Abstract
We have recently demonstrated that CD8+ T cells provide a critical contribution to the antineoplastic activity of 2 chemotherapeutic agents, i.e., doxorubicin and lapatinib, in a model of spontaneous mammary carcinogenesis. The activation of CD8+ T cells and their recruitment to neoplastic lesions turned out to rely on signal transduction and activator of transcription 1 (STAT1). Accordingly, STAT1-deficient tumor-bearing mice exhibited an impaired response to chemotherapy.
Keywords: antitumor immunity, CD8+ T cells, CXCR3 ligands, doxorubicin, mammary cancer, lapatinib, STAT1
Numerous reports have demonstrated that some conventional chemotherapeutic agents, which have originally been developed to target malignant cells, may enhance the infiltration of neoplastic lesions by immune cells, a process that may have impact on disease outcome.1 The mechanisms by which chemotherapeutics can influence the host immune system so to contribute to the elicitation of functional antitumor immune responses are subject of intense investigation. Experiments based on transplantation tumor models have revealed that anthracyclines as well as other DNA-damaging agents can trigger the immunogenic demise of malignant cells. This occurs through a series of molecular events that foster the presentation of tumor-associated antigens and hence elicit T-cell-dependent, interferon γ (IFNγ)-based antitumor immune response, thus substantially contributing to the efficacy of chemotherapy.2 One of the drawbacks of transplantation tumor models for the study of tumor-specific immunity elicited by chemotherapy is their rapid and aggressive growth after transplantation. In this setting, malignant cells bypass the early steps of oncogenesis as well as the progressive establishment of an immunosuppressive microenvironment through long-term tumor-host interactions, as it occurs in the development of the majority of autochtonous neoplasms in humans and mice. A well-studied model for tolerizing spontaneous carcinogenesis is provided by mouse mammary tumor virus (MMTV)/neu transgenic mice. In these animals, the rat variant of v-erb-b2 avian erythroblastic leukemia viral oncogene homolog 2 (Erbb2, also known as neu) is specifically overexpressed in mammary epithelial cells, leading to the development of palpable tumors with a latency of about 6 mo.3 Through their lifespan, MMTV-neu mice become tolerant to Erbb2, as demonstrated by their inability to mount a productive CD8+ T-cell response against cancer cells despite the presence of Erbb2-specific CD8+ T lymphocytes.4 CD4+CD25+ regulatory T cells (Tregs) have been implicated in the suppression of Erbb2-specific T-cell clones.5 Whether tumor-associated macrophages (TAMs), the major population of immune cells infiltrating spontaneous, fully-established Erbb2-driven tumors under steady-state conditions,6,7 are also involved in the maintenance of this state of tolerance/immunosuppression remains to be investigated. Of note, the neoplastic lesions developing in MMTV-neu mice are scarcely infiltrated with CD8+ T cells and are devoid of myeloid-derived suppressor cells (MDSCs).
We investigated the impact of 2 chemotherapeutic agents that are frequently employed for the treatment of breast cancer, namely, the anthracycline doxorubicin and the ERBB1/ERBB2 dual kinase inhibitor lapatinib, on the infiltration of Erbb2-driven tumors by immune cells.7 Both these drugs lead to an increase of the number of activated IFNγ-secreting CD4+ and CD8+ T cells within neoplastic lesions (Fig. 1). Moreover, the antineoplastic effects achieved with the combined administration of doxorubicin and lapatinib were significantly impaired upon the antibody-mediated depletion of CD8+ T cells, demonstrating a crucial role for this population in the overall outcome of therapy. By contrast, the depletion of CD4+ T cells augmented the anticancer efficacy of doxorubicin plus lapatinib, via a hitherto unknown mechanism. Thus, as in tumor transplantation settings,8 anthracyclines can induce a therapeutically relevant CD8+ T-cell response in spontaneous tumor models. These findings limit the conclusions of a previous publication based on similar tumor models,9 in which the doxorubicin response was found to be independent of adaptive immunity. These conflicting results may (at least in part) be explained by the differences in the dose and schedule of chemotherapy. Indeed, Ciampricotti et al. initiated therapy at a more advanced stage of tumor progression than us and, contrarily to what we did, they administered doxorubicin on a repetitive schedule, with possible detrimental effects on T-cell abundance and function.
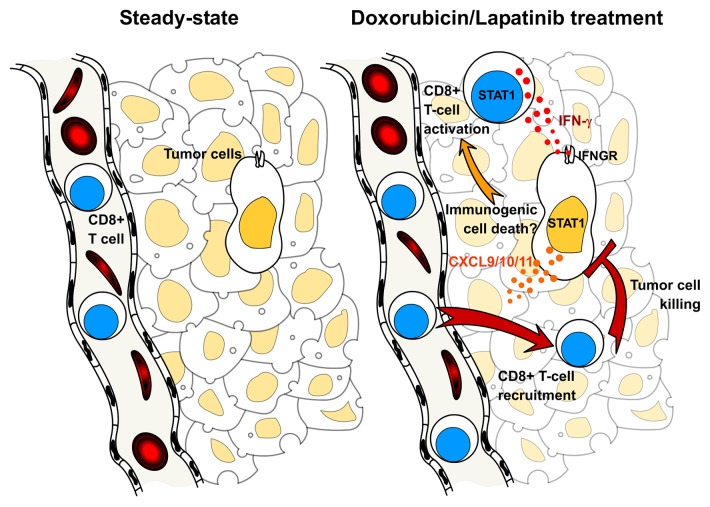
Figure 1. Impact of STAT1 and CXCR3-binding chemokines on the antitumor activity of doxorubicin and lapatinib. The neoplastic lesions developing in mouse mammary tumor virus (MMTV)-neu mice become infiltrated with activated interferon γ (IFNγ)-secreting CD8+ T cells in response to the administration of doxorubicin and/or lapatinib, significantly contributing to the antineoplastic effects of chemotherapy. In this setting, CD8+ T-cell priming is dependent on signal transducer and activator of transcription 1 (STAT1). However, whether STAT1 activation is triggered by the immunogenic demise of cancer cells in vivo remains to be investigated. In response to IFNγ, malignant cells express increased levels of 3 chemokine (C-X-C motif) receptor 3 (CXCR3)-binding chemokines, namely chemokine (C-X-C motif) ligand (CXCL)9, CXCL10 and CXCL11, in a IFNγ receptor (IFNGR)- and STAT1-dependent fashion, which may stimulate the local recruitment of CD8+ T cells.
An important question is how these 2 unrelated chemotherapeutic agents can break immunological tolerance and promote the activation of tumor-specific T cells in the MMTV-neu model. One possibility is that these agents are not only efficient at inducing immunogenic cell death in the neoplastic epithelium, but also deplete or disturb the function of immunosuppressive Tregs and TAMs. This attractive hypothesis remains to be investigated. Further insights into the mechanism whereby T cells are activated and recruited to the tumor and how this contributes to the efficacy of chemotherapy was provided by the study of MMTV-neu mice lacking signal transduction and activator of transcription 1 (STAT1). Strikingly, the growth of Erbb2-driven Stat1−/− tumors was insensitive to doxorubicin and lapatinib. Furthermore, these mice exhibited impaired T-cell activation and a reduced chemotherapy-elicited tumor infiltration as compared with their STAT1-proficient counterparts. The inefficient T-cell priming observed in Stat1−/− can be attributed to the cell-intrinsic role of STAT1 in T-cell expansion and maturation.10 As for the impaired tumor infiltration characterizing these animals, we propose a mechanism relying on the presence of STAT1 in the neoplastic epithelium. Indeed, we demonstrated that STAT1-deficient tumor cells fail to express chemokine (C-X-C motif) receptor 3 (CXCR3) ligands that operate as T-cell attractants, i.e., chemokine (C-X-C motif) ligand (CXCL)9, CXCL10, and CXCL11, in response to IFN-γ, thus exhibiting a reduced ability to recruit primed CD8+ T cells (Fig. 1).
Taken together, our findings point to the vital contribution of CD8+ T-cell mediated immune response to the efficacy of chemotherapy in spontaneous models of tumorigenesis. As these results were obtained in models that closely mimic tolerizing human malignancies, they may provide further insights into the mechanism of action of conventional chemotherapeutics and open a possibility for combining standard anticancer interventions with immunotherapy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Glossary
Abbreviations:
- CXCL
chemokine (C-X-C motif) ligand
- CXCR3
chemokine (C-X-C motif) receptor 3
- IFNγ
interferon γ
- IFNGR
IFNγ receptor
- MMTV
mouse mammary tumor virus
- STAT1
signal transducer and activator of transcription 1
- TAM
tumor-associated macrophage
Citation: Tymoszuk P, Doppler W. Impact of STAT1 and CD8+ T cells on the antineoplastic activity of lapatinib and doxorubicin against spontaneous mammary tumors. OncoImmunology 2013; 2:e26689; 10.4161/onci.26689
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/26689
References
- 1.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 2.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–8. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 3.Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci USA. 1992;89:10578–82. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uram JN, Black CM, Flynn E, Huang L, Armstrong TD, Jaffee EM. Nondominant CD8 T cells are active players in the vaccine-induced antitumor immune response. J Immunol. 2011;186:3847–57. doi: 10.4049/jimmunol.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ercolini AM, Ladle BH, Manning EA, Pfannenstiel LW, Armstrong TD, Machiels JP, Bieler JG, Emens LA, Reilly RT, Jaffee EM. Recruitment of latent pools of high-avidity CD8(+) T cells to the antitumor immune response. J Exp Med. 2005;201:1591–602. doi: 10.1084/jem.20042167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parajuli N, Müller-Holzner E, Böck G, Werner ER, Villunger A, Doppler W. Infiltrating CD11b+CD11c+ cells have the potential to mediate inducible nitric oxide synthase-dependent cell death in mammary carcinomas of HER-2/neu transgenic mice. Int J Cancer. 2010;126:896–908. doi: 10.1002/ijc.24805. [DOI] [PubMed] [Google Scholar]
- 7.Hannesdóttir L, Tymoszuk P, Parajuli N, Wasmer MH, Philipp S, Daschil N, Datta S, Koller JB, Tripp CH, Stoitzner P, et al. Lapatinib and doxorubicin enhance the Stat1-dependent antitumor immune response. Eur J Immunol 2013; ; [DOI] [PubMed]
- 8.Mattarollo SR, Loi S, Duret H, Ma Y, Zitvogel L, Smyth MJ. Pivotal role of innate and adaptive immunity in anthracycline chemotherapy of established tumors. Cancer Res. 2011;71:4809–20. doi: 10.1158/0008-5472.CAN-11-0753. [DOI] [PubMed] [Google Scholar]
- 9.Ciampricotti M, Hau CS, Doornebal CW, Jonkers J, de Visser KE. Chemotherapy response of spontaneous mammary tumors is independent of the adaptive immune system. Nat Med. 2012;18:344–6, author reply 346. doi: 10.1038/nm.2652. [DOI] [PubMed] [Google Scholar]
- 10.Quigley M, Huang X, Yang Y. STAT1 signaling in CD8 T cells is required for their clonal expansion and memory formation following viral infection in vivo. J Immunol. 2008;180:2158–64. doi: 10.4049/jimmunol.180.4.2158. [DOI] [PubMed] [Google Scholar]