Abstract
Objective
This study aimed to evaluate cervical lesions by the Swede coloscopy system, histologic finding, liquid-based cytology, and human papillomavirus (HPV) in women who resulted positive for visual inspection of the cervix with acetic acid (VIA) by using a pocket-sized battery-driven colposcope, the Gynocular (Gynius AB, Sweden).
Methods
This study was a crossover, randomized clinical trial at the colposcopy clinic of Bangabandhu Sheikh Mujib Medical University in Dhaka, Bangladesh, with 540 VIA-positive women. Swede scores were obtained by the Gynocular and stationary colposcope, as well as samples for liquid-based cytology, HPV, and cervical biopsies. The Swede scores were compared against the histologic diagnosis and used as criterion standard. The percentage agreement and the κ statistic for the Gynocular and standard colposcope were also calculated.
Results
The Gynocular and stationary colposcope showed high agreement in Swede scores with a κ statistic of 0.998, P value of less than 0.0001, and no difference in detecting cervical lesions in biopsy. Biopsy detected cervical intraepithelial neoplasia (CIN) 2+ (CIN2, CIN3, and invasive cancer) in 38 (7%) of the women, whereas liquid-based cytology detected CIN2+ in 13 (2.5%) of the women. Forty-four (8.6%) women who were tested resulted positive for HPV; 20 (3.9%) women had HPV-16, 2 (0.4%) had HPV-18, and 22 (4.3%) had other high-risk HPV.
Conclusions
Our study showed that few VIA-positive women had CIN2+ lesions or HPV infection. Colposcopy by Swede score identified significantly more CIN2+ lesions than liquid-based cytology and could offer a more accurate screening and selection for immediate treatment of cervical lesions in low-resource settings.
Key Words: Cervical cancer, HPV, Cytology, Gynocular, Colposcope
It is estimated that most global cervical cancer burdens occur in developing countries,1 where cervical cancer is the leading cause of cancer-related death in women and commonly affects women of reproductive age.1 Survival rates are also lower in developing countries and attributed to patients presenting at a more advanced stage of the disease because many women in low-resource settings do not receive or complete treatment because of insufficient access or the inability to afford the treatment.1
In developed countries, nationwide screening programs using cervical cytology have successfully reduced the incidence of cervical cancer.2 Cytology-based screening programs have been difficult to implement in many low-resource settings because it is laboratory based and requires expensive equipment with technician support and skilled personnel to prepare and interpret the slides.3,4 In addition, to be effective, cytologic screening needs to be repeated regularly.5,6 Consequently, opportunistic unaided visual inspection of the cervix after applying 3% to 5% acetic acid (VIA) and examination under a 100-W lamp observing for color changes on the cervix by trained health personnel has been suggested as an alternative to cervical cytology screening in low-resource settings.5,6 Visual inspection of the cervix with acetic acid is inexpensive, its results are immediately available, and the treatment can be administered on-site or by referral to a colposcopy clinic for biopsy and/or treatment.6 However, there have been concerns over the reproducibility and accuracy of VIA raised during the recent years.4,7,8
The optimal screening cervical cancer screening strategy should identify those women who are at the greatest risk of developing invasive cervical cancer.9 Technological improvements are unlikely to improve screening outcomes if they do not reach the population. To reduce the burden of cervical cancer incidence and mortality, access to screening is the most important step, regardless of method.9
Colposcopic evaluation and guided biopsy are important diagnostic steps and standard management for abnormal cytology smear findings in developed countries.8 Conventional colposcopes are large microscopes with strong illumination and optics adjusted for vaginal examination. Directed by visually suspected lesions, biopsies are taken and sent for histopathologic examination. It has been suggested that in VIA-positive women, colposcopy may be used to identify women who are likely to benefit from immediate treatment10 or to be used as a primary cervical screening tool in low-resource settings by using the Swede score colposcopy method,11–13 where each of the 5 colposcopic variables (acetowhiteness, margins plus surface, vessel pattern, lesion size, and iodine staining) are given a score of 0, 1, or 2 points, where the total score gives an indication of the severity of the visual impression of the cervix.
To date, widespread colposcopy screening has been difficult to implement because current colposcopes are expensive, heavy, and difficult to transport, need technician support service, and require an electrical grid. Taking in to account the limits of infrastructure and available resources in low-resource settings, the technology used in these areas needs to be simple to use, robust, and in need of minimal technical maintenance. A pocked-sized battery-driven colposcope (the Gynocular [Gynius AB, Sweden]) was developed to provide criterion standard examination suitable for any infrastructural setting, thus enabling access to high-quality colposcopy in low-resource settings.13
The aim of this study was to investigate if the Swede score colposcopy method using a pocket-sized battery-driven colposcope, the Gynocular, may improve detection of cervical lesions and selection for immediate treatment in low-resource settings.
MATERIALS AND METHODS
This study was a crossover, randomized clinical trial for assessing accuracy of the Swede scores11–13 by colposcopy performed by standard colposcopes and the Gynocular, using biopsy as a criterion standard. The inclusion criteria were (1) women who resulted positive for VIA at opportunistic screening by trained family welfare visitors, senior staff nurses, and doctors in the Dhaka region,6 Bangladesh, referredfor colposcopy at the colposcopy clinic of Bangabandhu Sheikh Mujib Medical University (BSMMU) from June 2012 to September 2012. In Bangladesh, 2.3% of the women have been screened with VIA up to date. Among the women who had VIA of approximately 5% are VIA positive.6 A woman is considered to be VIA positive when a trained doctor or nurse noticed sharp, distinct, well-defined, dense acetowhite areas on the cervix, with or without raised margins, close to the squamocolumnar junction in the transformation zone.6 Other inclusion criteria were (2) ability to understand written and oral information and (3) women signing an informed consent to participate in the study after receiving oral and written information from a social worker. Exclusion criteria were the following: (1) on-going vaginal bleeding, (2) any previous gynecologic examinations for at least 1 week before, and (3) pregnancy. Women who chose not to participate in the study had a standard colposcopy examination. In total, 540 women were included in the study after written consent. All women in the study were examined by 1 of the 6 colposcopy specialists in the colposcopy clinic of BSMMU. The colposcopy specialists were physicians or gynecologists who were trained in colposcopy, cold coagulation, and loop electrical excision procedure at the colposcopy clinic of BSMMU.6
Colposcopy was performed using 1 of the 2 standard colposcopes (Karl Kaps Som 52, Karl Kaps GmbH & Co.KG, Asslar/Wetzlar, Germany or Leisegang 1DF, Leisegang Feinmechanik-Optik GmbH, Berling, Germany) and the Gynocular. The Gynocular was mounted on a camera tripod during the examination. Women were randomly allocated in blocks of 50 to start the examination with either standard colposcope or Gynocular examination of the cervix. A total of 298 women started the examination with colposcope and 242 women with the Gynocular. All women were inspected with a standard colposcope and the Gynocular by the same examiner in a crossover design to assess the performance of agreement between the standard colposcope and the Gynocular. This crossover design was selected to reduce potential observer variability.14–16
Performing the Swede score, each of the 5 colposcopic variables (acetowhiteness, margins plus surface, vessel pattern, lesion size, and iodine staining) was given a score of 0, 1,or 2 points.11–13 A nonlubricated self-holding speculum was inserted into the vagina and the cervix was visualized. The examination started with inspection of cervical vessel patterns with colposcope or the Gynocular as randomized using the red-free (green filter) mode. A cervical cell sample was then collected with a soft spatula from the cervix and cytobrush from the cervical canal for liquid-based cytology (ThinPrep; Hologic Inc, Marlborough, Mass). Then, the cervix was wiped with 5% acetic acid for 1 minute, followed by completion of the first colposcopic examination. After each examination, the 4 Swede score variables (acetowhiteness, margins plus surface, vessel pattern, and lesion size) were scored by the colposcopist and documented by the study coordinator. The examiner then changed instruments and repeated the examination procedure, and the new 4 Swede score variables were documented by the study coordinator. Next, the cervix was swabbed with 5% Lugol iodine solution, and the colposcopist scored the Swede score’s fifth variable (iodine staining) with both instruments as randomized. The examination was completed with 1 or more biopsies taken from areas of suspected cervical lesions. Punch biopsies of the cervix were done in all women with Swede score of 4 and above.11,12
The cervical biopsies were analyzed at the histopathology laboratory of BSMMU. The ThinPrep (Hologic Inc) tests were analyzed at the Laboratory of Clinical Pathology and Cytology in Karolinska University Hospital at Karolinska Institutet in Stockholm, Sweden. The human papillomavirus (HPV) tests were analyzed in the Laboratory of Clinical Microbiology and the Laboratory of Viruses of Karolinska University Hospital at Karolinska Institutet in Stockholm, Sweden, using the Cobas HPV Test (Roche Molecular Systems Inc, Pleasanton, Calif). The test specifically identifies (types) HPV-16 and HPV-18 while detecting the rest of the high-risk HPV types (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68) at clinically relevant infection levels.
The final diagnosis was the histopathology result, using the cervical intraepithelial neoplasia (CIN) system,17 or other histopathologic diagnosis in situation of such outcome. Women with CIN1 lesions were given the choice of being treated directly or a follow-up appointment after 6 months. Women with lesions grade of CIN2 or higher underwent a loop electrical excision procedure. Women with invasive cancers were referred to the Department of Gynecology and Oncology at BSMMU for further management.
The study was approved by the local ethics committees in Bangladesh and in Sweden: the institutional review board of BSMMU (Dnr BSMMU/2012/3176) and the Stockholm Regional Ethical Review Board (Dnr 2012/545-31/1).
The Gynocular
The Gynocular (Gynius AB, Stockholm, Sweden) has similar specifications to traditional colposcopes (Table 1). The Gynocular is a monocular with 300mm focal distance and 3 magnifications: 5× 8× and 12×. It is a handheld device measuring 50 × 33 × 166 mm and comes with a tripod-mounting clip that screws into a standard tripod, enabling the medical professional to also perform colposcopy hands-free mode for ease of biopsy (Fig. 1). The Gynocular uses high-intensity LEDs for warm white illumination, has a green filter light, and is powered by a rechargeable lithium-ion battery (Fig. 2). The Gynocular is an approved product by the Swedish National Drug Authority as a noninvasive medical diagnostic class I tool and CE marked.
TABLE 1.
Technical characteristics of the Gynocular and colposcopes Leisegang IDF and Karl Kaps SOM 52
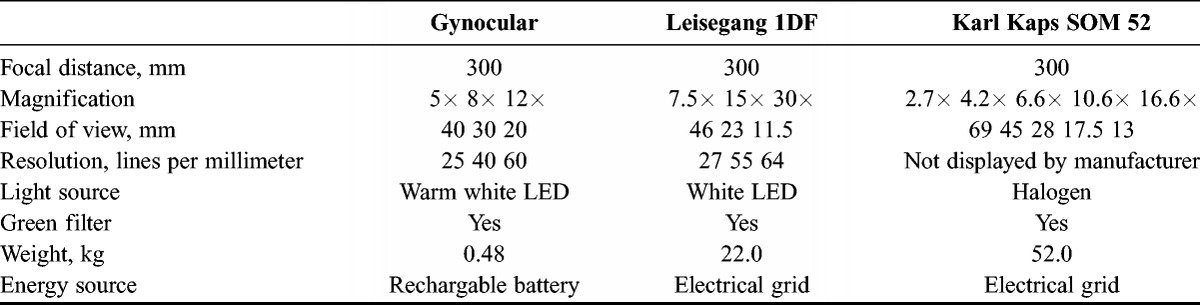
FIGURE 1.

The Gynocular with charger and mounting clip for camera tripod.
FIGURE 2.

The Gynocular showing lens, green filter, and warm white LED illumination.
Statistical Analysis
All statistical analyses have been performed using R version 2.14.1, Kurt Hornik, Free Software Foundation, INC, Vienna, Switzerland,. The baseline patient characteristics of the women were summarized using means (SD) and frequencies (%; Table 1).To test the level of agreement between the colposcopy and the Gynocular, we calculated the percentage agreement and the weighted κ statistic.15 Cervical lesions were classified by the Swede scores system using the Gynocular and a standard colposcope.10,11 Swede scores of 4 and above were the cutoff scores for cervical biopsy by any of the instruments. We calculated detection rates of CIN1, CIN2, CIN3, CIN3+ (invasive cancer), cervicitis, cervical tuberculosis, HPV-16, HPV-18, and the other high-risk HPV types (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68). A positive biopsy result was defined as CIN1, CIN2, CIN3, or CIN3+, and we calculated the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the Swede score using biopsy as a criterion standard for all cutoff levels of Swede score between 4 and 10. The results are presented in tables and as receiver operating characteristic curves.
RESULTS
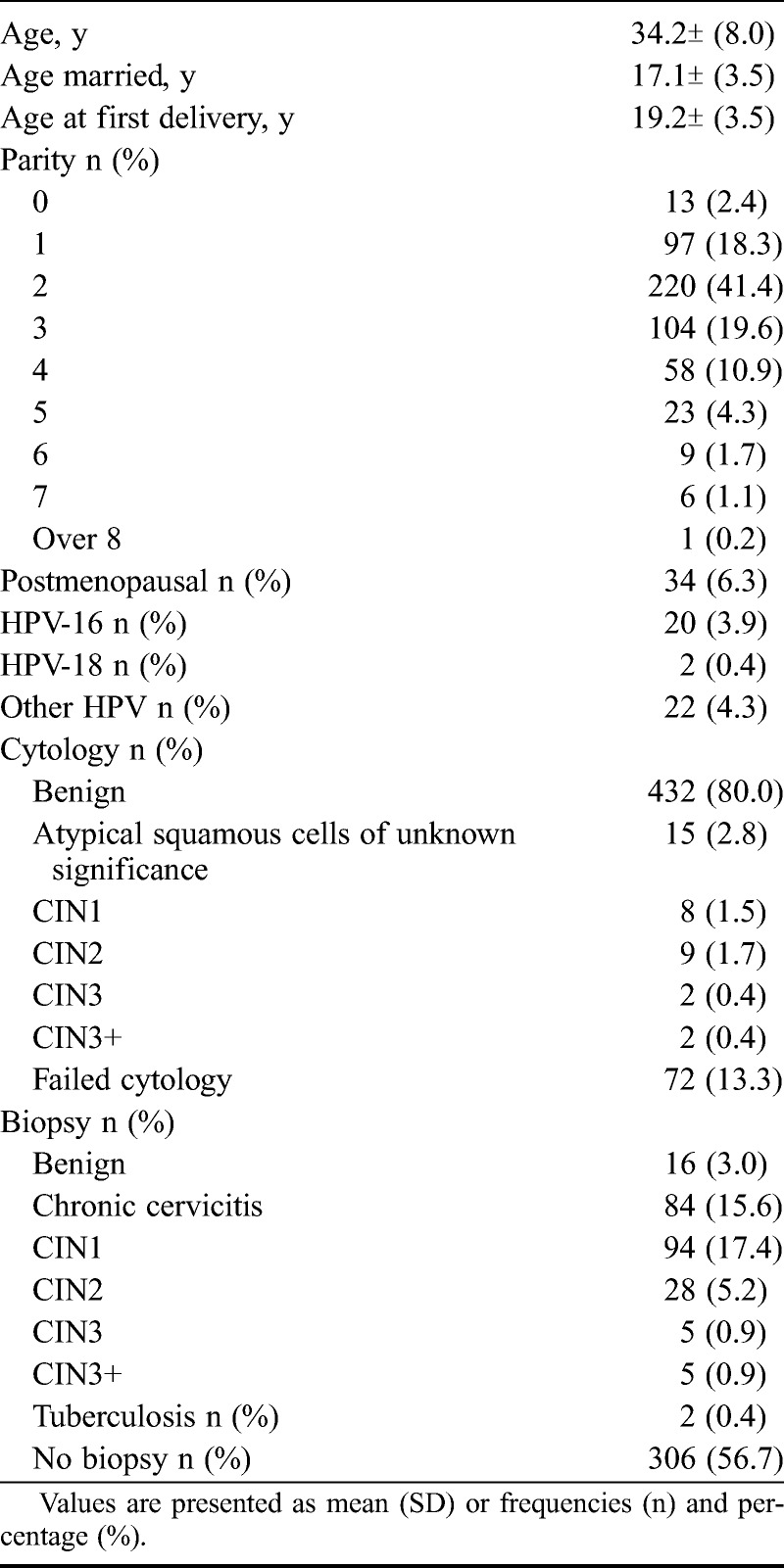
The women’s baseline characteristics are presented in Table 2. The mean (SD) age was 34.2 (8.0) years. Most women (506 [93.7%]) were of reproductive age. The mean (SD) age of first marriage was 17.1 (3.5) years, and the mean age at first delivery was 19.2 (3.5) years.
TABLE 2.
Baseline characteristics
In our study, HPV-16 was present in 20 (3.9%) women, HPV-18 was found in 2 (0.4%) women, and other high-risk HPV (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68) were detected in 22 (4.3%) women.
Liquid-based cytology was normal in 432 (80.0%) women and detected 15 (2.8%) women with atypical squamous cells of unknown significance, 8 (1.5%) women with CIN1, 9 (1.7%) women with CIN2, 2 (0.4%) women with CIN3, and 2 (0.4%) women with CIN3+ (invasive cancer).
Biopsy was normal in 16 (3.0%) women and identified 85 (15.6%) women with chronic cervicitis, 94 (17.4%) women with CIN1, and 28 (5.2%) women with CIN2. Two (0.4%) women had CIN3, and 2 (0.4%) women had invasive cervical cancer (CIN3+). In 2 (0.4%) women, biopsy result showed cervical tuberculosis. Thus, Swede score–directed biopsy diagnosed CIN2+ (CIN2, CIN3, and invasive cancer) in 38 (7.1%) women, whereas cytology detected CIN2+ in 13 (2.4%) of the women.
Swede scores were obtained by cervical examination with colposcope and the Gynocular. A cross tabulation of Swede scores by colposcope versus the Gynocular showed high agreement in 521 measurements, with a κ coefficient of 0.998 and P value of less than 0.001 (Fig. 3). There were no significant differences between the Gynocular and the colposcope in identifying cervical lesions detected by biopsy (Fig. 4).
FIGURE 3.
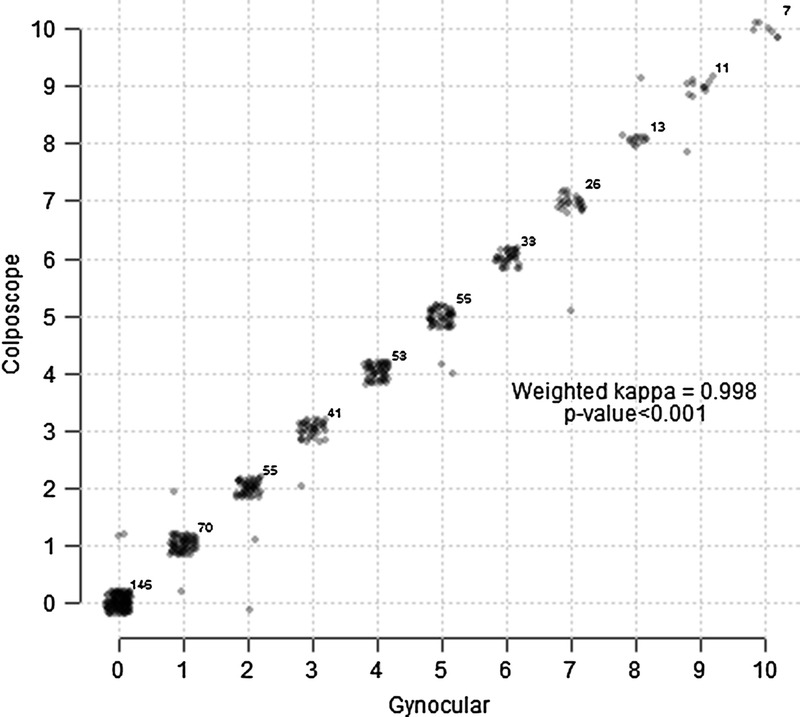
Agreement between Gynocular and colposcope.
FIGURE 4.
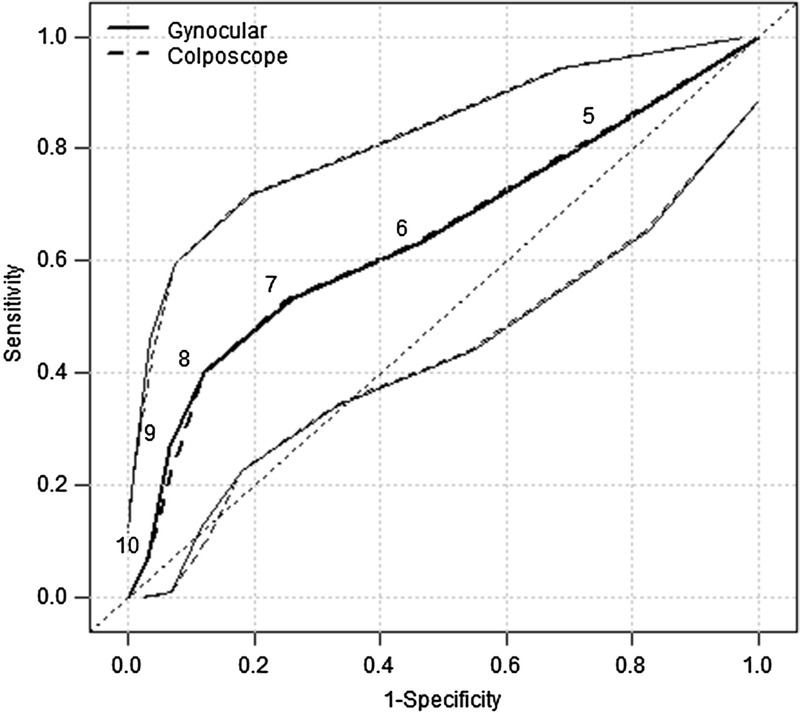
Receiver operating characteristic curves for predicting a positive biopsy result defined as CIN2, CIN3, or CIN3+.
To address the possibility that the Swede score taken by the first instrument has biased the Swede score taken by the second instrument, we reanalyzed the receiver operating characteristic curves excluding the Swede score taken by the second instrument (Fig. 5). Figure 5 compares the first Swede score taken by Gynocular or standard colposcope and presents the sensitivity and specificity for detecting CIN2+ and show that there are no significant differences between the Gynocular and the standard colposcope in detecting CIN2+ lesions.
FIGURE 5.

Comparison of Gynocular and colposcope only using first measurement.
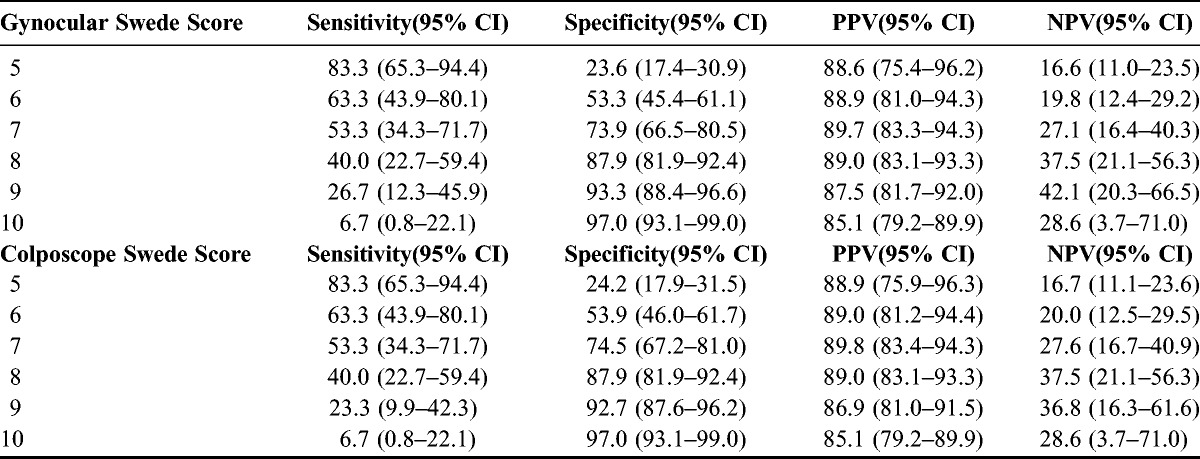
Using the cutoff value of 4 and above for Swede score and biopsy, it showed that colposcopy by the Gynocular had a sensitivity of 83.3% (95% CI [confidence interval], 65.3%–94.4%) and specificity of 23.6% (95% CI, 17.4%–30.9%) and a sensitivity of 83.3% (95% CI, 65.3%–94.7%) and specificity of 24.2% (95% CI, 17.9%–31.5%) for the colposcope (Tables 2 and 3). Positive predictive value was 88.6% (95% CI, 75.4%–96.2%) for the Gynocular and 88.9% (95% CI, 75.9%–96.3%) for the colposcope. Negative predictive value was 16.6% (95% CI, 11.0%–23.5%) for the Gynocular and 16.7% (95% CI, 75.9%–96.3%) for the colposcope (Table 3). With increased Swede score, the sensitivity decreased whereas the sensitivity increased, both for the Gynocular and colposcope, and further analysis of each individual item of the Swede score showed no differences between the different instruments (Table 3).
TABLE 3.
Sensitivity and specificity in percent for different cutoff levels for Gynocular and colposcope
DISCUSSION
The main finding of our study is that there were nodifferences between the Gynocular and the standard colposcope in detecting cervical lesions in biopsy, showing excellent agreement of Swedes scores by the Gynocular and colposcope, as well as high intraobserver agreement. A Swede score of above 4 in VIA-positive women gave a good indication for biopsy, whereas the possibility of a high-grade cervical lesion and a Swede score of 8 and above was highly correlated with CIN2+ and thus could serve as an aid to decide at site for direct removal of abnormal cervical areas. Another important finding was that very few of the referred VIA-positive women resulted positive for HPV infection or had a CIN2+ lesion on cytology or biopsy. This needs to be considered when VIA is used for cervical screening.
The main strength of our study is the large sample size and randomized crossover design, which reduced the risk of intraobserver variability. The fact that all patients had HPV and a cervical cytology test analyzed in an accredited laboratory increases the strength of the study.
The main weakness of our study is that biopsy was performed in patients with Swede score of 4 and above and thus not in all patients. This might have biased our results because we could not compare histopathologic results for Swede scores of lower than 4 with Swede scores of 4 and above. However, although all women had both a cervical cytology and HPV test, the low incidence of HPV and abnormal cytology supports the finding that few VIA-positive women had an increased Swede score and thus was not in need of a biopsy. Another weakness is that women who were included were referred as VIA positive, and we could not control for how the referrer assessed the woman as VIA positive.
Our finding that most referred VIA-positive women had no HPV infection nor cervical lesions is in line with VIA studies from Nigeria, Peru, and Uganda.4,7,13 In a pooled study from India, women who were never screened before for cervical cancer had a prevalence of 5.8% high-risk HPV.17 This finding is interesting to compare with the prevalence of 8.6% high-risk HPV among our study group of VIA-positive women from Bangladesh, whom thus did not differ much in HPV prevalence to women who were never screened before in a close-by region. These findings emphasize the need of a more accurate method for cervical cancer screening in low-resource settings to avoid overtreatment or unnecessary referrals and thus economic burden on already-restrained economies. In addition, the finding that 2 of the VIA-positive women had cervical tuberculosis on biopsy is important keep in mind, especially because it has only rarely been described in literature before.18
Pimple et al10 suggested a “single-visit approach” with colposcopy, which gives direct results and could be considered as a secondary testing tool to triage women who were found positive on VIA in settings where cytology and histopathology services are unavailable. In addition, a “see-and-treat” approach was a well-accepted management strategy of high-grade CIN in Bangladesh because it reduced the number of visits to the clinic and failure to receive treatment.19 Moreover, Bowring et al12 proposed that a modified Swede score in low-resource settings could predict cervical abnormalities and avoid overtreatment,11 and Strander et al11 suggested see-and-treat approach when the patient has a Swede score of 8 and above. It is reassuring to note that the observed high specificity for CIN2+ for Swede score of 8 and above in our study is well in line with the results from both Strander et al11 and Bowring et al12 and support the see-and-treat method when the patient has a Swede score of 8 and above.
A recent meta-analysis20 of the accuracy of colposcopy found that the pooled sensitivity for a punch biopsy–defined CIN2+ disease was 91.3% and the specificity was as low as 24.6%. In most of the studies included, most women had positive punch biopsies, and the authors concluded that the observed high sensitivity of the punch biopsy was probably the result of verification bias, whereas very few cases of negative punch biopsies were referred for colposcopy and thus lowering the specificity of colposcopy.20 Also, previous studies on the Swede score method took place in settings where most of the patients were referred for colposcopy because of abnormal cytology. In these studies, most patients with CIN2+ had Swede score of 5 and above.11,12 The study population in our study was different; cytology was not available before colposcopy, only the VIA result, and for many women included in the study, the current visit to the colposcopy clinic might have been their only lifetime opportunity to have a colposcopy examination. Therefore, it is reassuring to note that also in our study population of VIA-positive women, colposcopy with Swede score was able to identify those who are in need of further assessment with biopsy and also which women would benefit from direct treatment.
One alternative to screening by Swede score is cytology. In our study, the results from cytology showed that 2.5% of the VIA-positive women had CIN2+, whereas punch biopsy from Swede scores of 4 and above found CIN2+ in 7% of the women. These findings are comparable with the results from a multicenter study in India, where cytology for CIN2+ reported where colposcopy in VIA-positive women also detected more CIN2+ lesions in VIA-positive women than cytology.21
Increasing the Swede score cutoff decreased the sensitivity and increased the specificity. Lower specificity for a diagnostic test increases further burden on the health system in increased health care cost for treatment of the false positives. Thus, we recommend a colposcopy Swede score cutoff of 4 for biopsy in low-resource settings as an alternative cervical screening method.
In conclusion, Swede score by colposcopy using standard colposcope or the Gynocular might be an alternate cervical cancer screening method in low-resource settings, enabling the single-visit approach and avoiding overtreatment.
ACKNOWLEDGMENTS
First of all, we would like to thank all the women who participated in the study. We would also like to thank the doctors and nurses who examined all the women at the colposcopy clinic of Bangabandhu Sheikh Mujib Medical University (BSMMU) in Dhaka, Bangladesh. We would also like to thank the BSMMU in Bangladesh and Karolinska Institutet in Sweden for allowing us to perform the study at their premises and H&M Conscious Foundation for the economic support.
Footnotes
Address correspondence and reprint requests to Elisabeth Andrea Wikström Shemer, MD, PhD, Department of Obstetrics and Gynecology, Department of Clinical Sciences, Danderyd Hospital, 181 82 Danderyd, Karolinska Institutet, Stockholm, Sweden. E-mail: elisabeth@gynocular.com.
This study was supported by the H&M Conscious Foundation and the Gynius AB.
The authors declare no conflicts of interest.
REFERENCES
- 1. Sankaranarayanan R, Ferlay J. Worldwide burden of gynaecological cancer: the size of the problem. Best Pract Res Clin Obstet Gynaecol. 2006; 20: 207– 225 [DOI] [PubMed] [Google Scholar]
- 2.International Agency for Research on Cancer (IARC) WHO IARC Handbooks of Cancer Prevention: Cervix Cancer Screening, Chapter 2. Lyon, France: IARC Press; 2005 [Google Scholar]
- 3. Sankaranarayanan R, Sauvaget C, Ramadas K, et al. Clinical trials of cancer screening in the developing world and their impact on cancer healthcare. Ann Oncol. 2011; 22 (suppl 7): vii20– vii28 [DOI] [PubMed] [Google Scholar]
- 4. Ajenifuja KO, Gage JC, Adepiti AC, et al. A population-based study of visual inspection with acetic acid (VIA) for cervical screening in rural Nigeria. Int J Gynecol Cancer. 2013; 23: 507– 512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Deodhar K, Sankaranarayanan R, Jayant K, et al. Accuracy of concurrent visual and cytology screening in detecting cervical cancer precursors in rural India. Int J Cancer. 2012; 131: E954– E962 [DOI] [PubMed] [Google Scholar]
- 6. Nessa A, Hussain MA, Rahman, et al. Screening for cervical neoplasia in Bangladesh using visual inspection with acetic acid. Int J Gynaecol Obstet. 2010; 111: 115– 118 [DOI] [PubMed] [Google Scholar]
- 7. Almonte M, Ferreccio C, Winkler JL, et al. Cervical screening by visual inspection, HPV testing, liquid-based and conventional cytology in Amazonian Peru. Int J Cancer. 2007; 121: 796– 802 [DOI] [PubMed] [Google Scholar]
- 8. Cagle AJ, Hu SY, Sellors JW, et al. Use of an expanded gold standard to estimate the accuracy of colposcopy and visual inspection with acetic acid. Int J Cancer. 2010; 126: 156– 161 [DOI] [PubMed] [Google Scholar]
- 9. Saslow D, Solomon D, Lawson HW, et al. , American Cancer Society; American Society for Colposcopy and Cervical Pathology; American Society for Clinical Pathology American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. 2012; 137: 516– 542 [DOI] [PubMed] [Google Scholar]
- 10. Pimple SA, Amin G, Goswami S, et al. Evaluation of colposcopy vs cytology as secondary test to triage women found positive on visual inspection test. Indian J Cancer. 2010; 47: 308– 313 [DOI] [PubMed] [Google Scholar]
- 11. Strander B, Ellström-Andersson A, Franzén S, et al. The performance of a new scoring system for colposcopy in detecting high-grade dysplasia in the uterine cervix. Acta Obstet Gynecol Scand. 2005; 84: 1013– 1017 [DOI] [PubMed] [Google Scholar]
- 12. Bowring J, Strander B, Young M, et al. The Swede score: evaluation of a scoring system designed to improve the predictive value of colposcopy. J Low Genit Tract Dis. 2010; 14: 301– 305 [DOI] [PubMed] [Google Scholar]
- 13. Ngonzi J, Bajunirwe F, Wistrand C, et al. Agreement of colposcope and Gynocular in assessment of cervical lesions by Swede score: a randomized, crossover pilot trial. J Low Genit Tract Dis. 2013; 17: 372– 377 [DOI] [PubMed] [Google Scholar]
- 14. Cox JT. More questions about the accuracy of colposcopy: what does this mean for cervical cancer prevention? Obstet Gynecol. 2008; 111: 1266– 1267 [DOI] [PubMed] [Google Scholar]
- 15. Buckley CH, Butler EB, Fox H. Cervical intraepithelial neoplasia. J Clin Pathol. 1982; 35: 1– 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977; 33: 159– 174 [PubMed] [Google Scholar]
- 17. Basu P, Mittal S, Bhaumik S, et al. Prevalence of high-risk human papillomavirus and cervical intraepithelial neoplasias in a previously unscreened population—a pooled analysis from three studies. Int J Cancer. 2013; 132: 1693– 1699 [DOI] [PubMed] [Google Scholar]
- 18. Mandato VD, Sacchetti F, Costagliola L, et al. Primary tuberculosis of the uterine cervix: keep it in mind. J Low Genit Tract Dis. 2013 [DOI] [PubMed] [Google Scholar]
- 19. Nessa A, Rashid MH, E-Ferdous N, et al. Screening for and management of high-grade cervical intraepithelial neoplasia in Bangladesh: a cross-sectional study comparing two protocols. J Obstet Gynaecol Res. 2013; 39: 564– 571 [DOI] [PubMed] [Google Scholar]
- 20. Underwood M, Arbyn M, Parry-Smith W, et al. Accuracy of colposcopy-directed punch biopsies: a systematic review and meta-analysis. BJOG. 2012; 119: 1293– 1301 [DOI] [PubMed] [Google Scholar]
- 21. Sankaranarayanan R, Thara S, Sharma A, et al. Accuracy of conventional cytology: results from a multicentre screening study in India. J Med Screen. 2004; 11: 77– 84 [DOI] [PubMed] [Google Scholar]


