Abstract
Androgens are thought to cause prostate cancer, but the precise mechanisms by which they do so are unclear. Data, mostly from animal studies, suggest that for androgens to cause prostate cancer they must be aromatized to estrogen and act in concert with these estrogen metabolites. Androgen-receptor mediated activity of androgens and estrogen receptor-mediated effects of estrogen metabolites are likely to be necessary, but estrogen genotoxicity appears to be a probable critical factor as well. Only when all these mechanisms are active, may prostate carcinogenesis result. Convincing proof-of-concept studies are needed to definitively test this concept which, if proven, may lead to clinically feasible chemoprevention approaches interfering with these mechanisms.
Keywords: Prostate cancer, Estrogens, Androgens, Hormonal Carcinogenesis
1. Introduction
Prostate cancer is the leading non-skin malignancy detected in US males and the second cause of death due to male cancer in the US [1]. The causes of this major male malignancy are not entirely clear, but the idea that androgenic hormones play a major causative role in prostate carcinogenesis has been around for decades [2]. The basis for this assumption is that the prostate gland is an androgen-dependent tissue and that prostate cancer is an androgen-dependent malignancy [2]. The underlying mechanism has been postulated to be androgenic stimulation of cell proliferation resulting in an increased risk of oncogenic genetic alterations [3]. However, the human and biological evidence for this is indirect and very limited at best. There is no evidence that androgens cause sustained cell proliferation in the prostate. This is illustrated in rats that are surgically castrated, which causes involution of the prostate gland by apoptosis and cessation of secretory activity, and after a couple of weeks are given androgen back at physiological levels; this treatment causes a few waves of cell proliferation in the prostate, but after about four days, cell proliferation returns to levels found in intact control rats [4]. The further growth of the prostate upon continued androgen treatment is caused by increased secretion, not cell proliferation [4; 5]. There are no human data of the effects of androgen treatment on prostatic cell proliferation; this would be extremely difficult to investigate. There are only data on the effects of androgen treatment on serum levels of prostate specific antigen (PSA), but these do not necessarily reflect cell proliferation and are more likely to indicate effects at the level of PSA production by the prostate and prostate cancer cells [6]. Thus, if androgens indeed cause prostate cancer, the mechanisms by which they do this are currently not understood.
2. Androgens
There is no evidence that circulating hormone levels are associated with later risk of prostate cancer [7; 8]. Serum hormone levels provide no information about hormone concentrations in prostate tissue, which are controlled by intraprostatic metabolism of androgens [9; 10]. There is also no convincing evidence that functional polymorphisms in genes involved in intraprostatic metabolism of androgens are associated with risk of prostate cancer [11; 12; 13; 14; 15; 16; 17; 18; 19]. However, these genetic studies also do not address potentially important intra-prostatic factors affecting androgen metabolism and hormone concentrations in prostate tissue. Studies of genetic factors and serum hormone levels also do not reflect in which epithelial or stromal cell type androgens are metabolized or act on androgen receptors (AR) [9; 10].
Indirect evidence that androgens are involved in prostate carcinogenesis is derived from human studies with 5α-reductase inhibitors which reduce the formation of 5α-dihydrotestosterone (DHT) from testosterone (T) by this enzyme in the prostate and peripheral fat tissue. The 5α-reductase-type 2 inhibitor finasteride and dual 5α-reductase-type 1 & 2 inhibitor dutasteride have been tested in large clinical trials [20; 21] and both reduced risk of developing prostate cancer by 23-24% over a 4-7 year intervention period [22; 23]. Although these studies provide evidence in support of androgen action as an important factor of prostate cancer development, the duration of the intervention was short in view of the known slow growth of prostate cancer and the study subjects were middle-aged men who have a high frequency of small cancers in their prostates [24]. Thus, these studies are unlikely to provide much insight in whether androgens are involved in the process of carcinogenesis as such or only influence growth and progression of pre-existing cancer. It is not clear whether treatment of aging men with T to ameliorate effects of declining androgen levels increases risk of prostate cancer [25; 26]. Although meta-analyses of T-treated men did not indicate elevated risk [27; 28], there was a significant increased risk of any prostate-related problems identified in one of these studies [28]. It is important to note that the sample sizes of the studies included in these meta-analyses were small and the treatment duration short. Thus, the observed lack of elevated risk of prostate cancer in T-treated aging men should be considered very preliminary [6; 27; 28]. There are no adequate studies of exposure to anabolic steroids and prostate cancer risk; only some case reports of prostate cancer in anabolic steroid users exist [29].
The most direct and convincing evidence that androgens can cause prostate cancer comes from experiments with rats treated with T. Treatment of the inbred NBL rat strain (also known as Noble or Nb rats) with subcutaneously implanted cholesterol pellets containing T propionate at 6-8 week intervals caused grossly visible prostate adenocarcinomas in 19% of animals [30]. We extended this observation in an experiment with NBL rats treated with subcutaneously placed Silastic tubing implants containing T (not testosterone propionate often used by others) which hardly elevated circulating T and found that 11 of 30 rats (37%) developed histologically confirmed adenocarcinomas in the dorsolateral prostate [unpublished data]. We also applied the same treatment to outbred Wistar Cpb:WU rats and 18% developed prostate tumors [unpublished data]. Subcutaneous Silastic tubing implants containing T propionate (not T) induced prostate cancer in 7-15% of Lobund Wistar (LW) rats and some other rat strains [31; 32; 33; 34; 35]. T propionate is fairly rapidly released from Silastic tubing implants and results initially in high circulating T levels that later decline [33], while for unknown reasons T is far less rapidly released from Silastic implants and a sustained stable marginal elevation in circulating T is possible and has been used by us [36]. Thus, chronic T treatment, even when elevating circulating androgen levels only slightly, results in development of prostate adenocarcinomas in at least five different rat strains in incidences ranging from 7 to 37%, with the NBL rat being the most sensitive.
If androgen administration described above is preceded by treatment with a prostate-targeted chemical carcinogen, high prostate cancer incidences can be induced in rats, demonstrating that T is a strong tumor promoter [35; 36; 37; 38]. This tumor promoting effect of T in rats is evident even at circulating T concentrations that are well within the physiological range [31; 36; 39] and may be a significant factor in the carcinogenic activity of T by itself for the rat prostate summarized above.
3. Estrogens
T can be converted to 17β-estradiol (E2) by the enzyme aromatase (CYP 19), which is expressed in fat tissue and in the human and rodent prostate [40]. Therefore, estrogen may be involved in the aforementioned induction of prostate cancer by T in rat models. We have shown that when T treatment of NBL rats is combined with E2, prostate cancer incidence is increased from 35–40% with androgen alone to 90–100% [41; 42]. Even a short course of estrogen treatment is sufficient to result in a high incidence of prostate cancer in NBL rats if chronic low-dose T treatment is given, while the T metabolite DHT cannot be aromatized to estrogen and does not induce prostate cancer [unpublished data]. These results indicate that estrogen plays a critical role in prostate carcinogenesis, at least in the rat. Of note, estrogen treatment alone results in shutdown of luteinizing hormone (LH) production and endogenous androgen production, resulting in prostatic atrophy.
Interestingly, T plus E2 also induces cancer in BPH-1 human prostate epithelial cells that are grafted under the renal capsule of nude mice together with inductive rat or mouse urogenital sinus mesenchyme [43; 44]. These BPH-1 cells are immortalized by SV40-T-antigen, but are by themselves not tumorigenic (with or without the inductive mesenchyme) [43; 45]. The cancers that are induced by T plus E2 in these human prostate cells are capable of metastasis [43]. Besides the T+E2-treated NBL rat model, this is the only other model in which these two steroid hormones in concert have been shown to cause cancer in prostate tissue.
Aromatase knockout mice [46] and mice overexpressing aromatase [47; 48] suffer from androgen metabolism abnormalities that limit their potentially interesting use for carcinogenesis studies [49]. Aromatase knockout mice lack estrogen production, but have elevated circulating T levels and their prostate is enlarged but does not develop cancer [46]. In aromataseoverexpressing mice estrogen production is elevated, while T levels are considerably reduced and no neoplastic or preneoplastic prostate lesions develop [26; 50]. These observations are consistent with the idea that both hormones are necessary for prostate carcinogenesis.
In humans, however, there is no direct evidence of an association between circulating estrogens levels and risk of prostate cancer [7; 8; 51], with the possible exception of African American men [52]. There is also no evidence of an association of risk with single nucleotide polymorphisms (SNPs) in the aromatase (CYP19A1) gene that are associated with altered serum levels of total and free E2 [53]. Interestingly, the ratio of E2 to T increases with age in parallel with a decrease in T levels and an increasing prevalence of prostate cancer in men, which has been suggested to point to a role of estrogen in prostate carcinogenesis [54].
Both estrogen receptors (ER)-α and ER-β are expressed in the rat and human prostate and they may mediate some or all of the prostatic effects of estrogens [55; 56; 57]. Treatment of NBL rats with the antiestrogen ICI182,780 inhibits the induction by T plus E2 of development of prostatic dysplasia (a putative preneoplastic lesion comparable to human prostatic intraepithelial neoplasia or PIN) [58]. In contrast, the antiestrogen tamoxifen did not affect prostate cancer yield in rats treated with low-dose T after exposure to a prostate-targeted carcinogen [59] , but the effect of tamoxifen has not been examined in rats treated with T plus E2. Of note, the dysplasia in NBL rats treated with E2 plus T occurs in a different region of the prostate (dorsolateral prostate) than where carcinomas are found which originate from the periurethral prostatic ducts [41] and this dysplasia rarely progresses to cancer [unpublished data]. Mice lacking the ER-β have been reported to develop enlargement and focal hyperplasia of the ventral prostate [60; 61], but this has not been confirmed in other studies [49; 62; 63] and prostate enlargement by itself is not associated with prostate carcinogenesis [5]. We observed in immunohistochemical studies that the regions of the NBL rat prostate which are most susceptible to the carcinogenic effects of T+E2 have relatively low ER-α expression and very high ER-β expression [unpublished data]. Overall, these data suggest that estrogen receptors may play a role in the hormonal induction of prostate cancer in rats, but conclusive studies are lacking at present. In contrast, it has been suggested that in the human prostate ER-β, which is selectively expressed in epithelial cells, may mediate inhibition of the progression of cancer [28; 64], but this is not a generally accepted or validated concept. There are some studies suggesting associations between SNPs in the ER-α and ER-β genes [65], but their results still need confirmation.
4. Estrogens as Chemical Carcinogens
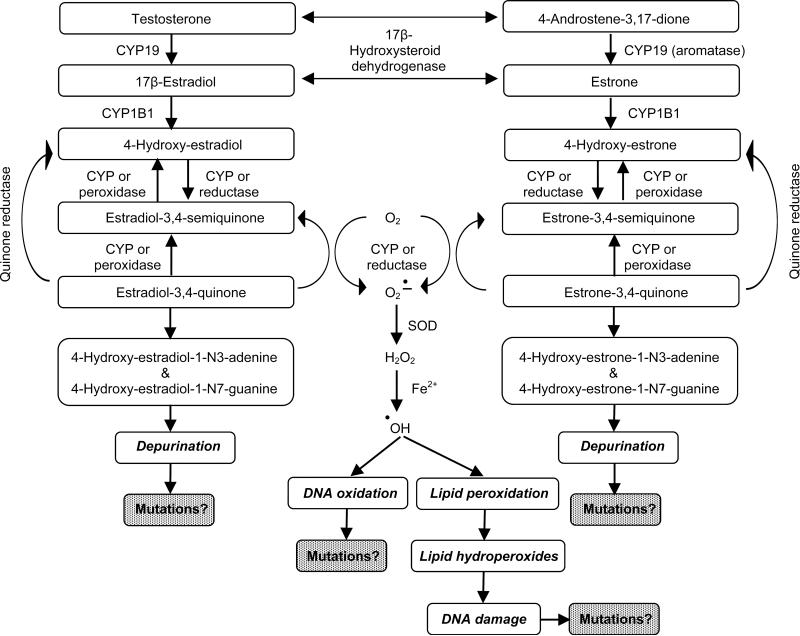
Evidence has been reported of enzymatic conversion of E2 and estrone to 2- and 4-hydroxyestradiol and -estrone mediated by CYP1A1 and CYP1B1 from studies of rodent [66] and human prostate tissue [E. Cavalieri & E. Rogan, personal communication] and analyses of levels of estrogen metabolites and adducts in the urine of men with or without prostate cancer [67]. These so-called catecholestrogens can be converted to highly reactive estrogen semiquinones and estrogen quinones by the process of redox cycling. These reactive intermediates can adduct DNA and redox cycling itself causes generation of reactive oxygen species (ROS) which, in turn, cause lipid peroxidation resulting in the formation of lipid hydroperoxides. Both ROS and reactive lipid hydroperoxides can also damage DNA and potentially lead to the formation of mutations [68]. The 4-hydroxyestradiol (4OH-E2)-quinone-DNA adducts rapidly depurinate, resulting in apurinic sites in the DNA. These apurinic sites can potentially lead to the mutations when repaired by error-prone DNA repair mechanisms [69], although such mutations have not been definitively demonstrated [68; 70]. A summary of this mechanism is provided in Figure 1 and for details of this complex mechanism, the reader is referred to Cavalieri et al. [66; 68] and Bolton and Thatcher [70]. Detection of 4OH-E2-quinone-DNA adducts has been problematic, because these are depurinating with a very short half-live leading to apurinic sites which are difficult to detect, but with highly sensitive analytical methods (LC-MS/MS) formation of such adducts has conclusively been demonstrated after estrogen treatment of DNA, cells, and tissues [70; 71; 72; 73; 74].
Fig. 1.
Summary of the metabolism of androgens and estrogens to reactive estrogen intermediates and the damage to DNA and lipids they can cause. (SOD = superoxide dismutase; CYP = a cytochrome P450 enzyme)
We have shown that these reactions can take place in the rat prostate in experiments in which we injected animals with 4OH-E2 or 4OH-E2-quinone and measured prostate tissue levels of E2, 4OH-E2, and detoxified methylated and glutathione conjugated 4OH-E2 metabolites [66]. Following treatment of NBL rats for 16 weeks with T plus E2, we identified a major DNA adduct by 32P-postlabeling selectively in the periurethral area of their prostates, the site of later cancer development [75]. A low level of this adduct was also found at this location in control animals, perhaps indicating the sensitivity of this tissue for DNA damage. Treatment of rats with only T caused moderately elevated levels of this adduct in the periurethral prostate [unpublished data]. Ho and Roy reported that T plus E2 treatment induced DNA strand breaks, and fluorescent lipid peroxidation products in the dorsolateral, but not ventral, prostate of NBL rats [76]. We measured the formation of (a) 8-hydroxydeoxyguanosine (8-OHdG), an indicator of oxidative DNA damage, and (b) DNA damaging lipid hydroperoxides in the prostate of NBL rats after treatment with T plus E2 for 16 weeks ; the highest levels of 8-OHdG and lipid hydroperoxides were found in the periurethral area of the prostate where cancer develops [68]. However, the contributions of oxidative DNA damage and lipid peroxidation to prostate carcinogenesis by T plus E2 are not clear, because dietary treatment with α-tocopherol and selenomethionine did not reduce the induction of prostate carcinomas [42].
Nevertheless, the above summarized data provide evidence indicating that estrogen treatment causes DNA damage in the NBL rat prostate and that this occurs prior to cancer development and at the exact same site within the rat prostate where carcinomas develop after treatment with T plus E2 [66; 68; 75]. We have also developed evidence that enzymes that provide protection against reactive estrogen metabolites, such as catechol-O-methyltransferase and glutathione reductase, are more active in the dorsolateral prostate region, which does not develop cancer in NBL rats treated with T plus E2, and less active in the periurethral prostate area, where carcinomas do develop [66].
6. Conclusions: Estrogenicity, Estrogen-Genotoxicity, and Androgenic Stimulation May Act in Concert in Hormonal Prostate Carcinogenesis
Collectively, the data, mostly from animal studies, summarized in this paper suggest that for androgens to cause prostate cancer they must be aromatized to estrogen and act in concert with these metabolites. Androgen-receptor mediated activity of the androgens and estrogen receptor-mediated effects of the estrogen metabolites are likely to be necessary, but estrogen genotoxicity appears to be a probable critical factor as well. Only when all these mechanisms are active, prostate carcinogenesis may be the result, at least in the NBL rat model. To explore whether this hypothesis holds true for human prostate carcinogenesis will require extensive tissue-based epidemiologic studies, but there is some experimental evidence that T plus E2 can induce malignant transformation of human prostate cells in xenograft experiments in nude mice mentioned earlier [44; 45; 56]. If all these factors, including aromatization of androgens, are required for androgenic hormones to be carcinogenic for the prostate, interference with any of these might be sufficient to yield a preventive effect and interference with a combination of these factors might have an even stronger preventive effect. With the NBL rat model available, it should be possible to critically test this idea as a first step towards developing new preventive strategies for prostate cancer that can be evaluated in clinical trials with agents that are very well tolerated and bioavailable upon oral administration at clinically feasible doses. However, convincing proof-of-concept studies using this model are needed to demonstrate conclusively that joint androgen-estrogen action and receptor-mediated and genotoxic effects are indeed all required for prostate carcinogenesis and that these mechanisms can be interfered with using chemopreventive treatments. While this challenge can be met with the NBL rat model, other preclinical models are desirable but not available at present and translation to human application will entail considerable multidisciplinary efforts and clinical trials.
Acknowledgements
Some of the work described in this paper was supported in part by NIH Grants No. CA159385, CA136027, CA104334, CA75293, and CA48084.
Abbreviations used
- 4OH-E2
4-hydroxyestradiol
- 8-OHdG
8-hydroxydeoxyguanosine
- AR
androgen receptors
- DHT
5α-dihydrotestosterone
- E2
17β-estradiol
- ER
estrogen receptor
- LH
luteinizing hormone
- PSA
prostate specific antigen
- ROS
reactive oxygen species
- SNP
single nucleotide polymorphism
- T
testosterone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
None
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA: a cancer journal for clinicians. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Bosland MC. The role of steroid hormones in prostate carcinogenesis. Journal of the National Cancer Institute. Monographs. 2000:39–66. doi: 10.1093/oxfordjournals.jncimonographs.a024244. [DOI] [PubMed] [Google Scholar]
- 3.Henderson BE, Feigelson HS. Hormonal carcinogenesis. Carcinogenesis. 2000;21:427–433. doi: 10.1093/carcin/21.3.427. [DOI] [PubMed] [Google Scholar]
- 4.Tuohimaa P. Control of cell prolifation in male accessory sex glands. In: Spring-Mills E, Hafez ESE, editors. Male Accessory Sex Glands. Elsevier/North-Holland Biomedical Press; Amsterdam: 1980. pp. 131–153. [Google Scholar]
- 5.Milman HA, Bosland MC, Walden PD, Heinze JE. Evaluation of the adequacy of published studies of low-dose effects of bisphenol A on the rodent prostate for use in human risk assessment. Regulatory toxicology and pharmacology : RTP. 2002;35:338–346. doi: 10.1006/rtph.2002.1553. [DOI] [PubMed] [Google Scholar]
- 6.Morgentaler A, Traish AM. Shifting the paradigm of testosterone and prostate cancer: the saturation model and the limits of androgen-dependent growth. Eur Urol. 2009;55:310–320. doi: 10.1016/j.eururo.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 7.Eaton NE, Reeves GK, Appleby PN, Key TJ. Endogenous sex hormones and prostate cancer: a quantitative review of prospective studies. Br J Cancer. 1999;80:930–934. doi: 10.1038/sj.bjc.6690445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roddam AW, Allen NE, Appleby P, Key TJ. Endogenous sex hormones and prostate cancer: a collaborative analysis of 18 prospective studies. J Natl Cancer Inst. 2008;100:170–183. doi: 10.1093/jnci/djm323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunha GR. Mesenchymal-epithelial interactions: past, present, and future. Differentiation; research in biological diversity. 2008;76:578–586. doi: 10.1111/j.1432-0436.2008.00290.x. [DOI] [PubMed] [Google Scholar]
- 10.Berry PA, Maitland NJ, Collins AT. Androgen receptor signalling in prostate: effects of stromal factors on normal and cancer stem cells. Mol Cell Endocrinol. 2008;288:30–37. doi: 10.1016/j.mce.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 11.Setiawan VW, Schumacher FR, Haiman CA, Stram DO, Albanes D, Altshuler D, Berglund G, Buring J, Calle EE, Clavel-Chapelon F, Cox DG, Gaziano JM, Hankinson SE, Hayes RB, Henderson BE, Hirschhorn J, Hoover R, Hunter DJ, Kaaks R, Kolonel LN, Kraft P, Ma J, Le Marchand L, Linseisen J, Lund E, Navarro C, Overvad K, Palli D, Peeters PH, Pike MC, Riboli E, Stampfer MJ, Thun MJ, Travis R, Trichopoulos D, Yeager M, Ziegler RG, Spencer Feigelson H, Chanock SJ. CYP17 genetic variation and risk of breast and prostate cancer from the National Cancer Institute Breast and Prostate Cancer Cohort Consortium (BPC3). Cancer Epidemiol Biomarkers Prev. 2007;16:2237–2246. doi: 10.1158/1055-9965.EPI-07-0589. [DOI] [PubMed] [Google Scholar]
- 12.Kraft P, Pharoah P, Chanock SJ, Albanes D, Kolonel LN, Hayes RB, Altshuler D, Andriole G, Berg C, Boeing H, Burtt NP, Bueno-de-Mesquita B, Calle EE, Cann H, Canzian F, Chen YC, Crawford DE, Dunning AM, Feigelson HS, Freedman ML, Gaziano JM, Giovannucci E, Gonzalez CA, Haiman CA, Hallmans G, Henderson BE, Hirschhorn JN, Hunter DJ, Kaaks R, Key T, Le Marchand L, Ma J, Overvad K, Palli D, Pike MC, Riboli E, Rodriguez C, Setiawan WV, Stampfer MJ, Stram DO, Thomas G, Thun MJ, Travis R, Trichopoulou A, Virtamo J, Wacholder S. Genetic variation in the HSD17B1 gene and risk of prostate cancer. PLoS genetics. 2005;1:e68. doi: 10.1371/journal.pgen.0010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pearce CL, Makridakis NM, Ross RK, Pike MC, Kolonel LN, Henderson BE, Reichardt JK. Steroid 5-alpha reductase type II V89L substitution is not associated with risk of prostate cancer in a multiethnic population study. Cancer Epidemiol Biomarkers Prev. 2002;11:417–418. [PubMed] [Google Scholar]
- 14.Pearce CL, Van Den Berg DJ, Makridakis N, Reichardt JK, Ross RK, Pike MC, Kolonel LN, Henderson BE. No association between the SRD5A2 gene A49T missense variant and prostate cancer risk: lessons learned. Human molecular genetics. 2008;17:2456–2461. doi: 10.1093/hmg/ddn145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chu LW, Reichardt JK, Hsing AW. Androgens and the molecular epidemiology of prostate cancer. Current opinion in endocrinology, diabetes, and obesity. 2008;15:261–270. doi: 10.1097/MED.0b013e3282febcf6. [DOI] [PubMed] [Google Scholar]
- 16.Chokkalingam AP, Stanczyk FZ, Reichardt JK, Hsing AW. Molecular epidemiology of prostate cancer: hormone-related genetic loci. Front Biosci. 2007;12:3436–3460. doi: 10.2741/2325. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Coates RJ, Gwinn M, Khoury MJ. Steroid 5-{alpha}-reductase Type 2 (SRD5a2) gene polymorphisms and risk of prostate cancer: a HuGE review. American journal of epidemiology. 2010;171:1–13. doi: 10.1093/aje/kwp318. [DOI] [PubMed] [Google Scholar]
- 18.Keshava C, McCanlies EC, Weston A. CYP3A4 polymorphisms--potential risk factors for breast and prostate cancer: a HuGE review. American journal of epidemiology. 2004;160:825–841. doi: 10.1093/aje/kwh294. [DOI] [PubMed] [Google Scholar]
- 19.Schleutker J. Polymorphisms in androgen signaling pathway predisposing to prostate cancer. Mol Cell Endocrinol. 2011 doi: 10.1016/j.mce.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Goodman PJ, Tangen CM, Crowley JJ, Carlin SM, Ryan A, Coltman CA, Jr., Ford LG, Thompson IM. Implementation of the Prostate Cancer Prevention Trial (PCPT). Controlled clinical trials. 2004;25:203–222. doi: 10.1016/j.cct.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Andriole G, Bostwick D, Brawley O, Gomella L, Marberger M, Tindall D, Breed S, Somerville M, Rittmaster R. Chemoprevention of prostate cancer in men at high risk: rationale and design of the reduction by dutasteride of prostate cancer events (REDUCE) trial. The Journal of urology. 2004;172:1314–1317. doi: 10.1097/01.ju.0000139320.78673.2a. [DOI] [PubMed] [Google Scholar]
- 22.Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, Lieber MM, Cespedes RD, Atkins JN, Lippman SM, Carlin SM, Ryan A, Szczepanek CM, Crowley JJ, Coltman CA., Jr. The influence of finasteride on the development of prostate cancer. The New England journal of medicine. 2003;349:215–224. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 23.Andriole GL, Bostwick DG, Brawley OW, Gomella LG, Marberger M, Montorsi F, Pettaway CA, Tammela TL, Teloken C, Tindall DJ, Somerville MC, Wilson TH, Fowler IL, Rittmaster RS. Effect of dutasteride on the risk of prostate cancer. The New England journal of medicine. 2010;362:1192–1202. doi: 10.1056/NEJMoa0908127. [DOI] [PubMed] [Google Scholar]
- 24.Sakr WA, Grignon DJ, Crissman JD, Heilbrun LK, Cassin BJ, Pontes JJ, Haas GP. High grade prostatic intraepithelial neoplasia (HGPIN) and prostatic adenocarcinoma between the ages of 20-69: an autopsy study of 249 cases. In Vivo. 1994;8:439–443. [PubMed] [Google Scholar]
- 25.Morgentaler A. Testosterone and prostate cancer: what are the risks for middle-aged men? The Urologic clinics of North America. 2011;38:119–124. doi: 10.1016/j.ucl.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Schultheiss D, Machtens S, Jonas U. Testosterone therapy in the ageing male: what about the prostate? Andrologia. 2004;36:355–365. doi: 10.1111/j.1439-0272.2004.00630.x. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez-Balsells MM, Murad MH, Lane M, Lampropulos JF, Albuquerque F, Mullan RJ, Agrwal N, Elamin MB, Gallegos-Orozco JF, Wang AT, Erwin PJ, Bhasin S, Montori VM. Clinical review 1: Adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2010;95:2560–2575. doi: 10.1210/jc.2009-2575. [DOI] [PubMed] [Google Scholar]
- 28.Calof OM, Singh AB, Lee ML, Kenny AM, Urban RJ, Tenover JL, Bhasin S. Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. The journals of gerontology. Series A, Biological sciences and medical sciences. 2005;60:1451–1457. doi: 10.1093/gerona/60.11.1451. [DOI] [PubMed] [Google Scholar]
- 29.Tentori L, Graziani G. Doping with growth hormone/IGF-1, anabolic steroids or erythropoietin: is there a cancer risk? Pharmacological research : the official journal of the Italian Pharmacological Society. 2007;55:359–369. doi: 10.1016/j.phrs.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 30.Noble RL. The development of prostatic adenocarcinoma in Nb rats following prolonged sex hormone administration. Cancer Res. 1977;37:1929–1933. [PubMed] [Google Scholar]
- 31.Pollard M, Luckert PH. Autochthonous prostate adenocarcinomas in Lobund-Wistar rats: a model system. Prostate. 1987;11:219–227. doi: 10.1002/pros.2990110303. [DOI] [PubMed] [Google Scholar]
- 32.Pollard M, Luckert PH, Schmidt MA. Induction of prostate adenocarcinomas in Lobund Wistar rats by testosterone. Prostate. 1982;3:563–568. doi: 10.1002/pros.2990030605. [DOI] [PubMed] [Google Scholar]
- 33.Hoover DM, Best KL, McKenney BK, Tamura RN, Neubauer BL. Experimental induction of neoplasia in the accessory sex organs of male Lobund-Wistar rats. Cancer Res. 1990;50:142–146. [PubMed] [Google Scholar]
- 34.Pollard M, Luckert PH. Prostate cancer in a Sprague-Dawley rat. Prostate. 1985;6:389–393. doi: 10.1002/pros.2990060407. [DOI] [PubMed] [Google Scholar]
- 35.Pour PM, Stepan K. Induction of prostatic carcinomas and lower urinary tract neoplasms by combined treatment of intact and castrated rats with testosterone propionate and N-nitrosobis(2-oxopropyl)amine. Cancer Res. 1987;47:5699–5706. [PubMed] [Google Scholar]
- 36.Bosland MC, Dreef-Van Der Meulen HC, Sukumar S, Ofner P, Leav I, Han X, Liehr JG. Multistage prostate carcinogenesis: the role of hormones. Princess Takamatsu Symp. 1991;22:109–123. [PubMed] [Google Scholar]
- 37.McCormick DL, Rao KV, Dooley L, Steele VE, Lubet RA, Kelloff GJ, Bosland MC. Influence of N-methyl-N-nitrosourea, testosterone, and N-(4-hydroxyphenyl)-all-trans retinamide on prostate cancer induction in Wistar-Unilever rats. Cancer Res. 1998;58:3282–3288. [PubMed] [Google Scholar]
- 38.Shirai T, Tamano S, Kato T, Iwasaki S, Takahashi S, Ito N. Induction of invasive carcinomas in the accessory sex organs other than the ventral prostate of rats given 3,2′-dimethyl-4-aminobiphenyl and testosterone propionate. Cancer Res. 1991;51:1264–1269. [PubMed] [Google Scholar]
- 39.Tsukamoto S, Akaza H, Onozawa M, Shirai T, Ideyama Y. A five-alpha reductase inhibitor or an antiandrogen prevents the progression of microscopic prostate carcinoma to macroscopic carcinoma in rats. Cancer. 1998;82:531–537. doi: 10.1002/(sici)1097-0142(19980201)82:3<531::aid-cncr15>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 40.Ellem SJ, Schmitt JF, Pedersen JS, Frydenberg M, Risbridger GP. Local aromatase expression in human prostate is altered in malignancy. J Clin Endocrinol Metab. 2004;89:2434–2441. doi: 10.1210/jc.2003-030933. [DOI] [PubMed] [Google Scholar]
- 41.Bosland MC, Ford H, Horton L. Induction at high incidence of ductal prostate adenocarcinomas in NBL/Cr and Sprague-Dawley Hsd:SD rats treated with a combination of testosterone and estradiol-17 beta or diethylstilbestrol. Carcinogenesis. 1995;16:1311–1317. doi: 10.1093/carcin/16.6.1311. [DOI] [PubMed] [Google Scholar]
- 42.Ozten N, Horton L, Lasano S, Bosland MC. Selenomethionine and alpha-tocopherol do not inhibit prostate carcinogenesis in the testosterone plus estradiol-treated NBL rat model. Cancer Prev Res (Phila) 2010;3:371–380. doi: 10.1158/1940-6207.CAPR-09-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ricke WA, Ishii K, Ricke EA, Simko J, Wang Y, Hayward SW, Cunha GR. Steroid hormones stimulate human prostate cancer progression and metastasis. Int J Cancer. 2006;118:2123–2131. doi: 10.1002/ijc.21614. [DOI] [PubMed] [Google Scholar]
- 44.Hayward SW, Wang Y, Cao M, Hom YK, Zhang B, Grossfeld GD, Sudilovsky D, Cunha GR. Malignant transformation in a nontumorigenic human prostatic epithelial cell line. Cancer Res. 2001;61:8135–8142. [PubMed] [Google Scholar]
- 45.Hayward SW, Dahiya R, Cunha GR, Bartek J, Deshpande N, Narayan P. Establishment and characterization of an immortalized but non-transformed human prostate epithelial cell line: BPH-1. In vitro cellular & developmental biology. Animal. 1995;31:14–24. doi: 10.1007/BF02631333. [DOI] [PubMed] [Google Scholar]
- 46.McPherson SJ, Wang H, Jones ME, Pedersen J, Iismaa TP, Wreford N, Simpson ER, Risbridger GP. Elevated androgens and prolactin in aromatase-deficient mice cause enlargement, but not malignancy, of the prostate gland. Endocrinology. 2001;142:2458–2467. doi: 10.1210/endo.142.6.8079. [DOI] [PubMed] [Google Scholar]
- 47.Fowler KA, Gill K, Kirma N, Dillehay DL, Tekmal RR. Overexpression of aromatase leads to development of testicular leydig cell tumors : an in vivo model for hormone-mediated TesticularCancer. Am J Pathol. 2000;156:347–353. doi: 10.1016/S0002-9440(10)64736-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li X, Nokkala E, Yan W, Streng T, Saarinen N, Warri A, Huhtaniemi I, Santti R, Makela S, Poutanen M. Altered structure and function of reproductive organs in transgenic male mice overexpressing human aromatase. Endocrinology. 2001;142:2435–2442. doi: 10.1210/endo.142.6.8211. [DOI] [PubMed] [Google Scholar]
- 49.Jarred RA, McPherson SJ, Bianco JJ, Couse JF, Korach KS, Risbridger GP. Prostate phenotypes in estrogen-modulated transgenic mice. Trends in endocrinology and metabolism: TEM. 2002;13:163–168. doi: 10.1016/s1043-2760(02)00575-1. [DOI] [PubMed] [Google Scholar]
- 50.Setlur SR, Chen CX, Hossain RR, Ha JS, Van Doren VE, Stenzel B, Steiner E, Oldridge D, Kitabayashi N, Banerjee S, Chen JY, Schafer G, Horninger W, Lee C, Rubin MA, Klocker H, Demichelis F. Genetic variation of genes involved in dihydrotestosterone metabolism and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:229–239. doi: 10.1158/1055-9965.EPI-09-1018. [DOI] [PubMed] [Google Scholar]
- 51.Yao S, Till C, Kristal AR, Goodman PJ, Hsing AW, Tangen CM, Platz EA, Stanczyk FZ, Reichardt JK, Tang L, Neuhouser ML, Santella RM, Figg WD, Price DK, Parnes HL, Lippman SM, Thompson IM, Ambrosone CB, Hoque A. Serum estrogen levels and prostate cancer risk in the prostate cancer prevention trial: a nested case-control study. Cancer causes & control : CCC. 2011;22:1121–1131. doi: 10.1007/s10552-011-9787-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rohrmann S, Nelson WG, Rifai N, Brown TR, Dobs A, Kanarek N, Yager JD, Platz EA. Serum estrogen, but not testosterone, levels differ between black and white men in a nationally representative sample of Americans. J Clin Endocrinol Metab. 2007;92:2519–2525. doi: 10.1210/jc.2007-0028. [DOI] [PubMed] [Google Scholar]
- 53.Travis RC, Schumacher F, Hirschhorn JN, Kraft P, Allen NE, Albanes D, Berglund G, Berndt SI, Boeing H, Bueno-de-Mesquita HB, Calle EE, Chanock S, Dunning AM, Hayes R, Feigelson HS, Gaziano JM, Giovannucci E, Haiman CA, Henderson BE, Kaaks R, Kolonel LN, Ma J, Rodriguez L, Riboli E, Stampfer M, Stram DO, Thun MJ, Tjonneland A, Trichopoulos D, Vineis P, Virtamo J, Le Marchand L, Hunter DJ. CYP19A1 genetic variation in relation to prostate cancer risk and circulating sex hormone concentrations in men from the Breast and Prostate Cancer Cohort Consortium. Cancer Epidemiol Biomarkers Prev. 2009;18:2734–2744. doi: 10.1158/1055-9965.EPI-09-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vermeulen A, Kaufman JM, Goemaere S, van Pottelberg I. Estradiol in elderly men. The aging male : the official journal of the International Society for the Study of the Aging Male. 2002;5:98–102. [PubMed] [Google Scholar]
- 55.Latil A, Bieche I, Vidaud D, Lidereau R, Berthon P, Cussenot O, Vidaud M. Evaluation of androgen, estrogen (ER alpha and ER beta), and progesterone receptor expression in human prostate cancer by real-time quantitative reverse transcription-polymerase chain reaction assays. Cancer Res. 2001;61:1919–1926. [PubMed] [Google Scholar]
- 56.Lau KM, Leav I, Ho SM. Rat estrogen receptor-alpha and -beta, and progesterone receptor mRNA expression in various prostatic lobes and microdissected normal and dysplastic epithelial tissues of the Noble rats. Endocrinology. 1998;139:424–427. doi: 10.1210/endo.139.1.5809. [DOI] [PubMed] [Google Scholar]
- 57.Leav I, Lau KM, Adams JY, McNeal JE, Taplin ME, Wang J, Singh H, Ho SM. Comparative studies of the estrogen receptors beta and alpha and the androgen receptor in normal human prostate glands, dysplasia, and in primary and metastatic carcinoma. Am J Pathol. 2001;159:79–92. doi: 10.1016/s0002-9440(10)61676-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thompson CJ, Tam NN, Joyce JM, Leav I, Ho SM. Gene expression profiling of testosterone and estradiol-17 beta-induced prostatic dysplasia in Noble rats and response to the antiestrogen ICI 182,780. Endocrinology. 2002;143:2093–2105. doi: 10.1210/endo.143.6.8846. [DOI] [PubMed] [Google Scholar]
- 59.McCormick DL, Johnson WD, Lubet RA, Steele VE, Bosland MC. Differential chemopreventive activity of the antiandrogen, flutamide, and the antiestrogen, tamoxifen, in the rat prostate. Proc Am Assoc Cancer Res. 2002;43:640. [Google Scholar]
- 60.Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci U S A. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weihua Z, Makela S, Andersson LC, Salmi S, Saji S, Webster JI, Jensen EV, Nilsson S, Warner M, Gustafsson JA. A role for estrogen receptor beta in the regulation of growth of the ventral prostate. Proc Natl Acad Sci U S A. 2001;98:6330–6335. doi: 10.1073/pnas.111150898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Couse JF, Curtis Hewitt S, Korach KS. Receptor null mice reveal contrasting roles for estrogen receptor alpha and beta in reproductive tissues. J Steroid Biochem Mol Biol. 2000;74:287–296. doi: 10.1016/s0960-0760(00)00105-9. [DOI] [PubMed] [Google Scholar]
- 63.Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- 64.Horvath LG, Henshall SM, Lee CS, Head DR, Quinn DI, Makela S, Delprado W, Golovsky D, Brenner PC, O'Neill G, Kooner R, Stricker PD, Grygiel JJ, Gustafsson JA, Sutherland RL. Frequent loss of estrogen receptor-beta expression in prostate cancer. Cancer Res. 2001;61:5331–5335. [PubMed] [Google Scholar]
- 65.Chen YC, Kraft P, Bretsky P, Ketkar S, Hunter DJ, Albanes D, Altshuler D, Andriole G, Berg CD, Boeing H, Burtt N, Bueno-de-Mesquita B, Cann H, Canzian F, Chanock S, Dunning A, Feigelson HS, Freedman M, Gaziano JM, Giovannucci E, Sanchez MJ, Haiman CA, Hallmans G, Hayes RB, Henderson BE, Hirschhorn J, Kaaks R, Key TJ, Kolonel LN, LeMarchand L, Ma J, Overvad K, Palli D, Pharaoh P, Pike M, Riboli E, Rodriguez C, Setiawan VW, Stampfer M, Stram DO, Thomas G, Thun MJ, Travis RC, Virtamo J, Trichopoulou A, Wacholder S, Weinstein SJ. Sequence variants of estrogen receptor beta and risk of prostate cancer in the National Cancer Institute Breast and Prostate Cancer Cohort Consortium. Cancer Epidemiol Biomarkers Prev. 2007;16:1973–1981. doi: 10.1158/1055-9965.EPI-07-0431. [DOI] [PubMed] [Google Scholar]
- 66.Cavalieri EL, Devanesan P, Bosland MC, Badawi AF, Rogan EG. Catechol estrogen metabolites and conjugates in different regions of the prostate of Noble rats treated with 4-hydroxyestradiol: implications for estrogen-induced initiation of prostate cancer. Carcinogenesis. 2002;23:329–333. doi: 10.1093/carcin/23.2.329. [DOI] [PubMed] [Google Scholar]
- 67.Yang L, Gaikwad NW, Meza J, Cavalieri EL, Muti P, Trock B, Rogan EG. Novel biomarkers for risk of prostate cancer: results from a case-control study. Prostate. 2009;69:41–48. doi: 10.1002/pros.20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cavalieri E, Frenkel K, Liehr JG, Rogan E, Roy D. Estrogens as endogenous genotoxic agents--DNA adducts and mutations. Journal of the National Cancer Institute. Monographs. 2000:75–93. doi: 10.1093/oxfordjournals.jncimonographs.a024247. [DOI] [PubMed] [Google Scholar]
- 69.Mailander PC, Meza JL, Higginbotham S, Chakravarti D. Induction of A.T to G.C mutations by erroneous repair of depurinated DNA following estrogen treatment of the mammary gland of ACI rats. J Steroid Biochem Mol Biol. 2006;101:204–215. doi: 10.1016/j.jsbmb.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 70.Bolton JL, Thatcher GR. Potential mechanisms of estrogen quinone carcinogenesis. Chemical research in toxicology. 2008;21:93–101. doi: 10.1021/tx700191p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liehr JG. Role of DNA adducts in hormonal carcinogenesis. Regulatory toxicology and pharmacology : RTP. 2000;32:276–282. doi: 10.1006/rtph.2000.1432. [DOI] [PubMed] [Google Scholar]
- 72.Li KM, Todorovic R, Devanesan P, Higginbotham S, Kofeler H, Ramanathan R, Gross ML, Rogan EG, Cavalieri EL. Metabolism and DNA binding studies of 4-hydroxyestradiol and estradiol-3,4-quinone in vitro and in female ACI rat mammary gland in vivo. Carcinogenesis. 2004;25:289–297. doi: 10.1093/carcin/bgg191. [DOI] [PubMed] [Google Scholar]
- 73.Saeed M, Rogan E, Fernandez SV, Sheriff F, Russo J, Cavalieri E. Formation of depurinating N3Adenine and N7Guanine adducts by MCF-10F cells cultured in the presence of 4-hydroxyestradiol. Int J Cancer. 2007;120:1821–1824. doi: 10.1002/ijc.22399. [DOI] [PubMed] [Google Scholar]
- 74.Zhang F, Swanson SM, van Breemen RB, Liu X, Yang Y, Gu C, Bolton JL. Equine estrogen metabolite 4-hydroxyequilenin induces DNA damage in the rat mammary tissues: formation of single-strand breaks, apurinic sites, stable adducts, and oxidized bases. Chemical research in toxicology. 2001;14:1654–1659. doi: 10.1021/tx010158c. [DOI] [PubMed] [Google Scholar]
- 75.Han X, Liehr JG, Bosland MC. Induction of a DNA adduct detectable by 32P-postlabeling in the dorsolateral prostate of NBL/Cr rats treated with estradiol-17 beta and testosterone. Carcinogenesis. 1995;16:951–954. doi: 10.1093/carcin/16.4.951. [DOI] [PubMed] [Google Scholar]
- 76.Ho SM, Roy D. Sex hormone-induced nuclear DNA damage and lipid peroxidation in the dorsolateral prostates of Noble rats. Cancer Lett. 1994;84:155–162. doi: 10.1016/0304-3835(94)90370-0. [DOI] [PubMed] [Google Scholar]