Abstract
Purpose
Wound care in a rehabilitation environment is a costly and difficult problem. The goal of this retrospective study is to evaluate differences in wound closure outcomes in acute and chronic wounds when treated with a microcurrent-generating wound care device as compared to standard wound care methods.
Methods
Data files of 38 patients who received either standard wound treatment (SOC; n = 20), or were treated with a microcurrent-generating wound device (MCD, n = 18), were retrospectively reviewed. Wounds were assessed until deemed clinically to have closed or healed with up to 100% epithelialization. All patients (18–99 years) with single wounds were included. The number of days to wound closure and the rate of wound volume reduction were compared across groups. Persistent reduction of wound size improvement was also examined.
Results
The wounds in the SOC group closed on average at 36.25 days (SD = 28.89), while the MCD group closed significantly faster in 19.78 days (SD = 14.45), p = 0.036. The rate of volume reduction per day was −3.83% for SOC vs. −9.82% volume reduction per day (p = 0.013) for the MCD group. The SOC group had 50% of its wounds close monotonically vs. 83.3% in the MCD group (p = 0.018).
Conclusion
This two-center retrospective study demonstrated a 45.4% faster, and more robust healing of wounds with the use of the MCD, when compared to SOC in a rehabilitation center environment. This translates into improved patient care, and potentially significant cost savings. Economic benefits for the use of MCD compared to other wound care methods are planned for future research.
Keywords: Wound closure, Electric stimulation, Antimicrobial dressings, Long-term care
Background
An estimated population of nine million Americans over the age of 65 required long-term care in 2012, with a projected increase to 27 million by 2050.1,2 Patients of long-term care, rehabilitation and nursing home centers frequently present with multiple co-morbidities and various underlying pathologies that have contributed to high incidence and prevalence of complex wounds of diverse etiologies, ranging from diabetes ulcerations, venous insufficiency ulcers and pressure ulcers.3 Of the 1.5 million US nursing home residents in 2004, 24.2% had diabetes and over 11% presented with a pressure ulcer.4,5 The development of acute and chronic wounds in a long-term care population poses a significant challenge in complexity, lack of viable options for their effective management, as well as the high cost of treatment associated with low quality of life for these patients. Treatment of wounds in patients with chronic illness or disability requires a specialized approach in the area of dressing selection and wound care management.
Debridement, standard dressings, and topical agents are currently used in the management of more complex wounds. Despite advances, the present options for wound care are costly, time-consuming, and may fail to address situational differences between the unique patient and the different types of wounds.6-9 Furthermore, patients are plagued with slow closure rates, infections, and often suffer with inadequate pain relief.10-12 Newer wound care options include; energy-based technologies that target wound repair and regeneration from cellular and clinical levels, and have the capacity to induce and stimulate wound healing processes.13
Among the various options for wound healing, electric fields in the form of direct microcurrents to wounds have been reported to result in a reduction in healing time, inflammation and pain.13-15 However, the delivery systems used on patients are often bulky, with limited clinical data reported.13-17 A microcurrent-generating device (MCD), consisting of a matrix of biocompatible micro-cells was developed in recent years. The self-contained device requires no external power source, generates a microcurrent in the presence of an electrolyte such as wound exudate, sterile saline or conductive hydrogel, and has been shown to facilitate chronic and acute wound repair.13,18-20 Findings from recent studies have pointed to mechanisms of faster re-epithelialization and reduced expression of inflammatory biomarkers such as cytokine Interleukin-1α.19,20 A retrospective study to assess the wound improvement capability of the MCD when compared to a diverse array of wound care options was conducted. The primary objective of this study was to determine differences in wound closure outcomes with the use of the MCD on patients with chronic and acute wounds in a long-term care and rehabilitation population.
Methods
Clinical Data Assessment
An IRB-approved retrospective, observational study was performed in a population of rehabilitation and long-term care patients with acute and chronic wounds of varied etiology. Overall time to attain a closed wound (100% epithelialization), and wound closure rates were collected. Data was retrospectively reviewed from a case series of patients treated between 2010 and 2012 for their acute and chronic wounds using regulatory approved steps at two rehabilitation centers, Bethany Health and Rehab Center as well as Trevecca Health and Rehab Center both located in Nashville, TN, USA. Both nursing home centers provide services ranging from intensive rehabilitative transitional care to palliative care, and offer wound care services to its patients and residents through a team of physicians, nurses, and therapists. Written consent was provided by patients upon facility admission for use of medical records and photographs.
Patients included in the study were part of an approved wound management program and all patients received wound care as appropriate to their specific case, including wound dressings, dressing changes, debridement, and etiology-specific care (i.e., offloading, compression, etc.) A retrospective chart review was performed to investigate differences in wound closure outcomes when the Procellera® Wound Dressing [(MCD; Vomaris Wound Care, Inc., Chandler, AZ)], was used for wound management as compared to a group of patients where wounds were treated with standard wound care (SOC) alone.
The IRB-approved protocol enabled the inclusion of all cases with wounds having the following characteristics: all male or female patients between the ages of 18–99 years, with no more than one wound on their body. Examples of wound types included diabetic, venous, and pressure ulcers, as well as burns and traumatic wounds. Wounds among these patients were either being treated using the MCD, or any other wound care method (SOC). The case inclusion criteria were broad in that wounds being managed with adjunctive therapies such as negative pressure wound therapy (NPWT), in combination with the MCD or with SOC were included in the analysis. Wounds included in the study for the SOC group were open to advanced wound management techniques such as collagen matrix dressings, enzymatic dressings, silver-based dressings, etc. Certain exclusions to the selection of cases for this research were: cases with known sensitivity to silver or zinc (although, no cases with either sensitivity were encountered in this study), active cancer, connective tissue disease, skin graft sites and participation in another trial.
Patient history files between April 2010 and October 2012 were reviewed to confirm inclusion in the study. Relevant patient characteristics such as demographics, wound etiology, history, categorization, chronicity, location, length of treatment co-morbidities, treatment regimen and procedures performed, interim wound dimensions (length, width, depth), number of visits and treatment completion observations were tabulated, along with digital photographs of wounds. All patient files reviewed had interim wound measurements and photographic documentation.
Wound Management
The wound management approach for the patients used in the retrospective data analysis is described here. All subjects were administered the best standard wound care appropriate to their specific etiologies. Patients treated with MCD received one application of the device per week, in some instances sooner in the presence of excessive wound exudate, per manufacturer's device instructions, in conjunction with the appropriate wound care management. The MCD was maintained moist by saturating with normal saline or conductive hydrogel on application, then was covered with double-layered saline-soaked gauze and was retained in place with semi-occlusive secondary dressing appropriate to its exudate level. Dressings were changed at weekly intervals or sooner, if necessary, and continued until full wound closure. Since wound care products such as enzymatic debriders are contraindicated in combination with the MCD, mechanical debridement was used. When not contraindicated, adjunctive therapies (such as NPWT, etc.) were administered with SOC or with the use of MCD, and according to facility protocol.
Participants not treated with the MCD received the standard of care dressings based on protocol and formulary used by the facilities and appropriate to the wound size and exudate level, i.e., antimicrobial dressings, barrier creams, alginates, silver dressings, absorptive foam dressings, hydrogel, enzymatic debridement ointment, NPWT, etc., used in the management of comparable wounds. Etiology-specific care was administered on a case-by-case basis, (i.e., compression, offloading and pressure redistribution). All dressings and topicals were used in accordance with the respective manufacturer's instructions. Dressings were applied at weekly intervals and changed sooner, if necessary. Advanced adjunctive therapies were also administered as appropriate, according to facility protocol. Wound care was administered until the wound healed or necessitated additional interventions.
Wound Size and Assessment
Wound dimensions and photographs were recorded at the beginning of the treatment, as well as interim and final patient visits. Linear wound dimensions, including length (l), width (w) and depth (d) were measured by paper ruler, with depth measured at the deepest point. Wound closure progression was also documented through digital photography under standard photographic requirements.
Determining an ellipsoidal area of the wound by using the length and width measurements was considered a better approximation of the wound surface area for each wound under consideration.21 Measurements of depth of wounds recorded in the patient forms were considered to estimate the volume of the wounds. This approach was quite pertinent for this study since the wound depths of the patients ranged from 0.1 to 3.6 cm. In order to perform a uniform wound analysis in terms of volume, when no recordable wound depth was listed in the patient chart, the wound depth was approximated to a clinically insignificant dimension of 0.05 cm. Assuming an ellipsoidal wound surface area, the wound volume was calculated using the following expression,
where l, w, d, are the length, width, and depth of the wound, respectively.
The “percent wound volume change per day” was calculated using the following expression,
where the difference in wound volume divided by the number of wound care days can be expressed as a percentage.
In addition to measurements, the treating clinician assessed the wound for the following parameters: (a) wound exudate (type and amount, per clinician's visual assessment); (b) wound bed tissue type (percent of granulation tissue vs. nonviable tissue); (c) peri-wound skin (healthy, intact, inflamed, or macerated); and (4) presence of undermining or tunneling within the wound. Closure was defined as 100% epithelialization with visible effacement of the wound. All wounds were assessed 1 week post-closure to ensure continued progress toward healing during its maturation and remodeling phase. Residents received weekly assessments for overall skin integrity.
Statistical Analysis
Data were abstracted from the charts and entered into a spreadsheet. This file was imported into SPSS, version 21 (SPSS, an IBM Company, Chicago, IL.). Prior to analysis, normality assumptions were checked, as appropriate, using the Kolmogorov–Smirnov test. Independent-samples t-tests were conducted for continuous variables if normality assumptions were met; otherwise, a Mann–Whitney (nonparametric) test was used. Chi-square or Fisher's exact test were used for categorical variables. A binomial (Z) test was used to test differences in proportions. Finally, Kaplan–Meier and log rank tests were used for time-to-event analysis. All tests were two-tailed, and a p-value of 0.05 was used to determine statistical significance.
Results
A total of 38 patients were included in the analysis in this study, 20 as part of the SOC arm, and 18 obtaining treatment with the MCD. Statistical analysis for various parameters describing the SOC and the MCD groups at inception of the study are provided in Table 1. The two groups did not differ significantly in gender, age, wound types or the length, width, and area of their wounds. Kolmogorov–Smirnov tests indicated that the only variable not meeting normality assumptions was the wound volume change, p = 0.02.
Table 1.
Summary of Patient Intake Data.
| Standard of Care (SOC) | Microcurrent-Generating Device (MCD) | |
|---|---|---|
| Number of cases | 20 | 18 |
| Females | 13 | 14 |
| Males | 7 | 4 |
| Age [years (SD)] | 81.5 ± 9.79 | 80.17 ± 10.24 |
| Wound size [volume, cc] | Min 0.01 | Min 0.03 |
| Max 312.1 | Max 224.1 | |
| Mean 30.4 ± 74.18 | Mean 21.1 ± 55.03 | |
| Wound types | Hematoma | Hematoma |
| Venous leg ulcer | Venous leg ulcer | |
| Skin tear III | Skin tear III | |
| Pressure ulcer II, IV | Pressure ulcer II, IV | |
| DFU | DFU | |
| Abrasion | Abrasion | |
| Non pressure blister, stage II blister | MRSA blister | |
| Surgical dehiscence | Surgical dehiscence | |
| Surgical site | Abdominal dehiscence | |
| Burn | ||
| Vascular |
Days to Complete Wound Closure
The wound types included in this study were diverse in etiology and dimensions. Therefore, the time to heal for wounds was distributed over a wide range (9–124 days for SOC, and 3–44 days for the MCD group). In addition, the patients in this study often had multiple co-morbidities, including diabetes, renal disease, and hypertension. The wounds were considered healed when the clinician indicated cessation of wound care in the patient notes, typically at 100% epithelialization. The average number of days to wound closure from the initial measurement was 36.25 (SD = 28.89) for the SOC group and 19.78 (SD = 14.45) for the MCD group, p = 0.036. On average, the wounds in the MCD treatment group attained closure 45.43% earlier than those in the SOC group. Examples in Fig. 1 show, one wound each from patients from the SOC (Fig. 1a and b) and MCD (Fig. 1c and d) groups, respectively. The wound treated with SOC closed in 39 days, and the other treated with the MCD closed in 21 days.
Figure 1.
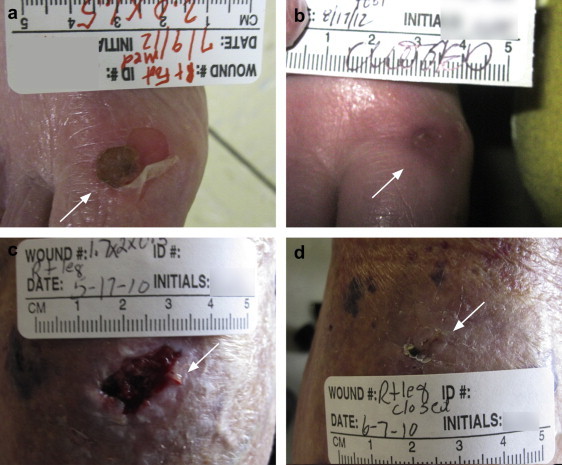
Example of a wound at Day 0 and end of treatment from the SOC (a) and (b), and MCD group (c) and (d). Note that a simple non-pressure foot blister required 39 days to close with SOC, possibly due to the compromised health status of the patient.
The mean percent volume changes per day of the two groups, SOC and MCD, were respectively, −3.83% and −9.82%, p = 0.013. This test was replicated using the Mann–Whitney test because data were slightly non-normal. The results of this test were quite similar to those of the t-test, p = 0.02.
Results of a time-to-event analysis of wounds as a function of number of days for the two groups, SOC and MCD, are shown in Fig. 2. Median estimated time-to-closure for the SOC group was 28 days (95% confidence interval: 8.28–47.72) vs. 14 days (95% confidence interval: 5.68–22.32) for the MCD group; log rank p-value = 0.024. The two curves separate early in the closure process and maintain their separation throughout the study.
Figure 2.
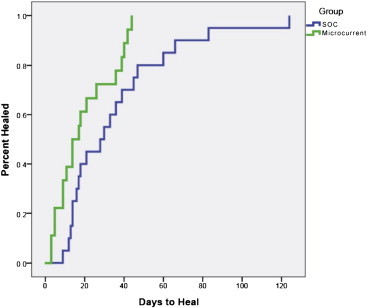
Cumulative wound survival trend as a function of days.
Interim Wound Dimensions As a Function of Time
As described earlier, the patients' wounds were periodically evaluated during the course of their treatment with various advanced wound care methods. During these evaluations, the wounds were measured and photographed. The frequency of interim wound assessments was recorded from the respective case report forms and tabulated. The interim wound assessments ranged from 2 days to 11 days. In both treatment groups, some wounds became worse in terms of wound volume while others showed persistent improvement. Fig. 3 shows sample graphs of percent changes in wound volume compared to respective initial volume. A negative trend represents wound improvement and a positive percentage represents worsening of the wound. Based on the volume calculated, some wounds improved persistently while others first increased in size before improving (“wax-and-wane”). The SOC and the MCD groups were compared to each other in terms of the number of instances when the dimensions of the patient wounds increased (i.e., wound treatment outcome degraded). In the SOC group, 10 wounds (50% for n = 20) became larger during at least one measurement interval, whereas 3 wounds (16.7% for n = 18) became larger in the MCD group (p = 0.018). Overall, despite wound care at the site of the injury, wounds in both groups responded positively. Response to treatment was observed to be slower during the initial phase, but was observed to improve as time progressed.
Figure 3.
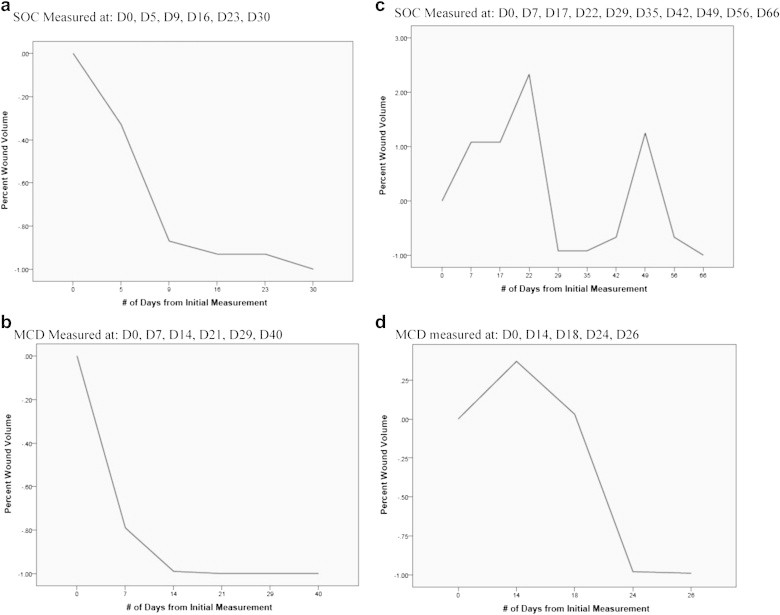
Wound trends: persistent (monotonic) and “wax-wane.” Wounds in both groups sometimes show a monotonic trend toward closure (a) and (b), or, tend to grow in size first and then improve (c) and (d).
Since the interim measurement points during the course of treatments were variable, it is difficult to compare the trends in wound closure between different patients. Hence, the wound dimensional measurements were interpolated at 5-day intervals for all wounds, by assuming a linear trend between two successive measurements made by the clinician. This approach enables a comparison of wound trajectories attained within the two wound groups. The 5-day interpolated wound interval was considered as a practical duration within the time course of the cases that were considered in this study, yet care was taken to not skew the wound course trends significantly due to interpolation. An estimate of the wound dimension at a fixed time interval makes a quantitative comparison of various wound measurement data much more feasible. The average, cumulative trajectory of percentage wound volume reduction for the SOC compared to the MCD group is shown in Fig. 4. In each case, the percentage reduction in wound volume was normalized to the volume for a particular wound at Day 0.
Figure 4.
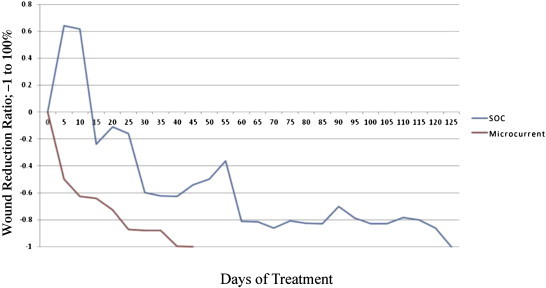
Cumulative average trend for percentage wound closure for the SOC and the MCD group, using the 5-day interpolated values. The overall trend for the wound trajectory is much steeper for the MCD group compared to SOC.
Discussion
This study is designed to investigate the healing and management of wounds of varying etiology in a rehabilitation center and long-term care environment. A retrospective study compared subjects receiving the wound care treatment deemed most appropriate by the clinician (SOC), to patients who received a microcurrent-generating wound device (Procellera®). Only patients presenting with a single wound were included. However, the inclusion criteria were broad, and patients with systemic co-morbidities except active cancer, connective tissue disease or graft wounds were evaluated. In addition to tracking the condition of the wounds at the initial time point and time of completion for wound treatment, several interim measurements and assessments were taken, and the wound volume estimated (Table 2).
Table 2.
Summary of All Statistics Comparing SOC With MCD Group.
| SOC | MCD | p value | |
|---|---|---|---|
| Average time for wound healing [mean (SD)] | 36.25 days (SD = 28.89) | 19.78 days (SD = 14.45) | 0.036 |
| Average volume reduction per day [mean (SD)] | −3.83% (SD = 2.9) | −9.82% (SD = 9.8) | 0.013 |
| Cumulative survival of wounds | 124 days | 43 days | |
| Number of patients (%) in which wound dimensions increased | 10 (50% of SOC group) | 3 (16.7% of microcurrent-generating dressing group) | 0.018 |
| Average wound trajectory at fixed 5-day intervals (interpolated) | Waxing and waning trend (Fig. 4) | Persistent waning trend (Fig. 4) |
Within these relatively broad inclusion criteria, the MCD wound treatment group demonstrated on average a 45.43% faster closure rate as compared to the SOC group. The average wound volume change per day in the MCD group was calculated to be −9.8% compared to −3.8% in the SOC group. These results suggest increased efficacy of the MCD. The interim measurements taken by the clinician have provided a good opportunity to evaluate the general course of wound closure within the two study groups. Patients with wounds receiving SOC were more likely to have their wounds follow a “waxing-and-waning” progression in wound closure compared to wounds in the MCD treatment group. This trend indicates a more robust healing response observed in the MCD group compared to current SOC approaches used in this study. The MCD is designed to provide a sustained microcurrent at the wound site to facilitate wound closure.13,18-20 Additionally, the antimicrobial efficacy of Ag and Zn, which are components of the MCD being investigated, may have also contributed to the more robust wound healing response compared to SOC.22-24
The results obtained in this study demonstrate some key aspects of efficacious wound closure when using the MCD. Compared to localized SOC treatments for wounds, the MCD (1) shortens the wound closure time, (2) has a steeper wound closure trajectory, and (3) has a more robust wound healing trend with fewer incidence of increased wound dimensions during the course of healing. Each of these criteria are used in assessing the effectiveness of a wound care strategy.6-9 These observations of overall improved capability for wound healing using the microcurrent approach are similar to those reported in other studies, for acute wounds,18-20 and provide further evidence of its benefits as a wound care modality for slow healing wounds.25
Microcurrent Stimulation and Wound Care
The wound care device used for the present study consists of a discrete matrix of silver and zinc dots, placed on a pliable polyester substrate. In the presence of an ionic fluid, such as normal saline, administered on the device prior to application, or the ionic environment by presence of wound exudate, a sustained voltage of approximately 0.8 V is generated between the Ag and Zn dots.13,18-20 The electric field generated at the device surface is measured to be 0.2–1.0 V, 10–50 μA. Such a field is comparable to the transcutaneous voltage potentials developed in injured skin.27-30 Microcurrents resulting from Ag electrodes have been reported to enhance wound healing in a number of studies in vivo and in the clinic. For example, Alvarez et al26 reported significantly faster healing of wounds in a porcine model when an Ag electrode delivering a direct current of 50 μA–300 μA was activated, compared to when the electrode was not energized. As another example, Huckfeldt et al14 reported a 39% improvement in wound closure for patients having full thickness skin burns in a 30-patient study, when an anodic microcurrent is delivered using a flexible silver electrode construct. The cathode was a return pad placed on uninjured skin.
In the context of the present study, in addition to the microelectric current from the Ag and Zn matrix covering the wound, the device provides all of the key prerequisites for standard wound care,12 namely, (1) a protective cover for the wound, from the polyester material, and with the addition of a semi-occlusive overlay, (2) a capability to keep the wound moist, using normal saline, and (3) an antimicrobial wound care environment with the Ag and Zn pattern.22-24
Despite the encouraging clinical results, there are a number of limitations to this investigation. The primary weaknesses are the retrospective and non-random nature of patient treatment in this clinical study, with a relatively small patient population in the SOC (n = 20) and the MCD group (n = 18). The ideal study design should be prospective and statistically powered to compare the groups based on individual disease etiology, e.g., diabetic, vascular etc., whereas the current study compares two general patient groups with diverse underlying wound etiologies. The interim measurement durations with the current patient population were not synchronized and the recorded follow-up visits fall within a wide range of 2–11 days. For analyzing the trends for wound improvement trajectories, interim wound dimensions therefore had to be interpolated at 5-day intervals.
In considering the broader implications of the clinical results of improved and faster wound closure using the MCD, the patients are being treated in a rehabilitation facility, a mainstay of community-based health care. The patients in these facilities require long-term care and present with multiple co-morbidities, and the superficial wounds are often greatly affected by systemic disease state(s). The patient population at such centers requiring health care is large, and the need for health care significant. It is therefore critical to kick-start healing of stalled wounds using an effective wound care treatment. Compared to the varied options with SOC, the MCD, when used in a community-based rehabilitation environment, may lead to significant cost benefit and effectiveness.
Conclusions
Results from this bi-center retrospective study suggest that application of the MCD may facilitate healing of wounds resulting from a wide range of etiologies in the aging population. In comparison to SOC methods, the MCD provides faster wound closure (45.4% compared to SOC), more robust trend of wound size reduction and a sharper wound closure trajectory. It is known that the presence of an electric field within a wound enhances wound healing. The present study demonstrates the efficacious clinical use of a self-contained, antimicrobial MCD composed of a discrete Ag–Zn matrix. Improved wound healing characteristics compared to SOC in the rehabilitation center patient population translates to greater patient comfort, quality of life, decreased resource utilization and potentially significant cost savings.
Acknowledgment
We wish to thank Prof. Curt Bay, PhD, A.T. Still University, Mesa, AZ, for his expertise and assistance in statistical analysis of the clinical data.
Footnotes
Disclaimer: Penny Campbell herewith certifies that she is a paid employee of Vomaris Wound Care, Inc.
References
- 1.Kaye H., Harrington C., LaPlante M. Long-term care: who gets it, who provides it, who pays, and how much? Health Aff. 2010;29(1):11–21. doi: 10.1377/hlthaff.2009.0535. [DOI] [PubMed] [Google Scholar]
- 2.The SCAN Foundation Fact Sheet. Growing demand for long term care in the US. Available at: http://www.thescanfoundation.org/sites/thescanfoundation.org/files/us_growing_demand_for_ltc_june_2012_fs.pdf; Accessed 25.01.13.
- 3.Gist S., Tio-Matos I., Falzgraf S., Cameron S., Beebe M. Wound care in the geriatric client. Clin Interv Aging. 2009;4:269–287. doi: 10.2147/cia.s4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Resnick H.E., Heineman J., Stone R., Shorr R.I. Diabetes in U.S. nursing homes, 2004. Diabetes Care. 2008;31(2):287–288. doi: 10.2337/dc07-1425. [DOI] [PubMed] [Google Scholar]
- 5.Park-Lee E., Caffrey C. 2009. Pressure Ulcers Among Nursing Home Residents: United States, 2004. National Health Care Survey (NHCS) Data Brief No. 14. [PubMed] [Google Scholar]
- 6.Campbell P.E., Smith G.S., Smith J.M. Retrospective clinical evaluation of gauze-based negative pressure wound therapy. Int Wound J. 2008;5(2):280–286. doi: 10.1111/j.1742-481X.2008.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kavros S.J., Liedl D.A., Boon A.J., Miller J.L., Hobbs J.A., Andrews K.L. Expedited wound healing with noncontact, low-frequency ultrasound therapy in chronic wounds: a retrospective study. Adv Skin Wound Care. 2008;21(9):416–423. doi: 10.1097/01.ASW.0000323546.04734.31. [DOI] [PubMed] [Google Scholar]
- 8.Vuerstaek J.D.D., Vainas T., Wuite J., Nelemans P., Neumann M.H.A., Veraart J.C.J.M. State-of-the-art treatment of chronic leg ulcers: a randomized controlled trial comparing vacuum-assisted closure (V.A.C.) with modern with modern wound dressings. J Vasc Surg. 2006;44(5):1029–1037. doi: 10.1016/j.jvs.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 9.Sheehan P., Jone P., Giurini J.M., Caselli A., Veves A. Percent change in wound area of diabetic foot ulcers over a 4-week period is a robust predictor of complete healing in a 12-week prospective trial. Plast Reconstr Surg. 2006;117(7 suppl):239S–244S. doi: 10.1097/01.prs.0000222891.74489.33. [DOI] [PubMed] [Google Scholar]
- 10.Franks P.J., Moffatt C.J. Quality of life in patients with chronic wounds. Wounds. 1998;10(suppl E):1E–9E. [Google Scholar]
- 11.Singer A.J., Clark R.A.F. Cutaneous wound healing. N Engl J Med. 1999;341(10):734–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 12.Fonder M.A., Lazarus G.S., Cowan D.A., Aronson-Cook B., Kohli A.R., Mamelak A.J. Treating the chronic wound: a practical approach to the care of nonhealing wounds and wound care dressings. J Am Acad Dermatol. 2008;58(2):185–206. doi: 10.1016/j.jaad.2007.08.048. [DOI] [PubMed] [Google Scholar]
- 13.Isseroff R.R., Dahle S.E. Electrical stimulation therapy and wound healing: where are we now? Adv Wound Care. 2012;1(6):238–243. doi: 10.1089/wound.2011.0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huckfeldt R., Flick A.B., Mikkelson D., Lowe C., Finley P.J. Wound closure after split-thickness skin grafting is accelerated with the use of continuous direct anodal micro current applied to silver nylon wound contact dressings. J Burn Care Res. 2007;28:703–707. doi: 10.1097/BCR.0B013E318148C945. [DOI] [PubMed] [Google Scholar]
- 15.Carley P.J., Wainapel S.F. Electrotherapy for acceleration of wound healing: low intensity direct current. Arch Phys Med Rehabil. 1985;66:443–446. [PubMed] [Google Scholar]
- 16.Kloth L.C., Feedar J.A. Acceleration of wound healing with high voltage, monophasic pulsed electrical current. Phys Ther. 1988;68:503–508. doi: 10.1093/ptj/68.4.503. [DOI] [PubMed] [Google Scholar]
- 17.Stromberg B.V. Effects of electrical currents on wound contraction. Ann Plast Surg. 1988;21(2):121–123. doi: 10.1097/00000637-198808000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Blount A.L., Foster S., Rapp D.A., Wilcox R. The use of bioelectric dressings in skin graft harvest sites: a prospective case series. J Burn Care Res. 2012;33(3):354–357. doi: 10.1097/BCR.0b013e31823356e4. [DOI] [PubMed] [Google Scholar]
- 19.Harding A.C., Gil J., Valdes J., Solis M., Davis S.C. Efficacy of a novel bio-electric dressing in healing deep partial-thickness wounds in a porcine model. Ostomy Wound Manage. 2012;58(9):50–55. [PubMed] [Google Scholar]
- 20.Davis S.C., Gil J., Valdes J., Perez R., Rivas Y. Assessment of the effects on wound healing and gene expression of a bioelectric dressing using a porcine wound model and real time reverse transcriptase-polymerase chain reaction. J Am Acad Dermatol. 2009;60:AB200. [Google Scholar]
- 21.Ahn C., Salcido R.S. Advances in wound photography and assessment methods. Adv Skin Wound Care. 2008;21(2):85–93. doi: 10.1097/01.ASW.0000305411.58350.7d. [DOI] [PubMed] [Google Scholar]
- 22.MacKeen P.C., Person S., Warner S.C., Snipes W., Stevens S.E., Jr. Silver-coated nylon fiber as an antibacterial agent. Antimicrob Agents Chemother. 1987;31:93–99. doi: 10.1128/aac.31.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parsons D., Bowler P.G., Myles V., Jones S. Silver antimicrobial dressings in wound management: a comparison of antimicrobial, physical, and chemical characteristics. Wounds. 2005;17(8):222–232. [Google Scholar]
- 24.Lansdown A.B.G., Mirastschijski U., Stubbs N., Scanlon E., Årgen S. Zinc in wound healing: theoretical, experimental, and clinical aspects. Wound Repair Regen. 2007;15:2–16. doi: 10.1111/j.1524-475X.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- 25.Sheftel S.N. The role of a bio-electric, antimicrobial dressing in the healing of acute and chronic wounds [abstract] Clin Symp Adv Skin Wound Care. Las Vegas, NV. October 2008;(suppl):217. [Google Scholar]
- 26.Alvarez O.M., Mertz P.M., Smerbeck R.V., Eaglstein W.H. The healing of superficial skin wounds is stimulated by external electrical current. J Invest Dermatol. 1983;81(2):144–148. doi: 10.1111/1523-1747.ep12543498. [DOI] [PubMed] [Google Scholar]
- 27.Barker A.T., Jaffe L.F., Vanable J.W., Jr. The glabrous epidermis of cavies contains a powerful battery. Am J Physiol. 1982;242:R358–R366. doi: 10.1152/ajpregu.1982.242.3.R358. [DOI] [PubMed] [Google Scholar]
- 28.Nuccitelli R. A role for endogenous electric fields in wound healing. Curr Top Dev Biol. 2003;58:1–26. doi: 10.1016/s0070-2153(03)58001-2. [DOI] [PubMed] [Google Scholar]
- 29.Zhao M., Song B., Pu J. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-γ and PTEN. Nature. 2006;442:447–450. doi: 10.1038/nature04925. [DOI] [PubMed] [Google Scholar]
- 30.Vanhaesebroeck B. Charging the batteries to heal wounds through P13K. Nat Chem Biol. 2006;2(9):453–455. doi: 10.1038/nchembio0906-453. [DOI] [PubMed] [Google Scholar]


