Abstract
Spermatogenesis, the process of spermatozoa production, is regulated by several endocrine factors, including testosterone, follicle stimulating hormone, luteinizing hormone and estradiol 17β. For spermatogenesis to reach completion, developing germ cells must traverse the seminiferous epithelium while remaining transiently attached to Sertoli cells. If germ cell adhesion were to be compromised for a period of time longer than usual, germ cells would slough the seminiferous epithelium and infertility would result. Presently, Sertoli-germ cell adhesion is known to be mediated largely by classical and desmosomal cadherins. More recent studies, however, have begun to expand long-standing concepts and to examine the roles of other proteins such as intercellular adhesion molecules. In this review, we focus on the biology of intercellular adhesion molecules in the mammalian testis, hoping that this information is useful in the design of future studies.
Keywords: ICAM, testis, Sertoli cell, germ cell, cell junction
1.1 Introduction
Spermatogenesis, the complex process of germ cell development, is comprised of a step-wise sequence of events that involves germ cell proliferation, maintenance and maturation. It takes place within the seminiferous epithelium, the functional unit of the testis, under the regulation of several endocrine factors, including testosterone (T), follicle stimulating hormone (FSH), luteinizing hormone (LH), and estradiol 17β [1–4]. The process begins with type A spermatogonia (diploid 2n), whose role is to either self-renew by mitosis or differentiate into type B spermatogonia. Type B spermatogonia lose all contact with the basement membrane and develop into primary spermatocytes (diploid 2n), namely preleptotene spermatocytes which traverse the blood-testis barrier (BTB) and enter the adluminal compartment of the seminiferous epithelium. This is followed by the formation of the short-lived secondary spermatocytes (haploid n) and then spermatids (haploid n). Spermatids undergo spermiogenesis, which is comprised of several steps that involve acrosome formation, nuclear changes, tail formation and maturation, and develop into spermatozoa that are subsequently released from the seminiferous epithelium during spermiation [5]. Spermiogenesis is comprised of 16, 19 and 12 steps in the mouse, rat and human, respectively.
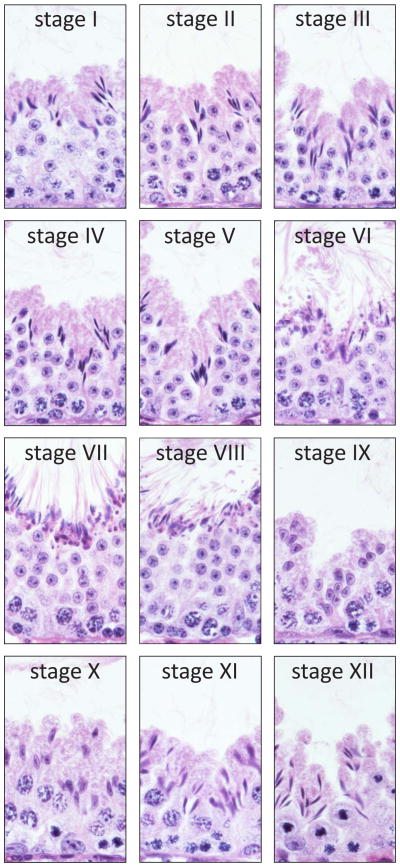
A typical cross-section of the adult mammalian (i.e., mouse, rat, human) testis shows several seminiferous tubules containing polarized Sertoli cells and differentiating germ cells with Leydig cells, which secrete T (essential for the completion of spermatogenesis) in the presence of the pituitary hormone LH, in the intertubular compartment. (Progenitor and immature Leydig cells also secrete androsterone and 5α-androstan-3α,17β-diol, respectively [6, 7]). Under normal physiological conditions, monocytes, macrophages, dendritic cells, T cells, natural killer cells and, in some species, mast cells (in rodent testes, mast cells are found in close vicinity of blood vessels or the tunica albuginea [8–10]) are also present in the interstitium. These cells together contribute to the spermatogenic process and to fertility. For example, activated macrophages can modulate Leydig cell steroidogenesis [11]. Quantitative histological studies using thick acrylic resin sections of the seminiferous epithelium in which cell nuclei were counted in consecutive focal planes revealed the number of germ cells to Sertoli cell to be approximately 35, 20 and 11 in mouse, rat and human, respectively [2], illustrating that the ability of Sertoli cells to nurse differentiating germ cells is enormous. Within the seminiferous epithelium, germ cells at different stages of development are grouped into highly organized associations, and these associations are defined as stages of the seminiferous epithelium [12, 13] (Fig. 1). Twelve, fourteen and six stages exist in the mouse, rat and human testis, respectively, and a cycle of the seminiferous epithelium is constituted by the completion of one sequence of stages (e.g., I through XII in the mouse). Different cellular events occur during different stages of the seminiferous epithelium. For example, spermiation occurs at late stage VIII in the mouse testis (Fig. 1), and it involves cyclic changes in mRNA and protein expression, coordinated interactions between different ultrastructures, and disruption of Sertoli cell–elongated spermatid adhesive contacts [5].
Fig. 1.
Stages of the seminiferous epithelial cycle in the adult mouse testis. A testis was embedded in paraffin, the tissue block was sectioned, and cross-sections were stained with hematoxylin and eosin. Stages of the seminiferous epithelial cycle are noted by Roman numerals. Each panel shows a portion of a seminiferous tubule at one of twelve spermatogenic stages in the mouse. Polarized Sertoli cells and differentiating germ cells are represented in each panel. Elongated spermatids line the lumenal edge in anticipation of spermiation at late stage VIII.
As mentioned previously, the seminiferous epithelium is divided into a basal and an adluminal compartment by the BTB (also known as the Sertoli cell barrier). Ultrastructurally, the BTB is constituted by different types of Sertoli cell junctions (i.e., tight junctions [TJs], basal ectoplasmic specializations [ESs], desmosomes and gap junctions [GJs]) that together contribute to the integrity of the seminiferous epithelium and to spermatogenesis. Throughout spermatogenesis, these junctions are restructured, especially during stages VIII–XI, in such a way that the homeostasis of the seminiferous epithelium and spermatogenesis are undisturbed [14]. Previous studies have shown BTB restructuring to involve changes in several proteins, including structural (e.g., claudin, occludin, cadherin), signaling (e.g., focal adhesion kinase), scaffolding (e.g., zonula occludens 1, catenin) and cytoskeletal (e.g., actin) components as well as to involve several regulatory pathways (e.g., p38 mitogen-activated protein kinase and focal adhesion kinase signaling cascades). More recent studies, however, have shown BTB function to involve other proteins such as drug transporters (e.g., P-glycoprotein) and cell adhesion molecules (e.g., intercellular adhesion molecule 1). The goal of this review is to discuss the role of intercellular adhesion molecule 1 (ICAM1), as well as the role of its biologically active soluble fragment, in spermatogenesis. Many new concepts are introduced in this review, and we hope that this information can form the basis of new studies in the future.
1.2 Structure and function of ICAM1
ICAMs are immunoglobulin (Ig)-like cell-cell and cell-extracellular matrix adhesion receptors expressed by leukocytes, macrophages, dendritic cells, fibroblasts, endothelial and epithelial cells, and some tumor cells. Five members (ICAMs1 to 5) have been identified thus far with each member showing distinct expression patterns [15–18]. Of these, ICAM1 (also defined as cluster of differentiation 54 [CD54]) is best studied with respect to the movement of leukocytes across the endothelium during inflammation [19]. ICAM1 is a highly-glycosylated single-membrane spanning protein possessing an N-terminal extracellular domain [consisting of domains (D) 1 to 5], a transmembrane domain and a C-terminal cytoplasmic domain, and seven ICAM1 isoforms resulting from alternative splicing have been identified thus far [20–23]. ICAM1 molecules are L-shaped, forming a sharp bend between D3 and D4 [24], whereas other crosslinking and ultrastructural studies have shown ICAM1 molecules to exist as non-covalently linked dimers via D4–D4 interactions [25–27]. It is also worth noting that the cytoplasmic domain contains a short stretch of sequence that appears to serve as a docking site for SRC homology 2 (SH2) domain-containing tyrosine phosphatases [28, 29]. Specifically, ICAM1 is defined as an adhesion protein because leukocyte adhesion to the endothelium was affected when ICAM1 function was blocked with specific monoclonal antibodies [30–32]. Moreover, ICAM1 binds several junction-associated, extracellular matrix and signaling proteins, including β2 integrin (e.g., lymphocyte function antigen-1 [LFA-1], macrophage-1 antigen [Mac-1]), JAM-A, occludin, zonula occludens-1 (ZO-1), cadherin, β-catenin, fibrinogen, mucin 1 (MUC1, a cell surface glycoprotein), hyaluronan (a large glycosaminoglycan and component of the extracellular matrix), Na+/H+ exchanger (NHE1), actin, ezrin, moesin, α-actinin, filamin, cortactin, β-tubulin, SRC, SRC homology domain 2-containing tyrosine phosphatase 2 (SHP-2) and proline-rich tyrosine kinase 2 (PYK2, a non-receptor protein tyrosine kinase of the focal adhesion kinase [FAK] family) [22, 29, 33–49]. Unlike most integrin-binding proteins, however, ICAM1 does not contain a RGD (Arg–Gly–Asp) motif to facilitate integrin binding [50]. In some instances of protein-protein binding (e.g., ICAM1 and fibrinogen), ICAM1 can be triggered to undergo phosphorylation on tyrosine residues 474 and 485 [51], illustrating the importance of ICAM1 post-translational modifications in cell function. ICAM1 is also known to facilitate outside-in and inside-out signaling [52, 53], although its ability to transduce signals resides within the cytoplasmic domain [54]. For example, transmigration of neutrophils was halted upon deletion of the cytoplasmic domain of ICAM1, illustrating that cell migration is triggered from within cells. Similar to other Ig-like proteins (e.g., JAM-A, CAR, nectin), ICAM1 is also known to function as a rhinovirus receptor in respiratory epithelial cells, which allows entry of human rhinoviruses (HRVs; e.g., causative virus of the common cold) and propagation of infection [55–57]. Interestingly, HRV uptake occurs via clathrin-dependent or -independent endocytosis, suggesting that ICAM1 may be internalized from the cell surface. On a final note relating to ICAM1 function, null mice were reported to be viable and fertile, but widespread defects in the inflammatory response (e.g., a decrease in inflammatory cell infiltration, a delay in wound healing) were observed when compared to wild-type littermates [58–63]. It is not known if other members of the ICAM protein family substitute for other cellular processes in null mice.
1.3 Regulation of ICAM1
1.3.a. Roles of cytokines and proteases in ICAM1 regulation
ICAM1 is either absent or present at low levels within different cells, but it can be upregulated by pro-inflammatory cytokines such as tumor necrosis factor α (TNFα), interleukins (IL-1, IL-11) and interferon-γ (IFN-γ) [64–74]. For instance, ICAM1 was upregulated by IL-11 in human chondrosarcoma cells via phosphoinositide 3-kinase (PI3-K) and AKT signaling pathways [75]. Other studies have shown cytokines to trigger proteolytic cleavage (i.e., ectodomain shedding) of the extracellular domain of ICAM1, thereby producing a biologically active soluble fragment (sICAM1) with the ability to affect cell function at multiple levels. First, sICAM1 binds integrin (i.e., LFA-1) and plays a role in cell migration [76–78]. Binding to integrins is mediated via the first and third Ig-like domains of sICAM1 [79, 80]. Second, sICAM1 may interfere with ICAM1–integrin interactions. Third, ectodomain shedding of ICAM1 may down-regulate ICAM1-mediated adhesion by rapidly decreasing its level at the cell surface, resulting in the formation of a negative feedback loop. Indeed, sICAM1 was found to reduce leukocyte adhesion to the endothelium during ischemia-reperfusion injury in mice [76]. In essence, ectodomain shedding can alter the biology and function of ICAM1. Ectodomain shedding of adhesion proteins (e.g., ICAM1, vascular cell adhesion molecule-1 [VCAM-1], platelet-endothelial cell adhesion molecule-1 [PECAM-1], selectin) involves matrix metalloproteases (MMPs) such as MMPs-9 and -14 [18, 81–86] and disintegrin metalloproteinases (ADAMs) such as ADAMs 10 and 17 (also known as tumor necrosis factor α-converting enzyme [TACE]) [87, 88]. For example, knockdown of MMP-9 by RNA interference (RNAi) in HT1080 cells (a human fibrosarcoma cell line) resulted in a decrease in sICAM1 [89], suggestive of a functional linkage between the two proteins. Indeed, previous studies have shown MMP-9 to co-immunoprecipitate with ICAM1 [84, 85]. In yet another study, knockdown of CD9, a tetraspanin, increased ADAM 17-mediated cleavage of ICAM1 and TNFα [90], suggesting that ICAM1-mediated adhesion may be regulated negatively by tetraspanins (note: ADAM17 cleaves precursor TNFα to yield bioactive TNFα [91]). Interestingly, these cytokine- and protease-mediated events involve upstream kinases. This is best exemplified by studies which showed extracellular signal regulated kinase (ERK) and p38 mitogen-activated protein kinase (p38 MAPK) to phosphorylate ADAM 17 at threonine residue 735, thereby upregulating ADAM 17-mediated ectodomain shedding of cytokine and growth factor receptors such as TNF receptor I and type I transforming growth factor (TGF) receptor [92–94]. ADAM 17 was found to associate with ERK as well [95]. The involvement of kinases such as SRC and PI3-K in ICAM1 cleavage has also been described [96]. Here, tyrosine residues 474 and 485 appeared to be critical when cleavage was shown to be drastically reduced following overexpression of either ICAM1 Y474A or ICAM1Y485A (and marginally reduced following overexpression of Y476A) [96]. Thus, in these ways, the levels of cytokines, growth factors, ICAM1 and sICAM1 can be controlled via specific kinases. At this point in our discussion, it is worth mentioning that proteases can further cleave the five Ig-like domains that constitute sICAM1, thereby producing several smaller biologically active fragments [97]. Robledo and colleagues also showed human leukocyte elastase and cathepsin G to cleave ICAM1 [97]. The remaining transmembrane and cytoplasmic domains may also be cleaved to provoke other cellular events. Additionally, the cytoplasmic domain may transit to the nucleus to activate transcription, similar to the cell adhesion protein CD44 [98, 99]. Finally, it is worth noting that a distinct mRNA encoding sICAM1 has been described to exist [100].
1.3.b. Role of stress in ICAM1 regulation
In addition to proinflammatory cytokines, many other forms of cell stress (e.g., reactive oxygen species [ROS], sheer stress, hypoxia, lipopolysaccharides) can upregulate ICAM1 and sICAM1. For instance, prolonged sheer stress was found to increase ICAM1, sICAM1 and MMPs-2 and -9 in endothelial cells isolated from human saphenous veins [84]. In other studies, overproduction of ROS in response to pro-inflammatory cytokines such as TNFα was found to result in oxidative stress and cellular damage, thereby increasing the level of ICAM1 [101–103]. Interestingly, the effects of ROS on ICAM1 expression were shown to be mediated by specific transcription factors such as nuclear factor-κB (NF-κB), activator protein-1 (AP-1, a heterodimer of c-Fos and c-Jun [104]) and E-twenty six (Ets) [105, 106], suggesting that de novo protein synthesis may be involved. The binding of TNFα to its receptors also activates NF-κB and AP-1, which consequently turns on the expression of genes implicated in inflammation such as ICAM1 [106]. Inactive NF-κB resides in the cytoplasm, sequestered by a family of proteins known as inhibitors of κB (IκB), and NF-κB can be activated rapidly because it does not require de novo protein synthesis. Upon phosphorylation and degradation of IκB, the NF-κB complex enters the nucleus where it turns on the expression of genes that contain DNA-binding sites for NF-κB, thereby resulting in a given cellular event. Transcriptional regulation of ICAM1 by CCAAT-enhancer-binding protein β (C/EBPβ) in spontaneously differentiated Caco-2 cells has also been reported [107]. Other molecules and possibly other mechanisms may be involved in the regulation of ICAM1 as well. For example, studies have shown kinases such as c-SRC, protein kinase C (PKC), PI3K/AKT and MAPKs to mediate the transcriptional regulation of Icam1 [101, 108]. When superoxide dismutase (SOD, an antioxidant enzyme) was overexpressed in TNFα-stimulated HAEC cells (a human aortic endothelial cell line) and the increase in Icam1 expression was blocked, the phosphorylation of c-Jun N-terminal kinase (JNK) and p38 was down-regulated [109]. Stress-activated MAPKs comprise a family of serine/threonine kinases that are activated by the step-wise phosphorylation of MAPK kinases kinases (MAP3Ks), MAP kinases (MAP2Ks) and MAPKs [110, 111]. In mammals, three major MAPK pathways are known to exist: (i) the ERK 1/2 pathway, (ii) the c-Jun N-terminal kinase (JNK) pathway and (iii) the p38 pathway. Of these, JNK (JNK-1, -2, -3) and p38 MAPK (p38α, β, γ, δ) are two MAPKs that can transit to the nucleus to phosphorylate transcription factors or other accessory proteins that may be involved in critical aspects of cell function. In another study, TNFα-treatment of endothelial cells resulted in ICAM1 ligation and SRC activation [112]. This was also accompanied by the activation of p38 MAPK, destabilization of microtubules, phosphorylation of ezrin, radixin and moesin (ERM), cytoskeletal changes and disruption of the endothelial permeability barrier [112–115]. ERM proteins are known to function as a scaffold by linking actin microfilaments to the plasma membrane as well as by connecting transmembrane receptors to downstream signaling proteins [116, 117]. Thus, it is possible that ERM proteins facilitate the clustering of ICAM1 at the surface of TNFα-treated cells. At this point, it is not entirely clear how TNFα can regulate microtubule dynamics, and if this can contribute to ICAM1 function. Nevertheless, these results illustrate that transcription factors and kinases are important regulators of ICAM1 function.
1.4 ICAM1 signaling
As discussed previously, ICAM1 is known to participate in outside-in and inside-out signaling. One of the best examples of outside-in signaling is the cascade of cellular events that is triggered by the binding of TNFα to its receptors. Several studies have shown TNFα to upregulate the expression and to induce clustering of ICAM1, which results in the recruitment of signaling proteins to the plasma membrane and in the activation of several cascades that regulate cell adhesion and movement either positively or negatively via inside-out signaling [118–121]. For instance, TNFα was shown to disrupt the integrity of junctions by inducing cytoskeletal rearrangement via myosin light chain kinase (MLCK)- and Rho kinase (ROCK)-dependent mechanisms [108, 122, 123]. MLCK is a ubiquitously expressed Ca2+/calmodulin-activated Ser/Thr kinase that phosphorylates the regulatory myosin light chain (MLC) in response to TNFα, thereby resulting in actin stress fiber formation, actomyosin contraction and junction dynamics [124, 125]. In addition to increasing kinase activity, TNFα also activated MLCK by increasing transcription [126]. ICAM1 clustering was also found to activate RhoA, another important regulator of actomyosin contraction and an activator of ROCK (a Ser/Thr kinase and downstream effector of RhoA) [127]. This resulted in the phosphorylation and in the inhibition of myosin light chain phosphatase (MLCP), as well as in the phosphorylation of MLC, which together facilitated actomyosin contraction [128, 129]. It is worth emphasizing that two distinct pools of actin stress fibers exist in non-muscle cells: (i) central, parallel stress fibers that run across cells and (ii) cortical stress fibers that are found at the periphery of cells [130, 131]. Central stress fiber dynamics are regulated by ROCK, and as discussed previously, their formation in cells is indicative of junction instability/disassembly. Cortical stress fibers, on the other hand, are regulated by MLCK, and they stabilize junctions [132, 133]. These observations reveal that there is a delicate balance between MLCK and ROCK. Furthermore, ICAM1 clustering was inhibited by both a dominant negative RhoA mutant (N19RhoA) and C3 transferase [an exoenzyme produced by Clostridium botulinum and an inhibitor of RhoA activity [134]] [121], demonstrating that a disruption of RhoA function downstream can affect ICAM1 dynamics at the cell surface. The cellular endpoint of these biochemical changes was the generation of an inward force that drives cadherin molecules residing on adjacent plasma membranes to dissociate from each other. This was followed by the internalization of junctional proteins and by the disassembly of junctions [135–137]. Tyrosine phosphorylation of junctional proteins following ICAM1 crosslinking has also been shown to disrupt junctions [120, 138–142]. For instance, clustering of ICAM1 on endothelial cells prompted SRC- and PYK2-mediated phosphorylation of cadherin on tyrosine residues 658 and 731 [142], thereby prohibiting the binding of p120 catenin and β-catenin to cadherin and disrupting adherens junctions [143]. Kinase activation following ICAM1 clustering may also result in the phosphorylation of other junction-associated proteins (e.g., paxillin, cortactin or ERM proteins), which may affect protein localization and/or protein-protein interactions, ultimately resulting in cytoskeletal rearrangement and junction dysfunction. Indeed, TNFα-mediated ICAM1 clustering triggered the activation of SRC in endothelial cells, which was accompanied by the phosphorylation of ezrin – a key organizer of cortical stress fibers –on tyrosine residue 146 [112].
ICAM1 engagement and downstream signaling can also be triggered experimentally by other ways, including treatment of cells with specific monoclonal antibodies, interaction of ICAM1 with integrin or other extracellular matrix proteins (e.g., collagen, fibronectin, hyaluronan) and addition of activated T-lymphocytes to previously cultured cells. For instance, many studies have sought to better understand the function of ICAM1 indirectly through the induction of β1 integrin clustering, which can also be induced by specific monoclonal antibodies, similar to ICAM1 [144]. Results emanating from these and other similar studies, however, have proved to be somewhat inconsistent across different cell types. In primary osteoblasts, crosslinking of β1 integrin was found to upregulate ICAM1 expression via a mechanism involving kinases [145]. When cells were treated with herbimycin A or genistein (tyrosine kinase inhibitors) or transfected with dominant-negative FAK mutants, the increase in ICAM1 was abolished [145]. In A904L cells (a human lung cancer cell line), on the other hand, crosslinking of β1 integrin was found to downregulate ICAM1 expression (but to increase sICAM1) and to reduce the adhesion of lung cancer cells to T cells [146]. Unexpectedly, these cellular events were also inhibited by tyrosine kinase inhibitors as well as by transfection of dominant and negative FAK mutants [146]. One of the dominant-negative FAK mutants used in both studies was FAK-related non-kinase (FRNK), a protein encoding the C-terminal non-catalytic domain of FAK [147, 148]. FRNK is believed to block FAK function by either replacing FAK within focal adhesions or by displacing critical FAK binding partners, thereby interrupting cell migration [147, 149–152]. FRNK can also inhibit the function of PYK2 [153, 154], a non-receptor protein tyrosine kinase of the FAK family known to be activated by integrin clustering as well as by integrin–ICAM1 interactions [155–157]. PYK2- and FAK-mediated integrin signaling was also found to result in the downstream activation of MAP kinase [158]. Taken collectively, these results substantiate the notion that phosphorylation of key proteins by kinases is essential for ICAM1-mediated cell adhesion and cell movement. The interaction of ICAM1 with integrin also involves other regulatory proteins, many of which are directly involved in cytoskeleton remodeling. For example, the binding of ICAM1 to integrin resulted in the redistribution of talin [159], a ubiquitous actin-binding protein [160, 161]. Talin is also known to bind the integrin β tail, which results in separation of α and β subunits (a hallmark of integrin activation) and in structural changes that increase its ligand binding affinity. Other proteins can also bind the integrin β subunit such as filamin A, which has been reported to associate with [41] and to compete with talin for binding to ICAM1 [162, 163], and receptor for activated C kinase 1 (RACK1, an adaptor protein), which interacts with kindlin 3 [164–166]. Kindlins (kindlin-1, -2 and -3) are cytoskeletal adaptor proteins known to function as co-activators of integrins, together with talin, and to regulate outside-in and inside-out signaling [167–169]. Interestingly, RACK1–kindlin-3 interactions were found to be mediated by ICAM1 [166]. The binding of ICAM1 to integrin also resulted in the recruitment of the ARP2/3 complex by talin [159]. The ARP2/3 complex, together with cortactin, is involved in the formation of branched actin filaments, which is crucial for cell migration [170–172], indicating that an important relationship exists between actin polymerization and cell movement. Taken collectively, these results clearly illustrate that ICAM1 engagement triggers a plethora of signaling events whose end result is cell movement.
1.5 ICAM1 and sICAM1 function in the testis
1.5.a. Role of ICAM1 in the testis
Presently, there is little information on the role of ICAM1 in mammalian spermatogenesis. For instance, early studies by flow cytometry showed the presence of ICAM1 on the surface of mouse Sertoli cells [173, 174]; however, the significance of this important finding probably went unknown to investigators at the time because cell junctions in the testis were largely uncharacterized structures in the early 1990s. In recent studies, we confirmed and expanded these initial findings by illustrating ICAM1 expression in both Sertoli and germ cells [49, 175]. Immunolocalization studies revealed ICAM1 to be present in all stages of seminiferous epithelial cycle, with ICAM1 staining surrounding the heads of elongating/elongated spermatids at stages IX–XIII of the seminiferous epithelial cycle where it likely participates in cell adhesion. At the BTB, ICAM1 staining was highest at stage VIII, coinciding with an early phase of BTB restructuring. When Sertoli cells were cultured under conditions that permitted for the assembly of a functional barrier, ICAM1 was found to colocalize partially with occludin and N-cadherin, both constituents of the BTB in vivo [49], indicating that ICAM1 is important for BTB function. The significance of ICAM1 in BTB dynamics was addressed in a subsequent experiment when ICAM1 was transiently overexpressed in Sertoli cells and barrier function was assessed by daily transepithelial electrical resistance readings [49]. Interestingly, ICAM1 overexpression was found to significantly enhance Sertoli cell barrier function, suggesting that ICAM1 may be involved in the assembly of the ‘new’ BTB below preleptotene spermatocytes at stage VIII (Fig. 2). The mechanism behind ICAM’s ability to strengthen BTB function is largely unknown, but it likely involves the recruitment of proteins to the plasma membrane and the activation of signaling cascades that regulate junction dynamics. As discussed previously, germ cells also expressed ICAM1; however, it is not known if preleptotene/leptotene spermatocytes express ICAM1. It would also be important to determine the precise role of preleptotene/leptotene spermatocytes in BTB dynamics because this information would improve our understanding of ICAM1 function. For instance, what role, if any, do germ cells have in BTB restructuring? Is ICAM1 expression upregulated in preleptotene/leptotene spermatocytes as they traverse the barrier? Which signaling cascades are directly linked with ICAM1 function at the BTB? In another report, coxsackie and adenovirus receptor (CAR), another Ig-like cell-cell adhesion receptor, was upregulated in migrating germ cells during BTB passage in the mouse [176], suggesting that ICAM1 may share a similar mechanism. Alternatively, cell movement may require synchronized cues from both proteins. At this point, additional studies are needed to identify bona fide ICAM1 binding partners that might facilitate transient adhesive interactions between Sertoli cells and preleptotene/leptotene spermatocytes. While ICAM1 was found to associate with occludin, ZO-1, N-cadherin and β-catenin (but surprisingly not with CAR) when testis or seminiferous tubule lysates were used for co-immunoprecipitation, the identities of the cell types mediating these interactions are not known. In other words, it is not clear if these interactions occurred on the same cell or across two different cell types in the seminiferous epithelium. Nevertheless, these results demonstrate ICAM1 to be important in BTB function.
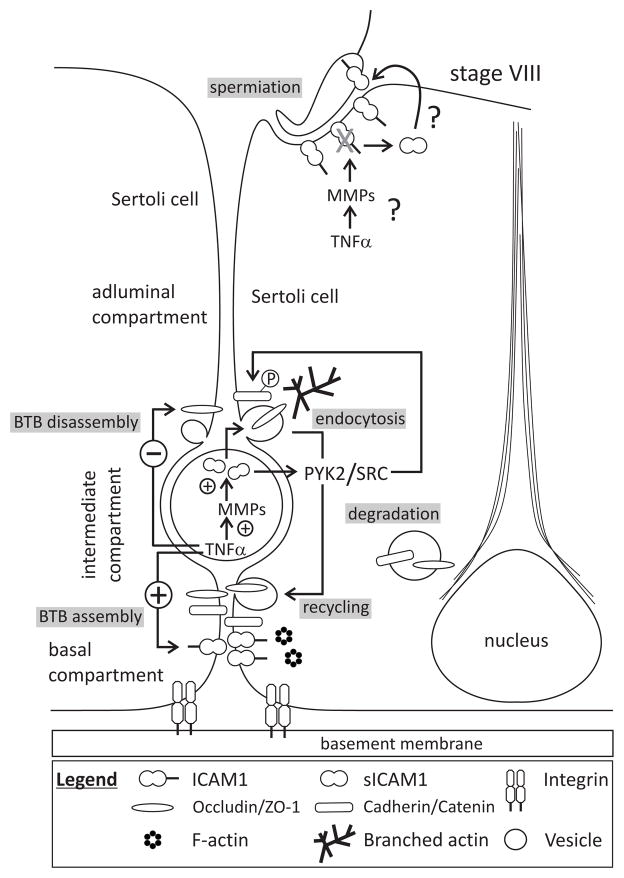
Fig. 2.
Role of ICAM1 and sICAM1 in the mammalian testis – a hypothetical model. This drawing of a stage VIII seminiferous tubule highlights the roles of ICAM1 and sICAM1 in BTB restructuring and spermiation. The BTB divides the seminiferous epithelium into a basal and an adluminal compartment, with a preleptotene/leptotene spermatocyte sequestered within a transient intermediate compartment created by two barriers. As this spermatocyte initiates entry into the adluminal compartment, the BTB above it is disassembled. BTB constituent proteins (i.e., occludin, N-cadherin) are endocytosed and either degraded or recycled to below spermatocytes to create a new barrier. In this way, the BTB is never completely disassembled nor assembled during germ cell migration. These cellular events are partly mediated by proinflammatory cytokines such as TNFα and IL-1α. TNFα can also up- or downregulate other proteins (i.e., ICAM1, MMPs) to bring about BTB assembly or disassembly directly or indirectly. For example, MMPs can cleave ICAM1, thereby generating a biologically active soluble fragment (i.e., sICAM1) which mediates BTB disassembly and possibly spermiation via changes in the actin cytoskeleton. Non-receptor protein tyrosine kinases such as PYK2 and SRC are also involved in these events. For the sake of simplicity, many elements such as the different types of cells junctions at the BTB were not highlighted in this drawing.
1.5.b. Role of sICAM1 in the testis
The role of the biologically active soluble fragment of ICAM1, sICAM1, in BTB function was also investigated in complementary experiments. When a cDNA encoding the entire extracellular domain of ICAM1 (i.e., D1–D5) was overexpressed in Sertoli cells in vitro or in vivo, Sertoli cell barrier/BTB function was disrupted, illustrating that ICAM1 and sICAM1 have opposing actions on barrier dynamics (Fig. 2). These results are in agreement with at least one report in another in vitro system which showed an increase in sICAM1 to associate with a decrease in cell adhesion [146]. Barrier disruption was mediated partly by non-receptor protein tyrosine kinases (i.e., PYK2/p-PYK2-Y402 and SRC/p-SRC-Y530) whose levels decreased following sICAM1 overexpression. Interestingly, no changes were observed in the levels of FAK, p-FAK-Y397 and p-FAK-Y407 following sICAM1 overexpression, and while FAK and PYK2 are related in sequence and structure, they are regulated differently in different cellular contexts [157]. In general, cell migration is impaired by an inhibition of FAK or PYK2 signaling; thus, a disruption in PYK2 signaling in Sertoli cells by sICAM overexpression likely affects germ cell movement across the BTB, although further studies are needed to test this hypothesis. Interestingly, macrophages isolated from Pyk2−/− mice failed to polarize [177]. A decrease in PYK2/p-PYK2-Y402 following sICAM1 overexpression in our system might result in similar effects. Changes in the localization of N-cadherin, γ-catenin and/or connexin 43 were also noted in Sertoli cells in vitro as well as at the BTB in vivo; however, it is not known whether these changes in protein localization were the end-result of changes in protein phosphorylation, proteolysis or de novo protein synthesis. γ-Catenin, the best-studied armadillo protein, tethers the intermediate filament network to desmosomes. It also plays an important role in cell signaling events [178, 179]. In an earlier study, we reported crosstalk between desmosomes and tight junctions at the Sertoli cell barrier following simultaneous knockdown of desmoglein-2 and desmocollin-3 by RNA interference [180]. Thus, a disruption in desmosome function via structural and/or signaling proteins can inadvertently affect tight junction dynamics. The downregulation in connexin 43 by sICAM1 overexpression in vitro and in vivo may also have affected the level of another armadillo protein, plakophilin-2. Firstly, connexin 43 binds plakophilin 2 in seminiferous tubule lysates, and secondly, simultaneous knockdown of connexin 43 and plakophilin 2 by RNA interference increased the permeability of the Sertoli cell barrier [181], indicating that sICAM1 perturbed BTB integrity in part by gap junctions and desmosomes. Taken collectively, these results reveal that sICAM1-mediated BTB restructuring involves a wide array of structural and signaling proteins found within distinct modules and that crosstalk among different modules results in germ cell migration across the barrier.
Overexpression of sICAM1 in vivo resulted in the sloughing of spermatocytes and round spermatids, and in the misorientation of elongated spermatids. These are very interesting findings because elongating/elongated spermatids generally deplete the seminiferous epithelium first after testis assault by environmental toxicants (e.g., cadmium) or other compounds (e.g., adjudin, CDB-4022) [182–186], revealing that the apical ectoplasmic specialization is especially susceptible to damage. At this point in our investigation, it is not entirely clear how sICAM1 affects adhesion between Sertoli cells and spermatocytes as well as between Sertoli cells and round spermatids. sICAM1 may have interfered with ICAM1 interactions between Sertoli and germ cells, leading to the sloughing of spermatocytes and round spermatids. Alternatively, sICAM1 may have disrupted desmosome function either directly or indirectly because this is the only junction type to mediate adhesion between Sertoli cells and pre-step 8 germ cells (i.e., spermatocytes and round spermatids). Although no changes in desmoglein-2 and desmocollin-3 were observed following sICAM1 overexpression by immunoblotting and/or immunohistochemistry, sICAM1 perturbed BTB integrity by affecting signaling and/or scaffolding proteins downstream (see previous discussion). Additional studies are needed to determine whether ICAM1 binds desmosomal proteins and whether there is crosstalk between these two adhesion modules at the BTB.
1.6 ICAM1 and disease
As discussed previously, ICAM1 has at least one additional function that is outside of its dual roles in cell adhesion and cell signaling. ICAM1 also functions as a receptor for human rhinoviruses (HRVs) in respiratory epithelial cells, thereby allowing viron entry and disease propagation [187–189]. When human tracheal epithelial cells were treated with levofloxacin (a fluoroquinolone antibiotic; brand name, LEVAQUIN®) and HRV infection was inhibited, a reduction in ICAM1 expression was noted [190]. These findings substantiate the assertion that ICAM1 serves as a port of entry for HRVs. ICAM1 also associated with Guillain-Barre syndrome (GBS) [191], an autoimmune disorder that attacks peripheral nerves resulting in weakness in the hands and feet, and in paralysis. GBS appears to be triggered by bacterial or viral infection. When peripheral blood mononuclear leukocytes from GBS patients were cocultured with primary human endoneurial endothelial cells derived from sciatic nerves, thereby creating an in vitro blood-nerve barrier, and treated with a function-neutralizing monoclonal ICAM1 antibody, leukocyte trafficking was reduced [191]. These results are interesting because an inhibition of leukocyte trafficking may abrogate neuro-inflammation in in vivo models.
The upregulation of ICAM1 or the presence of sICAM1 in tissues and/or biological fluids is also a hallmark of inflammatory and metabolic diseases such as coronary heart disease, neurodegeneration, tumorigenesis and diabetes [18, 77, 192–197]. For instance, the level of sICAM1 was higher in individuals having Alzheimer’s disease [194], a chronically progressive neurodegenerative disease accompanied by chronic inflammation, deposition of amyloid-β plaques, hyper-phosphorylation and aggregation of tau, and degeneration of neurons [198, 199]. Others have reported elevated levels of sICAM1 in systemic lupus erythematosus (SLE) [200] and human immunodeficiency virus (HIV) [201, 202] patients, suggesting that sICAM1 may be useful biomarker of chronic disease. Moreover, an increase in ICAM1 was noted during hypoxic-ischemic brain damage [203], suggesting that it, too, may be a useful biomarker. Since ICAM1 is crucial for normal immune function, its aberrant regulation may underlie the clinical manifestations of a wide array of diseases. For example, a deviation from its normal expression pattern may disrupt cell-cell interactions, trigger epithelial-mesenchymal transition and result in tumorigenesis. In a more recent investigation, ICAM1 was upregulated in several lung cancer cell lines by cannabidiol [204], a drug known to inhibit tumor angiogenesis and metastasis, cancer cell apoptosis and inflammation [205–207]. Interestingly, the increase in ICAM1 associated with an activation of p42/44 MAPK signaling and an induction of TIMP-1, resulting in an inhibition of invasion. While these results suggest that ICAM1 induction may suppress tumorigenesis, many studies have reported conflicting results. Regardless, a better understanding of the biology of ICAM1/sICAM1 may be key to understanding the pathology of several chronic diseases.
1.7 Future perspectives and concluding remarks
ICAMs are important cell adhesion proteins involved in cell movement. Based on our results, we conclude that ICAM1 and sICAM1 have opposing roles in the seminiferous epithelium, with ICAM1 and sICAM1 improving and worsening BTB function, respectively. These opposing roles are critical for the movement of germ cells across the BTB during spermatogenesis. Additional studies are needed to address the many questions that remain unanswered. For example, is ICAM1 simply guiding or actively mediating the migration of preleptotene/leptotene spermatocytes across the BTB? Are cytokines such as TNFα, TGF-β and IL-1 involved in these events? Does ICAM1 interact with cytokine-activated germ cells, and does this favor cell movement? Is SRC- or PYK2-mediated protein phosphorylation downstream of ICAM1 signaling critical for germ cell movement? Does ICAM1 interact with active MMP-9? Is ICAM1 under hormonal control? Is ICAM1 endocytosed via a caveolin-dependent mechanism? Which Sertoli and/or germ cell proteins does ICAM1 bind to? Does ICAM1 function require cooperation from vimentin- and/or microtubule-based cytoskeletons as well? Lastly, is ICAM1 function in the testis regulated by basement membrane constituents such as laminin 10 which can regulate leukocyte transmigration via its cleavage product [208]? Interestingly, biologically active fragments of laminin γ3 produced by proteolytic cleavage, possibly by MMP-2 [209], at the apical ectoplasmic specialization trigger a cascade of signaling events that result in BTB restructuring, suggesting that a similar mechanism might exist in the testis. However, cleavage of laminin γ3 by MMP-2 in the context of the testis has never been shown.
Studies are also needed to determine the precise roles of the other members of the ICAM protein family such as ICAM2 in the testis. In agreement with results from studies that used specific monoclonal antibodies to block ICAM1 function, loss of ICAM2 disrupted leukocyte transmigration in vitro and in vivo [32, 210, 211], illustrating that both proteins have a similar function in other biological systems. By immunohistochemistry, ICAM2 localized largely to Sertoli-germ cell contact sites, associating with the apical ectoplasmic specialization (Fig. 3) [212]. The presence of ICAM2 at the apical ectoplasmic specialization was substantiated by colocalization and/or co-immunoprecipitation experiments when ICAM2 was found to associate with apical ectoplasmic specialization constituent proteins such as β1-integrin, nectin 3 and actin [212]. Interestingly, ICAM2 did not localize to the BTB (Fig. 3). From our results, as well as from the results of others, we conclude that ICAM2 has important roles in cell adhesion and movement; however, the precise role of ICAM2 in spermatogenesis is not yet known. Studies in the future should determine whether ICAM2 knockdown destabilizes the Sertoli–germ cell ectoplasmic specialization and identify the cytoskeletal signaling cascades involved. Proteins such as Rho A or B, which mediate changes in cell shape, contractility and motility, or filamin A or B, which crosslink actin into a three-dimensional lattice, are likely to be involved. It is also important to determine whether other members of the ICAM protein family can substitute for the loss of ICAM1.
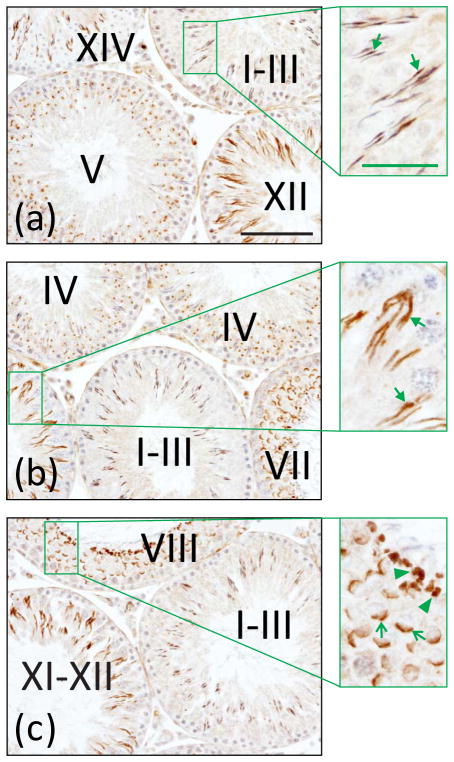
Fig. 3.
Localization of ICAM2 in the adult rat testis. A testis was embedded in paraffin, the tissue block was sectioned, and cross-sections were processed for immunohistochemistry using a monospecific ICAM2 antibody. ICAM2 localized largely to elongating and elongated spermatids [see
 in (a) and (b), insets] in all stages of the seminiferous epithelial cycle (noted by Roman numerals). Elongated spermatids in stages I–VIII were weakly immunoreactive for ICAM2. ICAM2 also localized to postacrosomal vesicles/acrosomes [see
in (a) and (b), insets] in all stages of the seminiferous epithelial cycle (noted by Roman numerals). Elongated spermatids in stages I–VIII were weakly immunoreactive for ICAM2. ICAM2 also localized to postacrosomal vesicles/acrosomes [see
 in (c), inset] and residual bodies [see ◀ in (c), inset]. Bar in (a), 100 μm; bar in (a), inset, 35 μm. These results are in agreement with a previously published report [212].
in (c), inset] and residual bodies [see ◀ in (c), inset]. Bar in (a), 100 μm; bar in (a), inset, 35 μm. These results are in agreement with a previously published report [212].
Several recent studies have showed microRNAs (miRNAs), including miRNAs-17-3p, -221/-222 and -339, to regulate ICAM1 expression in different cells cultured either in the presence or absence of cytokines (i.e., TNFα, INFγ) [213–215]. miRNAs are short, single-stranded fragments of RNA (i.e., 18–24 nucleotides) that bind mRNAs, resulting in translational repression and/or mRNA destabilization/cleavage [216, 217]. Approximately half of all identified miRNAs bind to the 3′-untranslated region (UTR) of mRNAs with partial complementarity; however, the remaining miRNAs bind to the 5′-UTR or open reading frame of target mRNAs [218]. At the writing of this article, miRBase (http://www.mirbase.org) was found to contain approximately 1,000 human miRNAs, with human miRNAs regulating approximately 60% of mammalian genes [219]. While studies have generally shown higher miRNA expression in germ cells than in Sertoli cells with developmental and stage-specific regulation throughout spermatogenesis [220–222], miRNA-mediated regulation of ICAM1 expression in the testis has yet to be explored. Because miRNA-mediated regulation of ICAM1 expression likely plays a major role in BTB dynamics, studies should focus on the identification of miRNAs that regulate ICAM1 expression and on the functional manipulation of these miRNAs. These studies may provide important insights on how BTB integrity can be modulated via ICAM1/sICAM1 and may contribute to the development of new approaches for male fertility control in mammals.
Highlights.
Intercellular adhesion molecules (ICAMs) are immunoglobulin-like cell-cell and cell-extracellular matrix adhesion receptors expressed by a variety of cells, including leukocytes, macrophages, fibroblasts, endothelial and epithelial cells (e.g., Sertoli cells), and germ cells. Five ICAMs have been identified thus far with each member showing distinct expression patterns.
In the rat testis, ICAM1 is present at the blood-testis barrier, colocalizing with blood-testis barrier constituent proteins (i.e., occludin, zonula occludens-1, N-cadherin and β-catenin).
In Sertoli cells, ICAM1 strengthens the tight junction permeability barrier upon overexpression, illustrating that ICAM1 contributes to blood-testis barrier function.
By contrast, overexpression of its biologically active soluble fragment, sICAM, compromises the integrity of the Sertoli cell barrier. These changes are mediated in part by non-receptor protein tyrosine kinases.
Acknowledgments
This work was supported in part by NICHD, NIH (R03 HD061401 to D.D.M.).
Abbreviations
- BTB
blood-testis barrier
- ES
ectoplasmic specialization
- GJ
gap junction
- ICAM
intercellular adhesion molecule
- TJ
tight junction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Kretser DM, Kerr JB. The cytology of the testis. In: Knobil E, Neill JB, Ewing LL, Greenwald GS, Markert CL, Pfaff DW, editors. The Physiology of Reproduction. New York: Raven Press; 1988. pp. 837–932. [Google Scholar]
- 2.Kerr JB, Loveland KL, O’Bryan MK, de Kretser DM. Cytology of the testis and intrinsic control mechanisms. In: Neill JD, editor. Knobil and Neill’s Physiology of Reproduction. New York: Elsevier; 2006. pp. 827–947. [Google Scholar]
- 3.Carreau S, Hess RA. Oestrogens and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1517–35. doi: 10.1098/rstb.2009.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Donnell L, Meachem SJ, Stanton PG, McLachlan RI. Endocrine regulation of spermatogenesis. In: Neill JD, editor. Knobil and Neill’s Physiology of Reproduction. New York: Elsevier; 2006. pp. 1017–69. [Google Scholar]
- 5.O’Donnell L, Nicholls PK, O’Bryan MK, McLachlan RI, Stanton PG. Spermiation: the process of sperm release. Spermatogenesis. 2011;1:14–35. doi: 10.4161/spmg.1.1.14525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moger WH. Production of testosterone, 5α-androstane-3α, 17β-diol and androsterone by dispersed testicular interstitial cells and whole testes in vitro. J Endocrinol. 1979;80:321–32. doi: 10.1677/joe.0.0800321. [DOI] [PubMed] [Google Scholar]
- 7.Akingbemi BT, Ge RS, Hardy MP. Leydig cells. In: Knobil E, Neill JD, editors. Encyclopedia of Reproduction. New York: Academic Press; 1999. pp. 1021–33. [Google Scholar]
- 8.Kerr JB. Ultrastructure of the seminiferous epithelium and intertubular tissue of the human testis. J Electron Microsc Tech. 1991;19:215–40. doi: 10.1002/jemt.1060190208. [DOI] [PubMed] [Google Scholar]
- 9.Anton F, Morales C, Aguilar R, Bellido C, Aguilar E, Gaytan F. A comparative study of mast cells and eosinophil leukocytes in the mammalian testis. Zentralbl Veterinarmed A. 1998;45:209–18. doi: 10.1111/j.1439-0442.1998.tb00819.x. [DOI] [PubMed] [Google Scholar]
- 10.Rival C, Lustig L, Iosub R, Guazzone VA, Schneider E, Meinhardt A, et al. Identification of a dendritic cell population in normal testis and in chronically inflammed testis of rats with autoimmune orchitis. Cell Tissue Res. 2006;324:311–8. doi: 10.1007/s00441-005-0129-5. [DOI] [PubMed] [Google Scholar]
- 11.Hales DB. Testicular macrophage modulation of Leydig cell steroidogenesis. J Reprod Immunol. 2002;57:3–18. doi: 10.1016/s0165-0378(02)00020-7. [DOI] [PubMed] [Google Scholar]
- 12.LeBlond CP, Clermont Y. Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann NY Acad Sci. 1952;55:548–73. doi: 10.1111/j.1749-6632.1952.tb26576.x. [DOI] [PubMed] [Google Scholar]
- 13.Hess RA, Renato de Franca L. Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol. 2008;636:1–15. doi: 10.1007/978-0-387-09597-4_1. [DOI] [PubMed] [Google Scholar]
- 14.Cheng CY, Mruk DD. The blood-testis barrier and its implication in male contraception. Pharmacol Rev. 2012;64:16–64. doi: 10.1124/pr.110.002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tian L, Yoshihara Y, Mizuno T, Mori K, Gahmberg CG. The neuronal glycoprotein telencephalin is a cellular ligand for the CD11a/CD18 leukocyte integrin. J Immunol. 1997;158:928–36. [PubMed] [Google Scholar]
- 16.Hermand P, Gane P, Huet M, Jallu V, Kaplan C, Sonneborn HH, et al. Red cell ICAM-4 is a novel ligand for platelet-activated alpha IIbbeta 3 integrin. J Biol Chem. 2003;278:4892–8. doi: 10.1074/jbc.M211282200. [DOI] [PubMed] [Google Scholar]
- 17.Toivanen A, Ihanus E, Mattila M, Lutz HU, Gahmberg CG. Importance of molecular studies on major blood groups–intercellular adhesion molecule-4, a blood group antigen involved in multiple cellular interactions. Biochim Biophys Acta. 2008;1780:456–66. doi: 10.1016/j.bbagen.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Lawson C, Wolf S. ICAM-1 signaling in endothelial cells. Pharmacol Rep. 2009;61:22–32. doi: 10.1016/s1734-1140(09)70004-0. [DOI] [PubMed] [Google Scholar]
- 19.Nourshargh S, Hordijk PL, Sixt M. Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nat Rev Mol Cell Biol. 2010;11:366–78. doi: 10.1038/nrm2889. [DOI] [PubMed] [Google Scholar]
- 20.Diamond MS, Staunton DE, Marlin SD, Springer TA. Binding of the integrin Mac-1 (CD11b/CD18) to the third immunoglobulin-like domain of ICAM-1 (CD54) and its regulation by glycosylation. Cell. 1991;65:961–71. doi: 10.1016/0092-8674(91)90548-d. [DOI] [PubMed] [Google Scholar]
- 21.Languino LR, Duperray A, Joganic KJ, Fornaro M, Thornton GB, Altieri DC. Regulation of leukocyte-endothelium interaction and leukocyte transendothelial migration by intercellular adhesion molecule-1-fibrinogen recognition. Proc Natl Acad Sci USA. 1995;92:1505–9. doi: 10.1073/pnas.92.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCourt PAG, Ek B, Forsberg N, Gustafson S. Intercellular adhesion molecule-1 is a cell surface receptor for hyaluronan. J Biol Chem. 1994;269:30081–4. [PubMed] [Google Scholar]
- 23.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 24.Kirchhausen T, Staunton DE, Springer TA. Location of the domains of ICAM-1 by immunolabeling and single-molecule electron microscopy. J Leukoc Biol. 1993;53:342–6. doi: 10.1002/jlb.53.3.342. [DOI] [PubMed] [Google Scholar]
- 25.Jun CD, Carman CV, Redick SD, Shimaoka M, Erickson HP, Springer TA. Ultrastructure and function of dimeric, soluble intercellular adhesion molecule-1 (ICAM-1) J Biol Chem. 2001;276:29019–27. doi: 10.1074/jbc.M103394200. [DOI] [PubMed] [Google Scholar]
- 26.Reilly PL, Woska JR, Jeanfavre DD, McNally E, Rothlein R, Bormann BJ. The native structure of intercellular adhesion molecule-1 (ICAM-1) is a dimer. Correlation with binding to LFA-1. J Immunol. 1995;155:529–32. [PubMed] [Google Scholar]
- 27.Yang Y, Jun CD, Liu JH, Zhang R, Joachimiak A, Springer TA, et al. Structural basis for dimerization of ICAM-1 on the cell surface. Mol Cell. 2004;14:269–76. doi: 10.1016/s1097-2765(04)00204-7. [DOI] [PubMed] [Google Scholar]
- 28.Neel BG, Gu H, Pao L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28:284–93. doi: 10.1016/S0968-0004(03)00091-4. [DOI] [PubMed] [Google Scholar]
- 29.Pluskota E, Chen Y, D’Souza SE. Src homology domain 2-containing tyrosine phosphatase 2 associates with intercellular adhesion molecule-1 to regulate cell survival. J Biol Chem. 2000;275:30029–36. doi: 10.1074/jbc.M000240200. [DOI] [PubMed] [Google Scholar]
- 30.Sadowska AM, van Overveld FJ, Luyten C, Germonpre P, De Backer WA. Use of ICAM-1 antibodies and antisense oligonucleotides to inhibit transmigration of neutrophils. Inflamm Res. 2004;53:143–9. doi: 10.1007/s00011-003-1237-x. [DOI] [PubMed] [Google Scholar]
- 31.Burns RC, Rivera-Nieves J, Moskaluk CA, Matsumoto S, Cominelli F, Ley K. Antibody blockade of ICAM-1 and VCAM-1 ameliorates inflammation in the SAMP-1/Yit adoptive transfer model of Crohn’s disease in mice. Gastroenterology. 2001;121:1428–36. doi: 10.1053/gast.2001.29568. [DOI] [PubMed] [Google Scholar]
- 32.Porter JC, Hall A. Epithelial ICAM-1 and ICAM-2 regulate the egression of human T cells across the bronchial epithelium. FASEB J. 2009;23:492–502. doi: 10.1096/fj.08-115899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molock KE, Lillehoj EP. Biochemical interactions among intercellular adhesion molecules expressed by airway epithelial cells. Biochem Biophys Res Commun. 2006;343:513–9. doi: 10.1016/j.bbrc.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Heiska L, Alfthan K, Gronholm M, Vilja P, Vaheri A, Carpen O. Association of ezrin with intercellular adhesion molecule-1 and -2 (ICAM-1 and ICAM-2). Regulation by phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 1998;273:21893–900. doi: 10.1074/jbc.273.34.21893. [DOI] [PubMed] [Google Scholar]
- 35.Carpen O, Pallai P, Staunton DE, Springer TA. Association of intercellular adhesion molecule-1 (ICAM-1) with actin-containing cytoskeleton and α-actinin. J Cell Biol. 1992;118:1223–34. doi: 10.1083/jcb.118.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carman CV, Jun CD, Salas A, Springer TA. Endothelial cells proactively form microvilli-like membrane projections upon intercellular adhesion molecule-1 engagement of leukocyte LFA-1. J Immunol. 2003;171:6135–44. doi: 10.4049/jimmunol.171.11.6135. [DOI] [PubMed] [Google Scholar]
- 37.Barreiro O, Yanez-Mo M, Serrador JM, Montoya MC, Vicente-Manzanares M, Tejedor R, et al. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J Cell Biol. 2002;157:1233–45. doi: 10.1083/jcb.200112126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Federici C, Camoin L, Hattab M, Strosberg AD, Couraud PO. Association of the cytoplasmic domain of intercellular adhesion molecule-1 with glyceraldehyde-3-phosphate dehydrogenase and β-tubulin. Eur J Biochem. 1996;238:173–80. doi: 10.1111/j.1432-1033.1996.0173q.x. [DOI] [PubMed] [Google Scholar]
- 39.Tilghman RW, Hoover RL. The Src-cortactin pathway is required for clustering of E-selectin and ICAM-1 in endothelial cells. FASEB J. 2002;16:1257–9. doi: 10.1096/fj.01-0969fje. [DOI] [PubMed] [Google Scholar]
- 40.Rahman A, Fazal F. Hug tightly and say goodbye: role of endothelial ICAM-1 in leukocyte transmigration. Antioxid Redox Signal. 2009;11:823–39. doi: 10.1089/ars.2008.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kanters E, van Rijssel J, Hensbergen PJ, Hondius D, Mul FP, Deelder AM, et al. Filamin B mediates ICAM-1-driven leukocyte transendothelial migration. J Biol Chem. 2008;283:31830–9. doi: 10.1074/jbc.M804888200. [DOI] [PubMed] [Google Scholar]
- 42.Casasnovas JM, Stehle T, Liu JH, Wang JH, Springer TA. A dimeric crystal structure for the N-terminal two domains of intercellular adhesion molecule-1. Proc Natl Acad Sci USA. 1998;95:4134–9. doi: 10.1073/pnas.95.8.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ostermann G, Weber KS, Zernecke A, Schroder A, Weber C. JAM-1 is a ligand of the β2 integrin LFA-1 involved in transendothelial migration of leukocytes. Nat Immunol. 2002;3:151–8. doi: 10.1038/ni755. [DOI] [PubMed] [Google Scholar]
- 44.Muller WA. Mechanisms of leukocyte transendothelial migration. Annu Rev Pathol. 2011;6:323–44. doi: 10.1146/annurev-pathol-011110-130224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van de Stolpe A, Jacobs N, Hage WJ, Tertoolen L, van Kooyk Y, Novakova IR, et al. Fibrinogen binding to ICAM-1 on EA. hy 926 endothelial cells is dependent on an intact cytoskeleton. Thromb Haemost. 1996;75:182–9. [PubMed] [Google Scholar]
- 46.Shen Q, Rahn JJ, Zhang J, Gunasekera N, Sun X, Shaw ARE, et al. MUC1 initiates Src-CrkL-Rac1/Cdc42-mediated actin cytoskeletal protrusive motility after ligating intercellular adhesion molecule-1. Mol Cancer Res. 2008;6:555–67. doi: 10.1158/1541-7786.MCR-07-2033. [DOI] [PubMed] [Google Scholar]
- 47.Regimbald LH, Pilarski LM, Longenecker BM, Reddish MA, Zimmermann G, Hugh JC. The breast mucin MUC1 as a novel adhesion ligand for endothelial intercellular adhesion molecule-1 in breast cancer. Cancer Res. 1996;56:4244–9. [PubMed] [Google Scholar]
- 48.Entwistle J, Hall CL, Turley EA. HA receptors: regulators of signalling to the cytoskeleton. J Cell Biochem. 1996;61:569–77. doi: 10.1002/(sici)1097-4644(19960616)61:4<569::aid-jcb10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 49.Xiao X, Cheng CY, Mruk DD. Intercellular adhesion molecule-1 is a regulator of blood-testis barrier function. J Cell Sci. 2012;125:5677–89. doi: 10.1242/jcs.107987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van de Stolpe A, van der Saag PT. Intercellular adhesion molecule-1. J Mol Med. 1996;74:13–33. doi: 10.1007/BF00202069. [DOI] [PubMed] [Google Scholar]
- 51.Pluskota E, D’Souza SE. Fibrinogen interactions with ICAM-1 (CD54) regulate endothelial survival. Eur J Biochem. 2000;267:1–13. doi: 10.1046/j.1432-1327.2000.01520.x. [DOI] [PubMed] [Google Scholar]
- 52.Durieu-Trautmann O, Chaverot N, Cazaubon S, Strosberg AD, Couraud PO. Intercellular adhesion molecule-1 activation induces tyrosine phosphorylation of the cytoskeleton-associated protein cortactin in brain microvessel endothelial cells. J Biol Chem. 1994;269:12536–40. [PubMed] [Google Scholar]
- 53.Rothlein R, Kishimoto TK, Mainolfi E. Cross-linking of ICAM-1 induces co-signaling of an oxidative burst from mononuclear leukocytes. J Immunol. 1994;152:2488–95. [PubMed] [Google Scholar]
- 54.Sans E, Delachanal E, Duberray A. Analysis of the roles of ICAM-1 in neutrophil transmigration using a reconstituted mammalian cell expression model: implication of ICAM-1 cytoplasmic domain and Rho-dependent signaling pathway. J Immunol. 2001;166:544–51. doi: 10.4049/jimmunol.166.1.544. [DOI] [PubMed] [Google Scholar]
- 55.Bella J, Rossman MG. Review: rhinoviruses and their ICAM receptors. J Struct Biol. 1999;128:69–74. doi: 10.1006/jsbi.1999.4143. [DOI] [PubMed] [Google Scholar]
- 56.Casasnovas JM. The dynamics of receptor recognition by human rhinoviruses. Trends Microbiol. 2000;8:251–4. doi: 10.1016/s0966-842x(00)01749-2. [DOI] [PubMed] [Google Scholar]
- 57.Dreschers S, Dumitru CA, Adams CL, Gulbins E. The cold case: are rhinoviruses perfectly adapted pathogens? Cell Mol Life Sci. 2007;64:181–91. doi: 10.1007/s00018-006-6266-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nagaoka T, Kaburagi Y, Hamaguchi Y, Hasegawa M, Takehara K, Steeber DA, et al. Delayed wound healing in the absence of intercellular adhesion molecule-1 or L-selectin expression. Am J Pathol. 2000;157:237–47. doi: 10.1016/S0002-9440(10)64534-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sligh JE, Jr, Ballantyne CM, Rich SS, Hawkins HK, Smith CW, Bradley A, et al. Inflammatory and immune responses are impaired in mice deficient in intracellular adhesion molecule-1. Proc Natl Acad Sci USA. 1993;90:8529–33. doi: 10.1073/pnas.90.18.8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hallahan DE, Geng L, Shyr Y. Effects of intercellular adhesion molecule 1 (ICAM-1) null mutation on radiation-induced pulmonary fibrosis and respiratory insufficiency in mice. J Natl Cancer Inst. 2002;94:733–41. doi: 10.1093/jnci/94.10.733. [DOI] [PubMed] [Google Scholar]
- 61.Kitagawa K, Matsumoto M, Mabuchi T, Yagita Y, Ohtsuki T, Hori M, et al. Deficiency of intercellular adhesion molecule 1 attenuates microcirculatory disturbance and infarction size in focal cerebral ischemia. J Cereb Blood Flow Metab. 1998;18:1336–45. doi: 10.1097/00004647-199812000-00008. [DOI] [PubMed] [Google Scholar]
- 62.Dunne JL, Collins RG, Beaudet AL, Ballantyne CM, Ley K. Mac-1, but not LFA-1, uses intercellular adhesion molecule-1 to mediate slow leukocyte rolling in TNFα-induced inflammation. J Immunol. 2003;171:6105–11. doi: 10.4049/jimmunol.171.11.6105. [DOI] [PubMed] [Google Scholar]
- 63.Steeber DA, Tang ML, Green NE, Zhang XQ, Sloane JE, Tedder TF. Leukocyte entry into sites of inflammation requires overlapping interactions between the L-selectin and ICAM-1 pathways. J Immunol. 1999;163:2176–86. [PubMed] [Google Scholar]
- 64.Dustin ML, Rothlein R, Bhan AK, Dinarello CA, Springer TA. Induction by IL 1 and interferon-γ: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1) J Immunol. 1986;137:245–54. [PubMed] [Google Scholar]
- 65.Hubbard AK, Rothlein R. Intercellular adhesion molecule-1 (ICAM-1) expression and cell signaling cascades. Free Radic Biol Med. 2000;28:1379–86. doi: 10.1016/s0891-5849(00)00223-9. [DOI] [PubMed] [Google Scholar]
- 66.Spoelstra FM, Postma DS, Hovenga H, Noordhoek JA, Kauffman HF. Interferon-γ and interleukin-4 differentially regulate ICAM-1 and VCAM-1 expression on human lung fibroblasts. Eur Respir J. 1999;14:759–66. doi: 10.1034/j.1399-3003.1999.14d06.x. [DOI] [PubMed] [Google Scholar]
- 67.Inoue T, Kobayashi K, Inoguchi T, Sonoda N, Fujii M, Maeda Y, et al. Reduced expression of adipose triglyceride lipase enhances tumor necrosis factor α-induced intercellular adhesion molecule-1 expression in human aortic endothelial cells via protein kinase C-dependent activation of nuclear factorκB. J Biol Chem. 2011;286:32045–53. doi: 10.1074/jbc.M111.285650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hosokawa Y, Hosokawa I, Ozaki K, Nakae H, Matsuo T. Cytokines differentially regulate ICAM-1 and VCAM-1 expression on human gingival fibroblasts. Clin Exp Immunol. 2006;144:494–502. doi: 10.1111/j.1365-2249.2006.03064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Defrere S, Donnez J, Moulin P, Befahy P, Gonzalez-Ramos R, Lousse JC, et al. Expression of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in human endometrial stromal and epithelial cells is regulated by interferon-γ but not iron. Gynecol Obstet Invest. 2008;65:145–54. doi: 10.1159/000110350. [DOI] [PubMed] [Google Scholar]
- 70.Thomson AJ, Greer MR, Young A, Boswell F, Telfer JF, Cameron IT, et al. Expression of intercellular adhesion molecules ICAM-1 and ICAM-2 in human endometrium: regulation by interferon-γ. Mol Hum Reprod. 1999;5:64–70. doi: 10.1093/molehr/5.1.64. [DOI] [PubMed] [Google Scholar]
- 71.Becker JC, Dummer R, Hartmann AA, Burg G, Schmidt RE. Shedding of ICAM-1 from human melanoma cell lines induced by INF-γ and TNF-α. Functional consequences on cell-mediated cytotoxicity. J Immunol. 1991;147:4398–401. [PubMed] [Google Scholar]
- 72.Fonsatti E, Altomonte M, Coral S, Cattarossi I, Nicotra MR, Gasparollo A, et al. Tumor-derived interleukin-1α (IL-1α) up-regulates the release of soluble intercellular adhesion molecule-1 (sICAM-1) by endothelial cells. Br J Cancer. 1997;76:1255–61. doi: 10.1038/bjc.1997.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Whiteman SC, Spiteri MA. IFN-γ regulation of ICAM-1 receptors in bronchial epithelial cells: soluble ICAM-1 release inhibits human rhinovirus infection. J Inflamm (Lond) 2008;5:8. doi: 10.1186/1476-9255-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jung J, Ko SH, Yoo DY, Lee JY, Kim YJ, Choi SM, et al. 5,7-Dihydroxy-3,4,6-trimethoxyflavone inhibits intercellular adhesion molecule 1 and vascular cell adhesion molecule 1 via the Akt and nuclear factor-κB-dependent pathway, leading to suppression of adhesion of monocytes and eosinophils to broncial epithelial cells. Immunology. 2012;137:98–113. doi: 10.1111/j.1365-2567.2012.03618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li TM, Wu CM, Huang HC, Chou PC, Fong YC, Tang CH. Interleukin-11 increases cell motility and up-regulates intercellular adhesion molecule-1 expression in human chondrosarcoma cells. J Cell Biochem. 2012;113:3353–62. doi: 10.1002/jcb.24211. [DOI] [PubMed] [Google Scholar]
- 76.Kusterer K, Bojunga J, Enghofer M, Heidenthal E, Usadel KH, Kolb H, et al. Soluble ICAM-1 reduces leukocyte adhesion to vascular endothelium in ischemia-reperfusion injury in mice. Am J Physiol. 1998;275:G377–G80. doi: 10.1152/ajpgi.1998.275.2.G377. [DOI] [PubMed] [Google Scholar]
- 77.Rieckmann P, Michel U, Albrecht M, Bruck W, Wockel L, Felgenhauer K. Soluble forms of intercellular adhesion molecule-1 (ICAM-1) block lymphocyte attachment to cerebral endothelial cells. J Neuroimmunol. 1995;60:9–15. doi: 10.1016/0165-5728(95)00047-6. [DOI] [PubMed] [Google Scholar]
- 78.Rothlein R, Mainolfi EA, Czajkowski M, Marlin D. A form of circulating ICAM-1 in human serum. J Immunol. 1991;147:3788–93. [PubMed] [Google Scholar]
- 79.Marlin SD, Springer TA. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1) Cell. 1987;51:813–9. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- 80.Diamond MS, Staunton DE, de Fougerolles AR, Stacker SA, Garcia-Aguilar J, Hibbs ML, et al. ICAM-1 (CD54): a counter-receptor for Mac-1 (CD11b/CD18) J Cell Biol. 1990;111:3129–39. doi: 10.1083/jcb.111.6.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Essick E, Sithu S, Dean W, D’Souza S. Pervanadate-induced shedding of the intercellular adhesion molecule (ICAM)-1 ectodomain is mediated by membrane type-1 matrix metalloproteinase (MT1-MMP) Mol Cell Biochem. 2008;314:151–9. doi: 10.1007/s11010-008-9776-7. [DOI] [PubMed] [Google Scholar]
- 82.Sithu SD, English WR, Olson P, Krubasik D, Baker AH, Murphy G, et al. Membrane-type 1 matrix metalloproteinase regulates intracellular adhesion molecule-1 (ICAM-1)-mediated monocyte transmigration. J Biol Chem. 2007;282:25010–9. doi: 10.1074/jbc.M611273200. [DOI] [PubMed] [Google Scholar]
- 83.Pino M, Galleguillos C, Torres M, Sovino H, Fuentes A, Boric MA, et al. Association between MMP1 and MMP9 activities and ICAM1 cleavage induced by tumor necrosis factor in stromal cell cultures from eutopic endometria of women with endometriosis. Reproduction. 2009;138:837–47. doi: 10.1530/REP-09-0196. [DOI] [PubMed] [Google Scholar]
- 84.Sultan S, Gosling M, Nagase H, Powell JT. Shear stress-induced shedding of soluble intercellular adhesion molecule-1 from saphenous vein endothelium. FEBS Lett. 2004;564:161–5. doi: 10.1016/S0014-5793(04)00337-0. [DOI] [PubMed] [Google Scholar]
- 85.Fiore E, Fusco C, Romero P, Stamenkovic I. Matrix metalloproteinase 9 (MMP-9/gelatinase B) proteolytically cleaves ICAM-1 and participates in tumor cell resistance to natural killer cell-mediated cytotoxicity. Oncogene. 2002;21:5213–23. doi: 10.1038/sj.onc.1205684. [DOI] [PubMed] [Google Scholar]
- 86.Lyons PD, Benveniste EN. Cleavage of membrane-associated ICAM-1 from astrocytes: involvement of a metalloprotease. Glia. 1998;22:103–12. doi: 10.1002/(sici)1098-1136(199802)22:2<103::aid-glia1>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 87.Black RA. Tumor necrosis factor α-converting enzyme. Int J Biochem Cell Biol. 2002;34:1–5. doi: 10.1016/s1357-2725(01)00097-8. [DOI] [PubMed] [Google Scholar]
- 88.Tsakadze NL, Sithu SD, Sen U, English WR, Murphy G, D’Souza SE. Tumor necrosis factor-α converting enzyme (TACE/ADAM-17) mediates the ectodomain cleavage of intercellular adhesion molecule-1 (ICAM-1) J Biol Chem. 2006;281:3157–64. doi: 10.1074/jbc.M510797200. [DOI] [PubMed] [Google Scholar]
- 89.Zhu X, Tai W, Shi W, Song Y, Zhang H, An G. Matrix metalloproteinase-9 silencing by RNA interference promotes the adhesive-invasive switch in HT1080 human fibrosarcoma cells. Clin Lab. 2012;58:313–22. [PubMed] [Google Scholar]
- 90.Gutierrez-Lopez MD, Gilsanz A, Yanez-Mo M, Ovalle S, Lafuente EM, Dominguez C, et al. The sheddase activity of ADAM17/TACE is regulated by the tetraspanin CD9. Cell Mol Life Sci. 2011;68:3275–92. doi: 10.1007/s00018-011-0639-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mohan MJ, Seaton T, Mitchell JS, Howe A, Blackburn K, Burkhart W, et al. The tumor necrosis factor α-converting enzyme (TACE): a unique metalloproteinase with highly defined substrate selectivity. Biochemistry. 2002;41:9462–9. doi: 10.1021/bi0260132. [DOI] [PubMed] [Google Scholar]
- 92.Xu P, Derynck R. Direct activation of TACE-mediated ectodomain shedding by p38 MAP kinase regulates EGF receptor-dependent cell proliferation. Mol Cell. 2010;37:551–66. doi: 10.1016/j.molcel.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Soond SM, Everson B, Riches DWH, Murphy G. ERK-mediated phosphorylation of Thr735 in TNFα-converting enzyme and its potential role in TACE protein trafficking. J Cell Sci. 2005;118:2371–80. doi: 10.1242/jcs.02357. [DOI] [PubMed] [Google Scholar]
- 94.Liu CF, Xu P, Lamouille S, Xu J, Derynck R. TACE-mediated ectodomain shedding of the type I TGF-β receptor downregulates TGF-β signaling. Mol Cell. 2009;35:26–36. doi: 10.1016/j.molcel.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Diaz-Rodriguez E, Montero JC, Esparis-Ogando A, Yuste L, Pandiella A. Extracellular signal-related kinase phosphorylates tumor necrosis factor α-converting enzyme at threonine 735: a potential role in regulated shedding. Mol Biol Cell. 2002;13:2031–44. doi: 10.1091/mbc.01-11-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tsakadze NL, Sen U, Zhao ZS, Sithu SD, English WR, D’Souza SE. Signals mediating cleavage of intercellular adhesion molecule-1. Am J Physiol Cell Physiol. 2004;287:55–63. doi: 10.1152/ajpcell.00585.2003. [DOI] [PubMed] [Google Scholar]
- 97.Robledo O, Papaioannou A, Ochietti B, Beauchemin C, Legault D, Cantin A, et al. ICAM-1 isoforms: specific activity and sensitivity to cleavage by leukocyte elastase and cathepsin G. Eur J Immunol. 2003;33:1351–60. doi: 10.1002/eji.200323195. [DOI] [PubMed] [Google Scholar]
- 98.Okamoto I, Kawano Y, Murakami D, Sasayama T, Araki N, Miki T, et al. Proteolytic release of CD44 intracellular domain and its role in the CD44 signaling pathway. J Cell Biol. 2001;155:755–62. doi: 10.1083/jcb.200108159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nagano O, Saya H. Mechanism and biological significance of CD44 cleavage. Cancer Sci. 2004;95:930–5. doi: 10.1111/j.1349-7006.2004.tb03179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wakatsuki T, Kimura K, Kimura F, Shinomiya N, Ohtsubo M, Ishizawa M, et al. A distinct mRNA encoding a soluble form of ICAM-1 molecule expressed in human tissues. Cell Adhes Commun. 1995;3:283–92. doi: 10.3109/15419069509081014. [DOI] [PubMed] [Google Scholar]
- 101.Lee IT, Yang CM. Role of NADPH oxidase/ROS in pro-inflammatory mediators-induced airway and pulmonary diseases. Biochem Pharmacol. 2012;84:581–90. doi: 10.1016/j.bcp.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 102.Satriano JA, Banas B, Luckow B, Nelson P, Schlondorff DO. Regulation of RANTES and ICAM-1 expression in murine mesangial cells. J Am Soc Nephrol. 1997;8:596–603. doi: 10.1681/ASN.V84596. [DOI] [PubMed] [Google Scholar]
- 103.Kim H, Hwang JS, Woo CH, Kim EY, Kim TH, Cho KJ, et al. TNFα-induced up-regulation of intercellular adhesion molecule-1 is regulated by a Rac-ROS-dependent cascade in human airway epithelial cells. Exp Mol Med. 2008;40:167–75. doi: 10.3858/emm.2008.40.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Okamoto H, Cejec TP, Yamanaka H, Kamatani N. Molecular aspects of rheumatoid arthritis: role of transcription factors. FEBS J. 2008;275:4463–70. doi: 10.1111/j.1742-4658.2008.06582.x. [DOI] [PubMed] [Google Scholar]
- 105.Ledebur HC, Parks TP. Transcriptional regulation of the intercellular adhesion molecule-1 gene by inflammatory cytokines in human endothelial cells. J Biol Chem. 1995;270:933–43. doi: 10.1074/jbc.270.2.933. [DOI] [PubMed] [Google Scholar]
- 106.Roebuck KA, Rahman A, Lakshminarayanan V, Janakidevi K, Malik AB. H2O2 and tumor necrosis factor α activate intracellular adhesion molecule-1 (ICAM-1) gene transcription through distinct cis-regulatory elements within the ICAM-1 promoter. J Biol Chem. 1995;270:18966–74. doi: 10.1074/jbc.270.32.18966. [DOI] [PubMed] [Google Scholar]
- 107.Astarci E, Sade A, Cimen I, Savas B, Banerjee S. The NF-κB target genes ICAM-1 and VCAM-1 are differentially regulated during spontaneous differentiation of Caco-2 cells. FEBS J. 2012;279:2966–86. doi: 10.1111/j.1742-4658.2012.08677.x. [DOI] [PubMed] [Google Scholar]
- 108.Marchiando AM, Shen L, Graham WV, Edelblum KL, Duckworth CA, Guan Y, et al. The epithelial barrier is maintained by in vivo tight junction expansion during pathologic intestinal epithelial shedding. Gastroenterology. 2011;140:1208–18. doi: 10.1053/j.gastro.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lin SJ, Shyue SK, Hung YY, Chen YH, Ku HH, Chen JW, et al. Superoxide dismutase inhibits the expression of vascular cell adhesion molecule-1 and intracellular cell adhesion molecule-1 induced by tumor necrosis factor α in human endothelial cells through the JNK/p38 pathways. Arterioscler Thromb Vasc Biol. 2005;25:334–40. doi: 10.1161/01.ATV.0000152114.00114.d8. [DOI] [PubMed] [Google Scholar]
- 110.Robinson MJ, Cobb MH. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180–6. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- 111.Tibbles LA, Woodgett JR. The stress-activated protein kinase pathways. Cell Mol Life Sci. 1999;55:1230–54. doi: 10.1007/s000180050369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang Q, Pfeiffer GR, II, Gaarde WA. Activation of SRC tyrosine kinases in response to ICAM-1 ligation in pulmonary microvascular endothelial cells. J Biol Chem. 2003;278:47731–43. doi: 10.1074/jbc.M308466200. [DOI] [PubMed] [Google Scholar]
- 113.Koss MK, Pfeiffer GR, II, Wang Y, Thomas ST, Yerukhimovich M, Gaarde WA, et al. Ezrin/radixin/moesin proteins are phosphorylated by TNFα and modulate permeability increases in human pulmonary microvascular endothelial cells. J Immunol. 2006;176:1218–27. doi: 10.4049/jimmunol.176.2.1218. [DOI] [PubMed] [Google Scholar]
- 114.Petrache I, Birukova AA, Ramirez SI, Garcia JGN, Verin AD. The role of microtubules in tumor necrosis factor α-induced endothelial permeability. Am J Respir Cell Mol Biol. 2003;28:574–81. doi: 10.1165/rcmb.2002-0075OC. [DOI] [PubMed] [Google Scholar]
- 115.Molony L, Armstrong L. Cytoskeletal reorganizations in human umbilical vein endothelial cells as a result of cytokine exposure. Exp Cell Res. 1991;196:40–8. doi: 10.1016/0014-4827(91)90454-3. [DOI] [PubMed] [Google Scholar]
- 116.Neisch AL, Fehon RG. Ezrin, radixin and moesin: key regulators of membrane-cortex interactions and signaling. Curr Opin Cell Biol. 2011;23:377–82. doi: 10.1016/j.ceb.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Arpin M, Chirivino D, Naba A, Zwaenepoel I. Emerging roles for ERM proteins in cell adhesion and migration. Cell Adh Migr. 2011;5:199–206. doi: 10.4161/cam.5.2.15081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Adamson P, Etienne S, Couraud PO, Calder V, Greenwood J. Lymphocyte migration through brain endothelial cell monolayers involves signaling through endothelial ICAM-1 via a Rho-dependent pathway. J Immunol. 1999;162:2964–73. [PubMed] [Google Scholar]
- 119.Etienne-Manneville S, Manneville JB, Adamson P, Wilbourn B, Greenwood J, Couraud PO. ICAM-1-coupled cytoskeletal rearrangements and transendothelial lymphocyte migration involve intracellular calcium signaling in brain endothelial cell lines. J Immunol. 2000;165:3375–83. doi: 10.4049/jimmunol.165.6.3375. [DOI] [PubMed] [Google Scholar]
- 120.Etienne S, Adamson P, Greenwood J, Strosberg AD, Cazaubon S, Couraud PO. ICAM-1 signaling pathways associated with Rho activation in microvascular brain endothelial cells. J Immunol. 1998;161:5755–61. [PubMed] [Google Scholar]
- 121.Wojciak-Stothard B, Williams L, Ridney AJ. Monocyte adhesion and spreading on human endothelial cells is dependent on Rho-regulated receptor clustering. J Cell Biol. 1999;145:1293–307. doi: 10.1083/jcb.145.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Petrache I, Verin AD, Crow MT, Birukova AA, Liu F, Garcia JG. Differential effect of MLC kinase in TNFα-induced endothelial cell apoptosis and barrier dysfunction. Am J Physiol Cell Mol Physiol. 2001;280:L1168–L78. doi: 10.1152/ajplung.2001.280.6.L1168. [DOI] [PubMed] [Google Scholar]
- 123.McKenzie JA, Ridley AJ. Roles of Rho/ROCK and MLCK in TNFα-induced changes in endothelial morphology and permeability. J Cell Physiol. 2007;213:221–8. doi: 10.1002/jcp.21114. [DOI] [PubMed] [Google Scholar]
- 124.Kamm KE, Stull JT. Dedicated myosin light chain kinases with diverse cellular functions. J Biol Chem. 2001;276:4527–30. doi: 10.1074/jbc.R000028200. [DOI] [PubMed] [Google Scholar]
- 125.Goeckeler ZM, Wysolmerski RB. Myosin light chain kinase-regulated endothelial cell contraction: the relationship between isometric tension, actin polymerization and myosin phosphorylation. J Cell Biol. 1995;130:613–27. doi: 10.1083/jcb.130.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-γ and tumor necrosis factor-α synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol. 2005;166:409–19. doi: 10.1016/s0002-9440(10)62264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Thompson PW, Randi AM, Ridley AJ. Intercellular adhesion molecule (ICAM)-1, but not ICAM-2, activates RhoA and stimulates c-fos and rhoA transcription in endothelial cells. J Immunol. 2002;169:1007–13. doi: 10.4049/jimmunol.169.2.1007. [DOI] [PubMed] [Google Scholar]
- 128.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–8. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 129.Ishizaki T, Maekawa M, Fujisawa K, Okawa K, Iwamatsu A, Fujita A, et al. The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO J. 1996;15:1885–93. [PMC free article] [PubMed] [Google Scholar]
- 130.Naumanen P, Lappalainen P, Hotulainen P. Mechanisms of actin stress fibre assembly. J Microsc. 2008;231:446–54. doi: 10.1111/j.1365-2818.2008.02057.x. [DOI] [PubMed] [Google Scholar]
- 131.Pellegrin S, Mellor H. Actin stress fibres. J Cell Sci. 2007;120:3491–9. doi: 10.1242/jcs.018473. [DOI] [PubMed] [Google Scholar]
- 132.Prasain N, Stevens T. The actin cytoskeleton in endothelial cell phenotypes. Microvasc Res. 2009;77:53–63. doi: 10.1016/j.mvr.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vandenbroucke E, Mehta D, Minshall R, Malik AB. Regulation of endothelial junctional permeability. Ann NY Acad Sci. 2008;1123:134–45. doi: 10.1196/annals.1420.016. [DOI] [PubMed] [Google Scholar]
- 134.Aktories K, Wilde C, Vogelsgesang M. Rho-modifying C3-like ADP-ribosyltransferases. Rev Physiol Biochem Pharmacol. 2004;152:1–22. doi: 10.1007/s10254-004-0034-4. [DOI] [PubMed] [Google Scholar]
- 135.Kowalczyk AP, Nanes BA. Adherens junction turnover: regulating adhesion through cadherin endocytosis, degradation and recycling. Subcell Biochem. 2012;60:197–222. doi: 10.1007/978-94-007-4186-7_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Green KJ, Getsios S, Troyanovsky S, Godsel LM. Intercellular junction assembly, dynamics and homeostasis. In: Nelson WJ, Fuchs E, editors. Cold Spring Harbor Perspectives in Biology. Woodbury: Cold Spring Harbor Laboratory Press; 2010. p. a000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.de Beco S, Amblard F, Coscoy S. New insights into the regulation of E-cadherin distribution by endocytosis. Int Rev Cell Mol Biol. 2012;295:63–108. doi: 10.1016/B978-0-12-394306-4.00008-3. [DOI] [PubMed] [Google Scholar]
- 138.Hirase T, Kawashima S, Wong EY, Ueyama T, Rikitake Y, Tsukita S, et al. Regulation of tight junction permeability and occludin phosphorylation by RhoA-p160ROCK-dependent and -independent mechanisms. J Biol Chem. 2001;276:10423–31. doi: 10.1074/jbc.M007136200. [DOI] [PubMed] [Google Scholar]
- 139.Wittchen ES. Endothelial signaling in paracellular and transcellular leukocyte transmigration. Front Biosci. 2009;14:2522–45. doi: 10.2741/3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Greenwood J, Amos CL, Walters CE, Couraud PO, Lyck R, Engelhardt B, et al. Intracellular domain of brain endothelial intercellular adhesion molecule-1 is essential for T lymphocyte-mediated signaling and migration. J Immunol. 2003;171:2099–108. doi: 10.4049/jimmunol.171.4.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang G, Pfeiffer GR, Gaarde WA. Activation of SRC tyrosine kinases in response to ICAM-1 ligation in pulmonary microvascular endothelial cells. J Biol Chem. 2003;278:47731–43. doi: 10.1074/jbc.M308466200. [DOI] [PubMed] [Google Scholar]
- 142.Allingham MJ, van Buul JD, Burridge K. ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration. J Immunol. 2007;179:4053–64. doi: 10.4049/jimmunol.179.6.4053. [DOI] [PubMed] [Google Scholar]
- 143.Potter MD, Barbero S, Cheresh DA. Tyrosine phosphorylation of VE-cadherin prevents binding of p120- and β-catenin and maintains the cellular mesenchymal state. J Biol Chem. 2005;280:31906–12. doi: 10.1074/jbc.M505568200. [DOI] [PubMed] [Google Scholar]
- 144.Whittard JD, Akiyama SK. Activation of β1 integrins induces cell-cell adhesion. Exp Cell Res. 2001;263:65–76. doi: 10.1006/excr.2000.5099. [DOI] [PubMed] [Google Scholar]
- 145.Nakayamada S, Okada Y, Saito K, Tamura M, Tanaka Y. β1 Integrin/focal adhesion kinase-mediated signaling induces intercellular adhesion molecule-1 and receptor activator of nuclear factor κB ligand on osteoblasts and osteoclast maturation. J Biol Chem. 2003;278:45368–74. doi: 10.1074/jbc.M308786200. [DOI] [PubMed] [Google Scholar]
- 146.Yasuda M, Tanaka Y, Tamura M, Fujii K, Sugaya M, So T, et al. Stimulation of β1 integrin down-regulates ICAM-1 expression and ICAM-1-dependent adhesion of lung cancer cells through focal adhesion kinase. Cancer Res. 2001;61:2022–30. [PubMed] [Google Scholar]
- 147.Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116:1409–16. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- 148.Hayasaka H, Simon K, Hershey ED, Masumoto KH, Parsons JT. FRNK, the autonomously expressed C-terminal region of focal adhesion kinase, is uniquely regulated in vascular smooth muscle: analysis of expression in transgenic mice. J Cell Biochem. 2005;95:1248–63. doi: 10.1002/jcb.20501. [DOI] [PubMed] [Google Scholar]
- 149.Richardson A, Parsons T. A mechanism for regulation of the adhesion-associated protein tyrosine kinase pp125FAK. Nature. 1996;380:538–40. doi: 10.1038/380538a0. [DOI] [PubMed] [Google Scholar]
- 150.Taylor JM, Mack CP, Nolan K, Regan CP, Owens GK, Parsons JT. Selective expression of an endogenous inhibitor of FAK regulated proliferation and migration of vascular smooth muscle cells. Mol Cell Biol. 2001;21:1565–72. doi: 10.1128/MCB.21.5.1565-1572.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hauck CR, Hsia DA, Puente XS, Cheresh DA, Schlaepfer DD. FRNK blocks v-Src-stimulated invasion and experimental metastases without effects on cell motility or growth. EMBO J. 2002;21:6289–302. doi: 10.1093/emboj/cdf631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sieg DJ, Hauck CR, Schlaepfer DD. Required roles of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J Cell Sci. 1999;112:2677–91. doi: 10.1242/jcs.112.16.2677. [DOI] [PubMed] [Google Scholar]
- 153.Govindarajan G, Eble DM, Lucchesi PA, Samarel AM. Focal adhesion kinase is involved in angiotensin II-mediated protein synthesis in cultured vascular smooth muscle cells. Circ Res. 2000;87:710–6. doi: 10.1161/01.res.87.8.710. [DOI] [PubMed] [Google Scholar]
- 154.Koshman YE, Chu M, Engman SJ, Kim TD, Iyengar R, Robia SL, et al. Focal adhesion kinase-related nonkinase inhibits vascular smooth muscle cell invasion by focal adhesion targeting, tyrosine 168 phosphorylation and competition for p130Cas binding. Arterioscler Thromb Vasc Biol. 2011;31:2432–40. doi: 10.1161/ATVBAHA.111.235549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gelman IH. Pyk 2 FAKs, any two FAKs. Cell Biol Int. 2003;27:507–10. doi: 10.1016/s1065-6995(03)00078-7. [DOI] [PubMed] [Google Scholar]
- 156.Orr AW, Murphy-Ullrich JE. Regulation of endothelial cell function by FAK and PYK2. Front Biosci. 2004;9:1254–66. doi: 10.2741/1239. [DOI] [PubMed] [Google Scholar]
- 157.Schaller MD. Cellular functions of FAK kinases: insight into molecular mechanisms and novel functions. J Cell Sci. 2010;123:1007–13. doi: 10.1242/jcs.045112. [DOI] [PubMed] [Google Scholar]
- 158.Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio JM, et al. Protein tyrosine kinase PYK2 involved in Ca2+-induced regulation of ion channel and MAP kinase functions. Nature. 1995;376:737–45. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 159.Mace EM, Zhang J, Siminovitch KA, Takei F. Elucidation of the integrin LFA-1-mediated signaling pathway of actin polarization in natural killer cells. Blood. 2010;116:1272–9. doi: 10.1182/blood-2009-12-261487. [DOI] [PubMed] [Google Scholar]
- 160.Anthis NJ, Campbell ID. The tail of integrin activation. Trends Biochem Sci. 2011;36:191–8. doi: 10.1016/j.tibs.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Critchley DR. Biochemical and structural properties of the integrin-associated cytoskeletal protein talin. Annu Rev Biophys. 2009;38:235–54. doi: 10.1146/annurev.biophys.050708.133744. [DOI] [PubMed] [Google Scholar]
- 162.Kiema T, Lad Y, Jiang P, Oxley CL, Baldassarre M, Wegener KL, et al. The molecular basis of filamin binding to integrins and competition with talin. Mol Cell. 2006;21:337–47. doi: 10.1016/j.molcel.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 163.Margadant C, Monsuur HN, Norman JC, Sonnenberg A. Mechanisms of integrin activation and trafficking. Curr Opin Cell Biol. 2011;23:607–14. doi: 10.1016/j.ceb.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 164.Liliental J, Chang DD. Rack1, a receptor for activated protein kinase C, interacts with integrin β subunit. J Biol Chem. 1998;273:2379–83. doi: 10.1074/jbc.273.4.2379. [DOI] [PubMed] [Google Scholar]
- 165.Buensuceso CS, Obergfell A, Soriani A, Eto K, Kiosses WB, Arias-Salgado EG, et al. Regulation of outside-in signaling in platelets by integrin-associated protein kinase Cβ. J Biol Chem. 2005;280:644–53. doi: 10.1074/jbc.M410229200. [DOI] [PubMed] [Google Scholar]
- 166.Feng C, Li YF, Yau YH, Lee HS, Tang XY, Xue ZH, et al. Kindlin-3 mediates integrin αLβ2 outside-in signaling, and it interacts with scaffold protein receptor for activated-C kinase 1 (RACK1) J Biol Chem. 2012;287:10714–26. doi: 10.1074/jbc.M111.299594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Larjava H, Plow EF, Wu C. Kindlins: essential regulators of integrin signalling and cell-matrix adhesion. EMBO Rep. 2008;9:1203–8. doi: 10.1038/embor.2008.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Meves A, Stremmel C, Gottschalk K, Fassler R. The kindlin protein family: new members to the club of focal adhesion proteins. Trends Cell Biol. 2009;19:504–13. doi: 10.1016/j.tcb.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 169.Malinin NL, Plow EF, Byzova TV. Kindlins in FERM adhesion. Blood. 2010;115:4011–7. doi: 10.1182/blood-2009-10-239269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Weed SA, Parson JT. Cortactin: coupling membrane dynamics to cortical actin assembly. Oncogene. 2001;20:6418–34. doi: 10.1038/sj.onc.1204783. [DOI] [PubMed] [Google Scholar]
- 171.Kirkbride KC, Sung BH, Sinha S, Weaver AM. Cortactin: a multifunctional regulator of cellular invasiveness. Cell Adh Migr. 2011;5:187–98. doi: 10.4161/cam.5.2.14773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pollard TD. Regulation of actin filament assembly by Arp2/3 complex and formins. Annu Rev Biophys Biomol Struct. 2007;36:451–77. doi: 10.1146/annurev.biophys.35.040405.101936. [DOI] [PubMed] [Google Scholar]
- 173.Ziparo E, Riccioli A, Filippini A, De Cesaris P, Barbacci E. TNFα induces surface modifications in mouse Sertoli cells: physiopathological implications. Ital J Anat Embryol. 1995;100:553–62. [PubMed] [Google Scholar]
- 174.Riccioli A, Filippini A, De Cesaris P, Barbacci E, Stefanini M, Starace G, et al. Inflammatory mediators increase surface expression of integrin ligands, adhesion to lymphocytes and secretion of interleukin 6 in mouse Sertoli cells. Proc Natl Acad Sci USA. 1995;92:5808–12. doi: 10.1073/pnas.92.13.5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lydka M, Bilinska B, Cheng CY, Mruk DD. Tumor necrosis factor α-mediated restructuring of the Sertoli cell barrier in vitro involves matrix metalloprotease 9 (MMP9), membrane-bound intercellular adhesion molecule-1 (ICAM-1) and the actin cytoskeleton. Spermatogenesis. 2012;2:294–303. doi: 10.4161/spmg.22602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mirza M, Petersen C, Nordqvist K, Sollerbrant K. Coxsackie- and adenovirus receptor (CAR) is up-regulated in migratory germ cells during passage of the blood-testis barrier. Endocrinology. 2007;148:5459–69. doi: 10.1210/en.2007-0359. [DOI] [PubMed] [Google Scholar]
- 177.Okigaki M, Davis CR, Falasca M, Harroch S, Felsenfeld DP, Sheetz MP, et al. Pyk2 regulates multiple signaling events crucial for macrophage morphology and migration. Proc Natl Acad Sci USA. 2003;100:10740–5. doi: 10.1073/pnas.1834348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Delva E, Tucker DK, Kowalczyk AP. The desmosome. Cold Spring Harb Perspect Biol. 2009;1:a002543. doi: 10.1101/cshperspect.a002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yin T, Green KJ. Regulation of desmosome assembly and adhesion. Semin Cell Dev Biol. 2004;15:665–77. doi: 10.1016/j.semcdb.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 180.Lie PPY, Cheng CY, Mruk DD. The desmoglein-2/desmocollin-2/Src kinase protein complex regulates blood-testis barrier dynamics. Int J Biochem Cell Biol. 2010;42:975–86. doi: 10.1016/j.biocel.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Li MWM, Mruk DD, Lee WM, Cheng CY. Connexin 43 and plakophilin-2 as a protein complex that regulates blood-testis barrier dynamics. Proc Natl Acad Sci USA. 2009;106:10213–8. doi: 10.1073/pnas.0901700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hild SA, Meistrich ML, Blye RP, Reel JR. Lupron Depot prevention of antispermatogenic/antifertility activity of the indenopyridine, CDB-4022, in the rat. Biol Reprod. 2001;65:165–72. doi: 10.1095/biolreprod65.1.165. [DOI] [PubMed] [Google Scholar]
- 183.Koduri S, Hild SA, Pessaint L, Reel JR, Attardi BJ. Mechanism of action of l-CDB-4022, a potential nonhormonal male contraceptive, in the seminiferous epithelium of the rat testis. Endocrinology. 2008;149:1850–60. doi: 10.1210/en.2007-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cheng CY, Silvestrini B, Grima J, Mo MY, Zhu LJ, Johansson E, et al. Two new male contraceptives exert their effects by depleting germ cells prematurely from the testis. Biol Reprod. 2001;65:449–61. doi: 10.1095/biolreprod65.2.449. [DOI] [PubMed] [Google Scholar]
- 185.Mruk DD, Wong CH, Silvestrini B, Cheng CY. A male contraceptive targeting germ cell adhesion. Nat Med. 2006;12:1323–8. doi: 10.1038/nm1420. [DOI] [PubMed] [Google Scholar]
- 186.Parizek J, Zahor Z. Effect of cadmium salts on testicular tissue. Nature. 1956;177:1036. doi: 10.1038/1771036b0. [DOI] [PubMed] [Google Scholar]
- 187.Casanovas JM, Springer TA. Pathway of rhinovirus disruption by soluble intercellular adhesion molecule 1 (ICAM-1): an intermediate in which ICAM-1 is bound and RNA is released. J Virol. 1994;68:5882–9. doi: 10.1128/jvi.68.9.5882-5889.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Greve JM, Davis G, Meyer AM, Forte CP, Yost SC, Marlor CW, et al. The major human rhinovirus receptor is ICAM-1. Cell. 1989;56:839–47. doi: 10.1016/0092-8674(89)90688-0. [DOI] [PubMed] [Google Scholar]
- 189.Tomassini JE, Graham D, DeWitt CM, Lineberger DW, Rodkey JA, Colonno RJ. cDNA cloning reveals that the major group rhinovirus receptor on HeLa cells is intercellular adhesion molecule 1. Proc Natl Acad Sci USA. 1989;86:4907–11. doi: 10.1073/pnas.86.13.4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yamaya M, Nishimura H, Hatachi Y, Yasuda H, Deng X, Sasaki T, et al. Levofloxacin inhibits rhinovirus infection in primary cultures of human tracheal epithelial cells. Antimicrob Agents Chemother. 2012;56:4052–61. doi: 10.1128/AAC.00259-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yosef N, Ubogu EE. αMβ2-Integrin-intercellular adhesion molecule-1 interactions drive the flow-dependent trafficking of Guillain-Barre syndrome patient derived mononuclear leukocytes at the blood-nerve barrier in vitro. J Cell Physiol. 2012;227:3857–75. doi: 10.1002/jcp.24100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zakynthinos E, Pappa N. Inflammatory biomarkers in coronary heart disease. J Cardiol. 2009;53:317–33. doi: 10.1016/j.jjcc.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 193.Peschon JJ, Slack JL, Reddy PS, Stocking KL, Sunnarborg SW, Lee DC, et al. An essential role for ectodomain shedding in mammalian development. Science. 1998;282:1281–4. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- 194.Rentzos M, Michalopoulou M, Nikolaou C, Cambouri C, Rombos A, Dimitrakopoulos A, et al. The role of soluble intercellular adhesion molecules in neurodegenerative disorders. J Neurol Sci. 2005;228:129–35. doi: 10.1016/j.jns.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 195.Christiansen I, Enblad G, Kalkner KM, Gidlof C, Glimelius B, Totterman TH. Soluble ICAM-1 in Hodgkin’s disease: a promising independent predictive marker for survival. Leuk Lymphoma. 1995;19:243–51. doi: 10.3109/10428199509107894. [DOI] [PubMed] [Google Scholar]
- 196.Tretjakovs P, Jurka A, Bormane I, Mikelsone I, Elksne K, Krievina G, et al. Circulating adhesion molecules, matrix metalloproteinase-9, plasminogen activator inhibitor-1 and myeloperoxidase in coronary artery disease patients with stable and unstable angina. Clin Chim Acta. 2012;413:25–9. doi: 10.1016/j.cca.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 197.Gu HF, Ma J, Gu KT, Brismar K. Association of intercellular adhesion molecule 1 (ICAM1) with diabetes and diabetic nephropathy. Front Endocrinol (Lausanne) 2012;3:179. doi: 10.3389/fendo.2012.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Claeysen S, Cochet M, Donneger R, Dumis A, Bockaert J, Giannoni P. Alzheimer culprits: cellular crossroads and interplay. Cell Signal. 2012;24:1831–40. doi: 10.1016/j.cellsig.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 199.Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148:1204–22. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Howe HS, Kong KO, Thong BY, Law WG, Chia FL, Lian TY, et al. Urine sVCAM-1 and sICAM-1 levels are elevated in lupus nephritis. Int J Rheum Dis. 2012;15:13–6. doi: 10.1111/j.1756-185X.2012.01720.x. [DOI] [PubMed] [Google Scholar]
- 201.Most J, Zangerle R, Herold M, Fuchs D, Wachter H, Fritsch P, et al. Elevated concentrations of circulating intercellular adhesion molecule 1 (ICAM-1) in HIV-1 infection. J Acquir Immune Defic Syndr. 1993;6:221–6. [PubMed] [Google Scholar]
- 202.Galea P, Vermot-Desroches C, Le Contel C, Wijdenes J, Chermann JC. Circulating cell adhesion molecules in HIV1-infected patients as indicator markers for AIDS progression. Res Immunol. 1997;148:109–17. doi: 10.1016/s0923-2494(97)82482-0. [DOI] [PubMed] [Google Scholar]
- 203.Wang Y, Cao M, Liu A, Liu W, Zhao F, Tian Y, et al. Changes in inflammatory cytokines and neutrophins emphasized their roles in hypoxic-ischemic brain damage. Int J Neurosci. 2013;123:191–5. doi: 10.3109/00207454.2012.744755. [DOI] [PubMed] [Google Scholar]
- 204.Ramer R, Bublitz K, Freimuth N, Merkord J, Rohde H, Haustein M, et al. Cannabidiol inhibits lung cancer cell invasion and metastasis via intercellular adhesion molecule-1. FASEB J. 2012;26:1535–48. doi: 10.1096/fj.11-198184. [DOI] [PubMed] [Google Scholar]
- 205.Hartwig W, Werner J, Warshaw AL, Antoniu B, Castillo CF, Gebhard MM, et al. Membrane-bound ICAM-1 is upregulated by trypsin and contributes to leukocyte migration in acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1194–G9. doi: 10.1152/ajpgi.00221.2004. [DOI] [PubMed] [Google Scholar]
- 206.Massi P, Solinas M, Cinquina V, Parolaro D. Cannabidiol as potential anticancer drug. Br J Clin Pharmacol. 2013;75:303–12. doi: 10.1111/j.1365-2125.2012.04298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Iuvone T, Esposito G, De Phillips D, Scuderi C, Steardo L. Cannabidol: a promising drug for neurodegenerative disorders. CNS Neurosci Ther. 2009;15:65–75. doi: 10.1111/j.1755-5949.2008.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Adair-Kirk TL, Atkinson JJ, Broekelmann TJ, Doi M, Tryggvason K, Miner JH, et al. A site on laminin α5, AQARSAASKVKVSMKF, induces inflammatory cell production of matrix metalloprotease-9 and chemotaxis. J Immunol. 2003;171:398–406. doi: 10.4049/jimmunol.171.1.398. [DOI] [PubMed] [Google Scholar]
- 209.Siu MKY, Cheng CY. Interactions of proteases, protease inhibitors, and the β1 integrin/laminin protein complex in the regulation of ectoplasmic specialization dynamics in the testis. Biol Reprod. 2004;70:945–64. doi: 10.1095/biolreprod.103.023606. [DOI] [PubMed] [Google Scholar]
- 210.Gerwin N, Gonzalo JA, Lloyd C, Coyle AJ, Reiss Y, Banu N, et al. Prolonged eosinophil accumulation in allergic lung interstitium of ICAM-2 deficient mice results in extended hyper-responsiveness. Immunity. 1999;10:9–19. doi: 10.1016/s1074-7613(00)80002-3. [DOI] [PubMed] [Google Scholar]
- 211.Huang MT, Larbi KY, Scheiermann C, Woodfin A, Gerwin N, Haskard DO, et al. ICAM-2 mediates neutrophil transmigration in vivo: evidence for stimulus specificity and a role in PECAM-1-independent transmigration. Blood. 2006;107:4721–7. doi: 10.1182/blood-2005-11-4683. [DOI] [PubMed] [Google Scholar]
- 212.Xiao X, Cheng CY, Mruk DD. Intercellular adhesion molecule-2 is involved in apical ectoplasmic specialization dynamics during spermatogenesis in the rat. J Endocrinol. 2013;216:73–86. doi: 10.1530/JOE-12-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Suarez Y, Wang C, Manes TD, Pober JS. TNF-induced microRNAs regulate TNF-induced expression of E-selectin and intercellular adhesion molecule-1 on human endothelial cells: feedback control of inflammation. J Immunol. 2010;184:21–5. doi: 10.4049/jimmunol.0902369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ueda R, Kohanbash G, Sasaki K, Fujita M, Zhu X, Kastenhuber ER, et al. Dicer-regulated microRNAs 222 and 339 promote resistence of cancer cells to cytotoxic T-lymphocytes by down-regulation of ICAM-1. Proc Natl Acad Sci USA. 2009;106:10746–51. doi: 10.1073/pnas.0811817106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Hu G, Gong AY, Liu J, Zhou R, Deng C, Chen XM. miR-221 suppresses ICAM-1 translation and regulates interferon-γ-induced ICAM-1 expression in human cholangiocytes. Am J Physiol Gastrointest Liver Physiol. 2010;298:G542–G50. doi: 10.1152/ajpgi.00490.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9:102–14. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 218.Moretti F, Thermann R, Hentze MW. Mechanism of translational regulation by miR-2 from sites in the 5′ untranslated region or the open reading frame. RNA. 2010;16:2493–502. doi: 10.1261/rna.2384610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Friedman RC, Farh KKH, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.McIver SC, Roman SD, Nixon B, McLaughlin EA. miRNA and mammalian male germ cells. Hum Reprod Update. 2012;18:44–59. doi: 10.1093/humupd/dmr041. [DOI] [PubMed] [Google Scholar]
- 221.Buchold GM, Coarfa C, Kim J, Milosavljevic A, Gunaratne PH, Matzuk MM. Analysis of microRNA expression in the prepubertal testis. PLoS ONE. 2010;5:e15317. doi: 10.1371/journal.pone.0015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Yan N, Lu Y, Sun H, Tao D, Zhang S, Liu W, et al. A microarray for microRNA profiling in mouse testis tissues. Reproduction. 2007;134:73–9. doi: 10.1530/REP-07-0056. [DOI] [PubMed] [Google Scholar]