Abstract
Couples often discuss genetic test results, and then manage their implications together. This interdependence can lead to common, shared experiences, similar intrapersonal processes to manage shared stressors, or interpersonal influences between spouses, leading to different outcomes. This study sought to reveal the intracouple, intrapersonal, and interpersonal influences of genetic stigma and negative feelings on spousal communication and perceived stress with 50 couples in which one spouse is a member of a genetic disease registry. The results were analyzed with dyadic analysis, including multilevel modeling. The findings showed that registered members and their spouses were not statistically different in their mean levels of perceived genetic stigma, negative feelings about alpha-1 antitrypsin deficiency (AATD), conversations with each other about the AATD test results, and their perceived stress. The findings also showed that their intracouple consistencies were not high, and their intrapersonal and interpersonal influences on communication and stress differed. The social implications of genetic research at the interpersonal level are discussed.
Keywords: Married couples, Dyadic analysis, Genetic Stigma, Negative Affect, Communication, Stress
Spouses often exhibit some level of similarity in their beliefs, attitudes, and behaviors. There are two common reasons for such interdependence: common fate and partner effects (Kenny, Kashy, & Cook, 2006). Common fate describes situations in which both spouses are exposed to the same experience. For example, they may both receive the news that one of them has inherited a genetic mutation associated with a health condition and both experience negative feelings as a result, which may be the case when couples undergo carrier testing. Partner effects occur when one person’s characteristics influence his or her spouse’s outcomes. For example, one spouse’s perceptions of a genetic stigma can influence his or her spouse’s level of stress.
When considering the social implications of genetic research, it is important to identify shared experiences and interpersonal influences. First, unaffected spouses may also experience negative feelings and stress when their spouse receives genetic test results. These unaffected partners may benefit by the development of support materials targeted to their needs in order to cope effectively. Second, if partners are influencing each other, then we may need to create different kinds of materials to assist their specific needs as a couple. Both shared and interpersonal effects may appear: spouses may talk with each other to cope with their shared reactions, and these spousal conversations may interpersonally influence their own levels of stress. While attention has been paid to genetic stigma, negative feelings, communication, and stress, to date, dyadic modeling has not been completed.
To fill this gap, this study models the intrapersonal and interpersonal influences in 50 couples in which one spouse is a registered member of the Alpha-1 Research Registry. We begin by describing AATD, which is followed by discussing influences in dyads.
Alpha-1 Antitrypsin Deficiency
Alpha-1 antitrypsin deficiency (AATD) is an autosomal codominant disorder caused by a mutation in the SERPINA1 gene, which predisposes people to diseases such as emphysema, cirrhosis, and lung or liver cancer (Laurell & Eriksson, 1963; Sharp, Bridges, Krivit, & Freier, 1969). Alpha-1 antitrypsin (AAT) is a serine protease inhibitor that is primarily produced in the liver. It then gets secreted into the blood stream and travels to the lungs where it protects the lungs from damage caused by neutrophil elastase released during periods of inflammation. Those individuals that are deficient in AAT, therefore have a higher risk for lung damage. Those that are homozygous for the M allele (PiMM) make normal amounts of AAT. However, the Z allele creates an AAT protein that polymerizes and accumulates inside the liver. With only about 15% of protein being released into the bloodstream, the remaining 80-90% can cause liver damage (ATS/ERS Statement, 2003). The clinical symptoms typically present when the person is an adult, with some children experiencing liver dysfunction. Age of onset and severity of symptoms vary along a wide spectrum with some living a lifetime without developing symptoms and others requiring a liver transplant antenatally. It is known that environmental factors, such as smoking increase the likelihood of developing symptoms. There are notable, unique features to AATD. For example, while homozygous results and particular phenotypes (e.g., PiZZ) are more strongly linked to severe symptom manifestations (i.e., wheezing, shortness of breath, chronic bronchitis, lung or liver deterioration), those with heterozygous results (e.g., PiMZ) can also develop serious symptoms through environmental exposure (e.g., pollutants) or health behaviors (e.g., smoking), which makes AATD different from many other genetic disorders (Klitzman, 2010; Tanash, Nilsson, Nilsson, & Piitulainen, 2010). Other unique features of AATD include its prevalence (1 in 2,500 in the US, according to the Alpha-1 Foundation), under-recognition (Campos, Wanner, & Zhang, & Sandhaus, 2005), delay in testing [five to eight years between symptom onset and genetic test results (Stoller et al., 2005)], and documentation associated with genetic discrimination (Jones & Sarata, 2008).
AATD fits into a class of genetic conditions in which the onset of clinical symptoms is adulthood, the likelihood of development is variable, and treatment and/or lifestyle modification can alter the onset or progression of clinical symptoms (Rolland & Williams, 2005). This is similar to other adult onset conditions like inherited breast and ovarian cancers associated with BRCA mutations and Alzheimer’s which has been associated with mutations in APOE. As testing for conditions of this type become more commonplace, knowing how to effectively counsel married couples will be crucial. AATD is already included on some expanded carrier screening panels due to the possibility of childhood liver disease. As a result, some people are now finding out that they are either homozygous or heterozygous for AATD in the context of a married relationship. This is further complicated by the impact this information could have on reproductive decision making.
Common Fate: Genetic Stigma and Negative Feelings
As noted earlier, one explanation for similarity between spouses is that they have both been exposed to the same experience. In the context of genetic test results, two variables—genetic stigma and negative feelings—fit within the common fate explanation. A genetic stigma is defined as the socialized, simplified, standardized image of the disgrace of a particular social group defined by shared genetics (Smith, 2007a, b). Stigmas are socialized stereotypes (Smith, 2007a, 2011), which suggests that everyone in a community, including both spouses in a couple, hold similar beliefs. While there is little research on AATD, studies of other genetic conditions show that psychological distress and negative feelings, such as guilt, frustration, sadness, and anger, are experienced by those receiving the genetic test results (Dohany, Gustafson, Ducaine, & Zakalik, 2012; Lippi, Favaloro, & Plebiani, 2011) and their spouses (Decruyenaere et al., 2004; Keenan, Simpson, Miedzybrodska, Alexander, & Semper, 2013; Metcalfe, Liede, Trinkaus, Hanna & Narod, 2002; Mireskandari et al., 2006; Richards & Williams, 2004). Indeed, communication privacy management (Petronio, 2002) argues that once a person discloses their health news to a confidant, the confidant may feel co-ownership of that information and its implications. Thus, spouses are likely to be similar in their genetic stigma beliefs and negative affect related to AATD, because the positive genetic test results make the genetic stigmas salient and evoke negative feelings.
Intrapersonal and Interpersonal Influences on Communication and Stress
Married persons receiving positive genetic tests often turn to their spouses for support (Metcalfe et al., 2002), and serve as support for their spouses (Keenan et al., 2013; Mireskandari et al., 2006). One reason may be to cope with genetic stigmas and negative affect (e.g., Wiseman, Dancyger, & Michie, 2010). Anticipating stigmatization and experiencing negative feelings are both stressful (Cohen et al., 1998; Goffman, 1963; Smith, 2011). Indeed, some couples experience stress when one of them has a genetic test, even if the results are negative (Richards & Williams, 2004). When experiencing such stressors, couples are likely to talk with each other. By talking with each other, spouses can provide emotional support and problem-focused support (Cohen, 2004). This rationale suggests an intrapersonal influence: as spouses report stronger genetic stigmas or negative affect, they may report more conversations with their partner about AATD and less overall stress as a result of these conversations. Spouses may not discuss AATD simply to satisfy their own needs.
A qualitative study of spouses of BRCA carriers (Mireskandari et al., 2006) suggests interpersonal influences on spousal communication: even though the husbands wanted to talk about the genetic results, they did not if the conversations or the topic upset their diagnosed wives. A strong concern of these husbands was to be a good support for their wife, which, for some, entailed avoiding sharing their own negative feelings about the test results. These dynamics resonate with research on two relationship-focused strategies couples use to cope with chronic illness: active engagement and protective buffering (Revenson & DeLongis, 2011). Active engagement occurs when spouses talk with each other about the problem in attempts to cope, solve, or manage it. Protective buffering, however, happens when a spouse hides his or her own concerns from their partner in order to protect them from upset and conflict. Active engagement can have positive outcomes, such as improving the patient’s wellbeing and reducing stress, by allowing the patient to gain control. Notably, research has also shown that when spouses engage in protective buffering, while their own stress may increase, there is no harm to the protected person (Revenson & DeLongis, 2011).
Genetic stigma and negative feelings, then, may affect spousal communication intrapersonally and interpersonally. Intrapersonally, as spouses report more genetic stigmas and negative feelings about AATD, they may talk with each other more and that communication may reduce their stress. Interpersonally, spouses may talk less to each other depending on their partner’s levels of stigma and affect. For example, ARR members may report more spousal communication when their spouses report more negative feelings about AATD, to help them cope. More spousal communication, associated more with one’s own needs or one’s partners’ needs, may reduce stress in the same ways that getting supported and being supportive can reduce stress and bring couples together (e.g., Mireskandari et al., 2006). These predictions are displayed in Figure 1. Thus, genetic stigma and negative feelings may influence spousal communication and perceived stress intrapersonally or interpersonally. It is important to identify which of these influences is more powerful and if it differs for ARR members and their spouses. Thus, the purpose of the present study was to investigate (1) how much genetic stigma and negative affect influence spousal communication and stress, and (2) the intrapersonal, interpersonal influences in these relations, and (3) the intracouple consistencies in their answers.
Figure 1.
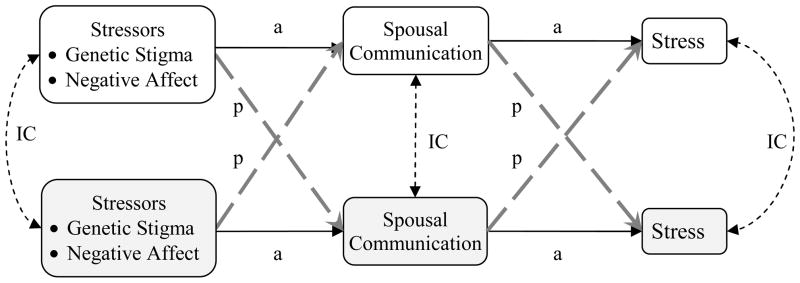
Modeling interpersonal communication about a genetic-based, adult-onset, chronic illness. Solid lines (marked with “a”) represent actor effects; grey, dashed lines (marked with “p”) represent partner effects; black, dashed double-headed arrows represent intraclass coefficients (marked with “IC”).
Method
Sample and Procedures
An institutional review board approved the study. Participants were recruited through the Alpha-1 Research Registry located at Medical University of South Carolina (MUSC) in August of 2012. The registry includes 1788 members (ARR members) who provided email addresses and indicated willingness to be contacted for research. The recruitment invitation (provided via email) told registered members that the study was interested in married couples’ experiences with the AATD test results, and provided the link to access our online questionnaire. Of the 1788 members, 179 members started the survey, and 130 completed it. We do not know how many of the ARR members are married, but the response rate (179/1788 or 10%) is likely an underestimate. Fifty-eight spouses of ARR members also completed the survey. Of the 130 ARR members and 58 spouses, 50 couples could be matched.
ARR members and their spouses participated in this study (n = 100; 50 couples). ARR members ranged in phenotypes (65% ZZ, 35% MZ); none of their spouses had tested positive. On average, members had known about their test results for over eight years (SD = 7.70, Median = 6.00, Minimum = 0, Maximum = 34). Participants on average were 57 years old (SD = 9.36, Median = 59, Minimum = 31, Maximum = 75), employed (49%), self-identified as White (95%), and had children (85%). These demographics are similar to those of the ARR registry overall.
After giving consent, participants were asked whether that they had a partner who could also complete the survey. Those without partners (n = 40 ARR members) were sent to a thank you page, and did not answer questions in the survey. Married participants were asked to report their marriage date and then state where they currently reside, which was used to link couples’ responses. Participants were then asked to complete measures related to genetic stigma, genetic testing, couple communication, emotions related to testing positive for AATD, and perceived stress, marital quality, diagnosed health conditions, and demographics. The survey was piloted with ten adults who were not members of the ARR, but were involved with AATD activities (e.g., education). A timestamp was in the survey, which showed that it took participants, on average, 20 minutes to complete the survey. Based on their feedback, we adjusted a few items in the spousal communication scales to make them clearer.
Measures
Genetic stigma
Eight items based on Link and colleagues (1989) were used to measure genetic stigma beliefs (e.g., Most people would feel that being diagnosed with a genetic mutation is a sign of personal failure). The response options were strongly disagree, disagree, neutral, agree and strongly agree, which were later coded for analysis (1 = strongly disagree to 5 = strongly agree). Responses were averaged into one score (α = .86, registered member; α = .84, spouse), with higher scores indicating stronger beliefs that AATD is a stigmatized condition.
Negative feelings
Seven items were used to measure negative emotional responses to the AATD test results, that is does thinking about the Alpha-1 test results make the participant feel particular emotions (e.g., anger; frustration; fear). The response options included not at all, at little, somewhat, moderately and very much, which were later coded for analysis (1 = not at all to 5 = very much). Responses were averaged into one score (α = .81, registered member; α = .84, spouse), with higher scores indicating stronger negative emotions regarding the AATD test results.
Spousal communication
Six items were used to measure the frequency of conversations couples currently have regarding AATD-related information (see the Appendix for the items and response options). Responses were coded (1=not at all to 5 = frequently) and averaged into one score (α = .85, registered member; α = .76, spouse), with higher scores indicating more frequent AATD-related couple communication.
Stress
Five items from the perceived stress scale (PSS, Cohen, Kamarck, & Mermelstein, 1983) were used to measure the degree to which people appraise their lives as stressful in the past month (e.g., In the last month, have you felt difficulties were piling up so high that you could not overcome them; 1 = yes, 0 = no). There is a ten-item and four-item version of the PSS. The four-item version does not include a few of the more emotional items found in the ten-item version. We retained an emotional item in the 10-item scale in our survey, specifically “Been upset because of something that happened unexpectedly?” when we used the 4-item scale. This item showed strong internal-consistency with the other items, so we retained it. Responses were summed into one score (α = .76, registered member; α = .72, spouse), with higher scores indicating higher levels of perceived stress.
Analysis Plan
First, interdependence in couples’ answers was assessed using Pearson-r correlations with a dyad structure, distinguishing participants as either the registered member (R) or the spouse (S) (Kenny et al., 2006). To test intrapersonal and interpersonal influences two strategies were pursued. First, multilevel modeling was used to estimate actor-partner interdependence models. Data were set up in an individual structure: each person has a row, and each row contains both person and partner’s data. Variables are created to distinguish each couple and their position in the couple (registered member or spouse), with effect codes (1= registered; −1 = spouse). Using SPSS syntax, distinguishing variable and interactions were modeled; the multilevel model was run allowing for compound symmetry (Kenny et al., 2006). Second, a path analysis was estimated with AMOS, which can be easier to interpret with distinguishable data (Kenny et al., 2006).
Results
Participants
Couples had been married, on average, 27 years (SD = 13.53, Minimum = 1.50, Maximum = 52); 50% of the participants were female. In the past year, ARR members reported being diagnosed with or experiencing chronic bronchitis (8%), emphysema (46%), chronic obstructive pulmonary disease (COPD, 42%), or liver disease (8%). The variable, disease status, was created by summing ARR members’ reported health conditions. ARR members reported no conditions (56%), one (18%), two (17%), three (8%), or all four conditions (1%). Notably, ARR members’ spouses reported on their own health conditions in the past year. In the past year, spouses reported being diagnosed with or experiencing chronic bronchitis (20%), emphysema (10%), COPD (16%), or liver disease (10%). Spouses varied in disease status: no conditions (70%), one (14%), two (8%), three (6%), or all four conditions (2%).
Descriptive Statistics
Table 1 presents the means and standard deviations for ARR members and spouses, and their intercorrelations. Spouses varied from disbelief to belief in a genetic stigma, from little to strong negative feelings, from rare to frequent spousal discussions, and from little to high levels of stress. The mean levels did not differ significantly between registered members and their spouses: paired-sample t(49) = −0.29, ns, for genetic stigma; paired-sample t(49) = −0.85, ns, for negative affect; paired-sample t(49) = 1.46, ns, for spousal communication, and paired-sample t(49) = 1.48, ns, for perceived stress.
Table 1.
Descriptive Statistics and Intercorrelations Among Variables (N = 50 dyads)
| M | SD | 1. | 2. | 3. | 4. | 5. | 6. | 7. | |
|---|---|---|---|---|---|---|---|---|---|
| 1. R Genetic stigma | 2.45 | 0.70 | -- | ||||||
| 2. R Negative affect | 2.12 | 0.74 | .36* | -- | |||||
| 3. R Spousal communication | 3.46 | 0.92 | .31* | .20 | -- | ||||
| 4. R Stress | 2.18 | 1.65 | .24 | .62* | .20 | -- | |||
| 5. S Genetic stigma | 2.48 | 0.60 | .31* | .18 | -.13 | .01 | -- | ||
| 6. S Negative affect | 2.23 | 0.83 | .19 | .36* | .30* | .25 | .27 | -- | |
| 7. S Spousal communication | 3.25 | 0.80 | .04 | .12 | .31* | .23 | -.27 | .04 | -- |
| 8. S Stress | 1.76 | 1.57 | .45* | .38* | .22 | .23 | .37* | .51* | .21 |
Note. Data structured dyadically; one row for each couple. R = registered member; S = spouse.
Intraclass coefficients are bolded. All of the scales range from 1 to 5.
p < .05
The intraclass coefficients (bolded in Table 1) were statistically significant for genetic stigma, negative affect, and spousal communication, but not for perceived stress. The significance test indicates whether the spouses are more similar to each other than what would be expected if they were paired with a random stranger (Kenny et al., 2006). The interdependence, while significant, is still not high for genetic stigma beliefs, current negative feelings about the genetic test results, or communication. Their stress is not significant, suggesting that there is not consistency between spouses’ stress levels.
Actor-partner effects
Multilevel modeling was used to estimate the intrapersonal and interpersonal effects of genetic stigma and negative feelings on spousal communication, and then all three variables on perceived stress. The results appear in Table 2.
Table 2.
Actor-Partner Interdependence Models Using Multilevel Modeling
| Spousal Communication | Stress | |||
|---|---|---|---|---|
| estimate | se | estimate | se | |
| Genetic stigma | 0.45* | 0.19 | 0.09 | 0.32 |
| Negative affect | 0.02 | 0.18 | 1.30* | 0.30 |
| P Genetic stigma | −0.50* | 0.22 | −0.24 | 0.38 |
| P Negative affect | 0.34* | 0.16 | 0.10 | 0.27 |
| Registered member | 0.92 | 0.74 | −3.25† | 2.01 |
| Genetic stigma* RM | −0.91* | 0.29 | 0.54 | 0.47 |
| Negative affect* RM | 0.05 | 0.24 | −0.62† | 0.38 |
| P Genetic stigma* RM | 0.61* | 0.29 | 0.86* | 0.48 |
| P Negative affect* RM | −0.22 | 0.24 | 0.09 | 0.38 |
| Spousal discussions | 0.01 | 0.25 | ||
| P Spousal discussions | 0.27 | 0.26 | ||
| Spousal discussions * RM | 0.49† | 0.34 | ||
| P Spousal discussions* RM | −0.35 | 0.34 | ||
|
| ||||
| Intraclass correlation | .23 | −.06 | ||
|
| ||||
| Pseudo R2 | .12 | .37 | ||
Notes. P denotes the partner’s answers. RM = registered member. Of note, the intraclass correlation computed from the multilevel model estimates controls for the effects of the independent variables (Kenny, Kashy, & Cook, 2006).
p < .10
p < .05
Spousal communication was predicted by intrapersonal and interpersonal variables (pseudo R2 = .12). Married spouses reported talking to their spouse more about the AATD test results if they perceived a stronger genetic stigma and their partner perceived a weaker one and felt less negative affect. There were also significant interactions for spousal position (registered member or spouse) with their own and their partner’s perceptions of genetic stigma.
Perceived stress was predicted by intrapersonal and interpersonal variables as well (pseudo R2 = .37). Married spouses reported greater stress when they felt stronger negative feelings about AATD, and their partner perceived a stronger genetic stigma. There were significant interactions for spousal position and the effects of negative affect, and reports of spousal discussions. These effects and interactions are easier to identify with path modeling when the dyad members have a distinguishing feature (Kenny et al., 2006), such as being a registered member or the spouse (versus identical twins). The path model is presented next.
Path Modeling
The path model depicted in Figure 1 was estimated using AMOS. The correlations among genetic stigma and negative affect within and across spouses were constrained to be the same, in accordance with a common fate prediction. The intraclass coefficient for genetic stigma, negative affect, spousal communication, and stress were allowed to estimate freely, as were the relationships from genetic stigma and negative affect to spousal communication and from spousal communication to stress.
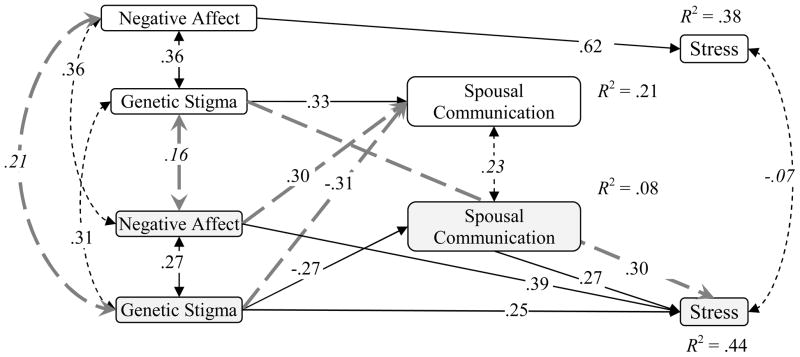
The model did not fit the data, X2 (11, N = 50) = 52.01, p = 0.00, CFI = 0.45, RMSEA = .28 (CI 90% .20, .35), SRMR = .18. Three issues were noted. First, the residual covariances showed strong, direct intrapersonal relationship from negative affect to stress for both spouses and registered members. Perceived stigma also showed a direct, intrapersonal relationship from genetic stigma to stress for spouses. A number of paths were not statistically significant, including intrapersonal paths from negative affect to spousal communication. Interpersonal paths from genetic stigma to spousal communication were also not statistically significant. The within-person relationships between genetic stigma and negative affect also had residual variances, so they were allowed to estimate freely. The paths were dropped, added, and unconstrained, and the model was re-estimated. The revised model fit the data, X2 (12, N = 50) = 5.59, p = 0.94, CFI = 1.00, RMSEA = .00 (CI 90% .00, .04), SRMR = .07. Paths were significant at p< .05 except for the intraclass coefficients for spousal communication and stress, and the interpersonal coefficients among genetic stigma and negative affect. The residuals were small. The final model is depicted in Figure 2.
Figure 2.
Empirical results of interpersonal Communication about a genetic-based, adult-onset, chronic Illness among Alpha-1 couples. Nonsignificant parameter estimates are italicized.
The findings suggest that there was intercouple consistency in negative affect and genetic stigma, supporting a common fate explanation. The relations from these stressors to spousal communication and perceived stress within person and across partners differed between registered members and their spouses. For registered members, both intrapersonal and interpersonal influences predicted spousal communication. When registered members reported talking more to their spouses about AATD as they perceived a stronger genetic stigma, their spouses perceived a weaker genetic stigma and felt more negative emotions. In contrast, spouses showed only an intrapersonal influence: they reported talking more with their spouses about AATD as they perceived a weaker genetic stigma.
The dynamics were the opposite for stress. Registered members showed only an intrapersonal influence: perceived stress was strongly predicted by stronger negative affect. For spouses, intrapersonal and interpersonal influences appeared. As spouses reported stronger negative affect, greater genetic stigma, and more spousal communication, and the registered members perceived a greater genetic stigma, spouses reported greater levels of stress.
Posthoc Analysis
A second multilevel modeling was used to estimate the intrapersonal and interpersonal effects of genetic stigma, negative feelings, and spousal communication on perceived stress after including additional couple-level covariates: gender of the registered member, disease status, time since tested, number of years together, and children present. The variables were not statistically significant. The parameter estimates for the predicted variables did not change significantly from those presented in Table 2.
Discussion
This study provides insights into the complexities in assessing intrapersonal and interpersonal influences in married couples’ experiences with genetic test results. ARR members and their spouses showed low intracouple consistencies in their perceptions of a genetic stigma, negative feelings about AATD, conversations with each other about the AATD test results, and their perceived stress. The intrapersonal and interpersonal influences on communication and stress differed between registered members and their spouses. These findings support the frequent claim that genetic tests influence carriers and their families in complex ways (Keenan et al., 2013; Metcalfe et al., 2002; Richards & Williams, 2004; Wiseman, Dancyger, & Michie, 2010), and insights into the form of those complexities.
Stress
Notably, couples showed the least similarity in their levels of perceived stress. This finding is important because it means that one person’s level of perceived stress should not be used to predict if their marital partner is also stressed. Both ARR members and spouses reported stress, which has been noted in other studies of couples receiving BRCA results (Metcalfe et al., 2002). As noted earlier, perceived stress overall, and specifically the measure used in this study, has been associated with physical stress responses (e.g., Cohen, Kessler, & Gordon, 1995) and negative behavioral responses (Cohen, 2004). The social implications of genetic research, then, may appear at macro-social levels, such as genetic stigmas and discrimination, as well as the intrapersonal and intrapersonal levels.
Notably, the model accounted for similar amounts of variance in perceived stress (R2 = .38 for registered members; R2 = .44 for spouses), but the covariates differ dramatically between registered members and spouses. For ARR members, there was one intrapersonal covariate: as they felt more negative emotions about AATD, they were more stressed. For spouses, intrapersonal and interpersonal influences appeared. As with ARR members, as spouses felt worse, they were more stressed. In addition, as spouses and ARR members perceived a stronger genetic stigma and spouses reported talking more about AATD with the ARR member, spouses were more stressed.
These findings suggest that interventions aiming to assist married adults with stress need to address their personal, negative feelings associated with the AATD test results. This finding resonates with research on other genetic conditions: negative feelings about positive test results are commonly expressed by those receiving the diagnosis and their partners (Mireskandari et al., 2006).
In addition, for spouses, communication with the ARR member about AATD and stress were positively related. Married adults who test positive for genetic mutations identify their spouses as their primary source of support (e.g., Metcalfe et al., 2002), and spouses express wanting to be supportive (Mireskandari et al., 2006). There are two possible reasons for the positive relationship among spousal communication and stress for spouses. First, spouses may be stressed because they do not feel like they are supporting their diagnosed partners well (Mireskandari et al., 2006). Second, spouses may be suppressing their own concerns in these conversations, and their secret-keeping creates stress (Keenan et al., 2013). The findings from this study suggest that spouses, in particular, may need assistance in having conversations with their registered husbands or wives that allow them to address their own negative feelings and concerns.
Genetic Stigma
Intrapersonally, genetic stigma was associated with spousal conversation for both ARR members and spouses. Indeed, genetic stigma was the only covariate of spousal conversations for spouses; spouses reported talking more with ARR members about AATD when spouses perceived a weaker genetic stigma. ARR members, in contrast, reported talking more to their spouses about AATD as they perceived a stronger genetic stigma, their spouses perceived a weaker genetic stigma, and their spouses felt more negative emotions. The findings support an active engagement strategy (Revenson & DeLongis, 2011) for registered members, but not just for themselves: this active engagement might also function to assist with their spouse’s negative feelings. The findings support a protective buffering effect (Revenson & DeLongis, 2011) for spouses, but there also could be a chilling effect. When spouses perceived a stronger genetic stigma, both the ARR member and the spouse reported that they talked less about AATD. Unfortunately, genetic stigma is a critical component to consider when developing support materials for spouses as well as creating interventions to help couples discuss and cope with test results.
It is possible that spouses who perceive a stronger genetic stigma refrain from participating in AATD-related spousal conversations as a means of buffering themselves from the genetic stigma they perceive to be associated with AATD. According to Goffman (1963), when a person has a social stigma, his or her associates—parents, siblings, friends, spouses—could also be extended the stigma and thereby experience stigmatization even though they themselves do not have the stigmatized condition, in this case AATD. Sometimes referred to as courtesy stigma (Goffman, 1963), this stigma-by-association can have socially damaging effects, and people often disassociate themselves from others with stigmatized conditions to avoid these negative effects. In this study, it is possible that while spouses do not entirely disassociate from their AATD-affected spouse (e.g., divorce, separate), they do disassociate from AATD in regard to communicating about the condition and thereby avoid acknowledging or accepting the stigma they perceive to be associated with the genetic condition.
Study Limitations and Future Research Recommendations
The findings are limited by the small sample, the response rate, and use of the registry. The small sample limited statistical power, which is around .6 (Preacher & Coffman, 2006). The response rate based on the entire membership was small (10%); even though this rate is an underestimate, because we do not know how many ARR members are married, it still suggests the possibility of a sampling bias in the results. The use of a registry, although it provided contact with persons with a mutation in the SERPINA1 gene, it may represent a selection bias of those willing to be registered. Indeed, the findings represent people with homozygous (ZZ) more than heterozygous (e.g., MZ) results. People considered carriers may be less likely to be involved in registries. Further, ARR members who have experienced problematic communication with their spouses may have decided not to join a research registry or to participate in our study. Future studies may benefit from including a measure of general communication within married spouses to assess how spousal conversations about the genetic test results may differ from their general marital patterns. The findings from this study should be treated cautiously until they have been replicated. For example, participants received the question in the same order, which could have introduced order effects. We also cannot confirm if the spouses followed our instructions and completed the surveys independently. Indeed, while this study provides insight into the frequency of different kinds of spousal conversations, the survey answers do not provide in depth descriptions of the nature of their conversations, which may be better complete through interviews.
In addition, although this study identified spousal communication, it did not attend to different communication patterns between couples. Fitzpatrick (1987, 1988) noted that couples’ communication patterns and decision-making styles differ. In a qualitative study of how spouses learned of a diagnosis, marital secret keeping by either diagnosed persons or their spouses aroused negative feelings and stress (Keenan et al., 2013). These different patterns may be important to further understand the interpersonal influences on wellbeing, and how it may be managed by different couples.
Conclusion
Couples often discuss genetic test results and manage their implications together. This interdependence can lead to common, shared experiences, similar intrapersonal processes to manage shared stressors, or interpersonal influences between spouses, leading to different outcomes. This study revealed the intracouple, interpersonal, and intrapersonal influences of genetic stigma and negative feelings on spousal communication and perceived stress. As we continue to understand the social implications of genetic results, further attention is needed at all social levels from communities to dyads. This study provided a start to identifying and understanding these interpersonal complexities. It also provides additional insights into the noted challenge of providing genetic counseling to married adults (Keenan et al., 2013).
Acknowledgments
This project was supported by an ELSI grant with the Alpha-1 Foundation and Award Number P50-DA010075-15 from the National Institute on Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Alpha-1 Foundation, the National Institute on Drug Abuse or the National Institutes of Health. We want to thank Roxanne Parrott, Michelle Baker, and Mary Poss for their feedback on earlier drafts of the paper. Most importantly, we are grateful to the members of the Alpha-1 Research Registry and their spouses for sharing their thoughts with us.
Appendix
Scale for Experienced Spousal Communication
Response options were not at all, rarely, sometimes, often, and frequently.
I have talked with my spouse about what Alpha-1 is.
I have talked with my spouse about how to treat conditions related to Alpha-1.
I have talked with my spouse about how the Alpha-1 results make me feel.
I have talked with my spouse about whether to share genetic results with insurance companies.
I have talked with my spouse about changing behavior (such as drinking, eating, exercise, or smoking) in order to avoid health conditions related to Alpha-1.
I have talked with my spouse about who else we will tell about the Alpha-1 diagnosis.
Footnotes
Conflict of Interest. The authors have no conflict of interest.
References
- American Thoracic Society/European Respiratory Society Statement. Standards for the diagnosis and management of individuals with alpha-1 antitrypsin deficiency. American Journal of Respiratory & Critical Care Medicine. 2003;168:818–900. doi: 10.1164/rccm.168.7.818. [DOI] [PubMed] [Google Scholar]
- Antoni MH, Schneiderman N, Fletcher MA, Goldstein DA, Ironson G, Laperriere A. Psychoneuroimmunology and HIV-1. Journal of Consulting and Clinical Psychology. 1990;58:38–49. doi: 10.1037//0022-006x.58.1.38. [DOI] [PubMed] [Google Scholar]
- Campos MA, Wanner A, Zhang G, Sandhaus RA. Trends in the diagnosis of symptomatic patients with α1-Antitrypsin Deficiency between 1968 and 2003. Chest. 2005;128:1179–1186. doi: 10.1378/chest.128.3.1179. [DOI] [PubMed] [Google Scholar]
- Cohen S. Psychosocial models of social support in the etiology of physical disease. Health Psychology. 1988;7:269–297. doi: 10.1037//0278-6133.7.3.269. [DOI] [PubMed] [Google Scholar]
- Cohen S. Social relationships and health. American Psychologist. 2004;59:676–684. doi: 10.1037/0003-066X.59.8.676. [DOI] [PubMed] [Google Scholar]
- Cohen S, Frank E, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM., Jr Types of stressors that increase susceptibility to the common cold in adults. Health Psychology. 1998;17:214–223. doi: 10.1037//0278-6133.17.3.214. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kessler RC, Gordon LU. Strategies for measuring stress in psychiatric and physical disorders. In: Cohen S, Kessler RC, Gordon LU, editors. Measuring stress. New York: Oxford University Press; 1995. pp. 3–28. [Google Scholar]
- Decruyenaere M, Evers-Kiebooms G, Cloostermans T, Boogaerts A, Demyttenaere K, Dom R, Fryns JP. Predictive testing for Huntington’s disease: Relationship with partners after testing. Clinical Genetics. 2004;65:24–31. doi: 10.1111/j..2004.00168.x. [DOI] [PubMed] [Google Scholar]
- Dohany L, Gustafson S, Ducaine W, Zakalik D. Psychological distress with direct-to-consumer genetic testing: A case report of an unexpected BRCA positive test result. Journal of Genetic Counseling. 2012 doi: 10.1007/s10897-011-9475-5. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick MA. Marriage and verbal intimacy. In: Derlaga VJ, Berg JH, editors. Self-disclosure: Theory, research, and therapy. New York, NY: Plenum Press; 1987. pp. 131–152. [Google Scholar]
- Fitzpatrick MA. Between husbands and wives: Communication in marriage. Newbury Park, CA: Sage; 1988. [Google Scholar]
- Goffman E. Stigma: Notes on the management of spoiled identity. Englewood Cliffs, NJ: Prentice Hall; 1963. [Google Scholar]
- Jones NL, Sarata AK. Report for Congress. Genetic Information: Legal Issues Relating to Discrimination and Privacy. 2008 Retrieved from http://biotech.law.lsu.edu/crs/RL30006_20080310.pdf on January 22 2013.
- Keenan KF, Simpson SA, Miedzybrodska Z, Alexander DA, Semper J. How do partners find out about the risk of Huntington’s Disease in couple relationships? Journal of Genetic Counseling. 2013 doi: 10.1007/s10897-012-9562-2. online. [DOI] [PubMed] [Google Scholar]
- Kenny DA, Kashy DA, Cook WL. Dyadic data analysis. New York, NY: The Guilford Press; 2006. [Google Scholar]
- Kiecolt-Glaser JK, Fisher LD, Ogrocki P, Stout JC, Speicher CE, Glaser R. Marital quality, marital disruption, and immune function. Psychosomatic Medicine. 1987;49:13–34. doi: 10.1097/00006842-198701000-00002. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R. Methodological issues in behavioral immunology research with humans. Brain, Behavior, and Immunology. 1988;2:67–78. doi: 10.1016/0889-1591(88)90007-4. [DOI] [PubMed] [Google Scholar]
- Klitzman R. Views of discrimination among individuals confronting genetic disease. Journal of Genetic Counseling. 2010;19:68–83. doi: 10.1007/s10897-009-9262-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurell CB, Eriksson S. The electrophoretic alpha-1 globulin pattern of serum in alpha-1 antitrypsin deficiency. Scandinavian Journal of Clinical Laboratory Investigation. 1963;15:132–140. [Google Scholar]
- Link BG, Cullen FT, Struening E, Shrout PE, Dohrenwend BP. A modified labeling theory approach to mental disorders: An empirical assessment. American Sociological Review. 1989;54:400–423. [Google Scholar]
- Lippi G, Favaloro EJ, Plebani M. Direct-to-consumer testing: More risks than opportunities. International Journal of Clinical Practice. 2011;65:1221–1229. doi: 10.1111/j.1742-1241.2011.02774.x. [DOI] [PubMed] [Google Scholar]
- Metcalfe KA, Liede A, Trinkaus M, Hanna D, Narod SA. Evaluation of the needs of spouses of female carriers of mutation in BRCA1 and BRCA2. Clinical Genetics. 2002;62:464–469. doi: 10.1034/j.1399-0004.2002.620607.x. [DOI] [PubMed] [Google Scholar]
- Mireskandari S, Meiser B, Sherman K, Warner BJ, Andrews L, Tucker KM. Evaluation of the needs and concerns of partners of women at high risk of developing breast/ovarian cancer. Psycho-Oncology. 2006;15:96–108. doi: 10.1002/pon.925. [DOI] [PubMed] [Google Scholar]
- Petronio S. Boundaries of privacy: Dialectics of disclosure. New York: State University of New York Press; 2002. [Google Scholar]
- Preacher KJ, Coffman DL. Computing power and minimum sample size for RMSEA. 2006 May; [Computer software]. Available from http://quantpsy.org/
- Revenson TA, DeLongis A. Couples coping with chronic illness. In: Folkman S, editor. Oxford handbook of stress, health, and coping. NY: Oxford University Press; 2011. pp. 101–123. [Google Scholar]
- Richards F, Williams K. Impact on couple relationships of predictive testing for Huntington Disease: A longitudinal study. American Journal of Medical Genetics. 2004;126a:161–169. doi: 10.1002/ajmg.a.20582. [DOI] [PubMed] [Google Scholar]
- Sharp H, Bridges R, Krivit W, Freier E. Cirrhosis associated with alpha-1 antitrypsin deficiency: A previously unrecognized inherited disorder. Journal of Laboratory and Clinical Medicine. 1969;73:934–939. [PubMed] [Google Scholar]
- Smith RA. Language of the lost: An explication of stigma communication. Communication Theory. 2007a;17:462–485. [Google Scholar]
- Smith RA. Picking a frame for communicating about genetics: Stigmas or challenges. Journal of Genetic Counseling. 2007b;16:289–298. doi: 10.1007/s10897-006-9075-y. [DOI] [PubMed] [Google Scholar]
- Smith RA. Stigma communication and health. In: Thompson TL, Parrott R, Nussbaum J, editors. Handbook of health communication. 2. London, UK: Taylor & Francis; 2011. pp. 455–468. [Google Scholar]
- Stoller JK, Sandhaus RA, Turino G, Dickson R, Rodgers K, Strange C. Delay in diagnosis of alpha-1 antitrypsin deficiency: A continuing problem. Chest. 2005;128:1989–94. doi: 10.1378/chest.128.4.1989. [DOI] [PubMed] [Google Scholar]
- Tanash HA, Nilsson PM, Nilsson JA, Piitulainen E. Survival in severe alpha-1 antitrypsin deficiency (PiZZ) Respiratory Research. 2010;11:44. doi: 10.1186/1465-9921-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman M, Dancyger C, Michie S. Communicating genetic risk information within families: A review. Familial Cancer. 2010;9:691–703. doi: 10.1007/s10689-010-9380-3. [DOI] [PubMed] [Google Scholar]