Neurosci. 1994;14:6160–6170. doi: 10.1523/JNEUROSCI.14-10-06160.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cirelli C. The genetic and molecular regulation of sleep: from fruit flies to humans. Nat Rev Neurosci. 2009;10:549–560. doi: 10.1038/nrn2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cirelli C, Gutierrez CM, Tononi G. Extensive and divergent effects of sleep and wakefulness on brain gene expression. Neuron. 2004;41:35–43. doi: 10.1016/s0896-6273(03)00814-6. [DOI] [PubMed] [Google Scholar]
- 41.Cirelli C, Pompeiano M, Tononi G. Neuronal gene expression in the waking state: A role for the locus coeruleus. Science. 1996;274:1211–1215. doi: 10.1126/science.274.5290.1211. [DOI] [PubMed] [Google Scholar]
- 42.Cirelli C, Tononi G. Differential expression of plasticity-related genes in waking and sleep and their regulation by the noradrenergic system. J Neurosci. 2000;20:9187–9194. doi: 10.1523/JNEUROSCI.20-24-09187.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clem RL, Barth A. Pathway-specific trafficking of native AMPARs by in vivo experience. Neuron. 2006;49:663–670. doi: 10.1016/j.neuron.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 44.Cohen-Cory S. The developing synapse: construction and modulation of synaptic structures and circuits. Science. 2002;298:770–776. doi: 10.1126/science.1075510. [DOI] [PubMed] [Google Scholar]
- 45.Cohen-Matsliah SI, Motanis H, Rosenblum K, Barkai E. A novel role for protein synthesis in long-term neuronal plasticity: maintaining reduced postburst afterhyperpolarization. J Neurosci. 2010;30:4338–4342. doi: 10.1523/JNEUROSCI.5005-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Collingridge GL, Peineau S, Howland JG, Wang YT. Long-term depression in the CNS. Nat Rev Neurosci. 2010;11:459–473. doi: 10.1038/nrn2867. [DOI] [PubMed] [Google Scholar]
- 47.Cooke SF, Bear MF. Stimulus-selective response plasticity in the visual cortex: an assay for the assessment of pathophysiology and treatment of cognitive impairment associated with psychiatric disorders. Biol Psychiatry. 2012;71:487–495. doi: 10.1016/j.biopsych.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 48.Crick F, Mitchison G. REM sleep and neural nets. Behav Brain Res. 1995;69:147–155. doi: 10.1016/0166-4328(95)00006-f. [DOI] [PubMed] [Google Scholar]
- 49.Czarnecki A, Birtoli B, Ulrich D. Cellular mechanisms of burst firing-mediated long-term depression in rat neocortical pyramidal cells. J Physiol. 2007;578:471–479. doi: 10.1113/jphysiol.2006.123588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Darsaud A, Dehon H, Lahl O, Sterpenich V, Boly M, Dang-Vu T, Desseilles M, Gais S, Matarazzo L, Peters F, et al. Does sleep promote false memories? J Cogn Neurosci. 2011;23:26–40. doi: 10.1162/jocn.2010.21448. [DOI] [PubMed] [Google Scholar]
- 51.Dash MB, Douglas CL, Vyazovskiy VV, Cirelli C, Tononi G. Long-term homeostasis of extracellular glutamate in the rat cerebral cortex across sleep and waking states. J Neurosci. 2009;29:620–629. doi: 10.1523/JNEUROSCI.5486-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Datta S. Avoidance task training potentiates phasic pontine-wave density in the rat: A mechanism for sleep-dependent plasticity. J Neurosci. 2000;20:8607–8613. doi: 10.1523/JNEUROSCI.20-22-08607.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davidson TJ, Kloosterman F, Wilson MA. Hippocampal replay of extended experience. Neuron. 2009;63:497–507. doi: 10.1016/j.neuron.2009.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davis S, Bozon B, Laroche S. How necessary is the activation of the immediate early gene zif268 in synaptic plasticity and learning? Behav Brain Res. 2003;142:17–30. doi: 10.1016/s0166-4328(02)00421-7. [DOI] [PubMed] [Google Scholar]
- 55.de Vivo L, Faraguna U, Nelson AB, Pfister-Genskow M, Klapperich ME, Tononi G, Cirelli C. Developmental patterns of sleep slow wave activity and synaptic density in adolescent mice. Sleep. 2013 in press. [Google Scholar]
- 56.Diba K, Buzsaki G. Forward and reverse hippocampal place-cell sequences during ripples. Nat Neurosci. 2007;10:1241–1242. doi: 10.1038/nn1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 58.Diekelmann S, Buchel C, Born J, Rasch B. Labile or stable: opposing consequences for memory when reactivated during waking and sleep. Nat Neurosci. 2011;14:381–386. doi: 10.1038/nn.2744. [DOI] [PubMed] [Google Scholar]
- 59.Diekelmann S, Landolt HP, Lahl O, Born J, Wagner U. Sleep loss produces false memories. PLoS One. 2008;3:e3512. doi: 10.1371/journal.pone.0003512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dijk DJ, Beersma DGM, Daan S, Bloem GM, Van den Hoofdakker RH. Quantitative analysis of the effects of slow wave sleep deprivation during the first 3 h of sleep on subsequent EEG power density. Eur Arch Psychiatr Neurol Sci. 1987;236:323–328. doi: 10.1007/BF00377420. [DOI] [PubMed] [Google Scholar]
- 61.Dong Z, Bai Y, Wu X, Li H, Gong B, Howland JG, Huang Y, He W, Li T, Wang YT. Hippocampal long-term depression mediates spatial reversal learning in the Morris water maze. Neuropharmacology. 2013;64:65–73. doi: 10.1016/j.neuropharm.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 62.Donlea JM, Ramanan N, Shaw PJ. Use-dependent plasticity in clock neurons regulates sleep need in Drosophila. Science. 2009;324:105–108. doi: 10.1126/science.1166657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Donlea JM, Thimgan MS, Suzuki Y, Gottschalk L, Shaw PJ. Inducing sleep by remote control facilitates memory consolidation in Drosophila. Science. 2011;332:1571–1576. doi: 10.1126/science.1202249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Doran SM, Van Dongen HP, Dinges DF. Sustained attention performance during sleep deprivation: evidence of state instability. Arch Ital Biol. 2001;139:253–267. [PubMed] [Google Scholar]
- 65.Dragoi G, Tonegawa S. Preplay of future place cell sequences by hippocampal cellular assemblies. Nature. 2011;469:397–401. doi: 10.1038/nature09633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ellenbogen JM, Hulbert JC, Stickgold R, Dinges DF, Thompson-Schill SL. Interfering with theories of sleep and memory: sleep, declarative memory, and associative interference. Curr Biol. 2006;16:1290–1294. doi: 10.1016/j.cub.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 67.Esser SK, Hill SL, Tononi G. Sleep homeostasis and cortical synchronization: I. Modeling the effects of synaptic strength on sleep slow waves. Sleep. 2007;30(12):1617–1630. doi: 10.1093/sleep/30.12.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Faraguna U, Vyazovskiy VV, Nelson AB, Tononi G, Cirelli C. A causal role for brain-derived neurotrophic factor in the homeostatic regulation of sleep. J Neurosci. 2008;28:4088–4095. doi: 10.1523/JNEUROSCI.5510-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Feldman DE. Synaptic mechanisms for plasticity in neocortex. Annu Rev Neurosci. 2009;32:33–55. doi: 10.1146/annurev.neuro.051508.135516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fenn KM, Gallo DA, Margoliash D, Roediger HL, 3rd, Nusbaum HC. Reduced false memory after sleep. Learn Mem. 2009;16:509–513. doi: 10.1101/lm.1500808. [DOI] [PubMed] [Google Scholar]
- 71.Foster DJ, Wilson MA. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature. 2006;440:680–683. doi: 10.1038/nature04587. [DOI] [PubMed] [Google Scholar]
- 72.Frank MG. Erasing synapses in sleep: is it time to be SHY? Neural Plast. 2012;2012:264378. doi: 10.1155/2012/264378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Frank MG, Issa NP, Stryker MP. Sleep enhances plasticity in the developing visual cortex. Neuron. 2001;30:275–287. doi: 10.1016/s0896-6273(01)00279-3. [DOI] [PubMed] [Google Scholar]
- 74.French RM. Catastrophic forgetting in connectionist networks. Trends Cogn Sci. 1999;3:128–135. doi: 10.1016/s1364-6613(99)01294-2. [DOI] [PubMed] [Google Scholar]
- 75.Ghilardi M, Ghez C, Dhawan V, Moeller J, Mentis M, Nakamura T, Antonini A, Eidelberg D. Patterns of regional brain activation associated with different forms of motor learning. Brain Res. 2000;871:127–145. doi: 10.1016/s0006-8993(00)02365-9. [DOI] [PubMed] [Google Scholar]
- 76.Gilestro GF, Tononi G, Cirelli C. Widespread changes in synaptic markers as a function of sleep and wakefulness in Drosophila. Science. 2009;324:109–112. doi: 10.1126/science.1166673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Giuditta A, Ambrosini MV, Montagnese P, Mandile P, Cotugno M, Grassi Zucconi G, Vescia S. The sequential hypothesis of the function of sleep. Behav Brain Res. 1995;69:157–166. doi: 10.1016/0166-4328(95)00012-i. [DOI] [PubMed] [Google Scholar]
- 78.Gouet C, Aburto B, Vergara C, Sanhueza M. On the mechanism of synaptic depression induced by CaMKIIN, an endogenous inhibitor of CaMKII. PLoS One. 2012;7:e49293. doi: 10.1371/journal.pone.0049293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Graves L, Pack A, Abel T. Sleep and memory: a molecular perspective. Trends Neurosci. 2001;24:237–243. doi: 10.1016/s0166-2236(00)01744-6. [DOI] [PubMed] [Google Scholar]
- 80.Grosmark AD, Mizuseki K, Pastalkova E, Diba K, Buzsaki G. REM sleep reorganizes hippocampal excitability. Neuron. 2012;75:1001–1007. doi: 10.1016/j.neuron.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grossberg S. Competitive learning: from interactive activation to adaptive resonance. Cognitive science. 1987;11:23–63. [Google Scholar]
- 82.Gruart A, Munoz MD, Delgado-Garcia JM. Involvement of the CA3-CA1 synapse in the acquisition of associative learning in behaving mice. J Neurosci. 2006;26:1077–1087. doi: 10.1523/JNEUROSCI.2834-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gupta AS, van der Meer MA, Touretzky DS, Redish AD. Hippocampal replay is not a simple function of experience. Neuron. 2010;65:695–705. doi: 10.1016/j.neuron.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Haider B, Duque A, Hasenstaub AR, McCormick DA. Neocortical network activity in vivo is generated through a dynamic balance of excitation and inhibition. J Neurosci. 2006;26:4535–4545. doi: 10.1523/JNEUROSCI.5297-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Haider B, Hausser M, Carandini M. Inhibition dominates sensory responses in the awake cortex. Nature. 2013;493:97–100. doi: 10.1038/nature11665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hanlon EC, Faraguna U, Vyazovskiy VV, Tononi G, Cirelli C. Effects of skilled training on sleep slow wave activity and cortical gene expression in the rat. Sleep. 2009;32:719–729. doi: 10.1093/sleep/32.6.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hanlon EC, Vyazovskiy VV, Faraguna U, Tononi G, Cirelli C. Synaptic potentiation and sleep need: clues from molecular and electrophysiological studies. Curr Top Med Chem. 2011;11:2472–2482. doi: 10.2174/156802611797470312. [DOI] [PubMed] [Google Scholar]
- 88.Hardt O, Nader K, Nadel L. Decay happens: the role of active forgetting in memory. Trends Cogn Sci. 2013;17:111–120. doi: 10.1016/j.tics.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 89.Harley C. Noradrenergic and locus coeruleus modulation of the perforant path-evoked potential in rat dentate gyrus supports a role for the locus coeruleus in attentional and memorial processes. Prog Brain Res. 1991;88:307–321. doi: 10.1016/s0079-6123(08)63818-2. [DOI] [PubMed] [Google Scholar]
- 90.Hashmi A, Nere A, Tononi G. Sleep Dependent Synaptic Down-Selection (II): Single Neuron Level Benefits for Matching, Selectivity, and Specificity. Frontiers in Sleep and Chronobiology. 2013;4:144. doi: 10.3389/fneur.2013.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hasselmo M. Neuromodulation: acetylcholine and memory consolidation. Trends Cognit Sci. 1999;3:351–359. doi: 10.1016/s1364-6613(99)01365-0. [DOI] [PubMed] [Google Scholar]
- 92.Herculano-Houzel S. Scaling of brain metabolism with a fixed energy budget per neuron: implications for neuronal activity, plasticity and evolution. PLoS One. 2011;6:e17514. doi: 10.1371/journal.pone.0017514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hill S, Tononi G, Ghilardi MF. Sleep improves the variability of motor performance. Brain Res Bull. 2008;76:605–611. doi: 10.1016/j.brainresbull.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hinard V, Mikhail C, Pradervand S, Curie T, Houtkooper RH, Auwerx J, Franken P, Tafti M. Key electrophysiological, molecular, and metabolic signatures of sleep and wakefulness revealed in primary cortical cultures. J Neurosci. 2012;32:12506–12517. doi: 10.1523/JNEUROSCI.2306-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hinton GE, Dayan P, Frey BJ, Neal RM. The “wake-sleep” algorithm for unsupervised neural networks. Science. 1995;268:1158–1161. doi: 10.1126/science.7761831. [DOI] [PubMed] [Google Scholar]
- 96.Holscher C, Anwyl R, Rowan MJ. Stimulation on the positive phase of hippocampal theta rhythm induces long-term potentiation that can Be depotentiated by stimulation on the negative phase in area CA1 in vivo. J Neurosci. 1997;17:6470–6477. doi: 10.1523/JNEUROSCI.17-16-06470.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hu H, Real E, Takamiya K, Kang MG, Ledoux J, Huganir RL, Malinow R. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell. 2007;131:160–173. doi: 10.1016/j.cell.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 98.Huber R, Esser SK, Ferrarelli F, Massimini M, Peterson MJ, Tononi G. TMS-induced cortical potentiation during wakefulness locally increases slow wave activity during sleep. PLoS ONE. 2007a;2:e276. doi: 10.1371/journal.pone.0000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huber R, Ghilardi MF, Massimini M, Ferrarelli F, Riedner BA, Peterson MJ, Tononi G. Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat Neurosci. 2006;9:1169–1176. doi: 10.1038/nn1758. [DOI] [PubMed] [Google Scholar]
- 100.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 101.Huber R, Maatta S, Esser SK, Sarasso S, Ferrarelli F, Watson A, Ferreri F, Peterson MJ, Tononi G. Measures of cortical plasticity after transcranial paired associative stimulation predict changes in electroencephalogram slow-wave activity during subsequent sleep. J Neurosci. 2008;28:7911–7918. doi: 10.1523/JNEUROSCI.1636-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huber R, Maki H, Rosanova M, Casarotto S, Canali P, Casali AG, Tononi G, Massimini M. Human Cortical Excitability Increases with Time Awake. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huber R, Tononi G, Cirelli C. Exploratory behavior, cortical BDNF expression, and sleep homeostasis. Sleep. 2007b;30:129–139. doi: 10.1093/sleep/30.2.129. [DOI] [PubMed] [Google Scholar]
- 104.Inostroza M, Born J. Sleep for preserving and transforming episodic memory. Annu Rev Neurosci. 2013;36:79–102. doi: 10.1146/annurev-neuro-062012-170429. [DOI] [PubMed] [Google Scholar]
- 105.Isaac JT, Buchanan KA, Muller RU, Mellor JR. Hippocampal place cell firing patterns can induce long-term synaptic plasticity in vitro. J Neurosci. 2009;29:6840–6850. doi: 10.1523/JNEUROSCI.0731-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Isomura Y, Sirota A, Ozen S, Montgomery S, Mizuseki K, Henze DA, Buzsaki G. Integration and segregation of activity in entorhinal-hippocampal subregions by neocortical slow oscillations. Neuron. 2006;52:871–882. doi: 10.1016/j.neuron.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 107.Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci. 2007;10:100–107. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- 108.Jouvet M. Paradoxical sleep as a programming system. J Sleep Res. 1998;7(Suppl 1):1–5. doi: 10.1046/j.1365-2869.7.s1.1.x. [DOI] [PubMed] [Google Scholar]
- 109.Jung-Beeman M, Bowden EM, Haberman J, Frymiare JL, Arambel-Liu S, Greenblatt R, Reber PJ, Kounios J. Neural activity when people solve verbal problems with insight. PLoS Biol. 2004;2:E97. doi: 10.1371/journal.pbio.0020097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Karlsson MP, Frank LM. Awake replay of remote experiences in the hippocampus. Nat Neurosci. 2009;12:913–918. doi: 10.1038/nn.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Karni A, Sagi D. Where practice makes perfect in texture discrimination: evidence for primary visual cortex plasticity. Proc Natl Acad Sci U S A. 1991;88:4966–4970. doi: 10.1073/pnas.88.11.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Karni A, Sagi D. The time course of learning a visual skill. Nature. 1993;365:250–252. doi: 10.1038/365250a0. [DOI] [PubMed] [Google Scholar]
- 113.Karni A, Tanne D, Rubenstein BS, Askenasy JJ, Sagi D. Dependence on REM sleep of overnight improvement of a perceptual skill. Science. 1994;265:679–682. doi: 10.1126/science.8036518. [DOI] [PubMed] [Google Scholar]
- 114.Kattler H, Dijk DJ, Borbely AA. Effect of unilateral somatosensory stimulation prior to sleep on the sleep EEG in humans. J Sleep Res. 1994;3:159–164. doi: 10.1111/j.1365-2869.1994.tb00123.x. [DOI] [PubMed] [Google Scholar]
- 115.Kavanau JL. Memory, sleep and the evolution of mechanisms of synaptic efficacy maintenance. Neuroscience. 1997;79:7–44. doi: 10.1016/s0306-4522(96)00610-0. [DOI] [PubMed] [Google Scholar]
- 116.Kennedy C, Gillin JC, Mendelson W, Suda S, Miyaoka M, Ito M, Nakamura RK, Storch FI, Pettigrew K, Mishkin M, Sokoloff L. Local cerebral glucose utilization in non-rapid eye movement sleep. Nature. 1982;297:325–327. doi: 10.1038/297325a0. [DOI] [PubMed] [Google Scholar]
- 117.Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Killgore WD. Effects of sleep deprivation on cognition. Prog Brain Res. 2010;185:105–129. doi: 10.1016/B978-0-444-53702-7.00007-5. [DOI] [PubMed] [Google Scholar]
- 119.Kim SJ, Linden DJ. Ubiquitous plasticity and memory storage. Neuron. 2007;56:582–592. doi: 10.1016/j.neuron.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 120.Kleim JA, Bruneau R, Calder K, Pocock D, VandenBerg PM, MacDonald E, Monfils MH, Sutherland RJ, Nader K. Functional organization of adult motor cortex is dependent upon continued protein synthesis. Neuron. 2003;40:167–176. doi: 10.1016/s0896-6273(03)00592-0. [DOI] [PubMed] [Google Scholar]
- 121.Knapska E, Kaczmarek L. A gene for neuronal plasticity in the mammalian brain: Zif268/Egr-1/NGFI-A/Krox-24/TIS8/ZENK? Prog Neurobiol. 2004;74:183–211. doi: 10.1016/j.pneurobio.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 122.Ko H, Cossell L, Baragli C, Antolik J, Clopath C, Hofer SB, Mrsic-Flogel TD. The emergence of functional microcircuits in visual cortex. Nature. 2013;496:96–100. doi: 10.1038/nature12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Korb E, Wilkinson CL, Delgado RN, Lovero KL, Finkbeiner S. Arc in the nucleus regulates PML-dependent GluA1 transcription and homeostatic plasticity. Nat Neurosci. 2013;16:874–883. doi: 10.1038/nn.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Korman M, Doyon J, Doljansky J, Carrier J, Dagan Y, Karni A. Daytime sleep condenses the time course of motor memory consolidation. Nat Neurosci. 2007;10:1206–1213. doi: 10.1038/nn1959. [DOI] [PubMed] [Google Scholar]
- 125.Kostin A, Stenberg D, Porkka-Heiskanen T. Effect of sleep deprivation on multi-unit discharge activity of basal forebrain. J Sleep Res. 2010;19:269–279. doi: 10.1111/j.1365-2869.2009.00791.x. [DOI] [PubMed] [Google Scholar]
- 126.Koukkou M, Lehmann D. EEG and memory storage in sleep experiments with humans. Electroencephalogr Clin Neurophysiol. 1968;25:455–462. doi: 10.1016/0013-4694(68)90155-7. [DOI] [PubMed] [Google Scholar]
- 127.Krueger JM, Obal F. A neuronal group theory of sleep function. J Sleep Res. 1993;2:63–69. doi: 10.1111/j.1365-2869.1993.tb00064.x. [DOI] [PubMed] [Google Scholar]
- 128.Krueger JM, Obal F., Jr Sleep function. Front Biosci. 2003;8:d511–519. doi: 10.2741/1031. [DOI] [PubMed] [Google Scholar]
- 129.Krueger JM, Tononi G. Local use-dependent sleep; synthesis of the new paradigm. Curr Top Med Chem. 2011;11:2490–2492. doi: 10.2174/156802611797470330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kubota S, Rubin J, Kitajima T. Modulation of LTP/LTD balance in STDP by an activity-dependent feedback mechanism. Neural Netw. 2009;22:527–535. doi: 10.1016/j.neunet.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 131.Kudrimoti HS, Barnes CA, McNaughton BL. Reactivation of hippocampal cell assemblies: effects of behavioral state, experience, and EEG dynamics. Journal Of Neuroscience. 1999;19:4090–4101. doi: 10.1523/JNEUROSCI.19-10-04090.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kuhl D, Kennedy TE, Barzilai A, Kandel ER. Long-term sensitization training in Aplysia leads to an increase in the expression of BiP, the major protein chaperon of the ER. J Cell Biol. 1992;119:1069–1076. doi: 10.1083/jcb.119.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Landsness EC, Crupi D, Hulse BK, Peterson MJ, Huber R, Ansari H, Coen M, Cirelli C, Benca RM, Ghilardi MF, Tononi G. Sleep-dependent improvement in visuomotor learning: a causal role for slow waves. Sleep. 2009;32:1273–1284. doi: 10.1093/sleep/32.10.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lante F, Toledo-Salas JC, Ondrejcak T, Rowan MJ, Ulrich D. Removal of synaptic Ca(2)+-permeable AMPA receptors during sleep. J Neurosci. 2011;31:3953–3961. doi: 10.1523/JNEUROSCI.3210-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Legenstein R, Maass W. Branch-specific plasticity enables self-organization of nonlinear computation in single neurons. J Neurosci. 2011;31:10787–10802. doi: 10.1523/JNEUROSCI.5684-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Leonard BJ, McNaughton BL, Barnes CA. Suppression of hippocampal synaptic plasticity during slow-wave sleep. Brain Res. 1987;425:174–177. doi: 10.1016/0006-8993(87)90496-3. [DOI] [PubMed] [Google Scholar]
- 137.Lewis PA, Durrant SJ. Overlapping memory replay during sleep builds cognitive schemata. Trends Cogn Sci. 2011;15:343–351. doi: 10.1016/j.tics.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 138.Lim J, Dinges DF. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol Bull. 2010;136:375–389. doi: 10.1037/a0018883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu ZW, Faraguna U, Cirelli C, Tononi G, Gao XB. Direct evidence for wake-related increases and sleep-related decreases in synaptic strength in rodent cortex. J Neurosci. 2010;30:8671–8675. doi: 10.1523/JNEUROSCI.1409-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Logothetis NK, Eschenko O, Murayama Y, Augath M, Steudel T, Evrard HC, Besserve M, Oeltermann A. Hippocampal-cortical interaction during periods of subcortical silence. Nature. 2012;491:547–553. doi: 10.1038/nature11618. [DOI] [PubMed] [Google Scholar]
- 141.Louie K, Wilson MA. Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron. 2001;29:145–156. doi: 10.1016/s0896-6273(01)00186-6. [DOI] [PubMed] [Google Scholar]
- 142.Lubenov EV, Siapas AG. Decoupling through Synchrony in Neuronal Circuits with Propagation Delays. Neuron. 2008;58:118–131. doi: 10.1016/j.neuron.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 143.Lubenov EV, Siapas AG. Hippocampal theta oscillations are travelling waves. Nature. 2009;459:534–539. doi: 10.1038/nature08010. [DOI] [PubMed] [Google Scholar]
- 144.Mackiewicz M, Shockley KR, Romer MA, Galante RJ, Zimmerman JE, Naidoo N, Baldwin DA, Jensen ST, Churchill GA, Pack AI. Macromolecule biosynthesis - a key function of sleep. Physiol Genomics. 2007;31:441–457. doi: 10.1152/physiolgenomics.00275.2006. [DOI] [PubMed] [Google Scholar]
- 145.Madsen PL, Vorstrup S. Cerebral blood flow and metabolism during sleep. Cerebrovascular and Brain Metabolism Reviews. 1991;3:281–296. [PubMed] [Google Scholar]
- 146.Maret S, Faraguna U, Nelson A, Cirelli C, Tononi G. Sleep and wake modulate spine turnover in the adolescent mouse cortex. Nature Neuroscience. 2011;14:1418–1420. doi: 10.1038/nn.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Markram H, Lubke J, Frotscher M, Roth A, Sakmann B. Physiology and anatomy of synaptic connections between thick tufted pyramidal neurones in the developing rat neocortex. J Physiol. 1997;500(Pt 2):409–440. doi: 10.1113/jphysiol.1997.sp022031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mascetti L, Foret A, Schrouff J, Muto V, Dideberg V, Balteau E, Degueldre C, Phillips C, Luxen A, Collette F, et al. Concurrent synaptic and systems memory consolidation during sleep. J Neurosci. 2013a;33:10182–10190. doi: 10.1523/JNEUROSCI.0284-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mascetti L, Muto V, Matarazzo L, Foret A, Ziegler E, et al. The impact of visual perceptual learning on sleep and local slow-wave initiation. Journal of Neuroscience. 2013b;33:3323–3331. doi: 10.1523/JNEUROSCI.0763-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Massimini M, Huber R, Ferrarelli F, Hill S, Tononi G. The sleep slow oscillation as a traveling wave. J Neurosci. 2004;24:6862–6870. doi: 10.1523/JNEUROSCI.1318-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Matsuo N, Reijmers L, Mayford M. Spine-type-specific recruitment of newly synthesized AMPA receptors with learning. Science. 2008;319:1104–1107. doi: 10.1126/science.1149967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.McCann CM, Tapia JC, Kim H, Coggan JS, Lichtman JW. Rapid and modifiable neurotransmitter receptor dynamics at a neuronal synapse in vivo. Nat Neurosci. 2008;11:807–815. doi: 10.1038/nn.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.McClelland J, O' Reilly R, McNaughton B. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- 154.Mednick SC, Cai DJ, Shuman T, Anagnostaras S, Wixted JT. An opportunistic theory of cellular and systems consolidation. Trends Neurosci. 2011;34:504–514. doi: 10.1016/j.tins.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Miyamoto H, Katagiri H, Hensch T. Experience-dependent slow-wave sleep development. Nat Neurosci. 2003;6:553–554. doi: 10.1038/nn1064. [DOI] [PubMed] [Google Scholar]
- 156.Molle M, Yeshenko O, Marshall L, Sara SJ, Born J. Hippocampal sharp wave-ripples linked to slow oscillations in rat slow-wave sleep. J Neurophysiol. 2006;96:62–70. doi: 10.1152/jn.00014.2006. [DOI] [PubMed] [Google Scholar]
- 157.Mongrain V, Hernandez SA, Pradervand S, Dorsaz S, Curie T, Hagiwara G, Gip P, Heller HC, Franken P. Separating the contribution of glucocorticoids and wakefulness to the molecular and electrophysiological correlates of sleep homeostasis. Sleep. 2010;33:1147–1157. doi: 10.1093/sleep/33.9.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Montgomery SM, Sirota A, Buzsaki G. Theta and gamma coordination of hippocampal networks during waking and rapid eye movement sleep. J Neurosci. 2008;28:6731–6741. doi: 10.1523/JNEUROSCI.1227-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Murphy M, Riedner BA, Huber R, Massimini M, Ferrarelli F, Tononi G. Source modeling sleep slow waves. Proc Natl Acad Sci U S A. 2009;106:1608–1613. doi: 10.1073/pnas.0807933106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nadasdy Z, Hirase H, Czurko A, Csicsvari J, Buzsaki G. Replay and time compression of recurring spike sequences in the hippocampus. J Neurosci. 1999;19:9497–9507. doi: 10.1523/JNEUROSCI.19-21-09497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Naidoo N, Giang W, Galante RJ, Pack AI. Sleep deprivation induces the unfolded protein response in mouse cerebral cortex. J Neurochem. 2005;92:1150–1157. doi: 10.1111/j.1471-4159.2004.02952.x. [DOI] [PubMed] [Google Scholar]
- 162.Nere A, Hashmi A, Cirelli C, Tononi G. Sleep Dependent Synaptic Down-Selection (I): Modeling the Benefits of Sleep on Memory Consolidation and Integration. Frontiers in Sleep and Chronobiology. 2013;4:143. doi: 10.3389/fneur.2013.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nere A, Olcese U, Balduzzi D, Tononi G. A neuromorphic architecture for object recognition and motion anticipation using burst-STDP. PLoS One. 2012;7:e36958. doi: 10.1371/journal.pone.0036958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nir Y, Staba RJ, Andrillon T, Vyazovskiy VV, Cirelli C, Fried I, Tononi G. Regional slow waves and spindles in human sleep. Neuron. 2011;70:153–169. doi: 10.1016/j.neuron.2011.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Nir Y, Tononi G. Dreaming and the brain: from phenomenology to neurophysiology. Trends Cogn Sci. 2010;14:88–100. doi: 10.1016/j.tics.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Okuno H, Akashi K, Ishii Y, Yagishita-Kyo N, Suzuki K, Nonaka M, Kawashima T, Fujii H, Takemoto-Kimura S, Abe M, et al. Inverse synaptic tagging of inactive synapses via dynamic interaction of Arc/Arg3.1 with CaMKIIbeta. Cell. 2012;149:886–898. doi: 10.1016/j.cell.2012.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Olcese U, Esser SK, Tononi G. Sleep and synaptic renormalization: a computational study. J Neurophysiol. 2010;104:3476–3493. doi: 10.1152/jn.00593.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pawlak V, Wickens JR, Kirkwood A, Kerr JN. Timing is not Everything: Neuromodulation Opens the STDP Gate. Front Synaptic Neurosci. 2010;2:146. doi: 10.3389/fnsyn.2010.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Payne JD, Schacter DL, Propper RE, Huang LW, Wamsley EJ, Tucker MA, Walker MP, Stickgold R. The role of sleep in false memory formation. Neurobiol Learn Mem. 2009;92:327–334. doi: 10.1016/j.nlm.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pennartz CM, Lee E, Verheul J, Lipa P, Barnes CA, McNaughton BL. The ventral striatum in off-line processing: ensemble reactivation during sleep and modulation by hippocampal ripples. J Neurosci. 2004;24:6446–6456. doi: 10.1523/JNEUROSCI.0575-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Perfetti B, Moisello C, Landsness EC, Kvint S, Lanzafame S, Onofrj M, Di Rocco A, Tononi G, Ghilardi MF. Modulation of gamma and theta spectral amplitude and phase synchronization is associated with the development of visuo-motor learning. J Neurosci. 2011;31:14810–14819. doi: 10.1523/JNEUROSCI.1319-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pfeiffer BE, Foster DJ. Hippocampal place-cell sequences depict future paths to remembered goals. Nature. 2013;497:74–79. doi: 10.1038/nature12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Poe GR, Walsh CM, Bjorness TE. Cognitive neuroscience of sleep. Prog Brain Res. 2010;185:1–19. doi: 10.1016/B978-0-444-53702-7.00001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Portnoff G, Baekeland F, Goodenough DR, Karacan I, Shapiro A. Retention of verbal materials perceived immediately prior to onset of non-REM sleep. Percept Mot Skills. 1966;22:751–758. doi: 10.2466/pms.1966.22.3.751. [DOI] [PubMed] [Google Scholar]
- 175.Qin Y, Zhu Y, Baumgart JP, Stornetta RL, Seidenman K, Mack V, van Aelst L, Zhu JJ. State-dependent Ras signaling and AMPA receptor trafficking. Genes Dev. 2005;19:2000–2015. doi: 10.1101/gad.342205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Quirk GJ, Pare D, Richardson R, Herry C, Monfils MH, Schiller D, Vicentic A. Erasing fear memories with extinction training. J Neurosci. 2010;30:14993–14997. doi: 10.1523/JNEUROSCI.4268-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rao Y, Liu ZW, Borok E, Rabenstein RL, Shanabrough M, Lu M, Picciotto MR, Horvath TL, Gao XB. Prolonged wakefulness induces experience-dependent synaptic plasticity in mouse hypocretin/orexin neurons. J Clin Invest. 2007;117:4022–4033. doi: 10.1172/JCI32829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Rasch B, Born J. About sleep's role in memory. Physiol Rev. 2013;93:681–766. doi: 10.1152/physrev.00032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rasch B, Buchel C, Gais S, Born J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science. 2007;315:1426–1429. doi: 10.1126/science.1138581. [DOI] [PubMed] [Google Scholar]
- 180.Reynolds AM, Malow BA. Sleep and autism spectrum disorders. Pediatr Clin North Am. 2011;58:685–698. doi: 10.1016/j.pcl.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 181.Ribeiro S, Mello CV, Velho T, Gardner TJ, Jarvis ED, Pavlides C. Induction of hippocampal long-term potentiation during waking leads to increased extrahippocampal zif-268 expression during ensuing rapid-eye- movement sleep. J Neurosci. 2002;22:10914–10923. doi: 10.1523/JNEUROSCI.22-24-10914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ribeiro S, Shi X, Engelhard M, Zhou Y, Zhang H, Gervasoni D, Lin SC, Wada K, Lemos NA, Nicolelis MA. Novel experience induces persistent sleep-dependent plasticity in the cortex but not in the hippocampus. Front Neurosci. 2007;1:43–55. doi: 10.3389/neuro.01.1.1.003.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Robins A, McCallum S. The consolidation of learning during sleep: comparing the pseudorehearsal and unlearning accounts. Neural Netw. 1999;12:1191–1206. doi: 10.1016/s0893-6080(99)00056-8. [DOI] [PubMed] [Google Scholar]
- 184.Roffwarg HP, Muzio JN, Dement WC. Ontogenetic development of the human sleep-dream cycle. Science. 1966;152:604–619. doi: 10.1126/science.152.3722.604. [DOI] [PubMed] [Google Scholar]
- 185.Rosanova M, Ulrich D. Pattern-specific associative long-term potentiation induced by a sleep spindle-related spike train. J Neurosci. 2005;25:9398–9405. doi: 10.1523/JNEUROSCI.2149-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Roth T, Roehrs T, Zwyghuizen-Doorenbos A, Stepanski E, Wittig R. Sleep and memory. Psychopharmacology Series. 1988;6:140–145. doi: 10.1007/978-3-642-73288-1_10. [DOI] [PubMed] [Google Scholar]
- 187.Rudoy JD, Voss JL, Westerberg CE, Paller KA. Strengthening individual memories by reactivating them during sleep. Science. 2009;326:1079. doi: 10.1126/science.1179013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–88. doi: 10.1126/science.1103944. Epub 2005 Mar 2003. [DOI] [PubMed] [Google Scholar]
- 189.Sagaspe P, Taillard J, Chaumet G, Moore N, B B, Philip P. Aging and Nocturnal Driving: Better with Coffee or a Nap? A Randomized Study. Sleep. 2007;30:1808–1813. doi: 10.1093/sleep/30.12.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sale A, De Pasquale R, Bonaccorsi J, Pietra G, Olivieri D, Berardi N, Maffei L. Visual perceptual learning induces long-term potentiation in the visual cortex. Neuroscience. 2011;172:219–225. doi: 10.1016/j.neuroscience.2010.10.078. [DOI] [PubMed] [Google Scholar]
- 191.Sandberg D, Anund A, Fors C, Kecklund G, Karlsson JG, Wahde M, Akerstedt T. The characteristics of sleepiness during real driving at night--a study of driving performance, physiology and subjective experience. Sleep. 2011;34:1317–1325. doi: 10.5665/SLEEP.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sanes JR, Yamagata M. Many paths to synaptic specificity. Annu Rev Cell Dev Biol. 2009;25:161–195. doi: 10.1146/annurev.cellbio.24.110707.175402. [DOI] [PubMed] [Google Scholar]
- 193.Sanhueza M, Lisman J. The CaMKII/NMDAR complex as a molecular memory. Mol Brain. 2013;6:10. doi: 10.1186/1756-6606-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sejnowski TJ, Destexhe A. Why do we sleep? Brain Res. 2000;886:208–223. doi: 10.1016/s0006-8993(00)03007-9. [DOI] [PubMed] [Google Scholar]
- 195.Self MW, Kooijmans RN, Super H, Lamme VA, Roelfsema PR. Different glutamate receptors convey feedforward and recurrent processing in macaque V1. Proc Natl Acad Sci U S A. 2012;109:11031–11036. doi: 10.1073/pnas.1119527109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Seol GH, Ziburkus J, Huang S, Song L, Kim IT, Takamiya K, Huganir RL, Lee HK, Kirkwood A. Neuromodulators control the polarity of spike-timing-dependent synaptic plasticity. Neuron. 2007;55:919–929. doi: 10.1016/j.neuron.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Shannon BJ, Dosenbach RA, Su Y, Vlassenko AG, Larson-Prior LJ, Nolan TS, Snyder AZ, Raichle ME. Morning-evening variation in human brain metabolism and memory circuits. J Neurophysiol. 2013;109:1444–1456. doi: 10.1152/jn.00651.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sheth BR, Varghese R, Truong T. Sleep shelters verbal memory from different kinds of interference. Sleep. 2012;35:985–996. doi: 10.5665/sleep.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Shinohara Y, Hirase H. Size and Receptor Density of Glutamatergic Synapses: A Viewpoint from Left-Right Asymmetry of CA3-CA1 Connections. Front Neuroanat. 2009;3:10. doi: 10.3389/neuro.05.010.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21:1123–1128. doi: 10.1016/s0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- 201.Siegel JM. The REM sleep-memory consolidation hypothesis. Science. 2001;294:1058–1063. doi: 10.1126/science.1063049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Simon CW, Emmons WH. Responses to material presented during various levels of sleep. J Exp Psychol. 1956;51:89–97. doi: 10.1037/h0043637. [DOI] [PubMed] [Google Scholar]
- 203.Singer AC, Carr MF, Karlsson MP, Frank LM. Hippocampal SWR Activity Predicts Correct Decisions during the Initial Learning of an Alternation Task. Neuron. 2013;77:1163–1173. doi: 10.1016/j.neuron.2013.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sirota A, Csicsvari J, Buhl D, Buzsaki G. Communication between neocortex and hippocampus during sleep in rodents. Proc Natl Acad Sci U S A. 2003;100:2065–2069. doi: 10.1073/pnas.0437938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Smith GB, Heynen AJ, Bear MF. Bidirectional synaptic mechanisms of ocular dominance plasticity in visual cortex. Philos Trans R Soc Lond B Biol Sci. 2009;364:357–367. doi: 10.1098/rstb.2008.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sokoloff L. Metabolism of the central nervous system in vivo. In: Field J, Magoun HW, editors. Handbook of Physiology Neurophysiology. Washington, DC: American Physiological Society; 1960. pp. 1843–1864. [Google Scholar]
- 207.Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 208.Standing L. Learning 10,000 pictures. Q J Exp Psychol. 1973;25:207–222. doi: 10.1080/14640747308400340. [DOI] [PubMed] [Google Scholar]
- 209.Steriade M, Timofeev I. Neuronal plasticity in thalamocortical networks during sleep and waking oscillations. Neuron. 2003;37:563–576. doi: 10.1016/s0896-6273(03)00065-5. [DOI] [PubMed] [Google Scholar]
- 210.Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol. 2001;85:1969–1985. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- 211.Stickgold R. To sleep: perchance to learn. Nat Neurosci. 2012;15:1322–1323. doi: 10.1038/nn.3223. [DOI] [PubMed] [Google Scholar]
- 212.Stickgold R, Walker MP. Sleep-dependent memory triage: evolving generalization through selective processing. Nat Neurosci. 2013;16:139–145. doi: 10.1038/nn.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Tononi G. Integrated information theory of consciousness: an updated account. Arch Ital Biol. 2012;150:56–90. doi: 10.4449/aib.v149i5.1388. [DOI] [PubMed] [Google Scholar]
- 214.Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62:143–150. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 215.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 216.Tononi G, Cirelli C. Time to Be SHY? Some Comments on Sleep and Synaptic Homeostasis. Neural Plast. 2012;2012:415250. doi: 10.1155/2012/415250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Tononi G, Edelman GM, Sporns O. Complexity and coherency: integrating information in the brain. Trends Cogn Sci. 1998;2:474–484. doi: 10.1016/s1364-6613(98)01259-5. [DOI] [PubMed] [Google Scholar]
- 218.Tononi G, Massimini M, Riedner BA. Sleepy dialogues between cortex and hippocampus: who talks to whom? Neuron. 2006;52:748–749. doi: 10.1016/j.neuron.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 219.Tononi G, Sporns O, Edelman GM. A measure for brain complexity: relating functional segregation and integration in the nervous system. Proc Natl Acad Sci U S A. 1994;91:5033–5037. doi: 10.1073/pnas.91.11.5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Tononi G, Sporns O, Edelman GM. A complexity measure for selective matching of signals by the brain. Proc Natl Acad Sci U S A. 1996;93:3422–3427. doi: 10.1073/pnas.93.8.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Tse D, Takeuchi T, Kakeyama M, Kajii Y, Okuno H, Tohyama C, Bito H, Morris RG. Schema-dependent gene activation and memory encoding in neocortex. Science. 2011;333:891–895. doi: 10.1126/science.1205274. [DOI] [PubMed] [Google Scholar]
- 222.Turrigiano G. Homeostatic synaptic plasticity: local and global mechanisms for stabilizing neuronal function. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a005736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Tye KM, Stuber GD, de Ridder B, Bonci A, Janak PH. Rapid strengthening of thalamo-amygdala synapses mediates cue-reward learning. Nature. 2008;453:1253–1257. doi: 10.1038/nature06963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Uddin LQ, Supekar K, Menon V. Typical and atypical development of functional human brain networks: insights from resting-state FMRI. Front Syst Neurosci. 2010;4:21. doi: 10.3389/fnsys.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Ulloor J, Datta S. Spatio-temporal activation of cyclic AMP response element-binding protein, activity-regulated cytoskeletal-associated protein and brain-derived nerve growth factor: a mechanism for pontine-wave generator activation-dependent two-way active-avoidance memory processing in the rat. J Neurochem. 2005;95:418–428. doi: 10.1111/j.1471-4159.2005.03378.x. [DOI] [PubMed] [Google Scholar]
- 226.Van Der Werf YD, Altena E, Schoonheim MM, Sanz-Arigita EJ, Vis JC, De Rijke W, Van Someren EJ. Sleep benefits subsequent hippocampal functioning. Nat Neurosci. 2009;12:122–123. doi: 10.1038/nn.2253. [DOI] [PubMed] [Google Scholar]
- 227.van Welie I, van Hooft JA, Wadman WJ. Homeostatic scaling of neuronal excitability by synaptic modulation of somatic hyperpolarization-activated Ih channels. Proc Natl Acad Sci U S A. 2004;101:5123–5128. doi: 10.1073/pnas.0307711101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Vandenberghe W, Nicoll RA, Bredt DS. Interaction with the unfolded protein response reveals a role for stargazin in biosynthetic AMPA receptor transport. J Neurosci. 2005;25:1095–1102. doi: 10.1523/JNEUROSCI.3568-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Vladyslav VV, Olcese U, Lazimy YM, Faraguna U, Esser SK, Williams JC, Cirelli C, Tononi G. Firing of cortical neurons reflects sleep homeostasis. 2009 doi: 10.1016/j.neuron.2009.08.024. in preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11:200–208. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- 231.Vyazovskiy VV, Harris KD. Sleep and the single neuron: the role of global slow oscillations in individual cell rest. Nat Rev Neurosci. 2013;14:443–451. doi: 10.1038/nrn3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Vyazovskiy VV, Olcese U, Hanlon EC, Nir Y, Cirelli C, Tononi G. Local sleep in awake rats. Nature. 2011;472:443–447. doi: 10.1038/nature10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Vyazovskiy VV, Olcese U, Lazimy YM, Faraguna U, Esser SK, Williams JC, Cirelli C, Tononi G. Cortical firing and sleep homeostasis. Neuron. 2009;63:865–878. doi: 10.1016/j.neuron.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Vyazovskiy VV, Welker E, Fritschy JM, Tobler I. Regional pattern of metabolic activation is reflected in the sleep EEG after sleep deprivation combined with unilateral whisker stimulation in mice. Eur J Neurosci. 2004;20:1363–1370. doi: 10.1111/j.1460-9568.2004.03583.x. [DOI] [PubMed] [Google Scholar]
- 235.Wagner U, Gais S, Haider H, Verleger R, Born J. Sleep inspires insight. Nature. 2004;427:352–355. doi: 10.1038/nature02223. [DOI] [PubMed] [Google Scholar]
- 236.Werk CM, Chapman CA. Long-term potentiation of polysynaptic responses in layer V of the sensorimotor cortex induced by theta-patterned tetanization in the awake rat. Cereb Cortex. 2003;13:500–507. doi: 10.1093/cercor/13.5.500. [DOI] [PubMed] [Google Scholar]
- 237.Werk CM, Klein HS, Nesbitt CE, Chapman CA. Long-term depression in the sensorimotor cortex induced by repeated delivery of 10 Hz trains in vivo. Neuroscience. 2006;140:13–20. doi: 10.1016/j.neuroscience.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 238.Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- 239.Wilhelm I, Prehn-Kristensen A, Born J. Sleep-dependent memory consolidation--what can be learnt from children? Neurosci Biobehav Rev. 2012;36:1718–1728. doi: 10.1016/j.neubiorev.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 240.Winnubst J, Lohmann C. Synaptic clustering during development and learning: the why, when, and how. Front Mol Neurosci. 2012;5:70. doi: 10.3389/fnmol.2012.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Winocur G, Moscovitch M. Memory transformation and systems consolidation. J Int Neuropsychol Soc. 2011;17:766–780. doi: 10.1017/S1355617711000683. [DOI] [PubMed] [Google Scholar]
- 242.Wixted JT. The psychology and neuroscience of forgetting. Annu Rev Psychol. 2004;55:235–269. doi: 10.1146/annurev.psych.55.090902.141555. [DOI] [PubMed] [Google Scholar]
- 243.Wyatt JK, Bootzin RR, Anthony J, Bazant S. Sleep onset is associated with retrograde and anterograde amnesia. Sleep. 1994;17:502–511. doi: 10.1093/sleep/17.6.502. [DOI] [PubMed] [Google Scholar]
- 244.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O'Donnell J, Christensen DJ, Nicholson C, Iliff JJ, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Yang G, Gan WB. Sleep contributes to dendritic spine formation and elimination in the developing mouse somatosensory cortex. Dev Neurobiol. 2012;72:1391–1398. doi: 10.1002/dneu.20996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Yoo SS, Hu PT, Gujar N, Jolesz FA, Walker MP. A deficit in the ability to form new human memories without sleep. Nat Neurosci. 2007;10:385–392. doi: 10.1038/nn1851. [DOI] [PubMed] [Google Scholar]
- 247.Zatorre RJ, Fields RD, Johansen-Berg H. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci. 2012;15:528–536. doi: 10.1038/nn.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zhou Q, Tao HW, Poo MM. Reversal and stabilization of synaptic modifications in a developing visual system. Science. 2003;300:1953–1957. doi: 10.1126/science.1082212. [DOI] [PubMed] [Google Scholar]
- 249.Zhou X, Ferguson SA, Matthews RW, Sargent C, Darwent D, Kennaway DJ, Roach GD. Dynamics of neurobehavioral performance variability under forced desynchrony: evidence of state instability. Sleep. 2011;34:57–63. doi: 10.1093/sleep/34.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Zikopoulos B, Barbas H. Changes in prefrontal axons may disrupt the network in autism. J Neurosci. 2010;30:14595–14609. doi: 10.1523/JNEUROSCI.2257-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]