Abstract
Charcot spine is a progressive and destructive process that affects the vertebral bodies, intervertebral discs, and posterior facets. It is the result from repetitive microtrauma in patients who have decreased joint protective mechanisms due to loss of deep pain and proprioceptive sensation, typically because of spinal cord injury. The objective of the study is to report an unusual case of Charcot spine, as a late complication of traumatic spinal cord injury, treated by a circumferential arthrodesis performed with a single staged posterolateral costotransversectomy approach.
Keywords: Charcot spine, Spinal cord injury, Circumferential arthrodesis, Posterolateral costotransversectomy approach
INTRODUCTION
Charcot spine, also known as neuropathic spinal arthropathy, is a relatively rare, a progressive and destructive process that affects the vertebral bodies, intervertebral discs, and posterior facets11). It is the result from repetitive microtrauma in patients who have decreased joint protective mechanisms due to loss of deep pain and proprioceptive sensation caused by primary disease such as spinal cord injury, diabetic neuropathy, tertiary syphilis, anesthetic leprosy, syringomyelia, or congenital absence of pain syndrome3,5).
Generally, Charcot spine is difficult to manage because destruction and instability of one neuropathic joint can transfer to adjacent joints. Historically, the treatment of Charcot spine was conservative because surgical treatment had poor outcomes9,10). Recent advancements in instrumentation have facilitated the successful treatment of Charcot spine with surgical management.
The operative treatment of Charcot spine has conventionally been a combination of anterior and posterior surgery. But the morbidity associated with these surgical procedures can be considerable. A single staged posterolateral costotransversectomy approach to the problem would avoid the additional morbidity associated with an anterior approach5,19).
The objective of the study is to report an unusual case of Charcot spine, as a late complication of traumatic spinal cord injury, treated by a circumferential arthrodesis performed with a single staged posterolateral costotransversectomy approach.
CASE REPORT
The patient was a 57-year-old man with a 26 year history of complete T8 paraplegia. He suffered a T7, 8 burst fracture in a motor vehicle accident. At that time, he was treated at another institution. In 2010, 26 years after the injury, the patient presented to our institution because of a crunching noise inside his back when transferring from the wheelchair to the bed. He was unable to maintain the sitting posture.
Physical examination showed a kyphotic deformity of the thoracolumbar junction. He had flaccid, areflexic lower extremities. He had no significant contractures at the hips and knees. Laboratory analysis revealed no signs of infectious spondylitis or urinary tract infection.
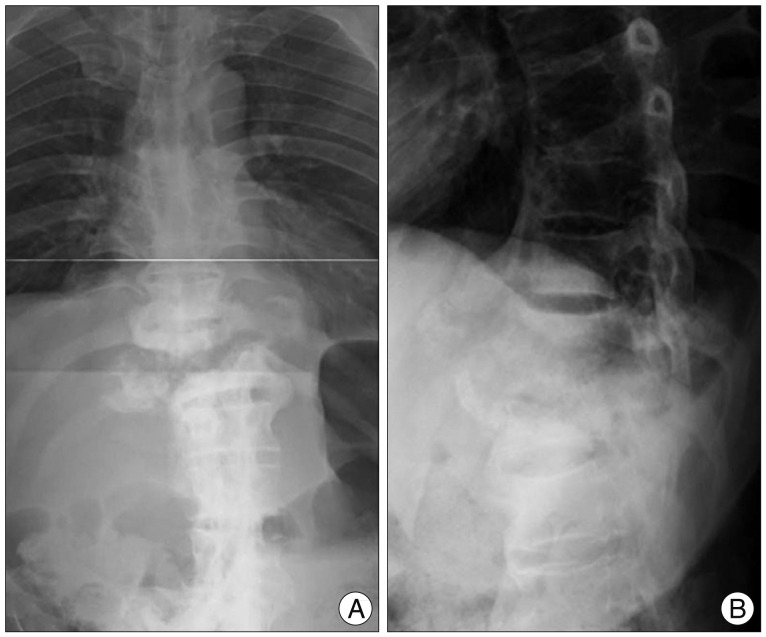
On thoracolumbar spine radiographs, there was old fracture of T7, 8 by trauma, and deformity at the T11, 12 segments : right ward translation of T11 with respect to the distal segments; marked destruction of the T11, 12 vertebral body; productive bony changes including sclerosis and bulky osteophytes; and destruction of the posterior elements (Fig. 1).
Fig. 1.
Thoracolumbar spine (A) AP and (B) lateral radiograph show old fracture of T7, 8 and right ward translation of T11 by extensive destruction of the T11, 12 vertebral columns.
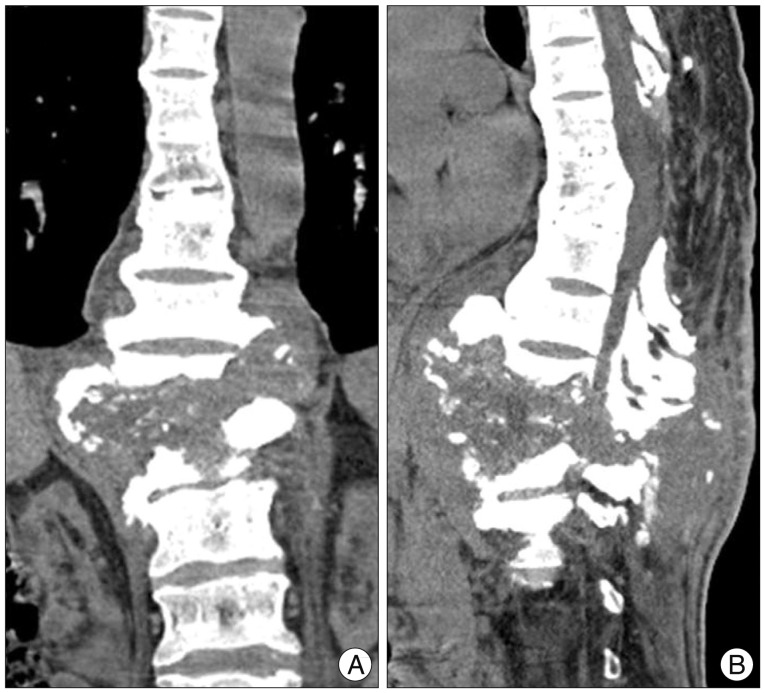
CT scan confirmed the radiographic findings of an extensive destructive. Additionally, CT revealed a large complex paraspinal soft tissue process with areas of fluid attenuation, ossifications, and peripheral bony debris extending as far as the posterior paraspinal soft tissues (Fig. 2).
Fig. 2.
Thoracolumbar (A) coronal and (B) sagittal CT image show three-column bony destruction, and a large complex paraspinal soft tissue with areas of ossifications and peripheral bony debris.
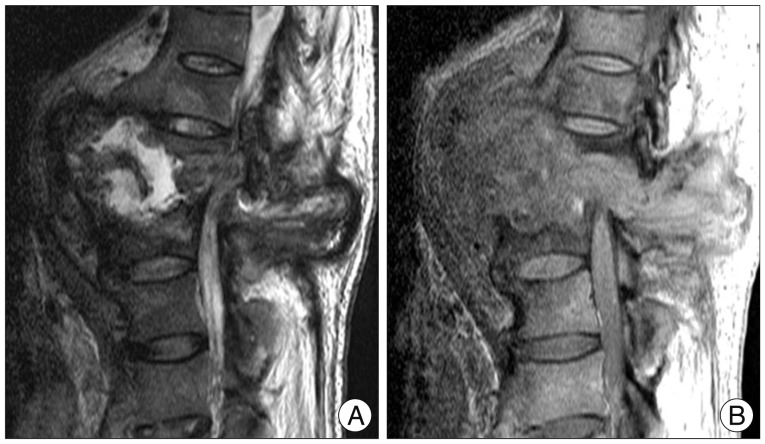
MRI further demonstrated the complexity of the vertebral destruction and paraspinal mass with associated fluid collections. The mass was intermediate signal intensity on T1 weighted images, complex with mildly hyperintense areas on T2 weighted images, and demonstrated peripheral enhancement (Fig. 3).
Fig. 3.
Magnetic resonance imaging scans showing the complexity of the vertebral destruction and paraspinal mass with associated fluid collections. The mass is intermediate signal intensity on T1 weighted image (A), complex with mildly hyperintense areas on T2 weighted image (B).
In view of this radiological evidence, the possibility of a chronic infection or a cancerous process was nevertheless envisaged and prompted us to perform a surgical exploration and biopsy for ruling out these differential diagnoses. Cultures of liquid samples were negative. Histological examination revealed the presence of fibrous tissue with sequestered bone but no signs of malignancy (Fig. 4). The findings were typical of Charcot spine. Therefore, the authors could make a diagnosis Charcot spine.
Fig. 4.
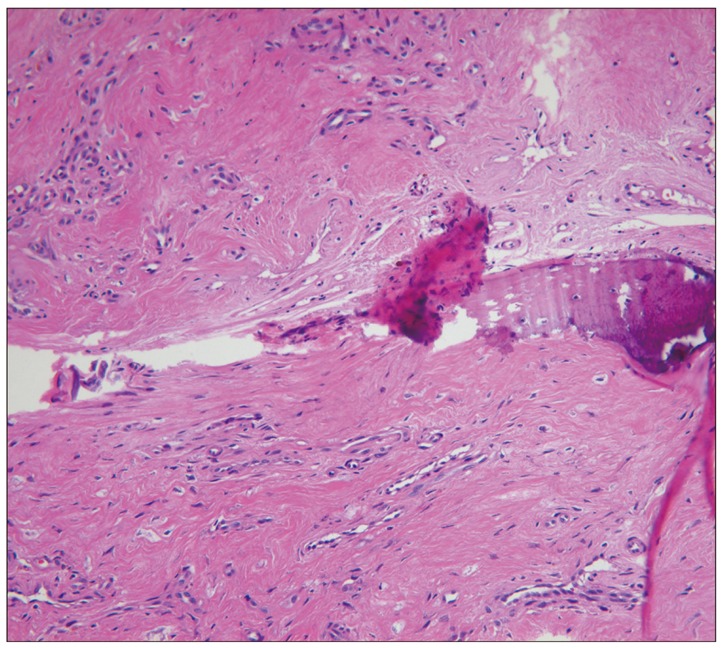
Photomicrograph shows fibrous and bony tissue with hemosiderin pigment and hyalinization (H&E stain ×100).
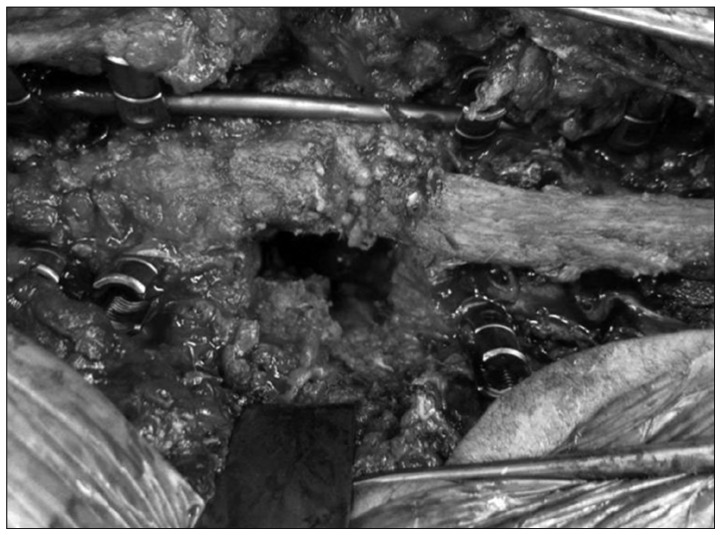
One week after a surgical exploration and biopsy, the authors performed a circumferential arthrodesis via a single staged posterolateral costotransversectomy approach for correction T11, 12 severe deformities. The thoracolumbar spine was exposed and pedicle screws were inserted into T8-T10 and L1-L3. Because of gross instability and collapse across the Charcot segment, a unilateral rod was used for temporary stability and distraction. Laminectomy was performed at T10 and L1 to gain access to the normal epidural space above and below. Next, the scar and hypertrophic reactive tissue around the left T11, 12 roots and the residual T11, 12 posterior elements was removed. The left T11, 12 roots were then ligated and divided to give access to the lateral and anterior aspects of the destroyed vertebral bodies (Fig. 5). Bony resection of the residual T11, 12 bodies and adjacent disc was then carried out, taking care to keep within the confines of the anterior pseudocapsule of the Charcot joint. The end plates of T10 and L1 were then prepared. A mesh case with auto-iliac bone graft, placed within the concavity of the endplates. The lateral posterior elements of T10 (inferior facet) and L1 (superior facet) were decorticated and packed with morselized local bone and allograft. The patient tolerated the surgical procedure well, there were no intraoperative complications, and estimated blood loss was 500 mL. After surgery, the patient was mobilized as tolerated without brace. A prophylactic antibiotic was used for 48 hours after surgery. There were no postoperative complications.
Fig. 5.
Intraoperative photograph shows the posterolateral exposure of the Charcot segment, with a temporary stabilizing rod in place before distraction.
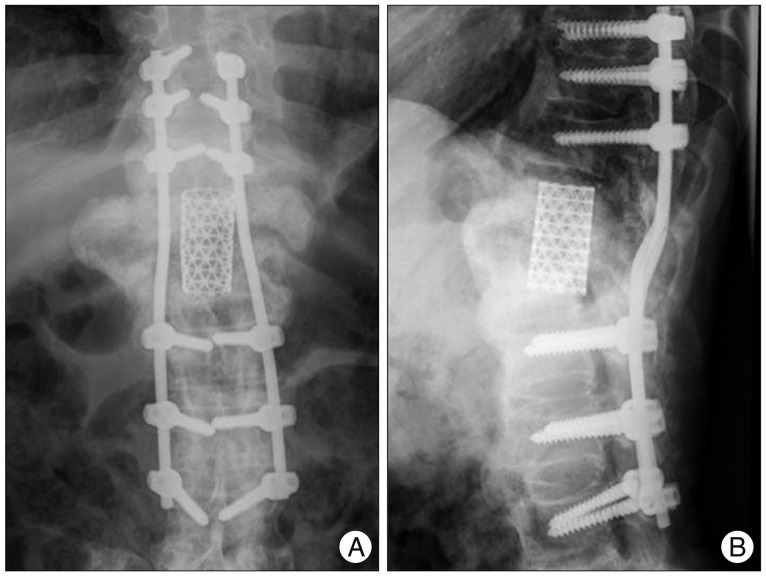
Two year after surgery, the patient returned to independent life including driving to work. And the fusion appeared solid without evidence of hardware loosening or change in spinal alignment on the plain radiographs (Fig. 6). However, the follow-up CT imagies revealed inadequate preparation of T10 lower and L1 upper end plate, and incorrect placement of a mesh cage. A floating mesh cage (not placed within the concavity of the endplates) generally cannot guarant solid fusion. We thought that causes of inadequate endplate preparation were limited visualization of posterior approach and vague boundary between bone and disc by the scar and hypertrophic reactive tissue around the Charcot segment. Instead of getting fusion between T10 and L1, we luckily got solid fusion between the residual T11, 12 bodies by a mesh cage with auto-iliac bone graft (Fig. 7).
Fig. 6.
The two years later follow up thoracolumbar sitting (A) AP and (B)lateral radiograph shows solid fusion and good alignment without evidence of hardware loosening.
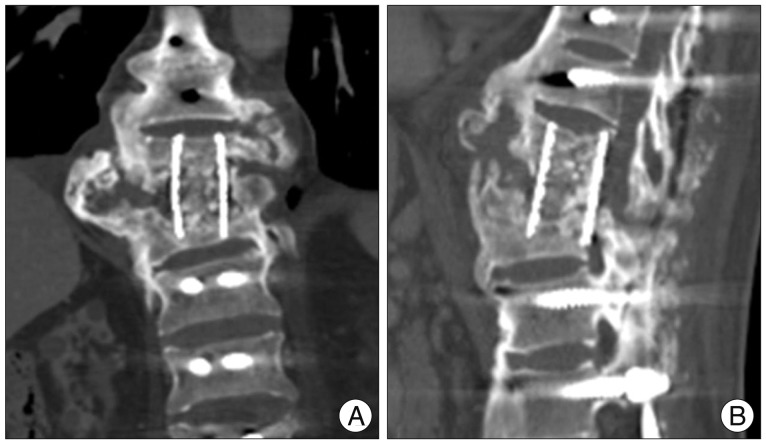
Fig. 7.
Thoracolumbar (A) coronal and (B) sagittal CT image shows inadequate preparation of T10 lower and L1 upper end plate, and solid fusion between the residual T11, 12 bodies by a mesh cage.
DISCUSSION
In 1868, Jean Charcot described neuropathic arthropathies that developed in patients with tabes dorsalis8,14). Since that time, neuropathic arthropathy, neuroarthropathy, and Charcot joint have been synonymous terms used to describe a progressive and destructive arthropathy that develops secondary to a neurological lesion. Any joint in the body may be affected by a Charcot joint; the lower extremity is most commonly involved14). Spinal involvement, first reported by Kronig in 1884, is less common and has been reported to occur in 6-21% of patients with neuropathic arthropathy14).
The most classical underlying cause of Charcot spine has traditionally been tabes dorsalis, and other causes include traumatic injury of the spinal cord, diabetic polyneuropathy, syringomyelia, congenital analgesia, and peripheral nerve injury. In recent years, as the number of patients with tabes dorsalis has decreased markedly, because of the low incidence of syphilis, the number of patients with Charcot spine caused by traumatic injury of the spinal cord is on the increase3,14).
The main disease mechanism in Charcot spine is impairment of joint innervation with loss of proprioception and sensitivity to pain. Impaired innervation induces loss of the joint's defence mechanisms which otherwise help avoid excessive distraction of the disc and ligaments and enable a more homogeneous distribution of the mechanical stresses7,8). This causes further repetitive trauma, fractures, and progressive instability. The overall destruction of joint structural elements predisposes the patient to kyphotic deformities, retrolisthesis, and frank dislocation. Often, gross spinal instability is seen with the formation of a ball and socket joint.
Another mechanism is the neurovascular theory, which assumes that neurologic changes produced by an underlying medical disorder that results in a hypervascular region in the subchondral bone characterized by increased osteoclastic resorption and osteoporosis1,6). This state leads to pathologic microfractures and eventual subchondral collapse, followed by joint destruction.
In Charcot spine, mechanical factors appear to be critical. In paraplegic patients, spinal stress in the position sitting and during transfers (which repeatedly expose the mobile spine below the fused area to excessive stress) is significant. Then, the risk of early occurrence of Charcot spine is greater in very active spinal cord injured patients3). In previously operated patients, Charcot spine typically develops either within the operated area (e.g., a non-instrumented laminectomy) or below an extensively instrumented area5). According to several authors, laminectomy appears to be an aggravating factor17,19).
The time lag between the onset of neurological impairment and a diagnosis of Charcot spine is usually long (17.3 years, on average)5). The time lag between the first symptoms of Charcot spine and its diagnosis is also long in many cases, due to the relatively non-specific symptoms. In paraplegic patients with complete neurologic impairment, the most frequent symptoms are a feeling of instability in the sitting position and spinal deformity (usually with a thoracolumbar gibbosity and an audible cracking noise)5,10).
On radiological studies, the findings of Charcot spine reflect the underlying pathology. Plain films can show destruction of the articular facets as one of the earliest signs along with disc space narrowing and end-plate sclerosis. Some authors distinguish atrophic and hypertrophic forms16,17).
Atrophic forms resulting in osteolysis and bony destruction occur less frequently. Typically, these are related to peripheral nerve lesions. More commonly, there are hypertrophic forms in which there is sclerosis, large osteophyte formation, disc space destruction, and pseudoarthrosis formation. Hypertrophic forms are more closely related to lesions of the central cord and more often observed around the spine.
A CT examination is useful for assessing the severity of vertebral body bone destruction and the extent of paravertebral bone formation. MR findings are similar to those on CT, with better delineation and characterization of the soft tissue mass15-18).
The differential diagnosis to consider includes infection (bacterial, fungal, and tuberculosis), osteoarthritis, tumor, and Paget's disease. But the differential diagnoses are very difficult because there are no Charcot spine specific signs. The following signs are non-discriminant : bone erosion, osteophytosis, narrowing of the intervertebral space and a paravertebral mass. This explains the high frequency of disc and vertebra biopsies in these patients (36% of cases) with a view to ruling out these differential diagnoses and confirming the diagnosis of Charcot spine1,19,20). In our case, vertebra biopsy was done for confirming the diagnosis of Charcot spine.
Although natural progression of the disease is slow, it is clearly related to aggravation of disc and vertebral lesions1,8). It is also important to emphasize the risk of secondary neurological aggravation in incompletely paraplegic patients10). The various therapeutic options consist of monitoring, immobilization with a body jacket and surgery.
Conservative treatment may be reasonable in patients without neurologic deficit or severe symptoms. However, if conservative therapy fails or there is already instability, then surgical management is an option.
The aim of surgery is to stabilize the diseased spinal segment by obtaining high quality fusion. Debridement of necrotic or inflamed tissue (above all sclerosed and hypovascularized bone) is usually preferable, in order to optimize conditions for bone fusion. The debridement is best performed via an anterior approach13). In cases with mild bony involvement, a posterior only approach may be effective5,18). However, in more advanced disease, most surgical strategies have involved anterior approaches to the spine, excision of diseased segments followed by anterior reconstruction, followed by supplemental posterior instrumentation via a separate approach15,18).
This two-stage procedure is often associated with considerable patient morbidity, surgical time, and blood loss. Unfortunately, the previous reports of two-stage procedures for Charcot spine have not included any details of operative time or intraoperative blood loss to enable a comparative analysis of anterior and posterior versus posterior approach alone17,18).
A single staged posterolateral costotransversectomy approach would avoid the additional morbidity associated with an anterior approach in patients with single level disease. A situation where there is a complete transection of the spinal cord with absence of neural elements at the site of the Charcot joint would also facilitate direct access to the anterior column through a single posterior incision2,4,12).
The other surgical option is a posterior three-column shortening procedure. A posterior three-column shortening procedure has been used with success in patients with multilevel disease and a major fixed frontal or sagittal plane deformity. A posterior three-column shortening procedure has the benefits of enhancing bony contact, thus obviating the need of a sizeable anterior cage or a long segment structural allograft.
The boundaries of the instrumented zone are subject to much debate. After fusion, there is a risk of developing damage below the operated spinal segment and above it1,13).
Some authors recommend the systematic extension of the operation to the pelvis, in order to eliminating the risk of seeing additional Charcot spine develops below the instrumentation. Hans et al.9) suggest that patients whose Charcot spine is fused to the lumbar spine have a higher long term risk of developing a second Charcot joint than those patients whose were fused to the sacrum or pelvis. An additional advantage of fusion to the pelvis is that it may be lead to a higher fusion rate. Therefore, in patients with Charcot involvement of the lumbar spine, extensive fusion to the pelvis was recommended9). But, Morita et al. reported the limitation of extensive fusion to the pelvis with the case of a patient who could no longer perform self catherization after surgery, due to post-arthrodesis spinal ankylosis13). Thus, it is important to evaluate the functional impact of arthrodesis including pelvis. Although solid fusion to the sacrum or pelvis may prevent the development of an additional Charcot spine and facilitate union, it may also significantly decrease function. One patient had excellent lumbar flexibility because of the gross motion in the Charcot spine but had limited flexion at the hips because of bilateral heterotopic ossification. After the spine was fused to the sacrum or pelvis, the patient lost the capacity to perform activities of daily living. This case demonstrates that fusion to the sacrum or pelvis in the setting of limited hip range of motion can lead to a significant loss of function9). Although in most cases it is preferable to extend the fusion to the sacrum or pelvis, treatment should therefore be individualized. Surgeons discussing treatment with patients should explain that, if flexibility is to be preserved by fusing to the lumbar spine rather than the sacrum or pelvis, there is an increased likelihood of a recurrence and need for more complicated revision surgery in the future.
References
- 1.Barrey C, Massourides H, Cotton F, Perrin G, Rode G. Charcot spine : two new case reports and a systematic review of 109 clinical cases from the literature. Ann Phys Rehabil Med. 2010;53:200–220. doi: 10.1016/j.rehab.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Choi DY, Kim YB, Yeo SJ, Park SW, Hwang SN. One stage spondylodesis for the bursting fracture of the thoracolumbar spine through unilateral posterior approach. J Korean Neurosurg Soc. 2004;35:379–382. [Google Scholar]
- 3.Crim JR, Bassett LW, Gold RH, Mirra JM, Mikulics M, Dawson EG, et al. Spinal neuroarthropathy after traumatic paraplegia. AJNR Am J Neuroradiol. 1988;9:359–362. [PMC free article] [PubMed] [Google Scholar]
- 4.David KS, Agarwala AO, Rampersaud YR. Charcot arthropathy of the lumbar spine treated using one-staged posterior three-column shortening and fusion. Spine (Phila Pa 1976) 2010;35:E657–E662. doi: 10.1097/BRS.0b013e3181e03577. [DOI] [PubMed] [Google Scholar]
- 5.Devlin VJ, Ogilvie JW, Transfeldt EE, Boachie-Adjei O, Bradford DS. Surgical treatment of neuropathic spinal arthropathy. J Spinal Disord. 1991;4:319–328. doi: 10.1097/00002517-199109000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Eloesser L. On the nature of neufopathic affections of the joints. Ann Surg. 1917;66:201–207. doi: 10.1097/00000658-191708000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta R. A short history of neuropathic arthropathy. Clin Orthop Relat Res. 1993;(296):43–49. [PubMed] [Google Scholar]
- 8.Harrison MJ, Sacher M, Rosenblum BR, Rothman AS. Spinal Charcot arthropathy. Neurosurgery. 1991;28:273–277. doi: 10.1097/00006123-199102000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Haus BM, Hsu AR, Yim ES, Meter JJ, Rinsky LA. Long-term follow-up of the surgical management of neuropathic arthropathy of the spine. Spine J. 2010;10:e6–e16. doi: 10.1016/j.spinee.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 10.Hoppenfeld S, Gross M, Giangarra C. Nonoperative treatment of neuropathic spinal arthropathy. Spine (Phila Pa 1976) 1990;15:54–56. doi: 10.1097/00007632-199001000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs WB, Bransford RJ, Bellabarba C, Chapman JR. Surgical management of Charcot spinal arthropathy : a single-center retrospective series highlighting the evolution of management. J Neurosurg Spine. 2012;17:422–431. doi: 10.3171/2012.7.SPINE111039. [DOI] [PubMed] [Google Scholar]
- 12.Kim YS, Cho YE. One stage three column fixation by posterior approach in thoracolumbar junction lesion. J Korean Neurosurg Soc. 1998;27:222–228. [Google Scholar]
- 13.Morita M, Miyauchi A, Okuda S, Oda T, Yamamoto T, Iwasaki M. Charcot spinal disease after spinal cord injury. J Neurosurg Spine. 2008;9:419–426. doi: 10.3171/SPI.2008.9.11.419. [DOI] [PubMed] [Google Scholar]
- 14.Park YH, Taylor JA, Szollar SM, Resnick D. Imaging findings in spinal neuroarthropathy. Spine (Phila Pa 1976) 1994;19:1499–1504. doi: 10.1097/00007632-199407000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Rose DM, Hilton AI, Tucker SK. Charcot spinal arthropathy in a paraplegic weight lifter : case report. Spine (Phila Pa 1976) 2006;31:E339–E341. doi: 10.1097/01.brs.0000217651.73372.66. [DOI] [PubMed] [Google Scholar]
- 16.Staloch MA, Hatem SF. Charcot spine. Emerg Radiol. 2007;14:265–269. doi: 10.1007/s10140-007-0615-z. [DOI] [PubMed] [Google Scholar]
- 17.Standaert C, Cardenas DD, Anderson P. Charcot spine as a late complication of traumatic spinal cord injury. Arch Phys Med Rehabil. 1997;78:221–225. doi: 10.1016/s0003-9993(97)90267-7. [DOI] [PubMed] [Google Scholar]
- 18.Suda Y, Saito M, Shioda M, Kato H, Shibasaki K. Infected Charcot spine. Spinal Cord. 2005;43:256–259. doi: 10.1038/sj.sc.3101687. [DOI] [PubMed] [Google Scholar]
- 19.Vialle R, Mary P, Tassin JL, Parker F, Guillaumat M. Charcot's disease of the spine : diagnosis and treatment. Spine (Phila Pa 1976) 2005;30:E315–E322. doi: 10.1097/01.brs.0000164283.01454.9f. [DOI] [PubMed] [Google Scholar]
- 20.Wagner SC, Schweitzer ME, Morrison WB, Przybylski GJ, Parker L. Can imaging findings help differentiate spinal neuropathic arthropathy from disk space infection? Initial experience. Radiology. 2000;214:693–699. doi: 10.1148/radiology.214.3.r00mr16693. [DOI] [PubMed] [Google Scholar]