Abstract
Objective:
To investigate whether short-term systemic effects of wood smoke occurred in atopic subjects after experimental wood smoke exposures.
Methods:
A double-blind climate chamber study was conducted on 20 healthy atopic subjects with exposures to filtered air and wood smoke. Pneumoproteins, coagulation and adhesion factors, and cytokines were measured. Heart rate was monitored with pulse monitors. Data were analyzed with mixed models.
Results:
Few differences in the outcomes were observed. Plasma tissue factor remained elevated during filtered air exposure (P = 0.002). P-selectin declined independent of exposure (P = 0.0006). Interleukin-6 increased after filtered air (P = 0.03).
Conclusions:
The study confirmed previous observations among nonatopics of limited changes after a 3-hour wood smoke exposure.
Air pollution is a well-documented cause of morbidity and mortality. Cardiovascular and pulmonary effects associated with short- as well as long-term pollution exposure have been known for long1,2 and confirmed in recent studies.3 Studies have been conducted in a wide range of cities and regions of the world, confirming the adverse effect of polluted air from a variety of sources on cardiopulmonary health. The strongest associations are most often ascribed to fine particulate matter,4 but effects of gases such as ozone and SO2 have also been observed.5,6 Air pollution is a complex mixture of these and numerous other pollutants of which many stem from combustion sources. Indeed, evidence is growing that combustion-derived pollutants (ie, smoke) especially from traffic are the major drivers of adverse health effects.7
In less affluent regions of the World, smoke from the combustion of wood (and other biomass) is a very important contributor to both ambient and indoor air pollution (eg, from open fireplaces). Severe health effects have been shown in relation to such exposures, in particular on respiratory health and less so on cardiovascular health.8,9 In more affluent regions, wood smoke contributes less to the air pollution although some evidence exists for local wood smoke contributions to both ambient and indoor air pollution as well as to health effects.10 Associations between wood smoke and respiratory and cardiovascular effects have been demonstrated in both epidemiological10–12 and experimental studies.13 Because of decreases in other air pollutants and increases in the combustion of wood and other biomass in recent years, there is a need for better understanding of health effects of wood smoke. People with chronic respiratory disease and cardiovascular diseases may be particularly prone to adverse effects of wood smoke.14
A handful of short-term experimental wood smoke exposure studies have been conducted in healthy subjects so far.13,15–19 In these studies, effects on pneumoproteins—in particular, Clara cell protein (CC16), an anti-inflammatory protein secreted in large amounts in the bronchioli of healthy humans—exhaled NO, and symptoms of mucosal irritation have repeatedly been demonstrated, though no single effect was observed in all of the studies.
In previous publications from the present study on atopic subjects, we have reported on short-term wood smoke-induced increase in symptoms20 without finding any effects on the airways,21 the microvascular function, or a range of markers of oxidative stress and inflammation in peripheral blood mononuclear cells.22
Yet inflammation23 and changes in heart rate variability (HRV)24,25 have been shown to occur with exposure to ambient air pollution including wood smoke.13 Both mechanisms may be relevant in the causal pathway to cardiovascular and pulmonary disease.23 Pollution-induced inflammation in the airways causes the formation of reactive oxidative species and the release of numerous cellular mediators, such as cytokines into the bloodstream, where they can affect coagulation and endothelial function. In association with deceased HRV, these mechanisms are plausibly involved in the development of cardiovascular disease such as myocardial infarction.
Our aim was to investigate whether short-term systemic effects of wood smoke could be observed in a population of atopic volunteers. We hypothesized that at optimal wood burning conditions emitting fine particle concentrations above 200 μg/m3 blood coagulability and heart rate variability would be affected and that these effects would be accorded by an increase in circulating markers of epithelial damage such as pneumoproteins and cytokines. In addition, we hypothesized that controlled experimental conditions, blinding, and randomization would enable us to observe even small changes in the outcomes. Finally, we hypothesized that increased effects could be observed at higher concentrations.
MATERIALS AND METHODS
Study Population
Details on recruitment, clinical testing, design, and exposure are provided in a previous publication and are only briefly described here.20 Nonsmoking atopic volunteers with normal lung function and without bronchial hyperresponsiveness were enrolled in the study. Atopy was determined by at least 2 positive (≥3 mm) reactions by skin-prick testing to 11 common inhalant allergens. Exclusion criteria were pregnancy or a medical history of suggesting a risk for the participant. Bronchial responsiveness was determined by a methacholine provocation test.
Before any exposure session during the study, the participants were required to be without signs of infections or airway symptoms for at least 1 week, and not to have taken any drug during at least 48 hours. The study protocol was approved by the Aarhus County Human Study Review Board in accordance with the regulations for the protection of the participants (Ref. no. 20070097), and written consent was obtained from all the participants.
Design
Exposures were delivered using a randomized, double-blind, crossover design with six different Latin squares to ensure that all possible exposure orders were represented in a group of 24 subjects and that learning or carryover effects were avoided. In groups of four, the participants were allocated to a total of three exposure sessions: high particle concentration, low particle concentration, and filtered air with at least 2 weeks between each exposure session as described elsewhere.20 Before the exposures, the participants entered the chamber and spent a 30-minute acclimatization period with filtered air exposure. After acclimatization, approximately 30 minutes was used to build up the exposure, followed by 3 hours of maintained exposure. Each exposure session and all clinical samples were conducted at the same times of the day on every exposure day.
During the exposure sessions, participants were seated at a desk at rest. They were seated at different positions in the chamber during each of the three sessions to outbalance possible exposure variations because of the location of the inflow of air to the chamber. The climate chamber was thoroughly cleaned before each exposure session, and participants wore disposable coverall (Kleen Guard T65XP, Kimberly-Clark, Dallas, TX) over their clothes to avoid unintended contamination of the air.
Exposures
The study was conducted in a 79-m3 stainless steel climate chamber at the Section of Environmental and Occupational Health, University of Aarhus, as described in more detail elsewhere.20 The wood smoke was generated in a wood stove Morsø model 7110 (Morsø Jernstøberi, Nykøbing Mors, Denmark) from which it entered the chambers through a ventilation duct. The flue gas emissions were mixed with filtered ambient air (HEPA filter, McLeod Russel Filter AG, LUWA, United Kingdom) and aged to allow particle condensation, chemical reactions, and changes of water content and temperature, mimicking the changes normally occurring to smoke from a chimney mixing with ambient air. The stove had primary, secondary, and tertiary air inflow, window, and convection heating. The tertiary airflow was switched off in order to be representative of an average wood stove in Denmark, but otherwise the wood was burned under optimal conditions. Burning was done with Danish beech wood, which had been stored under controlled atmospheric conditions to ensure that it was dry (relative humidity <20%). On days with smoke exposure, the 30-minute buildup period was not started until the fire in the stove was stably burning. During exposure sessions, combustion was kept stable with insertion of one piece of wood every 30 to 40 minutes. During insertion of wood, the connection of the chamber to the stove was temporarily closed to prevent large particle boosts. Combustion procedures were the same for all exposure sessions, although during clean air exposures the inlet to the climate chamber was kept closed and all smoke left the stove through an outside chimney. To ensure the safety of the participants, the CO concentration was continuously monitored with an X-am2000 CO-monitor (Drägerwerk AG & Co, Lübeck, Germany) and the clean air inflow was increased by the technician if CO concentrations reached 35 ppm. Concentrations between 35 and 50 ppm CO were accepted for a total of 30 minutes per session. Additional exposure conditions monitored included ventilation, air temperature and humidity (LinaxA310, Camille Bauer AG, Wohlen AG, Switzerland; HMT100 Thermo-hydrograph, Vaisala Oyj, Helsinki, Finland), light (Panlux, Gossen, Erlangen, Germany), sound (CR:822B, Cirrus Research plc, Hunmanby, United Kingdom), and stationary sampled gravimetric particle concentrations using a Harvard impactor operated at 10 L/min equipped with polytetrafluoroethylene W/ring 37-mm, 2.0-μm filters for each size fraction (R2PJ037, Pall, Port Washington, NY). The mass of sampled particles on the filters was determined by weighing them before and after sampling.
Clinical Measurements and Biomarkers
Just before each exposure session, baseline clinical investigations were performed. The investigations were repeated immediately after exposure (0 hour post), 6 hours after exposure (6 hours post), and the next morning (20 hours post). All clinical investigations were timed, so that they were performed at the same time of the day during and after each exposure session.
The clinical investigations consisted of symptom questionnaires, spirometry, acoustic rhinometry, nasal lavage, exhaled NO measurements, venous blood sampling, urine sampling, measurements of blood pressure, heart rate, and endothelial vascular function. Only the methods relevant to the topic of this article are described herein. Details of the remaining methods were published previously.
Venous blood sampling was done at baseline and at 0, 6, and 20 hours after exposure. To avoid inducing artifacts in the outcomes of interest, it was done with a 21-gauge needle (Multifly needle for monovette tubes, Sarstedt, Nümbrecht, Germany) and without the use of a tourniquet when possible. Plasma samples were collected in S-Monovette (Sarstedt, Nümbrecht, Germany) vacuum tubes for serum or with added citrate or ethylene diamine tetraacetic acid. Citrate tubes used for the tissue factor (TF), selectins, and von Willebrand factor antigen (VWF:Ag) were never the first tubes filled to avoid the blood sampling procedure to influence the concentration of the markers. All tubes were centrifuged within 2 hours at 4000 rpm for 25 minutes at 12°C. From the centrifuged tubes, the supernatant was transferred to Eppendorf tubes in specified amounts. Urine sampling was performed only at baseline and after 24 hours. Men were informed to discard the first 100 mL of urine before sampling in polyethylene tubes All samples were stored in a −80°C freezer until analysis.
Primary endpoints CC16 and surfactant protein D (SP-D) in serum, CC16 in urine, and the plasma TF, soluble (s)P-Selectin, and sE-selectin were measured with commercial enzyme-linked-immunosorbent serologic assays (ELISAs). The TF was analyzed with IMUBIND ELISAkit Product No. 845 (American Diagnostica Inc, Stamford, CT) and the selectins with Parameter human sP- and sE-Selectin (R&D Systems, Abingdon, United Kingdom), and CC16 and SP-D with kits from Biovendor (BioVendor Laboratory Medicine Inc, Brno, Czech Republic). Urinary CC16 was adjusted for urinary creatinine. von Willebrand factor antigen was measured with an in-house ELISA using antihuman VWF (from Dako A/S, Glostrup, Denmark).26 Lower limits of detection were 0.35 U/mL for VWF:Ag, 18 ng/mL for P-selectin, 13 ng/mL for E-selectin, and unknown for the TF. Day-to-day coefficient of variation varied between 2.1% and 9.7%. Surfactant protein A (SP-A) was determined using a homemade ELISA using two different antibodies against human SP-A, one polyclonal and the other monoclonal.27
After thawing, the ethylene diamine tetraacetic acid plasma was analyzed with an in-house assay as described by Skogstrand et al.28 In short, 50 μL of plasma (diluted 1:10) and 50 μL of a suspension of capture antibody-conjugated beads were mixed in plate wells. After 1½ hours of incubation, the beads were washed twice and subsequently reacted for 1½ hours with a mixture (50 μL) of corresponding biotinylated detection antibodies, each diluted 1:1000. Streptavidin–phycoerythrin (50 μL) was added to the wells, and the incubation was continued for an additional 30 minutes. Finally, the beads were washed twice and resuspended in 125 μL of buffer and analyzed on the Luminex 100™ platform. All samples were measured in duplicates. By this method, the concentrations of the following proteins were measured: interleukin-1β (IL-1β), −4, −5, −6, −8, −10, −12, −18; tumor necrosis factor (TNF); interferon-γ; granulocyte-macrophage colony stimulating factor (GM-CSF); transforming growth factor-β1; monocyte chemoattractant protein-1, macrophage inflammatory protein-1α, regulated upon activation, normal T-cell expressed and secreted (RANTES).
Heart rate variability was assessed as intervals between R peaks from the electrocardiogram signal (R–R intervals). Measurements were performed with a Polar RS800 pulse monitor (Polar Electro Oy, Kempele, Finland) during a 20-minute resting period in the supine position 9 to 10 hours after the start of the exposures. Data were extracted from the first 4 minutes of the resting period using Polar ProTrainer 5 software. For sensitivity analysis, data from the last 4 minutes of the resting period were also used.
The plasma cytokines and HRV were considered secondary endpoints of the study.
Power Considerations
Calculations of study power showed that even with only 10 participants (ie, in the case of loss of more than 50% of the participants) we would be able to detect standardized residuals in the order of 2.5 with a power of 80%.
Statistics
A mixed model was developed using SAS software (SAS 9.2, SAS Institute Inc, Cary, NC), with a significance level of 0.05. As random effects it included patient, patient–time, and patient–exposure interactions, and as fixed effects it included time, exposure, carryover, period, seat in the chamber, and time–exposure interaction. Time was divided into baseline, 0, 6, and 20 hours. Carryover was a variable created to indicate what had been the previous exposure (if any). Period was a variable indicating during which of the six 2-week periods of the entire experiment that any particular exposure session was performed. The primary outcome of interest was the interaction term time–exposure as an effect of this term indicated a difference in the change from baseline associated with the exposures. In case of nonnormal distributions, analyses were performed on ln-transformed outcome variables. HRV data were also ln-transformed and analyzed with a one-way analysis of variance test with SPSS (SPSS Inc, PASW Statistics 18, Chicago, IL).
RESULTS
A total of 20 atopic (female–male ratio 1:1) participants with a mean age of 25.1 years completed the study. The mean concentrations of fine particles (<2.5 μm) were 13 μg/m3 during filtered air, 222 μg/m3 during low, and 385 μg/m3 during high wood smoke exposures. The mean temperature was 22.9 to 23.0°C, and the relative humidity varied from 22% during filtered air to 32% during high wood smoke exposure.
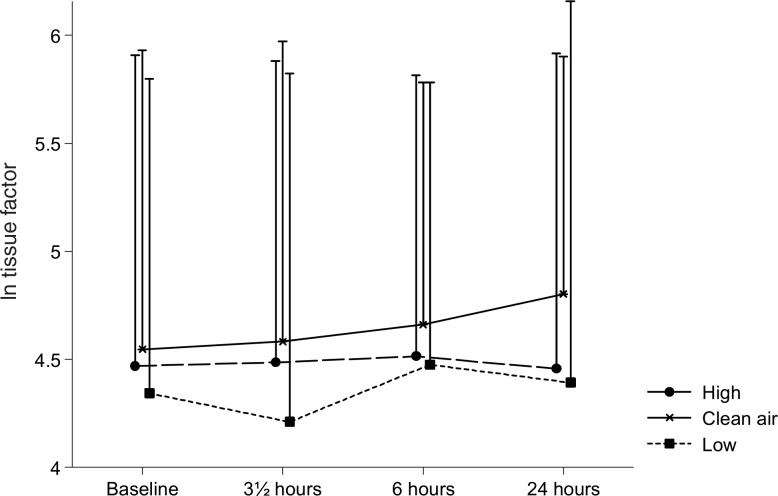
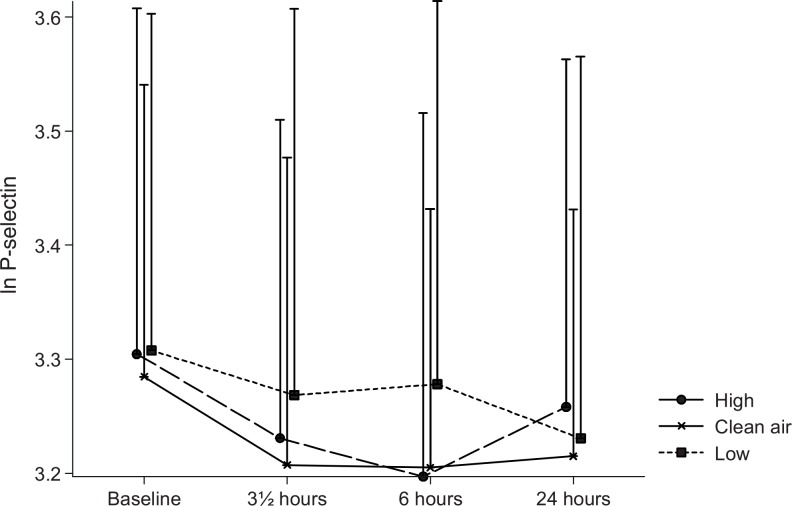
The plasma TF varied statistically significant between the three exposures (P = 0.002). As shown in Fig. 1, the TF was statistically significant higher during the filtered air exposure than during the low and high concentration wood smoke exposures (P = 0.03 and 0.0005, respectively). Nevertheless, a P value of 0.38 for a time–exposure interaction indicated that the difference between the exposures did not change with time (ie, it was present already before the smoke exposure was initiated). Plasma sP-selectin, as can be seen in Fig. 2, declined with time (P = 0.0006). The concentration of sP-selectin was statistically significant higher at baseline compared with 0 hours (P = 0.0002) and 6 hours (P = 0.0009) but not 20 hours after exposure (P = 0.61). The P value for a time–exposure interaction was 0.66, thus indicating that the difference in sP-selectin between the exposures did not change with time. No statistically significant changes were observed in sE-selectin or VWF:Ag.
FIGURE 1.
Plasma tissue factor. Means of the ln-transformed plasma tissue factor concentrations at baseline, and 0, 6, and 20 hours after the three exposures to filtered air, low, and high concentrations of wood smoke. Error bars represent +1 standard deviation.
FIGURE 2.
Plasma sP-selectin. Means of the ln-transformed plasma sP-selectin concentrations at baseline, and 0, 6, and 20 hours after the three exposures to filtered air, low, and high concentrations of wood smoke. Error bars represent +1 standard deviation.
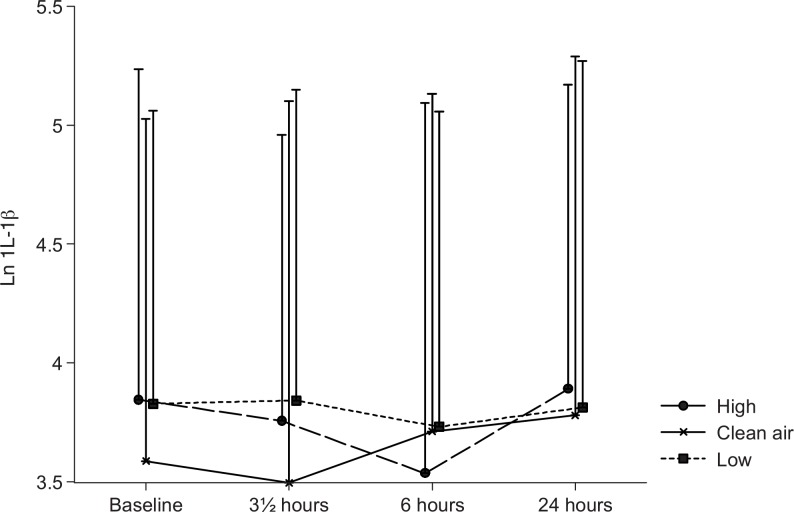
In most cases the cytokines had to be ln-transformed before statistical analysis because of deviations from normality. A difference in IL-1β levels between the exposures was observed (P = 0.03), as shown in Fig. 3. During the wood smoke exposures, IL-1β was elevated (GM between 42.8 and 44.8 ng/L) compared with the filtered air exposure (GM 38.2 ng/L). With a P value of 0.09 for the time–exposure interaction, a tendency to a difference in the development of the IL-1β concentrations over time between the exposures was indicated.
FIGURE 3.
Plasma interleukin-1β. Means of the ln-transformed plasma IL-1β concentrations at baseline, and 0, 6, and 20 hours after the three exposures to filtered air, low, and high concentrations of wood smoke. Error bars represent +1 standard deviation.
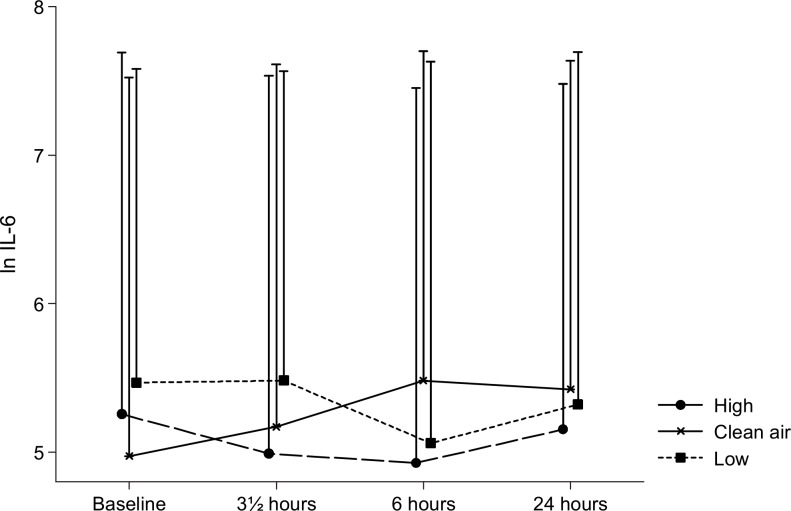
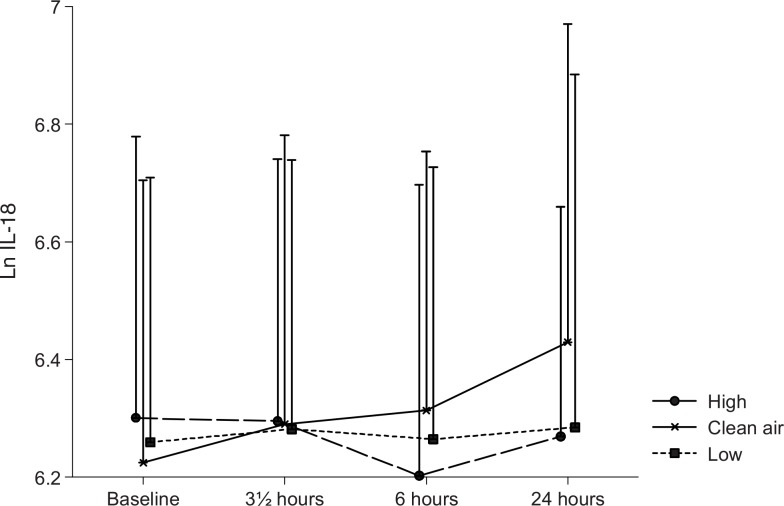
The time–exposure interaction was statistically significant for IL-6 (P = 0.03), indicating that a differential change in the plasma concentration of this cytokine occurred during the three different exposure sessions. As illustrated in Fig. 4, the difference appeared to be due to an increase in IL-6 from baseline during the filtered air exposure sessions, which did not appear during the wood smoke exposures. Overall, the cytokine IL-18 did not vary statistically significant with time or exposure, but in the paired analyses the level of the cytokine was found to be elevated during filtered air exposure compared with the two wood smoke exposures. Figure 5 illustrates this and is suggestive of an elevation with time in IL-18 only during the filtered air exposure.
FIGURE 4.
Plasma interleukin-6. Means of the ln-transformed plasma IL-6 concentrations at baseline, and 0, 6, and 20 hours after the three exposures to filtered air, low, and high concentrations of wood smoke. Error bars represent +1 standard deviation.
FIGURE 5.
Plasma interleukin-18. Means of the ln-transformed plasma IL-18 concentrations at baseline, and 0, 6, and 20 hours after the three exposures to filtered air, low, and high concentrations of wood smoke. Error bars represent +1 standard deviation.
In contrast, the majority of the measured plasma cytokines did not show any variations related to time or exposure. This was true for IL-10, IL-12, TNF, interferon-γ, GM-CSF, transforming growth factor-β1, monocyte chemoattractant protein-1, macrophage inflammatory protein-1α, and RANTES. The vast majority of IL-4, IL-5, and IL-8 measurements were below the lower limit of detection, and data were not analyzed. The serum pneumoproteins CC16, SP-D, and SP-A showed levels in line with those reported previously, with a diurnal variation for CC16. There was no significant impact of wood smoke exposure. Males had higher urine CC16 than females. Data are not shown as no differences in relation to exposure were observed.
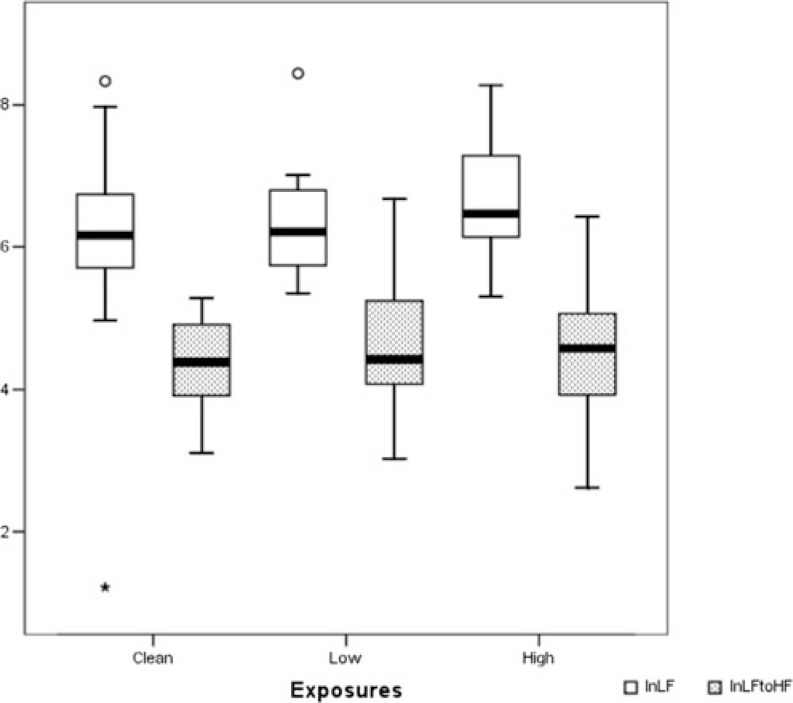
HRV data were complete for 93.3% of sessions. Errors were detected in 22 of the total of 14,652 R–R intervals that were recorded in the 4-minutes intervals used for the analysis. Mean heart rate during testing was 65.4 beats/min (range, 50–83) with no difference between exposures. The high frequency power (HF), low frequency power (LF), LF/HF ratio, root mean square of successive differences in normal-to-normal intervals, and the percentage of successive normal R–R interval differences greater than 50 ms were all in the same range during the 3 exposures. Box plots of the LF and LF/HF ratio are shown in Fig. 6. Using heart rate measurements from the last 4 minutes of the resting period did not alter the results.
FIGURE 6.
Heart rate variability. Box plots of the ln-transformed low-frequency power (white) and low-to-high-frequency power ratio (dotted) from the heart rate monitoring after the three exposures. Error bars represent +1 standard deviation.
DISCUSSION
Despite mean fine particle concentrations during the wood smoke session varying from 222 to 385 μg/m3 and occurrence of symptoms of airway mucosal irritation as reported in detail elsewhere,20 very few effects were observable in this experimental exposure study.
No exposure-related changes were observed in other primary (coagulation) and secondary (cytokines and HRV) endpoints. The plasma TF and IL-18 were both elevated during filtered air exposures compared with the wood smoke exposure sessions, but these differences were already apparent at baseline (ie, before any wood smoke entered the climate chamber). The decline in plasma sP-selectin, which was observed, did also not differ between the three exposures. Only for plasma IL-6 concentrations, we observed differential development with time between filtered air and wood smoke exposure. The diurnal variation in S-CC16 and the sex-related differences in U-CC16 we observed were similar to those observed by others.15,17,29,30
There has been a paucity of changes—other than in CC16 and exhaled NO—in markers of epithelial injury, airway, and systemic inflammation in the previous experimental wood smoke exposure studies in the literature15–17,19 in which healthy volunteers were exposed to fine particles in the 150 to 300 μg/m3 range. In those studies, no effects on lung function, blood, and bronchoalveolar lavage cell counts, plasma fibrinogen, factor VIIIc, VWF, TNF, or C-reactive protein were observed. Wood smoke did, however, induce increases in S-amyloid A, factor VIIIc/VWF ratio, urinary excretion of free 8-iso-prostaglandin2α, and glutathione concentrations in bronchoalveolar lavage. It is not known whether similar effects occurred in this study as we do not have data on these. We abstained from conducting serum amyloid A analyses. Considering that no increases were observed in IL-1β, TNF, or IL-6—three of the major inducers of hepatic amyloid A synthesis—any increase in serum amyloid A would be surprising. Because levels of VWF were found to be unaltered during the experiments, we abstained from measuring factor VIII:C, which represents a secondary acute-phase reactant to VWF. Furthermore, methods for recording of factor VIII:C are less reproducible than the ELISA method used for the estimation of VWF. Bronchial lavage was not performed and thus could not be analyzed. Thus, wood smoke in this concentration range, which can occur in homes with poor stoves or burning conditions,31 does not seem to have major effects during exposures lasting a few hours in healthy subjects at rest or mildly exercising. In contrast to this study, most,13,15,16,18,19 but not all,17 previous experimental studies have included exercise to increase breathing and thus exposure. We anticipated that the atopic status of the subjects rendered them more susceptible and investigated whether any systemic effects occurred even in the absence of exercise. Our study does not preclude effects in bronchoalveolar lavage, in mediators that were not measured, of higher concentrations, of prolonged exposure, or with exercising subjects. Indeed, a recent study of a 2-hour experimental wood smoke exposure of 485 μg/m3 revealed increased neutrophil counts in bronchoalveolar lavage and blood.18
Changes in HRV associated with air pollution have most often been documented among elderly subjects who are commonly considered more susceptible to air pollution although such changes were also documented among young adults exposed to urban air pollution.32 Previous studies on HRV after wood smoke exposures in which Holter monitor electrocardiographs (ECGs) were used for assessments have provided conflicting results. An intervention study of wood stove replacement in rural Guatemala found no evidence of an association between HRV and the stove intervention.33 In experiments with young healthy subjects, Ghio et al18 observed no change in HRV despite a higher wood smoke exposure than in our study, whereas Unosson et al13 found that wood smoke decreased several indices of HRV compatible with vagal inhibition. In the latter study, the exposures were comparable to the high exposures in our study but the burning conditions were poorer and the volunteers exercised intermittently during exposures. In our study, we assessed HRV with Polar RS800 pulse monitors intended primarily for use by athletes and not with ECG monitors recommended for this type of measurement. The advantages of the RS800 are low cost and simplicity of use in the setting where many clinical measurements were performed within limited time and space. HRV data were easily extracted in the desired 4-minute periods and graphical presentation of the data allowed for visual operator inspection and correction of the pulse data if needed. Thus, the Polar RS800 and Polar ProTrainer 5 software seemed feasible for the project. The Polar monitors and software have been directly compared with ECGs in one study.34 It was concluded that the Polar equipment did not identify errors satisfactorily, generally overestimated HRV, and that uncertainty increased with higher values. Agreement with ECG measurements was particularly poor among elderly women but improved considerably after visual inspection and manual removal of errors in the other age and sex groups. These are important flaws in a clinical setting but, as discussed by Quintana et al,35 are not likely to have seriously affected the ability to detect any HRV changes in this study of young healthy subjects. Without a direct comparison with ECG monitors, it cannot be excluded that HRV differences were present, which would have been discovered by such monitors. It seems more likely that the absence of exercise, the relatively low exposure levels, and the good burning conditions of the wood were insufficient to cause HRV change in the participants. Contrary to our hypothesis, atopic subjects may also be less susceptible to short-term effects of wood smoke.
Our finding of a decrease in plasma IL-6 after wood smoke exposure relative to an increase after filtered air confirms the finding in a previous experimental wood smoke exposure study.16 The timing of this relative decrease in IL-6 was comparable as in our study it was observed 6 hours and in the Swedish study 7 hours after the initiation of the exposures.16 The most likely reason for the increase in plasma IL-6 after the filtered air exposures is the normal afternoon increase caused by diurnal variation.36 The mechanism behind the absence of this increase after wood smoke exposure remains obscure. Surprisingly, the proinflammatory cytokines IL-1β and TNF were unaffected at 0 hour after wood smoke exposure. Either the reactions upstream of IL-6 occurred only locally and could not be observed in plasma as suggested by a tendency for an increase in nasal lavage reported elsewhere,20 involved other mediators than IL-1β or TNF as shown in relation to bioaerosol exposure,37 or they peaked earlier than 3.5 hours after the initiation of the exposure sessions.
The fact that sP-selectin decreased with time during all of the three exposures and continued this decrease in the morning after the exposure sessions was surprising. This adhesion molecule probably has a small diurnal variation with higher levels in the afternoon than in the morning,38 and an inflammatory reaction would tend to increase sP-selectin because of shedding of the membrane-bound form. It remains obscure whether our observation is coincidental or a result of conditions common to all three exposure sessions that led to increased baseline levels as a result of shedding in the hours before the study. The latter, that exposure conditions before the volunteers' arrival at the climate chamber caused common reactions unrelated to the exposure sessions, seems unlikely. Nevertheless, the fact that some outcomes such as the plasma TF reported here and lung function reported elsewhere21 differed between the exposure sessions at baseline also suggests that unintended differences in conditions before arrival at the climate chambers affected the results despite the balanced design, random order of, and 2-week intervals between exposures. Indications of such pre-entry differences in outcomes despite efforts to balance the design have previously been observed in climate chamber studies and may have affected the ability to detect effects of the outcomes.39
The design, randomization, organization, and blinding were the major assets of the study. In addition, we used an up-to-date climate chamber in which all conditions other than the exposures were kept very constant.20 Another strength was the application of mixed models with inclusion of dummy variables for possible carryover and period or seasonal effects.
The weaknesses of the study were the fact that it could not be conducted entirely according to the balanced design because of the lack of 4 of the planned 24 participants and some cases of respiratory illness among participants, which led to postponed exposure sessions compared with the schedule. The activities of the participating volunteers in the hours and days before the exposure sessions could not be standardized or completely controlled for and were likely to cause random effects (ie, decrease the signal-to-noise ratio). More importantly, the blinding was imperfect because of the smell of wood smoke, which was unmistakably stronger during the smoke exposure than during filtered air exposure sessions despite burning in the wood stove outside the chamber at all occasions. This most likely could have affected symptom reporting, as reported by Riddervold et al,20 and seem less likely to have caused effects in the objectively measured outcomes. In our opinion, the limited number of effects observed, combined with the observed changes in IL-6 in concordance with previous research, supports that imperfect blinding is unlikely to have resulted in spurious findings.
In this study, the healthy volunteers were atopic as defined by a skin-prick test but did not have symptoms of allergic disease such as rhinitis or asthma. Previously, asthmatic children have been shown to be particularly sensitive to wood smoke,40 and we speculated that this might also be the case for young adult atopics. The lack of stronger inflammatory reactions among the atopics in this study compared with the nonatopic subjects in other studies seems to indicate that atopy per se does not enhance susceptibility to short-term peak exposures to wood smoke. Nevertheless, this hypothesis remains to be tested in a study including both atopic and non-atopic subjects. In case atopics are not more sensitive to wood smoke than nonatopics, one might speculate that the increased sensitivity to wood smoke among asthmatics reported is related to the bronchial hyperresponsiveness rather than the (allergic) inflammation of the airways.
CONCLUSIONS
This experimental exposure study confirmed that only limited effects could be observed after a 3-hour wood smoke exposure in the 2 to 400 μg/m3 fine particle range in healthy atopic volunteers. A relative decrease in plasma IL-6 that has previously been described after wood smoke exposure was confirmed. The subjects in the study were atopic but did not seem to be more susceptible to cardiovascular and inflammatory effects of wood smoke compared with nonatopic subjects in the few previously published experimental wood smoke studies. Nevertheless, differences in exposure conditions render comparison with previous studies difficult.
ACKNOWLEDGMENT
We are indebted to Jørgen Ingerslev for suggestions on the coagulatory part of the study and together with his technical assistant Kirsten Christiansen for help with blood sampling and analyses. Vibeke H. Gutzke and Tine Bank are acknowledged for skillful technical assistance.
Footnotes
Address correspondence to: Jakob Hjort Bønløkke, MD, PhD, Section of Environment Work and Health, Institute of Public Health, University of Aarhus, Bartholins Allé 2, Building 1260, DK-8000 Aarhus C, Denmark (jb@mil.au.dk).
Dr Bønløkke was funded by the Center for Energy, Environment and Health, a center under the Danish Council for Strategic Research (contract no. 2104-06-0027) that also financially supported the study directly under their Program Commission on Sustainable Energy and Environment (Grant no. 2104-05-0045). The Danish Heart Association also provided financial support (08-4-R65-A 1999-B662-22436F).
The authors declare no conflicts of interest.
REFERENCES
- 1.Anderson HR, Ponce de Leon A, Bland JM, Bower JS, Strachan DP. Air pollution and daily mortality in London: 1987–92. BMJ. 1996;312:665–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pope CA, Burnett RT, Thurston GD, et al. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–77 [DOI] [PubMed] [Google Scholar]
- 3.Lipsett MJ, Ostro BD, Reynolds P, et al. Long-term exposure to air pollution and cardiorespiratory disease in the California teachers study cohort. Am J Respir Crit Care Med. 2011;184:828–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoek G, Krishnan RM, Beelen R, et al. Long-term air pollution exposure and cardio-respiratory mortality: a review. Environ Health. 2013;12:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hedley AJ, Wong C-M, Thach TQ, Ma S, Lam T-H, Anderson HR. Cardiorespiratory and all-cause mortality after restrictions on sulphur content of fuel in Hong Kong: an intervention study. Lancet. 2002;360:1646–1652 [DOI] [PubMed] [Google Scholar]
- 6.Jerrett M, Burnett RT, Pope CA, et al. Long-term ozone exposure and mortality. N Engl J Med. 2009;360:1085–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoek G, Brunekreef B, Goldbohm S, Fischer P, van den Brandt PA. Association between mortality and indicators of traffic-related air pollution in the Netherlands: a cohort study. Lancet. 2002;360:1203–1209 [DOI] [PubMed] [Google Scholar]
- 8.Martin WJ, Glass RI, Araj H, et al. Household air pollution in low- and middle-income countries: health risks and research priorities. PLoS Med. 2013;10:e1001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balmes JR. When smoke gets in your lungs. Proc Am Thorac Soc. 2010;7:98–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarnat JA, Marmur A, Klein M, et al. Fine particle sources and cardiorespiratory morbidity: an application of chemical mass balance and factor analytical source-apportionment methods. Environ Health Perspect. 2008;116:459–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larson TV, Koenig JQ. Wood smoke: emissions and noncancer respiratory effects. Annu Rev Public Health. 1994;15:133–156 [DOI] [PubMed] [Google Scholar]
- 12.Allen RW, Mar T, Koenig J, et al. Changes in lung function and airway inflammation among asthmatic children residing in a woodsmoke-impacted urban area. Inhal Toxicol. 2008;20:423–433 [DOI] [PubMed] [Google Scholar]
- 13.Unosson J, Blomberg A, Sandström T, et al. Exposure to wood smoke increases arterial stiffness and decreases heart rate variability in humans. Part Fibre Toxicol. 2013;10:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu L-JS, Box M, Kalman D, et al. Exposure assessment of particulate matter for susceptible populations in Seattle. Environ Health Perspect. 2003;111:909–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barregard L, Sallsten G, Andersson L, et al. Experimental exposure to wood smoke: effects on airway inflammation and oxidative stress. Occup Environ Med. 2008;65:319–324 [DOI] [PubMed] [Google Scholar]
- 16.Barregard L, Sällsten G, Gustafson P, et al. Experimental exposure to wood-smoke particles in healthy humans: effects on markers of inflammation, coagulation, and lipid peroxidation. Inhal Toxicol. 2006;18:845–853 [DOI] [PubMed] [Google Scholar]
- 17.Stockfelt L, Sallsten G, Olin AC, et al. Effects on airways of short-term exposure to two kinds of wood smoke in a chamber study of healthy humans. Inhal Toxicol. 2012;24:47–59 [DOI] [PubMed] [Google Scholar]
- 18.Ghio AJ, Soukup JM, Case M, et al. Exposure to wood smoke particles produces inflammation in healthy volunteers. Occup Environ Med. 2012;69:170–175 [DOI] [PubMed] [Google Scholar]
- 19.Sehlstedt M, Dove R, Boman C, et al. Antioxidant airway responses following experimental exposure to wood smoke in man. Part Fibre Toxicol. 2010;7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riddervold IS, Bønløkke JH, Mølhave L, et al. Wood smoke in a controlled exposure experiment with human volunteers. Inhal Toxicol. 2011;23:277–288 [DOI] [PubMed] [Google Scholar]
- 21.Riddervold IS, Bønløkke JH, Olin A-C, et al. Effects of wood smoke particles from wood-burning stoves on the respiratory health of atopic humans. Part Fibre Toxicol. 2012;9:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forchhammer L, Møller P, Riddervold IS, et al. Controlled human wood smoke exposure: oxidative stress, inflammation and microvascular function. Part Fibre Toxicol. 2012;9:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donaldson K, Stone V, Seaton A, MacNee W. Ambient particle inhalation and the cardiovascular system: potential mechanisms. Environ Health Perspect. 2001;109(suppl 4):523–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chuang K, Chan C, Su T, Lee C, Tang C. Urban air pollution on inflammation, oxidative stress, coagulation and autonomic dysfunction. Am J Respir Crit Care Med. 2007;176:370–376 [DOI] [PubMed] [Google Scholar]
- 25.Pope CA, Verrier RL, Lovett EG, et al. Heart rate variability associated with particulate air pollution. Am Heart J. 1999;138:890–899 [DOI] [PubMed] [Google Scholar]
- 26.Ingerslev J. A sensitive ELISA for von Willebrand factor (vWf:Ag). Scand J Clin Lab Invest. 1987;47:143–149 [PubMed] [Google Scholar]
- 27.Ellingsen DG, Ulvestad B, Andersson L, Barregard L. Pneumoproteins and inflammatory biomarkers in asphalt pavers. Biomarkers. 2010;15:498–507 [DOI] [PubMed] [Google Scholar]
- 28.Skogstrand K, Thorsen P, Nørgaard-Pedersen B, Schendel DE, Sørensen LC, Hougaard DM. Simultaneous measurement of 25 inflammatory markers and neurotrophins in neonatal dried blood spots by immunoassay with xMAP technology. Clin Chem. 2005;51:1854–1866 [DOI] [PubMed] [Google Scholar]
- 29.Blomberg A, Mudway I, Svensson M, et al. Clara cell protein as a biomarker for ozone-induced lung injury in humans. Eur Respir J. 2003;22:883–888 [DOI] [PubMed] [Google Scholar]
- 30.Andersson L, Lundberg P-A, Barregard L. Methodological aspects on measurement of Clara cell protein in urine as a biomarker for airway toxicity, compared with serum levels. J Appl Toxicol. 2007;27:60–66 [DOI] [PubMed] [Google Scholar]
- 31.Traynor GW, Apte MG, Carruthers AR, Dillworth JF, Grimsrud DT, Gundel LA. Indoor air pollution due to emissions from wood-burning stoves. Environ Sci Technol. 1987;21:691–697 [DOI] [PubMed] [Google Scholar]
- 32.Chuang K-J, Chan C-C, Su T-C, Lee C-T, Tang C-S. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am J Respir Crit Care Med. 2007;176:370–376 [DOI] [PubMed] [Google Scholar]
- 33.McCracken J, Smith KR, Stone P, Díaz A, Arana B, Schwartz J. Intervention to lower household wood smoke exposure in guatemala reduces ST-segment depression on electrocardiograms. Environ Health Perspect. 2011;119:1562–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wallen MB, Hasson D, Theorell T, Canlon B, Osika W. Possibilities and limitations of the Polar RS800 in measuring heart rate variability at rest. Eur J Appl Physiol. 2012;112:1153–1165 [DOI] [PubMed] [Google Scholar]
- 35.Quintana DS, Heathers JAJ, Kemp AH. On the validity of using the Polar RS800 heart rate monitor for heart rate variability research. Eur J Appl Physiol. 2012;112:4179–4180 [DOI] [PubMed] [Google Scholar]
- 36.Sothern RB, Roitman-Johnson B, Kanabrocki EL, et al. Circadian characteristics of circulating interleukin-6 in men. J Allergy Clin Immunol. 1995;95:1029–1035 [DOI] [PubMed] [Google Scholar]
- 37.Burvall K, Palmberg L, Larsson K. Expression of TNFalpha and its receptors R1 and R2 in human alveolar epithelial cells exposed to organic dust and the effects of 8-bromo-cAMP and protein kinase A modulation. Inflamm Res. 2005;54:281–288 [DOI] [PubMed] [Google Scholar]
- 38.Osmancik P, Kvasnicka J, Widimsky P, Tarnok A. Diurnal variation of soluble E- and P-selectin, and intercellular adhesion molecule-1 in patients with and without coronary artery disease. Cardiology. 2004;102:194–199 [DOI] [PubMed] [Google Scholar]
- 39.Bønløkke JH, Stridh G, Sigsgaard T, et al. Upper-airway inflammation in relation to dust spiked with aldehydes or glucan. Scand J Work Environ Health. 2006;32:374–382 [DOI] [PubMed] [Google Scholar]
- 40.Koenig JQ, Larson TV, Hanley QS, et al. Pulmonary function changes in children associated with fine particulate matter. Environ Res. 1993;63:26–38 [DOI] [PubMed] [Google Scholar]