Abstract
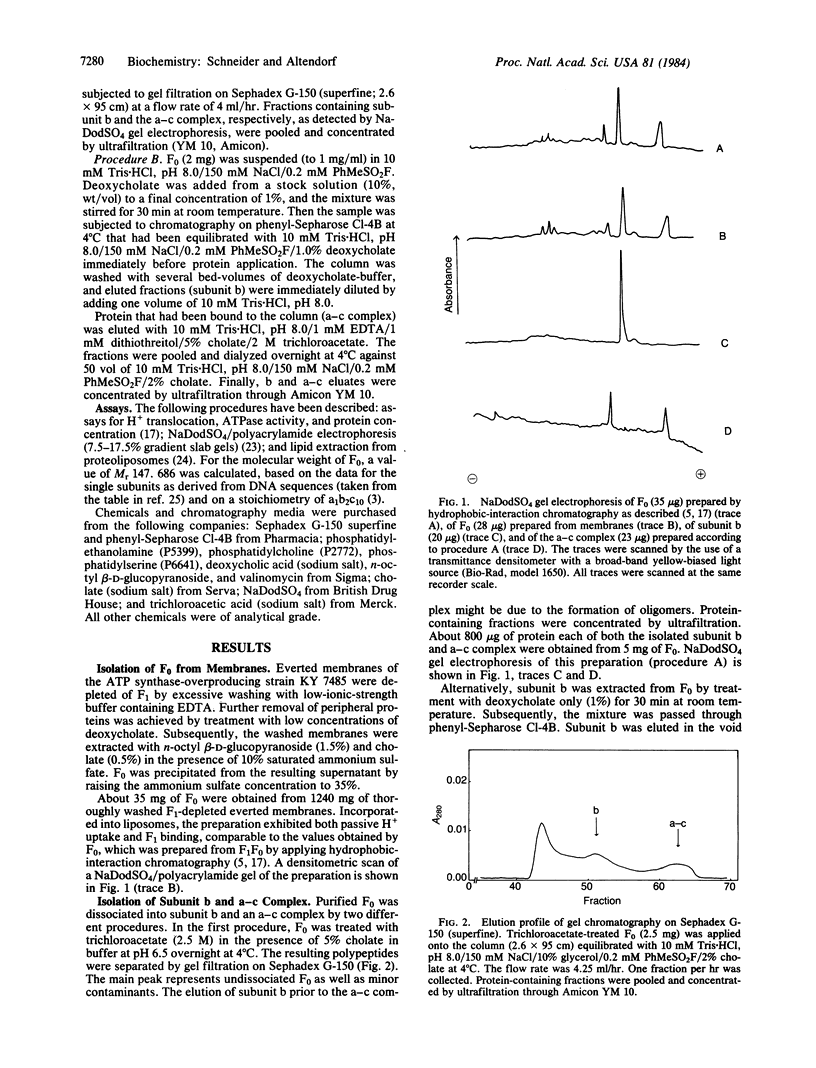
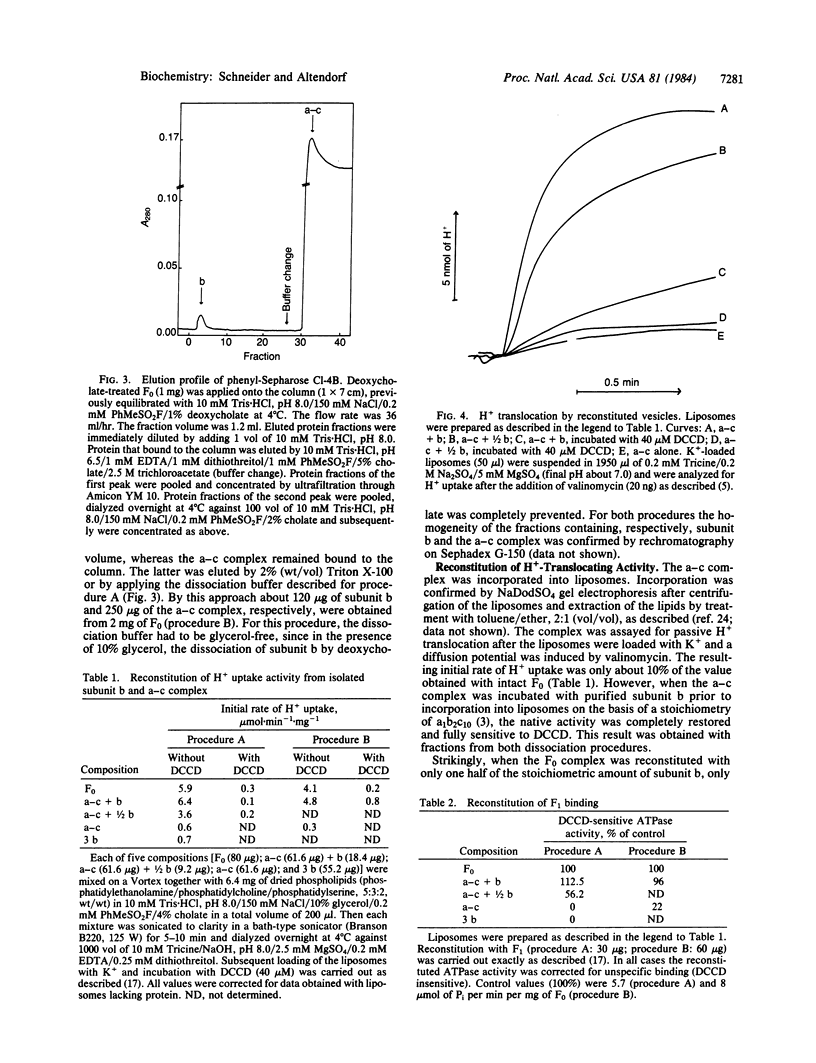
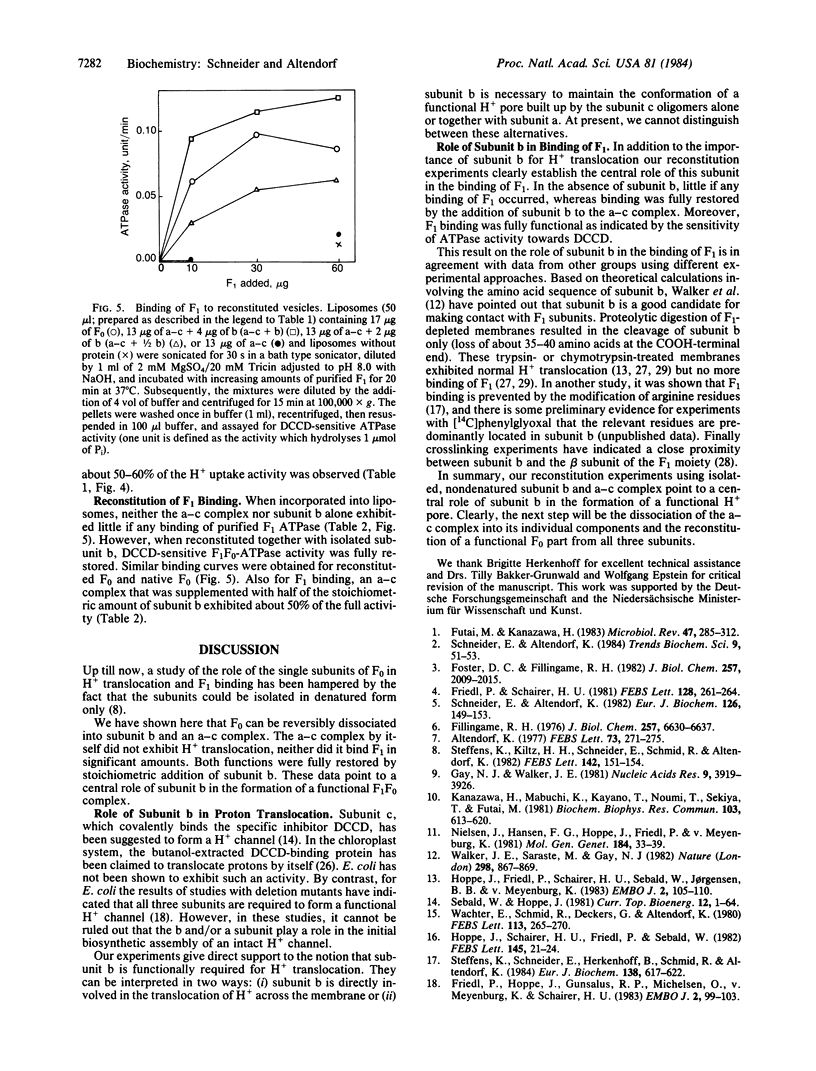
The ATP synthase complex, designated F1F0, of Escherichia coli is composed of a water-soluble portion (F1; membrane-associated ATPase, EC 3.6.1.3) with ATP-hydrolyzing activity and a membrane-integrated part (F0) with H+-translocating activity. F0 is built up from three kinds of subunits (a, b, and c). We have isolated the F0 portion directly from membranes of an E. coli strain (KY 7485) that overproduces the enzyme several fold. Subunit b was extracted from purified F0 by two methods. One method included prolonged incubation of the F0 complex in the presence of trichloroacetate (2.5 M) and the separation of subunit b and an a-c complex by gel filtration. Alternatively, subunit b was extracted by deoxycholate and separated from the a-c complex by hydrophobic-interaction chromatography. Integrated into liposomes, the a-c complex exhibited neither H+ uptake nor binding of F1. However, a functional F0 complex was reconstituted by adding stoichiometric amounts of subunit b to the a-c complex.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altendorf K. Purification of the DCCD-reactive protein of the energy-transducing adenosine triphosphatase complex from Escherichia coli. FEBS Lett. 1977 Feb 1;73(2):271–275. doi: 10.1016/0014-5793(77)80997-6. [DOI] [PubMed] [Google Scholar]
- Altendorf K., Zitzmann W. Identification of the DCCD-reactive protein of the energy transducing adenosinetriphosphatase complex from Escherichia coli. FEBS Lett. 1975 Nov 15;59(2):268–272. doi: 10.1016/0014-5793(75)80390-5. [DOI] [PubMed] [Google Scholar]
- Aris J. P., Simoni R. D. Cross-linking and labeling of the Escherichia coli F1F0-ATP synthase reveal a compact hydrophilic portion of F0 close to an F1 catalytic subunit. J Biol Chem. 1983 Dec 10;258(23):14599–14609. [PubMed] [Google Scholar]
- Douglas M., Finkelstein D., Butow R. A. Analysis of products of mitochondrial protein synthesis in yeast: genetic and biochemical aspects. Methods Enzymol. 1979;56:58–66. doi: 10.1016/0076-6879(79)56009-1. [DOI] [PubMed] [Google Scholar]
- Fillingame R. H. Purification of the carbodiimide-reactive protein component of the ATP energy-transducing system of Escherichia coli. J Biol Chem. 1976 Nov 10;251(21):6630–6637. [PubMed] [Google Scholar]
- Foster D. L., Fillingame R. H. Stoichiometry of subunits in the H+-ATPase complex of Escherichia coli. J Biol Chem. 1982 Feb 25;257(4):2009–2015. [PubMed] [Google Scholar]
- Friedl P., Hoppe J., Gunsalus R. P., Michelsen O., von Meyenburg K., Schairer H. U. Membrane integration and function of the three F0 subunits of the ATP synthase of Escherichia coli K12. EMBO J. 1983;2(1):99–103. doi: 10.1002/j.1460-2075.1983.tb01388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P., Schairer H. U. The isolated F0 of Escherichia coli aTP-synthase is reconstitutively active in H+-conduction and ATP-dependent energy-transduction. FEBS Lett. 1981 Jun 15;128(2):261–264. doi: 10.1016/0014-5793(81)80094-4. [DOI] [PubMed] [Google Scholar]
- Futai M., Kanazawa H. Structure and function of proton-translocating adenosine triphosphatase (F0F1): biochemical and molecular biological approaches. Microbiol Rev. 1983 Sep;47(3):285–312. doi: 10.1128/mr.47.3.285-312.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futai M., Sternweis P. C., Heppel L. A. Purification and properties of reconstitutively active and inactive adenosinetriphosphatase from Escherichia coli. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2725–2729. doi: 10.1073/pnas.71.7.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay N. J., Walker J. E. The atp operon: nucleotide sequence of the promoter and the genes for the membrane proteins, and the delta subunit of Escherichia coli ATP-synthase. Nucleic Acids Res. 1981 Aug 25;9(16):3919–3926. doi: 10.1093/nar/9.16.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermolin J., Gallant J., Fillingame R. H. Topology, organization, and function of the psi subunit in the F0 sector of the H+-ATPase of Escherichia coli. J Biol Chem. 1983 Dec 10;258(23):14550–14555. [PubMed] [Google Scholar]
- Hoppe J., Friedl P., Schairer H. U., Sebald W., von Meyenburg K., Jørgensen B. B. The topology of the proton translocating F0 component of the ATP synthase from E. coli K12: studies with proteases. EMBO J. 1983;2(1):105–110. doi: 10.1002/j.1460-2075.1983.tb01389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe J., Schairer H. U., Friedl P., Sebald W. An Asp-Asn substitution in the proteolipid subunit of the ATP-synthase from Escherichia coli leads to a non-functional proton channel. FEBS Lett. 1982 Aug 16;145(1):21–29. doi: 10.1016/0014-5793(82)81198-8. [DOI] [PubMed] [Google Scholar]
- Huang K. S., Bayley H., Khorana H. G. Delipidation of bacteriorhodopsin and reconstitution with exogenous phospholipid. Proc Natl Acad Sci U S A. 1980 Jan;77(1):323–327. doi: 10.1073/pnas.77.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa H., Mabuchi K., Kayano T., Noumi T., Sekiya T., Futai M. Nucleotide sequence of the genes for F0 components of the proton-translocating ATPase from Escherichia coli: prediction of the primary structure of F0 subunits. Biochem Biophys Res Commun. 1981 Nov 30;103(2):613–620. doi: 10.1016/0006-291x(81)90495-2. [DOI] [PubMed] [Google Scholar]
- Nielsen J., Hansen F. G., Hoppe J., Friedl P., von Meyenburg K. The nucleotide sequence of the atp genes coding for the F0 subunits a, b, c and the F1 subunit delta of the membrane bound ATP synthase of Escherichia coli. Mol Gen Genet. 1981;184(1):33–39. doi: 10.1007/BF00271191. [DOI] [PubMed] [Google Scholar]
- Perlin D. S., Cox D. N., Senior A. E. Integration of F1 and the membrane sector of the proton-ATPase of Escherichia coli. Role of subunit "b" (uncF protein). J Biol Chem. 1983 Aug 25;258(16):9793–9800. [PubMed] [Google Scholar]
- Schneider E., Altendorf K. ATP synthetase (F1F0) of Escherichia coli K-12. High-yield preparation of functional F0 by hydrophobic affinity chromatography. Eur J Biochem. 1982 Aug;126(1):149–153. doi: 10.1111/j.1432-1033.1982.tb06759.x. [DOI] [PubMed] [Google Scholar]
- Schneider E., Altendorf K. Reconstitution of the purified proton conductor (F0) of the adenosine triphosphatase complex from Escherichia coli. FEBS Lett. 1980 Jul 28;116(2):173–176. doi: 10.1016/0014-5793(80)80636-3. [DOI] [PubMed] [Google Scholar]
- Senda M., Kanazawa H., Tsuchiya T., Futai M. Conformational change of the alpha subunit of Escherichia coli F1 ATPase: ATP changes the trypsin sensitivity of the subunit. Arch Biochem Biophys. 1983 Feb 1;220(2):398–404. doi: 10.1016/0003-9861(83)90429-0. [DOI] [PubMed] [Google Scholar]
- Senior A. E., Wise J. G. The proton-ATPase of bacteria and mitochondria. J Membr Biol. 1983;73(2):105–124. doi: 10.1007/BF01870434. [DOI] [PubMed] [Google Scholar]
- Sigrist-Nelson K., Azzi A. The proteolipid subunit of the chloroplast adenosine triphosphatase complex. Reconstitution and demonstration of proton-conductive properties. J Biol Chem. 1980 Nov 25;255(22):10638–10643. [PubMed] [Google Scholar]
- Steffens K., Kiltz H. H., Schneider E., Schmid R., Altendorf K. ATP-synthetase complex (F1F0) from Escherichia coli. Purification and characterization of subunits A and B of the F0 part. FEBS Lett. 1982 Jun 1;142(1):151–154. doi: 10.1016/0014-5793(82)80240-8. [DOI] [PubMed] [Google Scholar]
- Steffens K., Schneider E., Herkenhoff B., Schmid R., Altendorf K. Chemical modification of the F0 part of the ATP synthase (F1F0) from Escherichia coli. Effects on proton conduction and F1 binding. Eur J Biochem. 1984 Feb 1;138(3):617–622. doi: 10.1111/j.1432-1033.1984.tb07959.x. [DOI] [PubMed] [Google Scholar]
- Wachter E., Schmid R., Deckers G., Altendorf K. Amino acid replacement in dicyclohexylcarbodiimide-reactive proteins from mutant strains of Escherichia coli defective in the energy-transducing ATPase complex. FEBS Lett. 1980 May 5;113(2):265–270. doi: 10.1016/0014-5793(80)80606-5. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Gay N. J. E. coli F1-ATPase interacts with a membrane protein component of a proton channel. Nature. 1982 Aug 26;298(5877):867–869. doi: 10.1038/298867a0. [DOI] [PubMed] [Google Scholar]



