Abstract
Dendritic cells (DCs), key players of immunity, are regulated by glycogen synthase kinase GSK3. GSK3 activity is suppressed by PKB/Akt and SGK isoforms, which are in turn stimulated by the PI3K pathway. Exposure to bacterial lipopolysaccharides increases cytosolic Ca2+-concentration ([Ca2+]i), an effect augmented in DCs isolated from mutant mice expressing PKB/SGK-resistant GSK3α,β (gsk3KI). Factors affecting [Ca2+]i include Ca2+-release from intracellular stores (CRIS), store-operated Ca2+-entry (SOCE) through STIM1/STIM2-regulated Orai1, K+-dependent Na+/Ca2+-exchangers (NCKX), K+-independent Na+/Ca2+-exchangers (NCX) and calbindin-D28k. The present study explored whether PKB/SGK-dependent GSK3α, β-activity impacts on CRIS, SOCE, NCKX, NCX or calbindin. DCs were isolated from gsk3KI mice and respective wild-type mice (gsk3WT), [Ca2+]i estimated from Fura2 fluorescence, Orai1, STIM1, STIM2 as well as calbindin-D28k protein abundance determined by Western blotting and mRNA levels quantified by real time PCR. As a result, thapsigargin-induced CRIS and SOCE were significantly blunted by GSK3-inhibitors SB216763 (1–10 µM, 30 min) or GSK-XIII (10 µM, 30 min) but were significantly lower in gsk3WT than in gsk3KIDCs. Orai1, STIM1 and STIM2 protein abundance was significantly lower and calbindin-D28k abundance significantly higher in gsk3KI than in gsk3WTDCs. Activity of NCKX and NCX was significantly higher in gsk3KI than in gsk3WTDCs and was significantly increased by SB216763 (1 µM, 30 min) or GSK-XIII (10 µM, 30 min). Treatment of gsk3WT DCs with SB216763 (1 µM, 4–24 h) or GSK-XIII (10 µM, 4–24 h) did not significantly modify the protein abundance of Orai1, STIM1 and STIM2. The present observations point to a dual role of GSK3 in the regulation of Ca2+ in DCs. Acute inhibition of GSK3 blunted the increase of [Ca2+]i following CRIS and SOCE and stimulated NCKX/NCX activity. However, expression of PKB/SGK-resistant GSK3α, β downregulated the increase of [Ca2+]i following CRIS and SOCE, an effect at least partially due to downregulation of Orai1, STIM1 and STIM2 expression as well as upregulation of Na+/Ca2+-exchanger activity and calbindin D28k expression.
Introduction
The function of dendritic cells (DCs), antigen-presenting cells critically important for primary immune responses and establishment of immunological memory [1], [2], is regulated by cytosolic Ca2+ concentration [3]. Increase of cytosolic Ca2+ concentration may be accomplished in part by store operated Ca2+ entry (SOCE) [4]–[7], which in DCs involves the pore forming channel proteins Orai1 or Orai2 and their regulators STIM1 or STIM2 [8], [9]. The increase of cytosolic Ca2+ concentration is further shaped by cytosolic Ca2+ extrusion mechanisms such as K+-independent (NCX) and K+-dependent (NCKX) Na+/Ca2+ exchangers [10], [11]. Moreover, at least in theory, alterations of free cytosolic Ca2+ concentration could be blunted by cytosolic Ca2+ binding proteins, such as calbindin-D28k [12].
Mechanisms involved in the regulation of cytosolic Ca2+ activity include glycogen synthase kinase 3 (GSK3α,β) [13]. Pharmacological inhibition of GSK3 by SB216763 (3-[2,4-Dichlorophenyl]-4-[1-methyl-1H-indol-3-yl]-1H-pyrrole-2,5-dione, 10 µM, 30 min) blunts lipopolysaccharide (LPS)-induced increase of cytosolic Ca2+ concentration [13]. SB216763 inhibits the activating phosphorylation site tyrosine 216 of GSK3 [14]. GSK3 is further inhibited by the aminopyrazole moiety of GSK-XIII, which blocks the ATP-binding site of GSK3 [15], [16].
GSK3 participates in the regulation of DC function [17]–[21], GSK3 is active in immature DCs and suppresses DCs maturation [20]. Upon toll-like receptor activation, GSK3 fosters the development of a proinflammatory phenotype [17]–[20] and GSK3 inhibitors may suppress immune responses [19]. GSK3 thus modifies the balance between pro- and anti-inflammatory cytokine production [17], [18], [22]–[30]. Beyond that GSK3 may counteract DC survival [31]. In other cell types, GSK3 (GSK3α,β) participates in the regulation of metabolism, cell proliferation, cell differentiation and cell survival [32]–[34] and deranged GSK3β activity contributes to the pathophysiology of diabetes, cancer, inflammation, mood disorders and neurodegeneration [35]–[37].
GSK3 activity could be suppressed by phosphorylation [38], [39]. GSK3 is phosphorylated by protein kinase B (PKB/Akt) [40], [41] and the serum and glucocorticoid inducible kinase SGK1 [42], [43], which are both activated through the phosphoinositide 3 (PI3) kinase pathway [44], [45]. Replacement of the respective PKB/SGK phosphorylation sites in GSK3α and GSK3β by alanine (GSK3α21A/21A, GSK3β9A/9A) renders GSK3 activity insensitive to inhibition by PKB/SGK [46]. Gene targeted mice carrying the respective mutations (gsk3KI) are resistant to the effect of insulin on muscle glycogen synthase [46]. Moreover, in those mice alterations of renal function [47], [48], gastric acid secretion [49], catecholamine release [50], behaviour [51] and IL-10 production following IFN-β treatment [17] have been observed.
Materials and Methods
Ethics Statement
All animal experiments were conducted according to the recommendations of the Guide for Care and Use of Laboratory Animals of the National Institutes of Health as well as the German law for welfare of animals, and the surgical procedures on the mice were reviewed and approved by the respective government authority of the state Baden-Württemberg (Regierungspräsidium) prior to the start of the study.
Mice
Mice were generated in which the codon of the GSK3β gene encoding Ser9 was changed to encode nonphosphorylatable alanine (GSK3β9A/9A), and simultaneously the codon encoding Ser21 of GSK3α was changed to encode alanine (GSK3α21A/21A) thus yielding the GSK3α/β21A/21A/9A/9A double knockin mouse (gsk3KI) as described previously [46]. The mice were compared to corresponding wild type mice (gsk3WT).
Cell Culture
Dendritic cells (DCs) were cultured from bone marrow of 7–12 week old mice. Bone marrow derived cells were flushed out of the cavities from the femur and tibia with PBS. Cells were then washed twice with RPMI and seeded out at a density of 2×106 cells/10 ml per 60-mm dish. Cells were cultured for 8 days in RPMI 1640 (GIBCO, Carlsbad) containing: 10% FCS, 1% penicillin/streptomycin, 1% glutamine, 1% non-essential amino acids (NEAA) and 0.05% β-mercaptoethanol. Cultures were supplemented with GM-CSF (35 ng/mL, Immunotools) and fed with fresh medium containing GM-CSF on days 3 and 6. Experiments were performed on DCs at days 7–9.
Western Blotting
The protein expression levels were analyzed by Western blotting. In brief, DCs from gsk3KI or gsk3WT mice were washed with ice cold phosphate-buffered saline (PBS) and cells were lysed with cell lysis buffer (Cell Signaling Technology, Inc., New England Biolabs). The extracts were centrifuged at 13,000 rpm for 20 min at 4°C and the protein concentration of the supernatant was determined. Total protein (30 µg) was subjected to 10% SDS-PAGE. Proteins were transferred to a nitrocellulose membrane (VWR) and the membranes were then blocked for 1 h at room temperature with 10% non- fat dried milk in tris-buffered saline (TBS) containing 0.1% Tween-20. For immunoblotting the membranes were incubated overnight at 4 °C with antibodies directed against GSK3α/β (D75D3, XP™ antibody, 1∶1000, Cell Signaling Technology, Inc., New England Biolabs, 46, 51 kDa), phospho-GSK3α/β (Ser21/9, 1∶1000, Cell Signaling Technology, Inc., New England Biolabs, 46, 51 kDa), Orai1 (1∶500, Proteintech, Manchester), STIM1 (1∶300, Cell Signaling Technology, Inc., New England Biolabs), STIM2 (1∶300 Cell Signaling Technology, Inc., New England Biolabs) or calbindin-D28k (1∶200, SWANT, Switzerland). A GAPDH antibody (1∶1000, Cell Signaling Technology, Inc., New England Biolabs) was used for a loading control. Specific protein bands were visualized after subsequent incubation with a 1∶3000 dilution of anti-rabbit IgG conjugated to horseradish peroxidase and a Super Signal Chemiluminescence detection procedure (GE Healthcare, UK). Specific bands were quantified by Quantity one software (Bio rad gel doc system, Chemidoc XRS). Levels of each protein were expressed as the ratio of signal intensity for the target protein relative to that of GAPDH.
Real-time PCR
Total RNA was extracted from mouse dendritic cells in TriFast (Peqlab, Erlangen, Germany) according to the manufacturer's instructions. After DNAse digestion reverse transcription of total RNA was performed using Transcriptor High Fidelity cDNA Synthesis kit (Roche). Polymerase chain reaction (PCR) amplification of the respective genes were set up in a total volume of 20 µl using 40 ng of cDNA, 500 nM forward and reverse primer and 2x GoTaq® qPCR Master Mix SYBR Green (Promega Corporation, Madison, WI, USA) according to the manufacturer's protocol. Cycling conditions were as follows: initial denaturation at 95°C for 2 min, followed by 40 cycles of 95°C for 15 sec, 55°C for 15 sec and 72°C for 20 sec. For the amplification the following primers were used (5′->3′orientation):
Orai1, fw CATGGTAGCGATGGTGGAAGTC rev TGCTfCATCGTCTTTAGTGCCT;
Orai2, fw ATGGTGGCCATGGTGGAGGT rev ATTGCCTTCAGCGCCTGCA;
STIM1, fw CTTGGCCTGGGATCTCAGAG rev TCAGCCATTGCCTTCTTGCC;
STIM2, fw GCAGGATCTTTAGCCAGAAG rev ACATCTGCTGTCACGGGTGA;
Calbindin, fw TCCCTCACCTAGAGATAGAAGCAGCGCAG
rev AGACAGCAGAATCGAGGAGTCTGCTGCTC;
Tbp, fw CAAGCTGGAGGTGATCATCG rev TCCACAGTGCTCTTGAATTCG.
Specificity of PCR products was confirmed by analysis of a melting curve. Real-time PCR amplifications were performed on a CFX96 Real-Time System (Bio-Rad). All experiments were done in duplicate. Amplification of the house-keeping gene Tbp (TATA binding protein) was performed to standardize the amount of sample RNA. Relative quantification of gene expression was achieved using the Δct method as described earlier [52].
Measurement of intracellular Ca2+
To determine cytosolic Ca2+ concentration, the cells were loaded with Fura-2/AM (2 µM, Molecular Probes, Goettingen, Germany) for 15 min at 37°C. Fluorescence measurements were carried out with an inverted phase-contrast microscope (Axiovert 100, Zeiss, Oberkochen, Germany). Cells were excited alternatively at 340 or 380 nm and the light was deflected by a dichroic mirror into either the objective (Fluar 40×/1.30 oil, Zeiss, Oberkochen, Germany) or a camera (Proxitronic, Bensheim, Germany). Emitted fluorescence intensity was recorded at 505 nm and data acquisition was accomplished by using specialized computer software (Metafluor, Universal Imaging Downingtown, USA). The corresponding ratios (F340/F380) were used to obtain intracellular Ca2+ concentrations. The following equation was used: [Ca2+]free = Kd x ((R-Rmin)/(Rmax-R)) x Sf (Kd = dissociation constant of Fura-2; R = ratio of emission intensity, exciting at 340 nm, to emission intensity, exciting at 380 nm; Rmin = ratio at zero free Ca2+; Rmax = ratio at saturating Ca2+; Sf = instrumental constant). As a measure for the increase of cytosolic Ca2+ concentration, the slope and peak of the changes in intracellular Ca2+ concentration were determined in each experiment.
To measure SOCE, changes in cytosolic Ca2+ were monitored upon depletion of the intracellular Ca2+ stores. Experiments were carried out prior to and during exposure of the cells to the Ca2+-free solution (see below). In the absence of Ca2+, the intracellular Ca2+ stores were depleted by inhibition of the vesicular Ca2+ pump by thapsigargin (1 µM; Molecular Probes). Readdition of Ca2+ allowed assessing the SOCE. To monitor the activity of K+ dependent (NCKX) or K+ independent (NCX) Na+/Ca2+ exchangers, changes in cytosolic Ca2+ were determined upon replacement of extracellular Na+ by N-methyl-D-glucamine (NMDG) in Ringer solution for NCX or NCKX. In some experiments DCs were treated with GSK3 inhibitors SB2167633 ([2,4-Dichlorophenyl]-4-[1-methyl-1H-indol-3-yl]-1H-pyrrole-2,5-dione, 100 nM - 10 µM, Enzo Life Sciences, Lausen, Switzerland) or GSK3 inhibitor XIII (GSK-XIII, 10 µM, company) [16] for 30 min before the experiment (15 min before addition of Fura-2A/M and then 15 min together with Fura-2A/M).
The Ringer solution contained (in mM): 125 NaCl, 5 KCl, 1.2 MgSO4, 32.2 Hepes, 2 Na2HPO4, 2 CaCl2, and 5 glucose at pH 7.4 (NaOH). Ca2+-free solution contained (in mM): 125 NaCl, 5 KCl, 1.2 MgSO4, 2 Na2HPO4, 32.2 Hepes, 0.5 EGTA, and 5 glucose at pH 7.4 (NaOH). Ringer solution for NCX contained (in mM): 130 NaCl, 2 CaCl2, 2 MgCl2, 10 HEPES, and 10 glucose at pH 7.4 (NaOH). Ringer solution for NCKX contained (in mM): 90 NaCl, 40 KCl, 2 CaCl2, 2 MgCl2, 10 HEPES, and 10 glucose at pH 7.4 (NaOH). The Na+-free solutions were identical, except that NaCl was replaced by 130 or 90 mM NMDG to measure NCX or NCKX, respectively.
Statistics
Data are provided as means ± SEM, n represents the number of independent experiments. All data were tested for significance using unpaired Student t-test, Mann–Whitney U test or ANOVA (Kruskal-Wallis Test, Dunnett test). Only results with p<0.05 were considered statistically significant.
Results
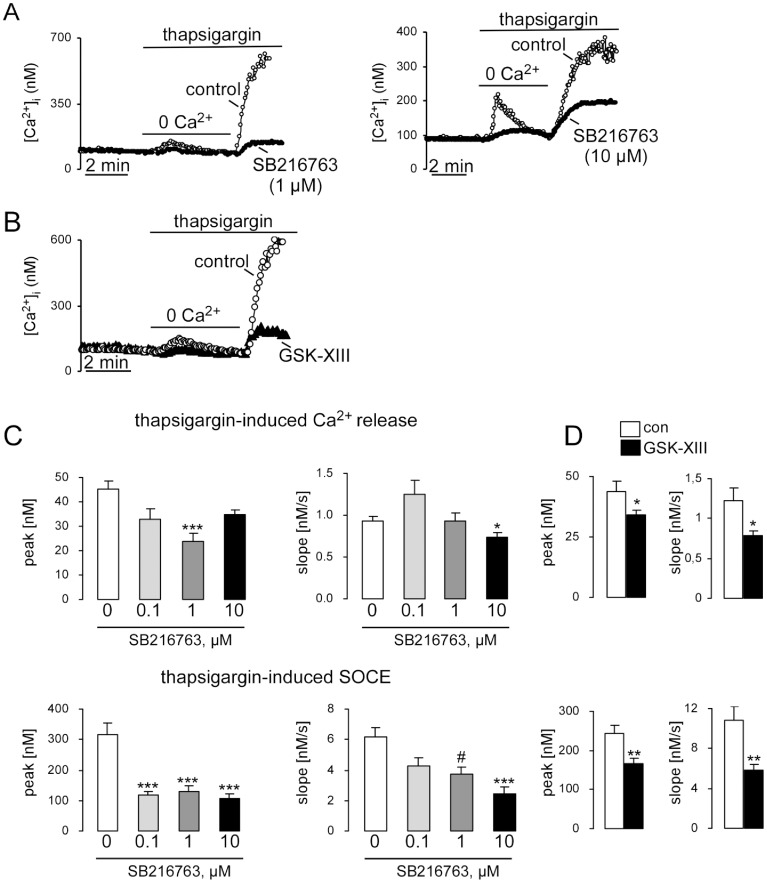
The present study explored whether glycogen synthase kinase 3 (GSK3) modifies the increase of cytosolic Ca2+ concentration ([Ca2+]i) in dendritic cells (DCs) following stimulation of intracellular Ca2+ release by inhibition of the sarco/endoplasmic Ca2+ ATPase (SERCA) with thapsigargin in the absence of extracellular Ca2+ and/or the increase of [Ca2+]i during store operated Ca2+ entry (SOCE) following readdition of extracellular Ca2+. The first series of experiments was performed in the presence and absence of the GSK3 inhibitor SB216763 (3-[2,4-Dichlorophenyl]-4-[1-methyl-1H-indol-3-yl]-1H-pyrrole-2,5-dione) added at different concentrations (100 nM – 10 µM) 30 min before the experiment. As illustrated in Fig. 1(A, C), treatment of DCs from wild type mice with thapsigargin in the absence of extracellular Ca2+ resulted in a transient increase of [Ca2+]i reflecting intracellular Ca2+ release. The subsequent readdition of extracellular Ca2+ was followed by a rapid increase of [Ca2+]i reflecting SOCE. Following addition of SB216763 the increase of [Ca2+]i upon thapsigargin treatment (Ca2+ release) and following readdition of extracellular Ca2+ (SOCE) were blunted by 1 µM and 10 µM of SB216763 (Fig. 1A, C). Those results were confirmed by using another GSK3 inhibitor, GSK-XIII (5-methyl-1Hpyrazol-3-yl-2-phenylquinazolin-4-yl-amine) [16]. Pretreatment of DCs wih GSK-XIII (10 µM, 30 min) resulted in a statistically significant decrease of Ca2+ release and SOCE (Fig. 1B, D).
Figure 1. Effect of GSK3 inhibitors SB216763 or GSK-XIII on thapsigargin-induced intacellular Ca2+ release and subsequent SOCE in DCs.
A. Representative original tracings showing intracellular Ca2+ concentrations ([Ca2+]i) in Fura-2/AM loaded wild type (gsk3WT) dendritic cells (DCs) prior to and following removal of extracellular Ca2+, addition of the sarco-endoplasmic Ca2+ ATPase (SERCA) inhibitor thapsigargin (1 µM) and readdition of extracellular Ca2+, all in the absence (open circles) and presence (closed circles) of GSK3 inhibitor SB216763 (3-[2,4-Dichlorophenyl]-4-[1-methyl-1H-indol-3-yl]-1H-pyrrole-2,5-dione, added 30 min before the experiment) 1 µM (left) or 10 µM (right). B. Representative original tracings showing [Ca2+]i in Fura-2/AM loaded gsk3WT without (open circles) and with (closed triangles) presence of GSK3 inhibitor GSK-XIII (10 µM, 30 min) DCs prior to and following removal of extracellular Ca2+, addition of SERCA inhibitor thapsigargin (1 µM) and readdition of extracellular Ca2+. C. Arithmetic means ± SEM (n = 16–83) of the peak (left) and slope (right) values of [Ca2+]i increase following addition of thapsigargin reflecting Ca2+ release from intracellular stores (upper bars) and of [Ca2+]i increase following readdition of extracellular Ca2+ reflecting store operated Ca2+ entry (SOCE, lower bars) in gsk3WT DCs incubated in the presence and absence of GSK3 inhibitor SB216763 (100 nM, 1 µM, 10 µM, 30 min). *(p<0.05), ***(p<0.001), ANOVA Kruskal-Wallis Test, #(p<0.05), Mann–Whitney U test. D. Arithmetic means ± SEM (n = 41–53) of the peak (left) and slope (right) values of [Ca2+]i increase upon Ca2+ release from intracellular stores (upper bars) and upon SOCE (lower bars) in gsk3WT DCs incubated in the presence and absence of GSK3 inhibitor GSK-XIII (10 µM, 30 min). *(p<0.05), **(p<0.001), unpaired t-test or Mann–Whitney U test.
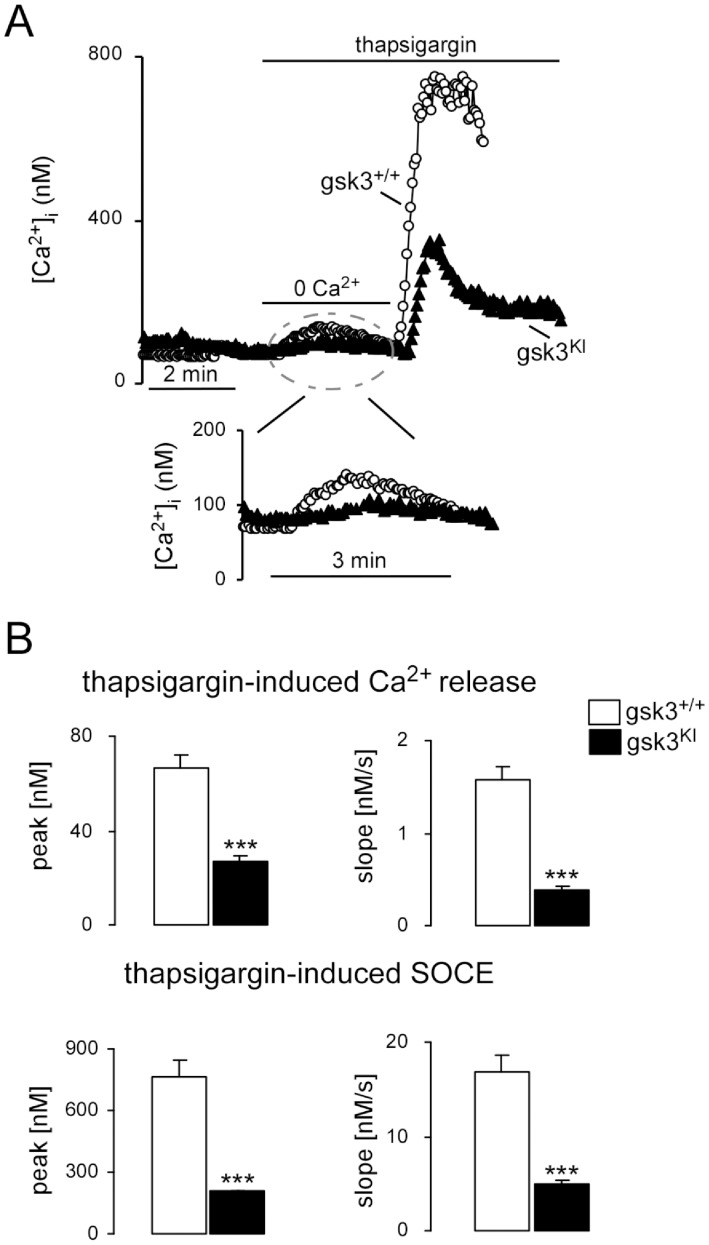
In a second series of experiments the increase of [Ca2+]i following intracellular Ca2+ release and subsequent SOCE was monitored in DCs isolated from mice expressing PKB/Akt and SGK insensitive GSK3α,β (gsk3KI) and DCs isolated from wild type mice (gsk3WT). As illustrated in Fig. 2, thapsigargin induced an increase of [Ca2+]i due to Ca2+ release and readdition of Ca2+ triggered SOCE, effects both significantly blunted in gsk3KI DCs as compared to gsk3WT DCs.
Figure 2. Thapsigargin-induced intacellular Ca2+ release and subsequent SOCE in DCs from gsk3KI and gsk3WT mice.
A. Representative original tracings showing [Ca2+]i in Fura-2/AM loaded gsk3WT (open circles) and gsk3KI (closed triangels) DCs prior to and following removal of extracellular Ca2+, addition of SERCA inhibitor thapsigargin (1 µM) and readdition of extracellular Ca2+. B. Arithmetic means ± SEM (n = 44–59) of the peak (left) and slope (right) values of [Ca2+]i increase upon Ca2+ release from intracellular stores (upper bars) and upon SOCE (lower bars) in gsk3WT DCs (white bars) and gsk3KI DCs (black bars). ***(p<0.001), unpaired t-test.
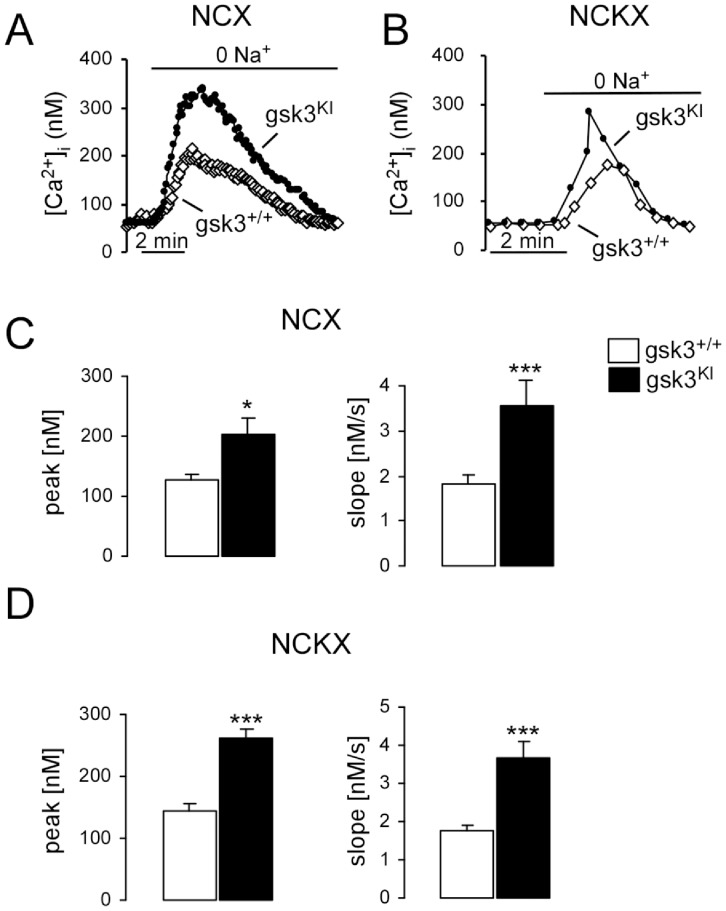
Further experiments were performed to elucidate underlying mechanisms. The increase of [Ca2+]i following intracellular Ca2+ release and/or SOCE is expected to be blunted by stimulation of Ca2+ extrusion, such as activation of Na+/Ca2+ exchangers. The increase of [Ca2+]i following removal of extracellular Na+ was taken as evidence for Na+/Ca2+ exchanger activity. In order to discriminate between K+-independent (NCX) and K+-dependent (NCKX) Na+/Ca2+ exchangers, experiments were performed in the absence (0 mM) or in the presence of high (40 mM) extracellular K+ concentrations. In the absence and in the presence of K+, the increase of [Ca2+]i following removal of extracellular Na+ was significantly higher in gsk3KI DCs than in gsk3WT DCs (Fig. 3). Accordingly, the activity of both, NCX and NCKX was significantly higher in gsk3KI DCs than in gsk3WT DCs.
Figure 3. K+ independent (NCX) and K+ dependent (NCKX) Na+/Ca2+ exchanger activity in DCs from gsk3KI and gsk3WT mice.
A,B. Representative original tracings showing [Ca2+]i in Fura-2/AM loaded gsk3WT (open diamonds) and gsk3KI (closed circles) DCs prior to and following removal of external Na+ (0 Na+) at 0 mM K+ (A) and at 40 mM K+ (B). C,D. Arithmetic means ± SEM of the peak (left) and slope (right) values of [Ca2+]i increase following removal of external Na+ at 0 mM K+ (C, n = 95–129) and at 40 mM K+ (D, n = 27–34) in gsk3WT DCs (white bars) and gsk3KI DCs (black bars). *(p<0.05), ***(p<0.001), unpaired t-test.
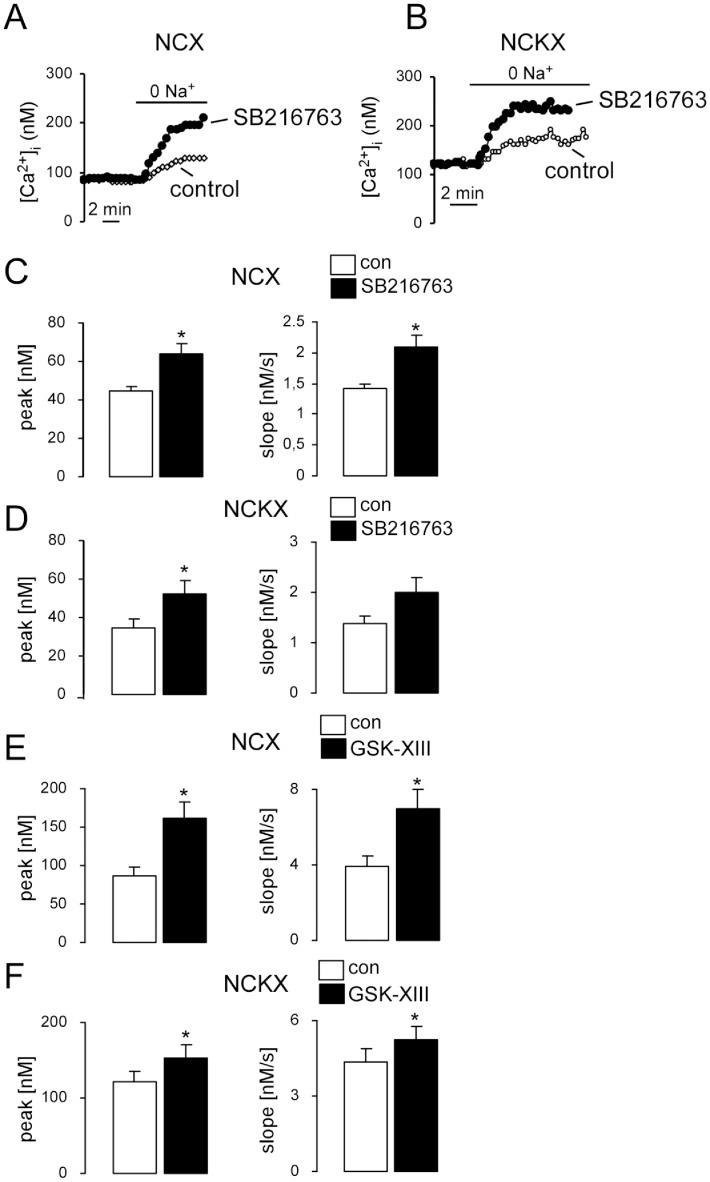
A short-term inhibition of GSK3 by either SB216763 (1 µM, 30 min, Fig. 4A–D) or GSK-XIII (10 µM, 30 min, Fig. 4E, F) also resulted in a significant upregulation of NCX and NCKX activity.
Figure 4. Effects of GSK3 inhibitors SB216763 or GSK-XIII on K+ independent (NCX) and K+ dependent (NCKX) Na+/Ca2+ exchanger activity in DCs.
A,B. Representative original tracings showing [Ca2+]i in Fura-2/AM loaded gsk3WT without (control, open circles) or with (closed circles) SB216763 treatment (1 µM, 30 min) DCs prior to and following removal of external Na+ (0 Na+) at 0 mM K+ (A) and at 40 mM K+ (B). C,D. Arithmetic means ± SEM of the peak (left) and slope (right) values of [Ca2+]i increase following removal of external Na+ at 0 mM K+ (C, n = 24–51) and at 40 mM K+ (D, n = 43–46) in gsk3WT DCs, without (white bars) or with (black bars) SB216763 treatment (1 µM, 30 min). *(p<0.05), unpaired t-test. E,F. Arithmetic means ± SEM of the peak (left) and slope (right) values of [Ca2+]i increase following removal of external Na+ at 0 mM K+ (E, n = 47–82) and at 40 mM K+ (F, n = 43–46) in gsk3WT DCs without (white bars) or with (black bars) GSK-XIII treatment (10 µM, 30 min). *(p<0.05), unpaired t-test or Mann–Whitney U test.
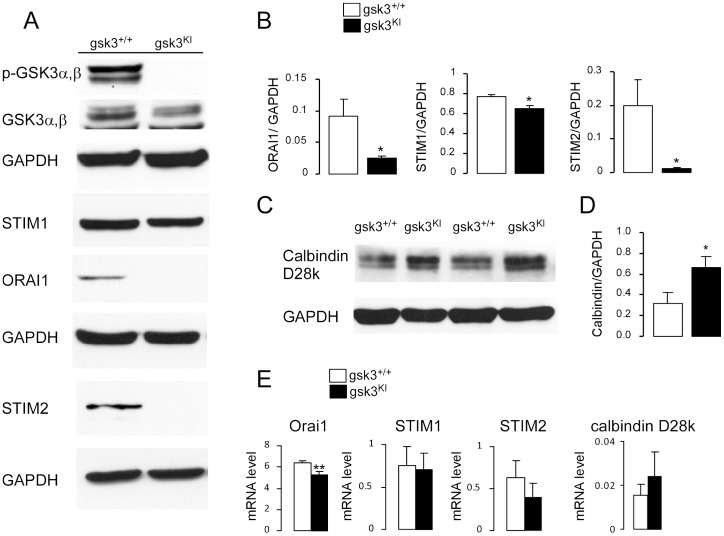
Western blotting and real time PCR were employed to quantify, respectively, the protein and mRNA abundance of the pore forming Ca2+ release activated Ca2+ channel (CRAC) moiety Orai1 and its regulators STIM1 and STIM2 in gsk3WT and gsk3KI DCs. As illustrated in Fig. 5 (A, B, E), the transcript abundance of Orai1 and the protein abundance of Orai1, STIM1 and STIM2 were all significantly lower in gsk3KI DCs than in gsk3WT DCs. The transcript abundance of STIM1, and STIM2 were not significantly different between genotypes. To confirm that the mutation in gsk3KI DCs disrupted GSK3α/β phosphorylation, phosphorylated GSK3 and total GSK3 were determined in gsk3WT and gsk3KI DCs. Indeed, phosphorylated GSK3 was observed in DCs from gsk3WT mice but not in DCs from gsk3KI mice (Fig. 5A).
Figure 5. Orai1, STIM1, STIM2 and calbindin-D28k mRNA and protein abundance in DCs from gsk3KI and gsk3WT mice.
A. Original western blot showing the protein abundance of phosphorylated (p, Ser21/9) GSK3α,β, total GSK3α,β and respective GAPDH, STIM1, Orai1 and respective GAPDH, STIM2 and respective GAPDH in DCs derived from bone marrow of gsk3KI and gsk3WT mice. Blots were stripped and reprobed with a GAPDH antibody to determine equal protein loading. Also the blot of p-GSK3 was stripped and reprobed with GSK3 antibody. B. Arithmetic means ± SEM (n = 4 independent experiments) of the relative (to GAPDH) protein abundance of Orai1, STIM1 and STIM2 in gsk3WT DCs (white bars) and gsk3KI DCs (black bars). *(p<0.05), unpaired t-test. C. Original western blot showing the protein abundance of calbindin-D28k (first lane) and GAPDH (2nd lane), in DCs derived from bone marrow of gsk3KI and gsk3WT mice. D. Arithmetic means ± SEM (n = 8 independent experiments) of the relative (to GAPDH) protein abundance of calbindin-D28k in gsk3WT DCs (white bar) and gsk3KI DCs (black bar). *(p<0.05), unpaired t-test. E. Arithmetic means (± SEM, n = 5–9) of the abundance of mRNA encoding Orai1, STIM1, STIM2, and calbindin-D28k in gsk3WT DCs (white bar) and gsk3KI DCs (black bar) as assessed by real-time PCR using TBP mRNA as a reference gene. **(p<0.01), unpaired t-test.
An increase of [Ca2+]i could further be modified by cytosolic Ca2+ buffering, a function of Ca2+ binding proteins such as calbindin-D28k. Accordingly, Western blotting and RT-PCR were employed to quantify calbindin-D28k expression in gsk3WT and gsk3KI DCs. As illustrated in Fig. 5 (C–E), the protein, but not transcript abundance of calbindin D28k was significantly higher in gsk3KI DCs than in gsk3WT DCs.
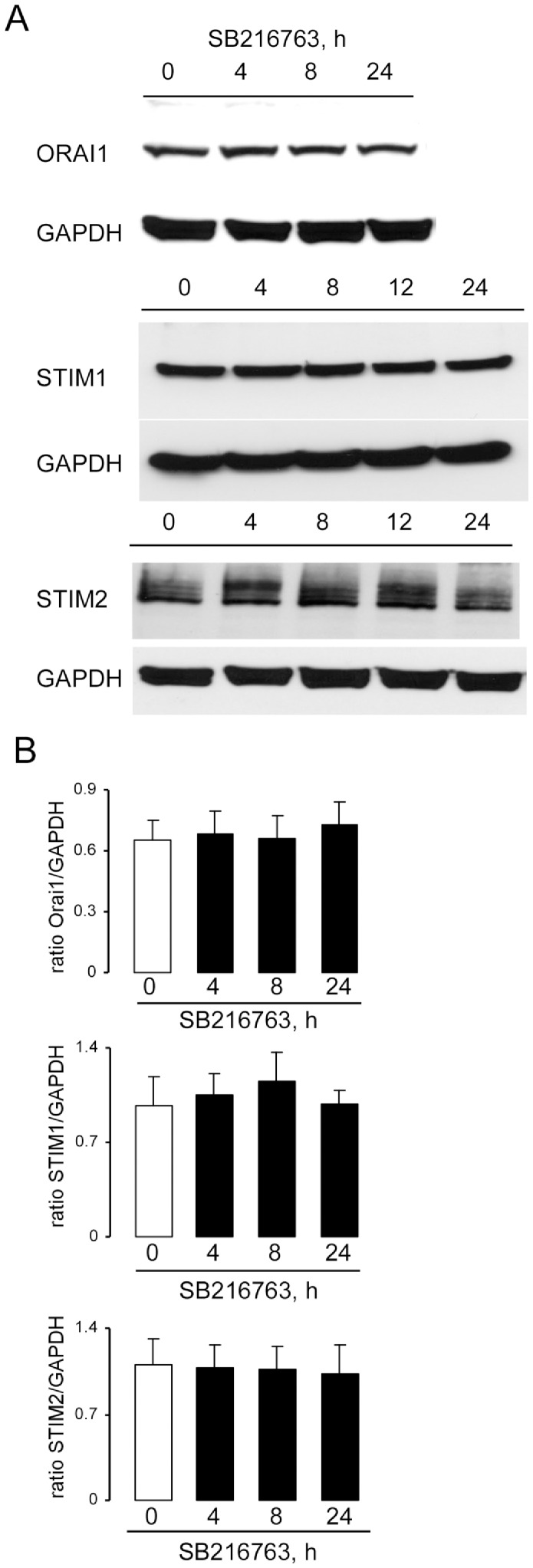
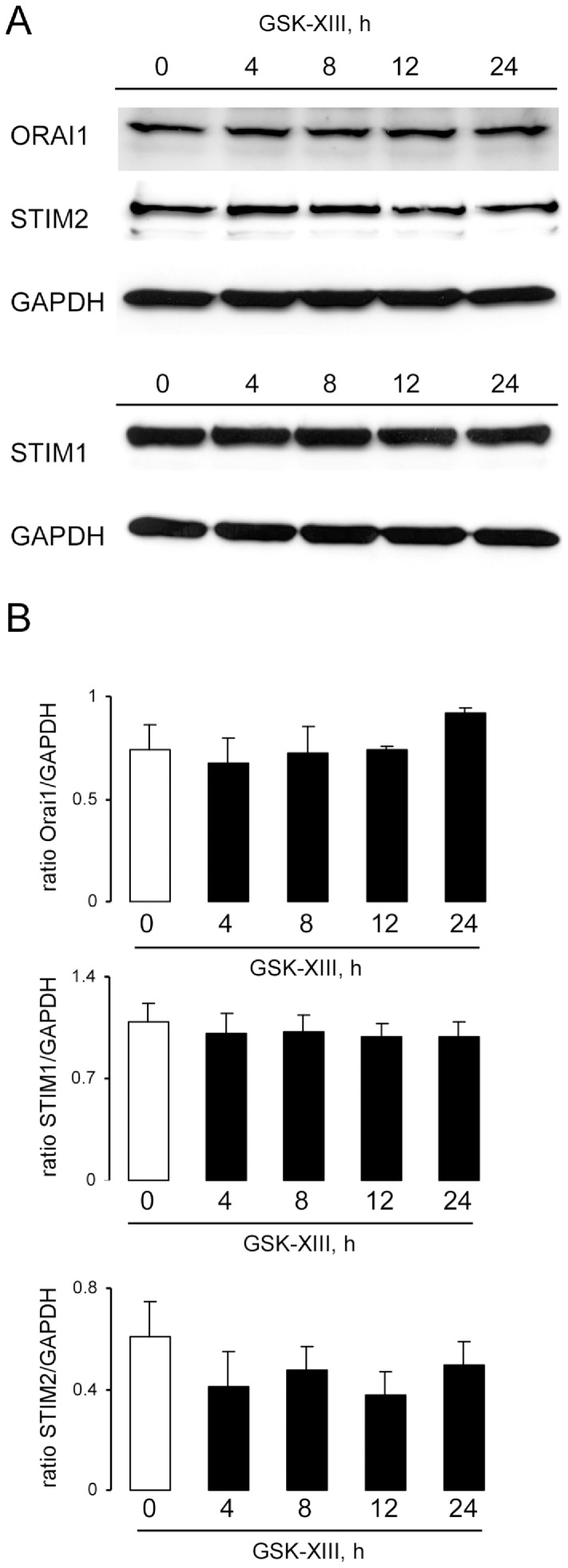
Treating gsk3WT DCs with GSK3 inhibitors SB216763 (1 µM, 4 h–24 h, Fig. 6) or GSK-XIII (10 µM, 4 h–24 h, Fig. 7) did not significantly modify Orai1, STIM1 and STIM2 protein abundance.
Figure 6. Effects of GSK3 inhibitor SB216763 on Orai1, STIM1, and STIM2 protein abundance in DCs.
A. Original western blot showing the protein abundance of Orai1 and respective GAPDH, STIM1 and respective GAPDH, STIM2 and respective GAPDH in DCs without (control) and with SB216763 treatment (1 µM, 4–24 h). Blots were stripped and reprobed with a GAPDH antibody to determine equal protein loading. B. Arithmetic means ± SEM (n = 4–5 independent experiments) of the relative (to GAPDH) protein abundance of Orai1, STIM1 and STIM2 in DCs without (white bar) and with SB216763 treatment (1 µM, 4–24 h, black bars).
Figure 7. Effects of GSK3 inhibitor GSK-XIII on Orai1, STIM1, and STIM2 protein abundance in DCs.
A. Original western blot showing the protein abundance of Orai1, STIM2 and respective GAPDH, STIM1 and respective GAPDH, in DCs without (control) and with GSK-XIII treatment (10 µM, 4–24 h). Blots were stripped and reprobed with a GAPDH antibody to determine equal protein loading. B. Arithmetic means ± SEM (n = 4–5 independent experiments) of the relative (to GAPDH) protein abundance of Orai1, STIM1 and STIM2 in DCs without (white bar) and with (black bars) GSK-XIII treatment (10 µM, 4–24 h).
Discussion
The present observations reveal a dual role of glycogen synthase kinase 3 (GSK3) in the regulation of dendritic cell (DC) Ca2+ signaling. At the one hand acute inhibition of GSK3 with the GSK3 inhibitors SB216763 or GSK-XIII blunts the increase of cytosolic Ca2+ concentration ([Ca2+]i) following inhibition of the sarco/endoplasmic Ca2+ ATPase (SERCA) with thapsigargin in the absence of extracellular Ca2+ as well as the store operated Ca2+ entry (SOCE) following readdition of extracellular Ca2+. On the other hand, disruption of PKB/Akt and SGK dependent GSK3α,β phosphorylation downregulates both, intracellular Ca2+ release and SOCE. Phosphorylation of GSK3 by PKB/Akt [40], [41] and SGK [42], [43] inhibits GSK3 activity. Accordingly, disruption of the PKB/Akt and SGK dependent phosphorylation is expected to prevent the inhibition of GSK3 following stimulation of the phosphoinositide 3 (PI3) kinase pathway [46].
GSK3 is apparently active in immature DCs [20]. The kinase is transiently phosphorylated upon lipopolysaccharide (LPS) stimulation [18], [19] but still contributes to the development of a proinflammatory phenotype of DCs [20]. Pharmacological inhibition of GSK3 has previously been shown to attenuate IL-12 [18]–[20], IL-6 and TNFα [20], [53] production and to enhance IL-10 production [18], [19]. Moreover, GSK3 inhibitors blunt the increase of IL12p70 secretion from monocyte-derived DCs following PI3 kinase inhibition with wortmannin [20].
As shown earlier [13], the increase of [Ca2+]i following treatment of DCs with bacterial LPS was virtually abolished in the presence of GSK3 inhibitor SB216763. Those observations are consistent with inhibition of SOCE by the GSK3 inhibitor. Notably, the inhibitor interferes with both, intracellular Ca2+ release and SOCE. Thus, GSK3 may be involved in the signaling leading to emptying of intracellular stores. Since the GSK3 inhibitor SB216763 was effective within a few minutes it apparently disrupted activation or inactivation of existing proteins. Moreover, as shown in the present study, both K+ dependent (NCKX) and K+ independent (NCX) Na+/Ca2+ exchangers are stimulated by short-term inhibition of GSK3 by either SB216763 or GSK-XIII. Enhanced extrusion of Ca2+ via these transporters could also underlie the reduced SOCE.
In contrast, the decreased SOCE in DCs isolated from gsk3KI mice is at least partially due to decreased expression of Orai1, STIM1 and STIM2. Moreover, the blunted increase of [Ca2+]i in DCs from gsk3KI mice during intracellular Ca2+ release and SOCE are in part due to enhanced Ca2+ buffering due to increased expression of calbindin-D28k. The differences between DCs from gsk3KI mice and DCs from gsk3WT mice is thus partially due to altered gene expression. The blunted increase of [Ca2+]i during intracellular Ca2+ release and SOCE is further in part due to enhanced activity of both K+ dependent (NCKX) and K+ independent (NCX) Na+/Ca2+ exchangers. In contrast, inhibition of GSK3 by either SB216763 or GSK-XIII did not modify expression of Orai1, STIM1 and STIM2.
At least in theory, the differences between DCs from gsk3KI mice and DCs from gsk3WT mice may be an indirect result of the altered regulation of GSK3 activity in other cell types, which in turn influence gene expression in DCs. Along those lines PKB/Akt and SGK1 resistance of GSK3 influences steroid hormone release [47], catecholamine release [50], and function of lymphocytes [54]. To the extent that those or further alterations of hormonal or cellular environment do imprint DCs in vivo prior to isolation, the DCs could remain altered following isolation and subsequent culture.
In conclusion, GSK3 plays a dual role in the regulation of cytosolic Ca2+ concentration in dendritic cells. Acutely, GSK3 activity is required for the full effect of LPS stimulation and SERCA inhibition on cytosolic Ca2+ activity. On the other hand, disruption of PKB/Akt and SGK dependent phoshorylation of GSK3 downregulates Orai1, STIM1 and STIM2 expression, upregulates calbindin-D28k expression and enhances the activity of K+ dependent (NCKX) and K+ independent (NCX) Na+/Ca2+ exchangers in dendritic cells.
Acknowledgments
The authors acknowledge the meticulous preparation of the manuscript by Lejla Subasic.
Funding Statement
This study was supported by the Deutsche Forschungsgemeinschaft (GK 1302) (http://www.dfg.de/) and the Open Access Publishing Fund of Tuebingen University. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392: 245–252. [DOI] [PubMed] [Google Scholar]
- 2. Dubsky P, Ueno H, Piqueras B, Connolly J, Banchereau J, et al. (2005) Human dendritic cell subsets for vaccination. J Clin Immunol 25: 551–572. [DOI] [PubMed] [Google Scholar]
- 3. Shumilina E, Huber SM, Lang F (2011) Ca2+ signaling in the regulation of dendritic cell functions. Am J Physiol Cell Physiol 300: C1205–C1214. [DOI] [PubMed] [Google Scholar]
- 4. Schmid E, Bhandaru M, Nurbaeva MK, Yang W, Szteyn K, et al. (2012) SGK3 regulates Ca(2+) entry and migration of dendritic cells. Cell Physiol Biochem 30: 1423–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nurbaeva MK, Schmid E, Szteyn K, Yang W, Viollet B, et al. (2012) Enhanced Ca(2)(+) entry and Na+/Ca(2)(+) exchanger activity in dendritic cells from AMP-activated protein kinase-deficient mice. FASEB J 26: 3049–3058. [DOI] [PubMed] [Google Scholar]
- 6. Geng S, Gao YD, Yang J, Zou JJ, Guo W (2012) Potential role of store-operated Ca2+ entry in Th2 response induced by histamine in human monocyte-derived dendritic cells. Int Immunopharmacol 12: 358–367. [DOI] [PubMed] [Google Scholar]
- 7. Sharma S, Quintana A, Findlay GM, Mettlen M, Baust B, et al. (2013) An siRNA screen for NFAT activation identifies septins as coordinators of store-operated Ca2+ entry. Nature 499: 238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Felix R, Crottes D, Delalande A, Fauconnier J, Lebranchu Y, et al. (2013) The Orai-1 and STIM-1 complex controls human dendritic cell maturation. PLoS One 8: e61595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bandyopadhyay BC, Pingle SC, Ahern GP (2011) Store-operated Ca(2)+ signaling in dendritic cells occurs independently of STIM1. J Leukoc Biol 89: 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shumilina E, Nurbaeva MK, Yang W, Schmid E, Szteyn K, et al. (2013) Altered regulation of cytosolic Ca2+ concentration in dendritic cells from klotho hypomorphic mice. Am J Physiol Cell Physiol 305: C70–77. [DOI] [PubMed] [Google Scholar]
- 11. Heise N, Shumilina E, Nurbaeva MK, Schmid E, Szteyn K, et al. (2011) Effect of dexamethasone on Na+/Ca2+ exchanger in dendritic cells. Am J Physiol Cell Physiol 300: C1306–1313. [DOI] [PubMed] [Google Scholar]
- 12. Schwaller B (2010) Cytosolic Ca2+ buffers. Cold Spring Harb Perspect Biol 2: a004051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russo A, Schmid E, Nurbaeva MK, Yang W, Yan J, et al. (2013) PKB/SGK-dependent GSK3-phosphorylation in the regulation of LPS-induced Ca increase in mouse dendritic cells. Biochem Biophys Res Commun. [DOI] [PubMed]
- 14. Lochhead PA, Kinstrie R, Sibbet G, Rawjee T, Morrice N, et al. (2006) A chaperone-dependent GSK3beta transitional intermediate mediates activation-loop autophosphorylation. Mol Cell 24: 627–633. [DOI] [PubMed] [Google Scholar]
- 15. Pierce AC, ter Haar E, Binch HM, Kay DP, Patel SR, et al. (2005) CH…O and CH…N hydrogen bonds in ligand design: a novel quinazolin-4-ylthiazol-2-ylamine protein kinase inhibitor. J Med Chem 48: 1278–1281. [DOI] [PubMed] [Google Scholar]
- 16. Schutz SV, Cronauer MV, Rinnab L (2010) Inhibition of glycogen synthase kinase-3beta promotes nuclear export of the androgen receptor through a CRM1-dependent mechanism in prostate cancer cell lines. J Cell Biochem 109: 1192–1200. [DOI] [PubMed] [Google Scholar]
- 17. Wang H, Brown J, Garcia CA, Tang Y, Benakanakere MR, et al. (2011) The role of glycogen synthase kinase 3 in regulating IFN-beta-mediated IL-10 production. J Immunol 186: 675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martin M, Rehani K, Jope RS, Michalek SM (2005) Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol 6: 777–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ohtani M, Nagai S, Kondo S, Mizuno S, Nakamura K, et al. (2008) Mammalian target of rapamycin and glycogen synthase kinase 3 differentially regulate lipopolysaccharide-induced interleukin-12 production in dendritic cells. Blood 112: 635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rodionova E, Conzelmann M, Maraskovsky E, Hess M, Kirsch M, et al. (2007) GSK-3 mediates differentiation and activation of proinflammatory dendritic cells. Blood 109: 1584–1592. [DOI] [PubMed] [Google Scholar]
- 21. Ono T, Yanagawa Y, Iwabuchi K, Nonomura K, Onoe K (2007) Glycogen synthase kinase 3 activity during development of bone marrow-derived dendritic cells (DCs) essential for the DC function to induce T helper 2 polarization. Immunology 122: 189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Beurel E, Jope RS (2010) Glycogen synthase kinase-3 regulates inflammatory tolerance in astrocytes. Neuroscience 169: 1063–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Beurel E, Michalek SM, Jope RS (2010) Innate and adaptive immune responses regulated by glycogen synthase kinase-3 (GSK3). Trends Immunol 31: 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Beurel E, Yeh WI, Michalek SM, Harrington LE, Jope RS (2011) Glycogen synthase kinase-3 is an early determinant in the differentiation of pathogenic Th17 cells. J Immunol 186: 1391–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang H, Brown J, Martin M (2011) Glycogen synthase kinase 3: a point of convergence for the host inflammatory response. Cytokine 53: 130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang H, Brown J, Gu Z, Garcia CA, Liang R, et al. (2011) Convergence of the Mammalian Target of Rapamycin Complex 1- and Glycogen Synthase Kinase 3-{beta}-Signaling Pathways Regulates the Innate Inflammatory Response. J Immunol 186: 5217–5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hofmann C, Dunger N, Scholmerich J, Falk W, Obermeier F (2010) Glycogen synthase kinase 3-beta: a master regulator of toll-like receptor-mediated chronic intestinal inflammation. Inflamm Bowel Dis 16: 1850–1858. [DOI] [PubMed] [Google Scholar]
- 28. De Sarno P, Axtell RC, Raman C, Roth KA, Alessi DR, et al. (2008) Lithium prevents and ameliorates experimental autoimmune encephalomyelitis. J Immunol 181: 338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dugo L, Collin M, Allen DA, Patel NS, Bauer I, et al. (2005) GSK-3beta inhibitors attenuate the organ injury/dysfunction caused by endotoxemia in the rat. Crit Care Med 33: 1903–1912. [DOI] [PubMed] [Google Scholar]
- 30. Hu X, Paik PK, Chen J, Yarilina A, Kockeritz L, et al. (2006) IFN-gamma suppresses IL-10 production and synergizes with TLR2 by regulating GSK3 and CREB/AP-1 proteins. Immunity 24: 563–574. [DOI] [PubMed] [Google Scholar]
- 31. Escribano C, Delgado-Martin C, Rodriguez-Fernandez JL (2009) CCR7-dependent stimulation of survival in dendritic cells involves inhibition of GSK3beta. J Immunol 183: 6282–6295. [DOI] [PubMed] [Google Scholar]
- 32. Beurel E, Jope RS (2006) The paradoxical pro- and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog Neurobiol 79: 173–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cohen P, Frame S (2001) The renaissance of GSK3. Nat Rev Mol Cell Biol 2: 769–776. [DOI] [PubMed] [Google Scholar]
- 34. Frame S, Cohen P (2001) GSK3 takes centre stage more than 20 years after its discovery. Biochem J 359: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kaytor MD, Orr HT (2002) The GSK3 beta signaling cascade and neurodegenerative disease. Curr Opin Neurobiol 12: 275–278. [DOI] [PubMed] [Google Scholar]
- 36. Koros E, Dorner-Ciossek C (2007) The role of glycogen synthase kinase-3beta in schizophrenia. Drug News Perspect 20: 437–445. [DOI] [PubMed] [Google Scholar]
- 37. Jope RS, Yuskaitis CJ, Beurel E (2007) Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochem Res 32: 577–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hughes K, Ramakrishna S, Benjamin WB, Woodgett JR (1992) Identification of multifunctional ATP-citrate lyase kinase as the alpha-isoform of glycogen synthase kinase-3. Biochem J 288 (Pt 1): 309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Welsh GI, Proud CG (1993) Glycogen synthase kinase-3 is rapidly inactivated in response to insulin and phosphorylates eukaryotic initiation factor eIF-2B. Biochem J 294 (Pt 3): 625–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cross HR, Radda GK, Clarke K (1995) The role of Na+/K+ ATPase activity during low flow ischemia in preventing myocardial injury: a 31P, 23Na and 87Rb NMR spectroscopic study. Magn Reson Med 34: 673–685. [DOI] [PubMed] [Google Scholar]
- 41. Shaw M, Cohen P, Alessi DR (1997) Further evidence that the inhibition of glycogen synthase kinase-3beta by IGF-1 is mediated by PDK1/PKB-induced phosphorylation of Ser-9 and not by dephosphorylation of Tyr-216. FEBS Lett 416: 307–311. [DOI] [PubMed] [Google Scholar]
- 42. Sakoda H, Gotoh Y, Katagiri H, Kurokawa M, Ono H, et al. (2003) Differing roles of Akt and serum- and glucocorticoid-regulated kinase in glucose metabolism, DNA synthesis, and oncogenic activity. J Biol Chem 278: 25802–25807. [DOI] [PubMed] [Google Scholar]
- 43. Wyatt AW, Hussain A, Amann K, Klingel K, Kandolf R, et al. (2006) DOCA-induced phosphorylation of glycogen synthase kinase 3beta. Cell Physiol Biochem 17: 137–144. [DOI] [PubMed] [Google Scholar]
- 44. Hawkins PT, Anderson KE, Davidson K, Stephens LR (2006) Signalling through Class I PI3Ks in mammalian cells. Biochem Soc Trans 34: 647–662. [DOI] [PubMed] [Google Scholar]
- 45. Lang F, Bohmer C, Palmada M, Seebohm G, Strutz-Seebohm N, et al. (2006) (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev 86: 1151–1178. [DOI] [PubMed] [Google Scholar]
- 46. McManus EJ, Sakamoto K, Armit LJ, Ronaldson L, Shpiro N, et al. (2005) Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J 24: 1571–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Boini KM, Bhandaru M, Mack A, Lang F (2008) Steroid hormone release as well as renal water and electrolyte excretion of mice expressing PKB/SGK-resistant GSK3. Pflugers Arch 456: 1207–1216. [DOI] [PubMed] [Google Scholar]
- 48. Boini KM, Amann K, Kempe D, Alessi DR, Lang F (2009) Proteinuria in mice expressing PKB/SGK-resistant GSK3. Am J Physiol Renal Physiol 296: F153–F159. [DOI] [PubMed] [Google Scholar]
- 49.Rotte A, Pasham V, Eichenmuller M, Yang W, Qadri SM, et al. (2010) Regulation of basal gastric acid secretion by the glycogen synthase kinase GSK3. J Gastroenterol. [DOI] [PubMed]
- 50. Siraskar B, Volkl J, Ahmed MS, Hierlmeier M, Gu S, et al. (2011) Enhanced catecholamine release in mice expressing PKB/SGK-resistant GSK3. Pflugers Arch 462: 811–819. [DOI] [PubMed] [Google Scholar]
- 51. Ackermann TF, Kempe DS, Lang F, Lang UE (2010) Hyperactivity and enhanced curiosity of mice expressing PKB/SGK-resistant glycogen synthase kinase-3 (GSK-3). Cell Physiol Biochem 25: 775–786. [DOI] [PubMed] [Google Scholar]
- 52. Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Martin DR, Ritter CS, Slatopolsky E, Brown AJ (2005) Acute regulation of parathyroid hormone by dietary phosphate. Am J Physiol Endocrinol Metab 289: E729–E734. [DOI] [PubMed] [Google Scholar]
- 54. Bhavsar SK, Merches K, Bobbala D, Lang F (2012) AKT/SGK-sensitive phosphorylation of GSK3 in the regulation of L-selectin and perforin expression as well as activation induced cell death of T-lymphocytes. Biochem Biophys Res Commun 425: 6–12. [DOI] [PubMed] [Google Scholar]