Abstract
Glucagon-like peptide-1 (GLP-1) is an incretin hormone released from intestinal L-cells in response to food entering into the gastrointestinal tract. GLP-1-based pharmaceuticals improve blood glucose regulation and may hold promise for obesity treatment, as GLP-1 drugs reduce food intake and body weight in humans and animals. In an effort to improve GLP-1 pharmacotherapies, we focused our attention on macronutrients that, when present in the gastrointestinal tract, may enhance GLP-1 secretion and improve glycemic regulation and food intake suppression when combined with systemic administration of sitagliptin, a pharmacological inhibitor of DPP-IV (enzyme responsible for GLP-1 degradation). In particular, previous data suggest that specific macronutrient constituents found in dairy foods may act as potent secretagogues for GLP-1 and therefore may potentially serve as an adjunct dietary therapy in combination with sitagliptin. To directly test this hypothesis, rats received intraperitoneal injections of sitagliptin (6 mg/kg) or saline vehicle followed by intraduodenal infusions of either milk protein concentrate (MPC; 80/20% casein/whey; 4 kcal), soy protein (nondairy control infusate; 4 kcal), or 0.9% NaCl. Food intake was assessed 30 min postinfusion. In separate studies, regulation of blood glucose was examined via a 2-h oral glucose tolerance test (2 g/kg) following identical sitagliptin treatment and intraduodenal nutrient infusions. Collectively, results show that intraduodenal MPC, but not soy protein, significantly enhances both the food intake suppression and improved control of blood glucose produced by sitagliptin. These data support the hypothesis that dietary intake of dairy protein may be beneficial as an adjunct behavioral therapy to enhance the glycemic and food intake suppressive effects of GLP-1-based pharmacotherapies.
Keywords: obesity, diabetes, dairy, insulin, GLP-1
the incidence of obesity and Type II diabetes mellitus (T2DM) has risen dramatically in the United States (6, 12, 46). Leading theories suggest that for the vast majority of the population, treatments involving a combination of behavioral lifestyle modifications (e.g., dietary changes) and pharmacotherapy would be most effective in combating these diseases (50–53). However, the mechanisms and specific dietary and pharmacological treatments that make a combination approach more efficacious remain unclear. Management of T2DM often involves lifelong treatment with pharmaceuticals like those targeting the glucagon-like peptide-1 (GLP-1) system (10, 44), an incretin hormone produced in the distal intestine and the hindbrain. Several GLP-1-based pharmacotherapies are approved by the Food and Drug Administration (FDA) for the treatment of T2DM, including sitagliptin, which reduces the degradation of endogenously produced GLP-1 by inhibiting the endopeptidase dipeptidyl peptidase (DPP)-IV. Thus identification of dietary factors that can enhance the improved postprandial insulin secretion, food intake suppression, and weight loss by GLP-1-based pharmaceuticals is of critical importance. To this end, accumulating epidemiological data and basic science evidence support the perspective that regular consumption of dairy foods may have a beneficial role in body weight management and prevention of metabolic syndrome (1, 11, 34, 35, 49, 61), potentially through mechanisms involving GLP-1 signaling (39).
While all three macronutrient classes elicit GLP-1 secretion (16, 23, 27, 36, 40, 43, 55), it remains to be determined whether there are differences in the behavioral and physiological effects produced by GLP-1 signaling following intraintestinal exposure to an isolated macronutrient from one food group compared with another (i.e., protein derived from dairy versus soy). Thus one hypothesis regarding the relationship between regular dairy consumption and reduced body weight gain (11, 60) is that the unique macronutrient constituents found within dairy foods may enhance gastrointestinal (GI)-derived satiation signaling through increased secretions of specific gut peptides like GLP-1. Complete dairy protein [a.k.a. milk protein concentrate (MPC)] is composed of ∼80% casein and ∼20% whey, and as a result, dairy foods have a relatively high concentration of essential amino acids (11). The intraintestinal presence of specific bioactive components, whole proteins, and select amino acids found within MPC is linked with insulin and gut peptide secretions, as well as suppression of food intake (2, 3, 9, 11, 14, 18, 28). In fact, it is suggested that in animals maintained on an obesogenic high-fat diet, consumption of complete dairy protein attenuates weight gain and body fat mass accumulation more so than consumption of whey or casein alone (11). Thus it stands to reason that when combined with a pharmaceutical compound like sitagliptin that prevents the degradation of endogenous GLP-1, the glycemic and food intake suppressive effects produced by endogenous GLP-1 signaling may be enhanced by the presence of MPC within the small intestine. Therefore, the present experiments directly test the hypothesis that intraduodenal infusion of MPC enhances the food intake- and glycemic-suppressive effects of pharmacological DPP-IV inhibition by sitagliptin to a greater extent than isocaloric infusions of soy protein.
MATERIALS AND METHODS
Animals
Adult male Sprague-Dawley rats (Charles River Laboratories; 330–420 g, 11–13 wk of age at the beginning of testing) were housed individually in hanging wire-bottom metal cages and maintained on a 12-h light/12-h dark cycle (lights on at 0800 h). All rats had ad libitum access to rodent chow (Purina 5001 Rodent Diet) and water except as noted. All procedures conformed to and received approval by the institutional standards of the University of Pennsylvania Animal Care and Use Committee.
Surgery
As nutrients emptying from the stomach into the small intestine first enter the duodenum where digestion and absorption begin, we chose this as the site to experimentally infuse proteins. Briefly, rats were implanted with intraduodenal catheters under ketamine (90 mg/kg), xylazine (2.7 mg/kg), and acepromazine (0.64 mg/kg) anesthesia and analgesia (Metacam, 2 mg/kg). Briefly, as described previously (20, 21, 26, 59), intraduodenal catheter implantation consisted of the insertion of a 21-cm silicone rubber catheter (0.025′′ inner diameter, 0.047′′ outer diameter, VWR, Bridgeport, NJ) into the duodenum 2 cm distal to the pylorus and advancement 5 cm within the duodenal lumen in an aborad direction. The exposed end of the catheter exited subcutaneously through a skin incision between scapulas and was occluded with a stainless steel obturator, which was removed only for flushing of the catheter (0.3 ml of 0.9% NaCl) every other day and for experimental infusions. A minimum of 7 days was allowed for recovery before experimental infusions.
Drugs and Infusates
The DPP-IV inhibitor sitagliptin monophosphate, NaCl, and (+)-d-glucose were obtained from Sigma Aldrich. MPC 85 was obtained from Sports Supplements (Colchester, UK). Soy protein was obtained from Genisoy. Tween 80, the emulsifier for the intraduodenal protein and saline infusions, was obtained from Fisher Scientific.
Food Intake Studies
For all studies, rats were habituated to experimental procedures for 1 wk before testing. For all conditions, rats were deprived of food overnight (16 h). Each rat received counterbalanced intraperitoneal (ip) injections of the DPP-IV inhibitor sitagliptin (6 mg/kg; dose chosen from Ref. 47) or vehicle (0.9% NaCl; 1 ml/kg) followed immediately by an 8-ml intraduodenal infusion lasting 20 min (0.4 ml/min; 300 mosmol; pH 7.35–7.4) using a syringe infusion pump (Harvard Apparatus, South Natick, MA). The total 8-ml infusion volume and rate is within the physiological range of gastric distension and emptying (13, 22, 32, 57). In separate experiments, infusions of emulsified MPC (0.5 kcal/ml MPC in 0.9% NaCl with 1.5% Tween 80) were counterbalanced with vehicle (0.9% NaCl with 1.5% Tween 80), and infusions of emulsified soy protein (0.5 kcal/ml soy protein in 0.9% NaCl with 1.5% Tween 80) were counterbalanced with vehicle (0.9% NaCl with 1.5% Tween 80). The caloric concentration of 4 kcal per infusion was selected because it is subthreshold for effect alone on subsequent 30-min food intake (see Fig. 1), is <5% of the average total daily caloric intake of adult male rats, and is within the range of previous reports examining feeding effects of intraintestinal nutrient infusions in rats (8, 20, 26, 37). Preweighed food was returned 5 min after infusions and subsequent chow intake was measured at 30 min with spillage accounted for via crumb papers below the cages. All conditions were separated by a minimum of 48 h.
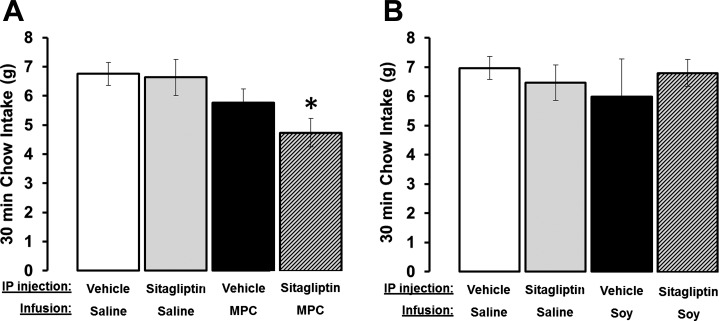
Fig. 1.
A: administration of the DPP-IV inhibitor sitagliptin (6 mg/kg, ip) in combination with intraduodenal infusion of 4 kcal of milk protein concentrate (MPC) suppressed 30 min chow intake. *P < 0.008 from vehicle-saline. B: there were no differences among treatments when the same dose of sitagliptin was delivered in combination with intraduodenal infusions of 4 kcal of soy protein. Data are means ± SE.
Glycemic Studies
The glycemic effects of combined DPP-IV inhibition with intraduodenal protein infusions were analyzed via an oral glucose tolerance test. Similar to the feeding studies, rats were overnight food deprived (16 h) to ensure an empty gastrointestinal tract before testing. At the start of experimental testing, water bottles were removed from the animals' home cages. Blood was collected from the tail tip of each rat, and blood glucose was measured by a standard glucometer (Accucheck, Roche Diagnostics) to determine baseline blood glucose concentrations (time = −20 min). Next, rats received an intraperitoneal injection of sitagliptin (6 mg/kg) or vehicle followed immediately by an intraduodenal infusion for 20 min as described for the feeding studies. Thus, in separate experiments, infusions of emulsified MPC (0.5 kcal/ml) were counterbalanced with vehicle, and separately, infusions of emulsified soy protein (0.5 kcal/ml) were counterbalanced with vehicle. Immediately after intraduodenal nutrient infusions, blood glucose concentrations were again assessed, and rats then received an oral glucose load (25%; 2 g/kg) via gavage (time = 0 min). Thus blood glucose was measured at −20, 0, 20, 40, 60, and 120 min in relation to the timing of the glucose load. Food and water were returned at the end of testing, and experimental treatments were separated by a minimum of 48 h.
Data and Statistical Analysis
All data are expressed as means ± SE. Data were analyzed by two-way repeated measures ANOVA, followed by Student-Newman-Keuls post hoc tests when main effects or interactions were significant. All statistical analyses were performed using Statistica Software. P < 0.05 was considered statistically significant.
RESULTS
Intraduodenal Infusion of Milk Protein Concentrate Enhances the Glycemic and Food Intake Suppressive Effects of DPP-IV Inhibition
Food intake (n = 7).
A main effect of MPC on food intake was observed at 30 min postfood presentation [main effect of MPC, F1,8 = 12.6693; P < 0.008]. However, planned comparisons between treatment conditions revealed that food intake was only significantly suppressed by the combination of an intraduodenal infusion of MPC and sitagliptin administration (Fig. 1A; vehicle-0.9% NaCl vs. sitagliptin-MPC, P < 0.05). Food intake after saline-MPC or sitagliptin-0.9% NaCl administration was similar to that observed after vehicle treatments (P > 0.05).
Glycemia (n = 33).
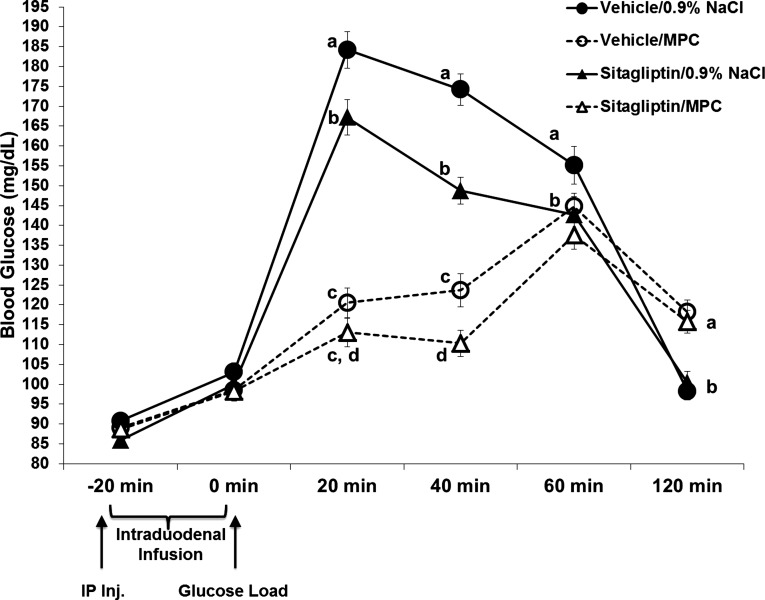
Sitagliptin alone significantly suppressed blood glucose at 20 and 40 min postglucose load [main effect of sitagliptin, at 20 min F1,32 = 9.615; P < 0.004; at 40 min: F1,32 = 50.772; P < 0.0001]. The glycemic effect of sitagliptin was enhanced by intraduodenal infusion of MPC at 40 min postglucose load (Fig. 2; F1,32 = 139.206; P < 0.01). In addition, MPC alone produced a suppression of blood glucose at 20 min postglucose load [main effect of MPC; F(1,32) = 269.176; P < 0.001].
Fig. 2.
Blood glucose concentrations (mg/dl) after intraperitoneal injection of vehicle or sitagliptin (6 mg/kg; time −20 min) in combination with intraduodenal infusions of 4 kcal of MPC or 0.9% NaCl in an oral glucose-tolerance test (2 g/kg of 25% glucose delivered via gavage at time 0). Sitagliptin alone significantly suppressed blood glucose levels compared with vehicle-saline at 20, 40, and 60 min postglucose load. MPC alone significantly suppressed blood glucose levels compared with vehicle-saline at 20 and 40 min postglucose load. When combined, MPC enhanced the glycemic suppressive effects of sitagliptin at 40 min postglucose load. Within a time point, data points with different letters are significantly different from each other (P < 0.05). Data are means ± SE.
Intraduodenal Infusion of Soy Protein Does Not Enhance the Glycemic or Food Intake Suppressive Effects of DPP-IV Inhibition
Food intake (n = 9).
To confirm that the enhanced suppression of food intake produced by the combination of sitagliptin and MPC was specific to dairy protein and to rule out the possibilities that the effect could be broadly attributed to any intraintestinal protein or caloric load, a separate group of rats received isocaloric intraduodenal infusions of soy protein in combination with sitagliptin. The combination of soy protein infusion with sitagliptin administration had no significant effect on 30 min chow intake (Fig. 1B; P > 0.05).
Glycemia (n = 24).
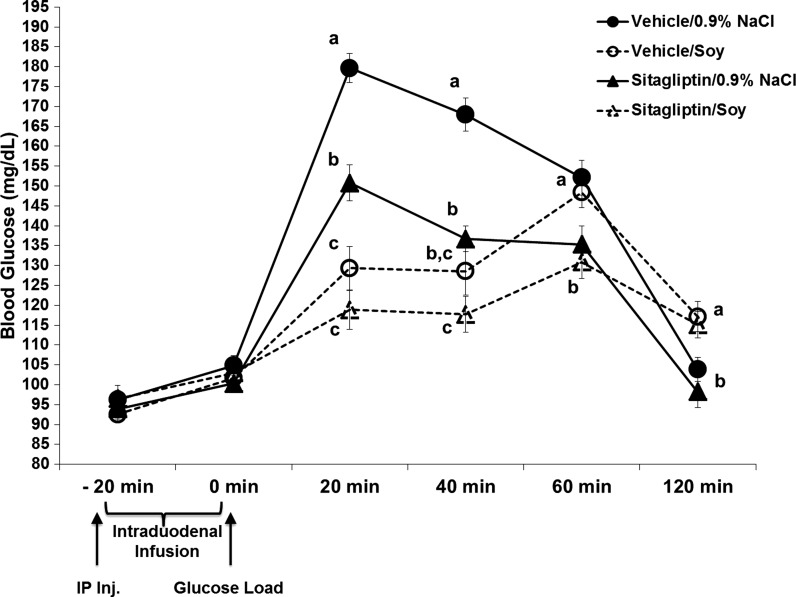
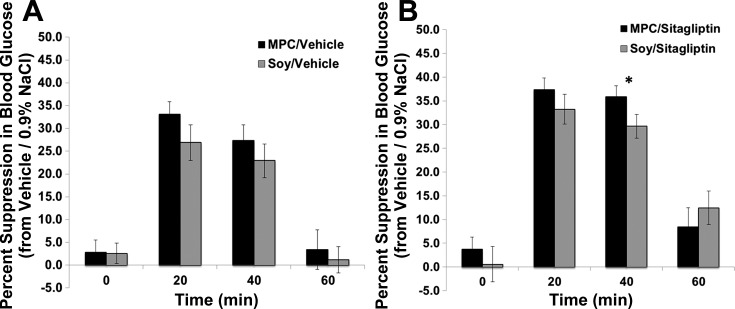
Similar to the feeding studies, we wanted to determine whether the improved glycemic regulation observed by MPC and sitagliptin combination was specific to dairy protein rather than any intraintestinal protein or caloric load. Thus a separate group of rats received intraduodenal infusions of isocaloric soy protein in combination with sitagliptin administration. As expected, sitagliptin alone produced a significant suppression of blood glucose at 20 and 40 min postglucose load [main effect of sitagliptin, at 20 min F1,23 = 35.824; P < 0.0001; at 40 min F1,23 = 29.845; P < 0.0001]. Soy protein alone reduced blood glucose at 20 and 40 min postglucose load [main effect of soy, at 20 min F1,23 = 51.114; P < 0.0001; at 40 min F1,23 = 46.393; P < 0.0001]. However, unlike the enhanced suppression of blood glucose observed with MPC, infusion of soy protein did not produce a significant enhancement in the glycemic suppressive effects of sitagliptin at any time point (Fig. 3; P > 0.05). Figure 4 shows the percent suppression of blood glucose concentrations following intraduodenal infusion of soy protein compared with MPC with and without sitagliptin administration. Between-subject analyses of this percent suppression shows that the combination of MPC with sitagliptin produced a significantly increased percent suppression in blood glucose compared with soy protein and sitagliptin administration at 40 min (P < 0.05).
Fig. 3.
Blood glucose concentrations (mg/dl) following intraperitoneal injection of vehicle or sitagliptin (6 mg/kg; time −20 min) in combination with intraduodenal infusions of 4 kcal of soy protein or 0.9% NaCl in an oral glucos-tolerance test (2 g/kg of 25% glucose delivered via gavage at time 0). Sitagliptin alone significantly suppressed blood glucose levels compared with vehicle-saline at 20, 40, and 60 min postglucose load. Soy protein alone significantly suppressed blood glucose levels compared with vehicle-saline at 20 and 40 min postglucose load. The combination of sitagliptin and soy protein did not produce any significant enhancement in the glycemic suppressive effects from either treatment alone. Within a time point, data points with different letters are significantly different from each other (P < 0.05). Data are means ± SE.
Fig. 4.
Within-subject percent suppression in blood glucose concentrations compared with vehicle-0.9% NaCl treatment after intraduodenal infusions of MPC or soy protein with (B) and without (A) sitagliptin administration. Between-subject analyses of this percent suppression shows that the combination of MPC with sitagliptin produced a significantly increased percent suppression in blood glucose compared with soy protein and sitagliptin administration at 40 min (P < 0.05).
DISCUSSION
Both DPP-IV inhibitors and GLP-1R agonists are FDA approved to treat T2DM by improving blood glucose regulation, but importantly, neither class of drug is a “cure” for T2DM. Given that with any pharmaceutical there is always the risk of tachyphylaxis (reduced response to a drug due to previous long-term exposure to that drug), identifying complementary and alternative/homeopathic therapies to GLP-1-based pharmaceuticals is a necessary step in the long-term control and treatment of T2DM. As accumulating data support the notion that regular consumption of dairy foods may have a beneficial role in the prevention of the metabolic syndrome (1, 11, 34, 35, 49, 60, 61), the rationale for identifying the specific dairy constituent(s) and mechanism of action for the putative beneficial effects of dairy products on glycemic regulation is clear. As dairy-enriched whole proteins and amino acids can elicit GLP-1 secretions in vitro (7, 15), as well as inhibit intestinal DPP-IV activity in vivo (17), we sought to determine whether the glycemic and feeding effects produced by acute pharmacological inhibition of DPP-IV by sitagliptin could be enhanced when combined with intraduodenal MPC infusion. Current findings show that intraduodenal infusion of MPC, but not soy protein, significantly enhances both the short-term food intake and glycemic suppressive effects of sitagliptin. These data support the hypothesis that dietary intake of dairy protein may be beneficial as an adjunct behavioral therapy to enhance the glycemic and food intake suppressive effects of select GLP-1-based pharmacotherapies.
The amount of endogenous postprandial GLP-1 released from intestinal L-cells in humans is correlated with the size and macronutrient composition of the meal (4, 43). This is an important concept when considering not only the dietary recommendations that should accompany prescribed DPP-IV inhibition for T2DM treatment, but also the relevant GLP-1R population(s) within the periphery that may respond to secreted GLP-1 to control for blood glucose utilization. Indeed, within the periphery there are distinct populations of GLP-1Rs relevant to glycemic control, including GLP-1R expressed on pancreatic β-cells, as well as vagal afferent fibers innervating the GI tract and/or hepatoportal bed (see Refs. 23 and 25 for review). Under normal physiological conditions, GLP-1 paracrine-like signaling and activation of vagal afferent fibers innervating the intestine appears to be the predominant mode of action controlling for intestinally derived GLP-1 glycemic effects (23–25, 54). An important limitation to the current findings is the absence of plasma GLP-1 and insulin measures. Nonetheless, with the presumed elevation in endogenous GLP-1 signaling produced by pharmacological DPP-IV inhibition in the present studies, it is possible that any of the aforementioned GLP-1R populations could mediate the enhanced suppression of blood glucose concentration by the combination of MPC with sitagliptin.
The strength of the peripheral GLP-1 system as a candidate for obesity treatment is highlighted by previous reports showing that endogenous peripherally secreted GLP-1 is physiologically important not only for glycemic control (24, 58) but also for the control of food intake (55). For example, systemic administration of the GLP-1R antagonist exendin-(9–39) in rats has been reported to impair glucose tolerance (24) and attenuate the intake-suppressive effects that follow voluntary consumption and intragastric infusion of a liquid meal (55). However, other reports indicate an absence of hyperphagia following systemic GLP-1R antagonist administration (41). While the discrepancies in the aforementioned antagonist-driven feeding effects may be a consequence of paradigmatic differences, the sustained glycemic- and food intake-suppressive effects that can be achieved by targeting the GLP-1 system with daily administration of DPP-IV inhibitors or GLP-1R agonists are clear (see Refs. 19, 29, 31, and 44 for review). Although less potent in their physiological and behavioral effects than the GLP-1R agonists, DPP-IV inhibitors offer humans the advantage of reduced incidence of adverse effects (e.g., nausea/vomiting) and the opportunity for an oral route of administration (10, 29). Interestingly, a previous report by Reimer et al. (38) showed that chronic elevation of endogenous GLP-1 signaling by DPP-IV inhibition produces a sustained suppression in food intake. Specifically, these authors reported that mice treated with the DPP-IV inhibitor NVP DPP728 in their drinking water had reduced weekly food intake when maintained on either standard rodent chow or high-fat diet (38). Together with the current acute feeding data, it seems logical to investigate whether the sustained suppression of weekly food intake observed in the aforementioned report could be further enhanced by the maintenance of animals on a diet high in MPC. Moreover, as DPP-IV inhibition does not selectively affect GLP-1 signaling but also can delay the degradation rate of other neuropeptides (e.g., gastric inhibitory polypeptide, vasoactive intestinal peptide, and substance P) (48), future research is also warranted to investigate potential alternative non-GLP-1-mediated pathways for the observed effects.
Perspectives and Significance
The collective set of data suggests that the presence of MPC within the small intestine can augment endogenous GLP-1 signaling, an effect important for both food intake and glycemic control, as the combination of acute DPP-IV inhibition together with an intraduodenal infusion of MPC resulted in a significant enhancement in the suppression of food intake and blood glucose concentrations. This hypothesis is also supported by clinical (5, 9, 18, 30, 42, 43) and basic science (45, 62) reports showing that ingestion or intraintestinal infusion of dairy proteins, oligopeptides, and isolated amino acids can elevate postprandial plasma GLP-1 and other intestinally derived gut peptide (e.g., cholecystokinin) concentrations. What remains to be determined is whether the putative increase in GLP-1 signaling following intraintestinal exposure to MPC is a consequence of increased GLP-1 secretions by L-cells and/or a consequence of reduced bioactive DPP-IV levels. Support for both possibilities is provided by evidence demonstrating that several oligopeptides can act as endogenous inhibitors of DPP-IV (17, 33), as well as a report by Gunnarsson et al. (17) showing that whey protein administration in mice reduces DPP-IV activity in the proximal small intestine, the predominant site of GLP-1 secretion in the intestine. Additionally, in vitro reports have shown that when GLP-1-producing L-cells are exposed to whole dairy proteins or specific dairy-enriched amino acids (i.e., leucine and isoleucine), there is an increase in GLP-1 secretion (7, 15). Thus as MPC is such a complex and diverse source of amino acids, oligopeptides, and complete proteins (i.e., casein and whey), it is not clear if the current results would be recapitulated with an isolated infusion of one or more of these dairy-based protein constituents. However, the absence of enhanced effects observed when sitagliptin was combined with intraduodenal infusion of soy protein suggests that the feeding and glycemic responses observed for MPC are not simply the consequence of intraduodenal protein in general or by the total caloric infusion, but rather represent some specific aspect of MPC. Thus further studies are certainly warranted to elucidate not only the physiological mechanisms by which dairy protein can enhance the glycemic and feeding effects of pharmacological DPP-IV inhibition, but also to investigate whether these effects would be recapitulated in hyperglycemic animal models and T2DM/prediabetic humans when treated daily with a DPP-IV inhibitor and maintained on a high dairy protein-based diet.
GRANTS
This research was supported by National Institutes of Health Grants NIH-DK085435 and DK096139, as well as an investigator-initiated research grant from “Dairy Management Inc.” and administered by the Dairy Research Institute (no. 1067).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.R.O., L.E.M., C.A.T., O.M., E.G.M.-B., and M.R.H. performed experiments; D.R.O., E.G.M.-B., and M.R.H. analyzed data; D.R.O., E.G.M.-B., and M.R.H. interpreted results of experiments; D.R.O. and M.R.H. prepared figures; D.R.O. and M.R.H. drafted manuscript; D.R.O., L.E.M., E.G.M.-B., and M.R.H. edited and revised manuscript; D.R.O., L.E.M., C.A.T., O.M., E.G.M.-B., and M.R.H. approved final version of manuscript; M.R.H. conception and design of research.
ACKNOWLEDGMENTS
Valuable technical assistance was provided by Derek Zimmer, Brianne Jeffrey, and Sky Prestowitz.
REFERENCES
- 1.Azadbakht L, Mirmiran P, Esmaillzadeh A, Azizi F. Dairy consumption is inversely associated with the prevalence of the metabolic syndrome in Tehranian adults. Am J Clin Nutr 82: 523–530, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Baum JI, O'Connor JC, Seyler JE, Anthony TG, Freund GG, Layman DK. Leucine reduces the duration of insulin-induced PI 3-kinase activity in rat skeletal muscle. Am J Physiol Endocrinol Metab 288: E86–E91, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Brenner LA, Ritter RC. Type A CCK receptors mediate satiety effects of intestinal nutrients. Pharmacol Biochem Behav 54: 625–631, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Calbet JA, Holst JJ. Gastric emptying, gastric secretion and enterogastrone response after administration of milk proteins or their peptide hydrolysates in humans. Eur J Nutr 43: 127–139, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Chang J, Wu T, Greenfield JR, Samocha-Bonet D, Horowitz M, Rayner CK. Effects of intraduodenal glutamine on incretin hormone and insulin release, the glycemic response to an intraduodenal glucose infusion, and antropyloroduodenal motility in health and type 2 diabetes. Diabetes Care 36: 2262–2265, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus–present and future perspectives. Nat Rev Endocrinol 8: 228–236, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Chen Q, Reimer RA. Dairy protein and leucine alter GLP-1 release and mRNA of genes involved in intestinal lipid metabolism in vitro. Nutrition 25: 340–349, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Covasa M, Ritter RC. Attenuated satiation response to intestinal nutrients in rats that do not express CCK-A receptors. Peptides 22: 1339–1348, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Diepvens K, Haberer D, Westerterp-Plantenga M. Different proteins and biopeptides differently affect satiety and anorexigenic/orexigenic hormones in healthy humans. Int J Obes (Lond) 32: 510–518, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Drucker DJ, Sherman SI, Bergenstal RM, Buse JB. The safety of incretin-based therapies–review of the scientific evidence. J Clin Endocrinol Metab 96: 2027–2031, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Eller LK, Reimer RA. Dairy protein attenuates weight gain in obese rats better than whey or casein alone. Obesity (Silver Spring) 18: 704–711, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 303: 235–241, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Fraser KA, Raizada E, Davison JS. Oral-pharyngeal-esophageal and gastric cues contribute to meal-induced c-fos expression. Am J Physiol Regul Integr Comp Physiol 268: R223–R230, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Frid AH, Nilsson M, Holst JJ, Bjorck IM. Effect of whey on blood glucose and insulin responses to composite breakfast and lunch meals in type 2 diabetic subjects. Am J Clin Nutr 82: 69–75, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Geraedts MC, Troost FJ, Fischer MA, Edens L, Saris WH. Direct induction of CCK and GLP-1 release from murine endocrine cells by intact dietary proteins. Mol Nutr Food Res 55: 476–484, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Giralt M, Vergara P. Glucagonlike peptide-1 (GLP-1) participation in ileal brake induced by intraluminal peptones in rat. Dig Dis Sci 44: 322–329, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Gunnarsson PT, Winzell MS, Deacon CF, Larsen MO, Jelic K, Carr RD, Ahren B. Glucose-induced incretin hormone release and inactivation are differently modulated by oral fat and protein in mice. Endocrinology 147: 3173–3180, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Hall WL, Millward DJ, Long SJ, Morgan LM. Casein and whey exert different effects on plasma amino acid profiles, gastrointestinal hormone secretion and appetite. Br J Nutr 89: 239–248, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Hayes MR. Neuronal and intracellular signaling pathways mediating GLP-1 energy balance and glycemic effects. Physiol Behav 106: 413–416, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayes MR, Bradley L, Grill HJ. Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology 150: 2654–2659, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayes MR, Chory FM, Gallagher CA, Covasa M. Serotonin type-3 receptors mediate cholecystokinin-induced satiation through gastric distension. Am J Physiol Regul Integr Comp Physiol 291: R115–R123, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Hayes MR, Covasa M. Gastric distension enhances CCK-induced Fos-like immunoreactivity in the dorsal hindbrain by activating 5-HT3 receptors. Brain Res 1088: 120–130, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Hayes MR, De Jonghe BC, Kanoski SE. Role of the glucagon-like-peptide-1 receptor in the control of energy balance. Physiol Behav 100: 503–510, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayes MR, Kanoski SE, De Jonghe BC, Leichner TM, Alhadeff AL, Fortin SM, Arnold M, Langhans W, Grill HJ. The common hepatic branch of the vagus is not required to mediate the glycemic and food intake suppressive effects of glucagon-like-peptide-1. Am J Physiol Regul Integr Comp Physiol 301: R1479–R1489, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev 87: 1409–1439, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Kanoski SE, Zhao S, Guarnieri DJ, Dileone RJ, Yan J, De Jonghe BC, Bence KK, Hayes MR, Grill HJ. Endogenous leptin receptor signaling in the medial nucleus tractus solitarius affects meal size and potentiates intestinal satiation signals. Am J Physiol Endocrinol Metab 303: E496–E503, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kokrashvili Z, Mosinger B, Margolskee RF. Taste signaling elements expressed in gut enteroendocrine cells regulate nutrient-responsive secretion of gut hormones. Am J Clin Nutr 90: 822S–825S, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liddle RA, Green GM, Conrad CK, Williams JA. Proteins but not amino acids, carbohydrates, or fats stimulate cholecystokinin secretion in the rat. Am J Physiol Gastrointest Liver Physiol 251: G243–G248, 1986 [DOI] [PubMed] [Google Scholar]
- 29.Lovshin JA, Drucker DJ. Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol 5: 262–269, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Ma J, Stevens JE, Cukier K, Maddox AF, Wishart JM, Jones KL, Clifton PM, Horowitz M, Rayner CK. Effects of a protein preload on gastric emptying, glycemia, and gut hormones after a carbohydrate meal in diet-controlled type 2 diabetes. Diabetes Care 32: 1600–1602, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madsbad S, Krarup T, Deacon CF, Holst JJ. Glucagon-like peptide receptor agonists and dipeptidyl peptidase-4 inhibitors in the treatment of diabetes: a review of clinical trials. Curr Opin Clin Nutr Metab Care 11: 491–499, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Mazda T, Yamamoto H, Fujimura M, Fujimiya M. Gastric distension-induced release of 5-HT stimulates c-fos expression in specific brain nuclei via 5-HT3 receptors in conscious rats. Am J Physiol Gastrointest Liver Physiol 287: G228–G235, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Mrestani-Klaus C, Fengler A, Faust J, Brandt W, Wrenger S, Reinhold D, Ansorge S, Neubert K. N-terminal HIV-1 Tat nonapeptides as inhibitors of dipeptidyl peptidase IV. Conformational characterization. Adv Exp Med Biol 477: 125–129, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Pfeuffer M, Schrezenmeir J. Milk and the metabolic syndrome. Obes Rev 8: 109–118, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Poddar KH, Hosig KW, Nickols-Richardson SM, Anderson ES, Herbert WG, Duncan SE. Low-fat dairy intake and body weight and composition changes in college students. J Am Diet Assoc 109: 1433–1438, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Raybould HE. Gut chemosensing: interactions between gut endocrine cells and visceral afferents. Auton Neurosci 153: 41–46, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reidelberger RD, Heimann D, Kelsey L, Hulce M. Effects of peripheral CCK receptor blockade on feeding responses to duodenal nutrient infusions in rats. Am J Physiol Regul Integr Comp Physiol 284: R389–R398, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Reimer MK, Holst JJ, Ahren B. Long-term inhibition of dipeptidyl peptidase IV improves glucose tolerance and preserves islet function in mice. Eur J Endocrinol 146: 717–727, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Reimer RA, Darimont C, Gremlich S, Nicolas-Metral V, Ruegg UT, Mace K. A human cellular model for studying the regulation of glucagon-like peptide-1 secretion. Endocrinology 142: 4522–4528, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Rocca AS, LaGreca J, Kalitsky J, Brubaker PL. Monounsaturated fatty acid diets improve glycemic tolerance through increased secretion of glucagon-like peptide-1. Endocrinology 142: 1148–1155, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Ruttimann EB, Arnold M, Geary N, Langhans W. GLP-1 antagonism with exendin (9–39) fails to increase spontaneous meal size in rats. Physiol Behav 100: 291–296, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Ryan AT, Feinle-Bisset C, Kallas A, Wishart JM, Clifton PM, Horowitz M, Luscombe-Marsh ND. Intraduodenal protein modulates antropyloroduodenal motility, hormone release, glycemia, appetite, and energy intake in lean men. Am J Clin Nutr 96: 474–482, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Ryan AT, Luscombe-Marsh ND, Saies AA, Little TJ, Standfield S, Horowitz M, Feinle-Bisset C. Effects of intraduodenal lipid and protein on gut motility and hormone release, glycemia, appetite, and energy intake in lean men. Am J Clin Nutr 300: 200–211, 2013 [DOI] [PubMed] [Google Scholar]
- 44.Sadry SA, Drucker DJ. Emerging combinatorial hormone therapies for the treatment of obesity and T2DM. Nat Rev Endocrinol 2013 [DOI] [PubMed] [Google Scholar]
- 45.Salehi A, Gunnerud U, Muhammed SJ, Ostman E, Holst JJ, Bjorck I, Rorsman P. The insulinogenic effect of whey protein is partially mediated by a direct effect of amino acids and GIP on beta-cells. Nutr Metab 9: 48, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sherwin R, Jastreboff AM. Year in diabetes 2012: The diabetes tsunami. J Clin Endocrinol Metab 97: 4293–4301, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tahara A, Matsuyama-Yokono A, Nakano R, Someya Y, Shibasaki M. Hypoglycaemic effects of antidiabetic drugs in streptozotocin-nicotinamide-induced mildly diabetic and streptozotocin-induced severely diabetic rats. Basic Clin Pharmacol Toxicol 103: 560–568, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Tarantola E, Bertone V, Milanesi G, Capelli E, Ferrigno A, Neri D, Vairetti M, Barni S, Freitas I. Dipeptidylpeptidase–IV, a key enzyme for the degradation of incretins and neuropeptides: activity and expression in the liver of lean and obese rats. Eur J Histochem 56: e41, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Teegarden D. The influence of dairy product consumption on body composition. J Nutr 135: 2749–2752, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Vetter ML, Faulconbridge LF, Webb VL, Wadden TA. Behavioral and pharmacologic therapies for obesity. Nat Rev Endocrinol 6: 578–588, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wadden TA, Berkowitz RI, Womble LG, Sarwer DB, Phelan S, Cato RK, Hesson LA, Osei SY, Kaplan R, Stunkard AJ. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med 353: 2111–2120, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Wadden TA, Butryn ML, Wilson C. Lifestyle modification for the management of obesity. Gastroenterology 132: 2226–2238, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Wadden TA, Fujioka K, Toubro S, Gantz I, Erondu NE, Chen M, Suryawanshi S, Carofano W, Johnson-Levonas AO, Shapiro DR, Kaufman KD, Heymsfield SB, Amatruda JM. A randomized trial of lifestyle modification and taranabant for maintaining weight loss achieved with a low-calorie diet. Obesity (Silver Spring) 18: 2301–2310, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Waget A, Cabou C, Masseboeuf M, Cattan P, Armanet M, Karaca M, Castel J, Garret C, Payros G, Maida A, Sulpice T, Holst JJ, Drucker DJ, Magnan C, Burcelin R. Physiological and pharmacological mechanisms through which the DPP-4 inhibitor sitagliptin regulates glycemia in mice. Endocrinology 152: 3018–3029, 2011 [DOI] [PubMed] [Google Scholar]
- 55.Williams DL, Baskin DG, Schwartz MW. Evidence that intestinal glucagon-like-1 peptide plays a physiological role in satiety. Endocrinology 150: 1680–1687, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Willing AE, Berthoud HR. Gastric distension-induced c-fos expression in catecholaminergic neurons of rat dorsal vagal complex. Am J Physiol Regul Integr Comp Physiol 272: R59–R67, 1997 [DOI] [PubMed] [Google Scholar]
- 58.Woerle HJ, Carneiro L, Derani A, Goke B, Schirra J. The role of endogenous incretin secretion as amplifier of glucose-stimulated insulin secretion in healthy subjects and patients with type 2 diabetes. Diabetes 61: 2349–2358, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yox DP, Brenner L, Ritter RC. CCK-receptor antagonists attenuate suppression of sham feeding by intestinal nutrients. Am J Physiol Regul Integr Comp Physiol 262: R554–R561, 1992 [DOI] [PubMed] [Google Scholar]
- 60.Zemel MB. Proposed role of calcium and dairy food components in weight management and metabolic health. Phys Sportsmed 37: 29–39, 2009 [DOI] [PubMed] [Google Scholar]
- 61.Zemel MB. The role of dairy foods in weight management. J Am Coll Nutr 24: 537S–546S, 2005 [DOI] [PubMed] [Google Scholar]
- 62.Zhou J, Keenan MJ, Losso JN, Raggio AM, Shen L, McCutcheon KL, Tulley RT, Blackman MR, Martin RJ. Dietary whey protein decreases food intake and body fat in rats. Obesity (Silver Spring) 19: 1568–1573, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]