Abstract
Drug-induced hypersensitivity syndrome is a systemic autoimmune disorder that results in mucocutaneous symptoms ranging in severity from mild pruritus to life-threatening skin and mucosal loss, with different nomenclature depending on the severity of the symptoms. The purpose of this article is to review the recent advances in understanding the pathology of drug-induced hypersensitivity syndrome, as well as current recommendations for both medical and wound management.
Keywords: DRESS syndrome, Drug-induced hypersensitivity syndrome, Erythema multiform, Stevens-Johnson syndrome, Toxic epidermal necrolysis
Introduction
Drug-induced hypersensitivity syndrome (DIHS) is a rare systemic autoimmune disorder that can cause mild to severe mucosal and cutaneous reactions. Discussion in the literature tends to focus on identifiable syndromes based on severity of symptoms (see Table 1); however, the underlying pathophysiology appears to be the same. The reported incidence varies: 0.4 per 1 million persons for drug reaction with eosinophilia and systemic symptoms (DRESS),1 1 to 1.4 per 1 million persons for toxic epidermal necrolysis (TEN),2 and 2.9 to 6.1 per 1 million persons for Stevens-Johnson syndrome (SJS).3-5 Predisposing factors include advanced age, polypharmacy, female sex, presence of infection (especially HIV), and genetic predisposition.6 Mortality rates are approximately 5% for SJS, 30% to 50% for TEN,7 and 10% for DRESS.
Table 1.
Drug-Induced Hypersensitivity Syndromes Based on Severity of Symptoms
| Name of Syndrome | Identifying Characteristics |
|---|---|
| Maculopapular exanthemas | Generalized, widespread rash with red macular (not elevated) or papular (elevated) skin eruptions |
| Erythema multiforme (EM) minor | Localized skin eruptions, usually on the lower extremities, that begin to heal in 7 days |
| Fixed drug eruption | One or more local annular or oval erythematous patches; resolve with hyperpigmentation; recur at the same location |
| Drug rash with eosinophilia and systemic systems | Three of the following: fever, exanthema, eosinophilia, atypical circulating lymphocytes, lymphadenopathy, hepatitis |
| Stevens-Johnson syndrome; also called EM major | Cutaneous lesions of erythematous papules, vesicles, bullae, or iris lesions covering < 10% of the body surface area; mucosal lesions or conjunctivitis |
| Toxic epidermal necrolysis; also referred to as Lyell syndrome | Cutaneous lesions of erythematous papules, vesicles, bullae, or iris lesions covering > 30% of the body surface area; mucosal lesions or conjunctivitis |
| Chemotherapy-induced acral erythema | Painful, symmetrical swelling and erythema of the palms and soles of patients on high-dose chemotherapy27 |
| Symmetrical drug-related intertriginous and flexural exanthema (Baboon's syndrome) | Bright-red, well-demarcated, anogenital lesions associated with a symmetrical eczematous eruption involving axillae, antecubital fossae, eyelids, and the sides of the neck28 |
| Drug-induced lupus erythematosus | Typical lupus-like symptoms, including skin signs associated with long-term use of the putative drug; symptoms resolve with the withdrawal of the drug.19 |
Pathophysiology
DIHS is an acute autoimmune reaction thought to be mediated by T cells and involving a variety of cytokines, inflammatory cells, and regulatory mechanisms, although not specifically understood. The mechanism appears to be activation of the immune system by the causative agents or their metabolites rather than a direct toxic effect on the keratinocytes.8
A study by Bellon et al. supported the T-cell–mediated hypothesis by identifying 85 genes that were differentially expressed during the acute phase of DIHS. Most of the genes upregulated in the acute phase were encoding proteins involved in cell cycle, apoptosis, and cell growth functions; 9 were involved in immune response and inflammation. Bellon et al. also found that histone messenger RNA levels were statistically significantly increased in severe and moderate reactions. Genes that were strongly upregulated in syndromes with both cutaneous and mucosal involvement were those involved in inflammation, now termed alarmins or endogenous damage-associated molecular patterns.9
In a study by Tohyama et al., immunostaining of cryosections from SJS and TEN lesions revealed CD14+ monocytes in the dermoepidermal junction, and CD14+ CD16+ cells present early in the disease process, before epidermal damage occurred, suggesting that the monocyte “infiltration is a cause, rather than a result, of epidermal damage.”10
Merk discusses the role of xenobiotica-metabolizing enzymes and transport proteins as a biochemical barrier that serves, in addition to the epidermal stratum corneum, as a protection from toxic chemical compounds. He describes 3 phases of xenobiotica metabolism mediation: Phase 1 is the activation of the parent compound by oxidizing enzymes to highly reactive intermediates; in phase 2 the intermediates are metabolized by other enzymes, such as transferases, to create more water-soluble metabolites that can leave the cells; and phase 3 is mediated by the influx and efflux of transporter proteins in cutaneous cells. An imbalance in the 3 phases of xenobiotica metabolism results in binding of the highly reactive intermediates to high–molecular weight molecules (such as proteins) and a subsequent toxic response. Merk uses studies of contact dermatitis to relate this action to DIHS.11
Clinical Presentation
Symptoms of DIHS usually occur 1 to 3 weeks after the first ingestion of the causative medication (Table 2). SJS and TEN begin with fever, sore throat, and stinging eyes for 1 to 3 days, followed by mucosal lesions involving conjunctiva, oral and genital mucosa, trachea, bronchi, and gastrointestinal tract. Cutaneous lesions develop next with erythematous macules, progressing to flaccid blisters that easily tear.12 The initial lesions are sometimes referred to as targetoid lesions because of the target appearance, with 2 zones of color.13 The rash usually involves the head, anterior and posterior torso, upper extremities, and lower extremities to above the knees and may progress to include lower back and gluteal area (see Figures 1 and 2).8 The most important signs of an impending severe cutaneous reaction are skin pain, epidermolysis, and a positive Nikolsky's sign (slight rubbing of the skin causes separation of the epidermis and dermis).14,15
Table 2.
Drugs that have been reported to cause DIHS
| Allipurinol |
| Antibiotics |
| Betalactams (pediatrics)29 |
| Ceftriaxone (check) |
| Cephalosporins |
| Ciprofloxacin |
| Clindamycin |
| Fluoroquinolones |
| Levofloxacin |
| Penicillin |
| Sulfanomides |
| Trimethoprim-sulfamethoxazole30 |
| Tetracyclines |
| Anticonvulsants |
| Carbamazepine |
| Phenobarbitone |
| Phenytoin |
| Anti-retroviral therapy |
| Nevirapine31 |
| Calcium-channel blockers |
| Dapsone |
| Oxicam NSAIDs |
| Cyclooxygenase-2 (COX-2) inhibitors |
Figure 1.
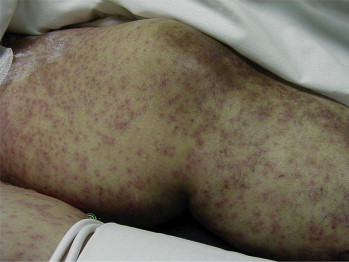
Skin Lesions on the Leg of a Patient With Toxic Epidermal Necrosis.
Figure 2.
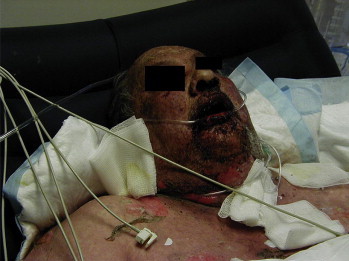
Oral Lesions and Epidermal Necrolysis Visible on a Patient With Toxic Epidermal Necrosis.
A retrospective study by Watanabe et al. suggested distinct differences between SJS and TEN and erythema multiforme major that can be helpful in making a definitive diagnosis. SJS and TEN patients were more likely to have mucous membrane involvement, higher C-reactive protein levels, and hepatic dysfunction. Erythema multiforme major patients had stronger mononuclear cell infiltration and required lower doses of systemic corticosteroids.16
The Score of Toxic Epidermal Necrosis (SCORTEN) scale is a severity-of-illness scale that can be used to determine the mortality rate of an individual patient.17 Although it was initially developed for patients with SJS and TEN, it has been validated and used for patients with burns and other exfoliative disorders. Calculations are advised within the first 24 hours after admission and on day 3.17 Tables 3 and 4 list the risk factors and mortality scores, showing that more risk factors result in a higher SCORTEN scale score, thereby indicating a higher mortality rate.
Table 3.
SCORTEN Scale Risk Factors for Determining Mortality Rates of Patients With Toxic Epidermal Necrolysis
| Risk Factor | 0 | 1 |
|---|---|---|
| Age | <40 years | >40 years |
| Associated malignancy | No | Yes |
| Heart rate (beats per minute) | <120 | >120 |
| Serum urea nitrogen (mg/dL) | <27 | >27 |
| Detached or compromised body surface | <10% | >10% |
| Serum bicarbonate (mEq/L) | >20 | <20 |
| Serum glucose (mg/dL) | <250 | >250 |
SCORTEN, score of toxic epidermal necrosis.
Table 4.
Interpretation of the SCORTEN Scale
| No. of risk factors | Mortality rate (%) |
|---|---|
| 0-1 | 3.2 |
| 2 | 12.1 |
| 3 | 35.3 |
| 4 | 58.3 |
| 5 or more | >90 |
SCORTEN, score of toxic epidermal necrosis.
Diagnostic laboratory values can play a role in prognosis of the disease, especially TEN and SJS. Neutropenia and lymphopenia can occur and may be a negative prognostic factor.18 The use of granulocyte colony-stimulating factor in the treatment of TEN has been shown to reverse the neutropenia with a corresponding increase in reepithelialization.15 Hyperferritinemia as a result of acute liver failure can be a useful marker for the severity of DIHS.19 Fujita and colleagues developed a rapid immunochromatographic test for detection of granulysin, a cytotoxic lipid-binding protein that causes apoptosis and is present in the blister fluid of patients with SJS and TEN. The granulysin was found to be elevated before skin and mucosal detachment occurred, suggesting that it may be a useful marker for detection of SJS and TEN in the early stages.20
Patch tests may be useful in most forms of DIHS, but not for SJS, TEN and vasculitis. The lymphocyte transformation test tends to test positive in maculopapular exanthemas, bullous exanthema, acute generalized exanthematous pustulosis, and DRESS, but rarely in TEN, cytopenias, and vasculitis.21 Drug provocation tests may also be useful in diagnosing the drug allergy.19
Medical Management
The first and foremost medical strategy is identification and cessation of the causative agent, usually the last one the patient initiated 1 to 3 weeks prior to onset of symptoms. Thereafter, treatment is predicated on the severity of the symptoms, both cutaneous and systemic. Corticosteroids are used for both treatment of symptoms and prevention of progression. For milder cases, systemic corticosteroids dosed at 0.5 to 1 mg/kg/day and tapered over 6 to 8 weeks are recommended; for SJS, 1 mg/kg/day of prednisolone or 1 to 2 mg/kg/day of methylprednisolone is recommended.21 Steroid therapy for TEN is reported as both controversial and no longer recommended; if used, it should be within the first 48 hours of treatment because of the increased risk of septic complications with an anti-inflammatory agent. Strict control of blood glucose levels is needed for patients with history of diabetes or on corticosteroids.22
For patients with extensive skin involvement, supportive care in an acute burn or intensive care unit is recommended for life support measures, pain management, and prevention of infection.23 Mechanical ventilation, fluid resuscitation with IV fluids or Ringer's solution for electrolyte balance, anticoagulation with heparin to prevent thromboembolism, and supplemental nutrition via a nasogastric tube may be needed in severe cases.2,12 Antibiotic therapy is not prophylactic but dependent on clinical symptoms, including positive skin cultures, sudden drop in temperature, or deterioration of patient's medical condition.2 In order to prevent caloric loss and an increase in metabolic rate, a room temperature of 30 °C to 32 °C is also recommended.2
Clinical studies on the use of intravenous immunoglobulin for patients with SJS and TEN have shown mixed results. Successful treatment appears to be dose dependent (1 g/kg/day for 3 days with a total of 3 g/kg over 3 consecutive days), with early treatment recommended.24 Other medications that have been studied and found beneficial include IV infliximab, cyclosporine, and IV N-acetylcysteine.12 Acyclovir has been suggested for herpetic lesions in the oral cavity.8
Wound Management
For severe cases involving loss of epidermis, wound management goals are to prevent fluid loss, prevent infection, and facilitate reepithelialization. Although patients with SJS and TEN are best treated in an acute burn center, there are some definite differences in their clinical presentation that affect treatment. For example, SJS and TEN epidermal involvement may continue to spread after admission; subcutaneous necrosis is deeper in burns, thereby creating subcutaneous edema that is not observed in SJS and TEN; fluid requirements for SJS and TEN are usually two-thirds to three-fourths those of burn patients with the same area involvement; and reepithelialization is usually faster in SJS and TEN because of more sparing of the hair follicles in the dermal layer.2 Skin lesions can be expected to heal in an average of 15 days; oral and pharyngeal lesions may take approximately 4 weeks longer.24
Debridement of detached epidermal tissue is controversial and usually not advisable in patients who have a positive Nikolsky sign.2 Collagen sheet dressings,13 Biobrane (Dow B. Hickam, Inc, Sugarland, TX, USA),8 and other occlusive nonadhesive wound coverings that prevent fluid loss and minimize pain with dressing changes have been recommended. These biological dressings create a physiological interface between the wound surface and the environment that is impermeable to bacteria, thus helping to prevent local wound infection.25 In addition, the collagen sheets are noninflammatory, facilitate fibroblast migration to the wound site, assist in extracellular matrix synthesis, are nontoxic, and minimize scarring.13
Conformant (Smith & Nephew, Inc, Largo, FL, USA) is a nonadherent contact layer that comes in rolls and can be applied to large involved areas outside the operating room. Secondary absorbent layers are applied over the contact layer and changed as needed, thereby reducing the number of direct dressing changes. The mesh is released from the wound as healing occurs and thus is easily removed. Because Conformant has no antimicrobial properties, it is recommended only on uninfected skin early in the disease process.8
Oral topical anesthetic gel (lignocaine 2%) and chlorhexidine mouth rinse have been used for oral lesions, and dexamethasone (0.1%) eye drops for ocular lesions.24 Post healing, artificial tears and lubricants may be needed.2
Skin care after full closure includes use of sunscreens and/or avoidance of sun exposure. Readministration of the causative medication should also be avoided. A second episode due to the same drug may have a shorter onset than did the first episode.26
Summary
In summary, DIHS usually occurs 2 to 6 weeks after initiation of the causative medication and can cause cutaneous and oral symptoms ranging from slight to life threatening. The most immediate care involves identification and cessation of the medication, followed by appropriate skin care and intensive medical care when indicated. A careful and thorough review of all medications, including start dates, is an integral component of the subjective and medical history of any patient presenting with dermatologic disorders.
Footnotes
Conflict of interest: The author reports no conflicts of interest.
References
- 1.Pereira de Silva N., Piquioni P., Kochen S., Saidon P. Risk factors associated with DRESS syndrome produced by aromatic and non-aromatic antiepileptic drugs. Eur J Clin Pharmacol. 2011;67(5):463–470. doi: 10.1007/s00228-011-1005-8. [DOI] [PubMed] [Google Scholar]
- 2.Ghislain P., Roujeau J.C. Treatment of severe drug reactions: Stevens-Johnson syndrome, toxic epidermal necrolysis, and hypersensitivity syndrome. Dermtol Online J. 2002;8(1):5. Available at: http://dermatology.cdlib.org/DOJvol8num1/reviews/drugrxn/ghislain.html. Accessed November 27, 2011. [PubMed] [Google Scholar]
- 3.Roujeau J.C., Kelly J.P., Naldi L. Medication use and risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. N Engl J Med. 1995;333:1600–1607. doi: 10.1056/NEJM199512143332404. [DOI] [PubMed] [Google Scholar]
- 4.Strom B.L., Carson J.L., Halpen A.C. A population-based study of Stevens-Johnson syndrome: incidence and antededent drug exposures. Arch Dermatol. 1991;127:831–838. [PubMed] [Google Scholar]
- 5.Chan H.L., Stern R.S., Arndt K.A. The incidence of erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis: a population-based study with particular reference to reactions caused by drugs among outpatients. Arch Dermatol. 1990;126:43–47. [PubMed] [Google Scholar]
- 6.Al-Niaimi F. Drug eruptions in dermatology. 2011;6(3):273-286.
- 7.Letko G., Papaliodis D.N., Papaliodis G.N., Daoud Y.J., Ahmed A.R., Foster C.S. Stevens-Johnson syndrome and toxic epidermal necrolysis: a review of the literature. Ann Allergy Asthma Immunol. 2005;94:419–436. doi: 10.1016/S1081-1206(10)61112-X. [DOI] [PubMed] [Google Scholar]
- 8.Digwood-Lettieri S., Reilly K.J., Linwood R.H. Levofloxacin-induced toxic epidermal necrolysis in an elderly patient. Pharmacotherapy. 2002;22(6) doi: 10.1592/phco.22.9.789.34074. http://www.medscape.com/viewarticle/438890 Available at: Accessed November 21, 2011. [DOI] [PubMed] [Google Scholar]
- 9.Bellon T., Alvarez L., Mayorga C. Differential gene expression in drug hypersensitivity reactions: induction of alarmins in severe bullous diseases. Br J Dermatol. 2010;162(5):1014–1022. doi: 10.1111/j.1365-2133.2009.09627.x. [DOI] [PubMed] [Google Scholar]
- 10.Tohyama M., Watanabe H., Murakami S. Possible involvement of CD14+ CD16+ monocyte lineage cells in the epidermal damage of Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol. 2012;166(2):322–330. doi: 10.1111/j.1365-2133.2011.10649.x. [DOI] [PubMed] [Google Scholar]
- 11.Merk H.F. Drug skin metabolites and allergic drug reactions. Curr Opin Allergy Clin Immunol. 2009;9(4):311–315. doi: 10.1097/ACI.0b013e32832dd13c. [DOI] [PubMed] [Google Scholar]
- 12.Chia F.L., Leong K.P. Severe cutaneous adverse reactions to drugs. Curr Opin Allergy Clin Immunol. 2007;7(4):304–309. doi: 10.1097/ACI.0b013e328216f54a. [DOI] [PubMed] [Google Scholar]
- 13.Bhattacharya S., Tripathi N., Gupta V., Nigam B., Khanna A. Collagen sheet dressings for cutaneous lesions of toxic epidermal necrolysis. Indian J Plast Surg. 2011;44(3):474–477. doi: 10.4103/0970-0358.90826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen S., Billig A. Ceftrixone-induced toxic epidermal necrolysis mimicking burn injury: a case report. J Med Case Reports. 2009;3:9323. doi: 10.1186/1752-1947-3-9323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bircher A.J. Symptoms and danger signs in drug hypersensitivity. Toxicology. 2005;209:201–207. doi: 10.1016/j.tox.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe R., Watanabe H., Sotozono C., Kokaze A., Iijima M. Critical factors differentiating erythema multiforme majus from Stevens-Johnson syndrome and toxic epidermal necrolysis. Eur J Dermatol. 2011;21(6):889–894. doi: 10.1684/ejd.2011.1510. [DOI] [PubMed] [Google Scholar]
- 17.Bastuji-Garin S., Fouchard N., Bertocchi M., Roujeau J.C., Revuz J., Wolkenstein P. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115:149–153. doi: 10.1046/j.1523-1747.2000.00061.x. [DOI] [PubMed] [Google Scholar]
- 18.Ang C.C., Tay Y.K. Hematological abnormalities and the use of granulocyte-colony-stimulating factor in patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. Int J Dermatol. 2011;50(12):1570–1578. doi: 10.1111/j.1365-4632.2011.05007.x. [DOI] [PubMed] [Google Scholar]
- 19.Miyazaki M., Tanaka M., Ueda A. Acute liver failure caused by drug-induced hypersensitivity syndrome associated with hyperferritinemia. World J Gastroenterol. 2011;17(44):4928–4931. doi: 10.3748/wjg.v17.i44.4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujita Y., Yoshioka N., Abe R. Rapid immunochromatographic test for serum granulysin is useful for the prediction of Stevens-Johnson syndrome and toxic epidermal necrolysis. J Am Acad Dermatol. 2011;65:65–68. doi: 10.1016/j.jaad.2010.04.042. [DOI] [PubMed] [Google Scholar]
- 21.Thong B.Y. Update on the management of antibiotic allergy. Allergy Asthma Immunol Res. 2010;2(2):77–86. doi: 10.4168/aair.2010.2.2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chave T.A., Mortimer N.J., Sladden M.J., Hall A.P., Hutchinson P.E. Toxic epidermal necrolysis: current evidence, practical management, and future directions. Br J Dermatol. 2005;153(2):241–253. doi: 10.1111/j.1365-2133.2005.06721.x. [DOI] [PubMed] [Google Scholar]
- 23.Chowta N.K., Chowta M.N., Ramapuram J., Kumar P., Fazil A. Carbamzepine-induced toxic epidermal necrolysis. Indian J Crit Care Med. 2011;15(2):123–125. doi: 10.4103/0972-5229.83018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mittmann N., Chan B.C., Knowles S., Shear N.H. IVIG for the treatment of toxic epidermal necrolysis. Skin Therapy Lett. 2007;12(1):7–9. http://www.medscape.com/viewarticle/554693 Available at: Accessed November 12, 2011. [PubMed] [Google Scholar]
- 25.Park S.N., Lee H.J., Lee K.H., Suh H. Biological characterization of EDC-crosslinked collagen-hyaluronic acid matrix in dermal tissue restoration. Biomaterials. 2003;24:1631–1641. doi: 10.1016/s0142-9612(02)00550-1. [DOI] [PubMed] [Google Scholar]
- 26.Grammer L.C. Stevens-Johnson syndrome: affects more than just skin. Medscape General Medicine. 1997;1(2) http://www.medscape.com/viewarticle/719223 Available at: Accessed November 23, 2011. [Google Scholar]
- 27.Coyle C., Wenhold V. Painful blistered hands and feet. Clin J Oncol Nurs. 2001;5(5):1–3. [PubMed] [Google Scholar]
- 28.Wolf R., Orion E., Matz H. The baboon syndrome or intertriginous drug eruption: a report of eleven cases and a second look at its pathomechanism. Dermatol Online J. 2003;9(3):2. http://dermatology.cdlib.org/93/original/baboon/wolf.html Available at: Accessed April 8, 2012. [PubMed] [Google Scholar]
- 29.Ponvert C., Perris Y., Bados-Albiero A. Allergy to betalactam antibiotics in children: results of a 20-year study based on clinical history, skin and challenge tests. Pediatr Allergy Immunol. 2011;22(4):411–418. doi: 10.1111/j.1399-3038.2011.01169.x. [DOI] [PubMed] [Google Scholar]
- 30.Momin S.B. Review of intravenous immunoglobulin in the treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis. J Clin Aesthetic Dermatol. 2009;2(2):51–58. [PMC free article] [PubMed] [Google Scholar]
- 31.Balasundaram S., Ranganathan K., Umadevi R. Oral lesions associated with nevirapine-related Stevens-Johnson syndrome: a report of four cases. J Oral Maxillofac Pathol. 2011;15(1):39–45. doi: 10.4103/0973-029X.80024. [DOI] [PMC free article] [PubMed] [Google Scholar]


