Abstract
Rats with bilateral excitotoxic lesions of the parabrachial nuclei (PBN) fail to acquire a conditioned taste aversion (CTA), yet they retain the ability to express a CTA learned prior to incurring the damage. Rats with bilateral electrolytic lesions of the lateral hypothalamus (LH) also have CTA learning deficits. The PBN have reciprocal neural connections with the LH. This suggests that these CTA deficits may be functionally related. Electrolytic lesions damage fibers of passage, as well as intrinsic neurons. Thus, these LH lesions might also interrupt reciprocal connections between the PBN and other ventral forebrain areas, such as the amygdala and bed nucleus of the stria terminalis. To distinguish the source of the LH-lesion deficit, we tested for CTA first after bilateral excitotoxic lesions of LH and subsequently with a second set of animals that had asymmetric excitotoxic PBN and LH lesions. The rats with bilateral excitotoxic LH lesions showed deficits when acquiring a postlesion CTA. The asymmetrical PBN-LH lesions not only slowed acquisition of a CTA but also sped up extinction. This implies that interaction between the two structures, at minimum, facilitates CTA learning and may have a role in its consolidation.
Keywords: conditioned taste aversion, lateral hypothalamus, learning, neural connections, parabrachial nuclei
rats with bilateral electrolytic or excitotoxic lesions of the parabrachial nuclei (PBN) can recall a prelesion conditioned taste aversion (CTA), but postlesion, they are unable to acquire new CTAs (1, 3–5, 9, 28, 37, 44). Rats with bilateral electrolytic lesions of the lateral hypothalamus (LH) exhibit a similar profile. They can retain a CTA learned prior to the lesions but fail to learn new CTAs (4, 34–36). The PBN has reciprocal neural connections to the LH, central nucleus of the amygdala (CNA), and the bed nucleus of the stria terminalis (BNST) (11, 12, 14, 16, 17, 21–24, 43).
Electrolytic lesions of the LH can damage the fibers of passage that link the PBN to the CNA and BNST (23, 24, 33). Thus, the CTA deficits displayed by rats with electrolytic LH lesions (LHx) could be due to the involvement of the intrinsic neurons of the LH or the functional isolation of PBN from the CNA, the BNST, or other anterior forebrain sites.
To assess the role of the LH neurons in CTA, we made bilateral lesions using the excitotoxin ibotenic acid to preserve the fibers of passage. These rats were tested for retention of a prelesion CTA and a postlesion CTA acquisition. These experiments can assess the role of LH neurons in CTA, but they cannot demonstrate a functional interaction between the PBN and LH in CTA acquisition.
Such an interaction has been demonstrated for salt appetite using rats with asymmetric PBN-LH lesions, i.e., unilateral PBN damage on one side of the brain and unilateral LH lesions on the other (2). A separate set of rats with asymmetrical PBN-LH lesions was used for the CTA acquisition and retention experiments summarized here. As in the salt appetite experiments, the controls consisted of unoperated rats and animals with unilateral PBN and LH damage but placed on the same side of the brain. Each of the experiments used essentially the same CTA paradigm. The exception was that in the asymmetric experiment, the postlesion CTA was extinguished with CS trials that were never followed by an injection of either LiCl or NaCl.
The animals used in this study were treated in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the experimental protocols were approved by the Institutional Animal Care and Use Committee of the College of Medicine of The Pennsylvania State University. Although the animals in experiment 1A and 1B were used in other experiments, the CTA experiments were conducted first. Thus, the other experiments could not have affected the CTA results. Because some of the experiments have been published (2), the description of the surgical procedures that were common to all of the experiments is abbreviated in what follows.
EXPERIMENT 1A
Materials and Methods
Animals and maintenance.
Male Sprague-Dawley rats (n = 36; Charles River, Wilmington, MA) weighing 230–430 g at the start of the experiment were assigned to groups by matching body weights. They were individually housed in stainless-steel hanging-wire-mesh cages in a colony room with automatically controlled temperature (21°C), humidity, ventilation, and light cycles (12:12-h light-dark periods; lights on at 0700). The CTA tests were conducted in the same cages during the light phase. The rats ate standard laboratory chow (rodent diet no. W8604; Harlan Teklad, Madison, WI) and drank deionized, distilled water (dH2O) in graduated cylinders ad libitum except during the CTA protocol. Their fluid intake (to 0.5 ml) and body weight were measured daily.
Prior to surgery, 20 rats were trained on a CTA. Of these, 14 subsequently received LH lesions (LHx); 1 died. Three others were full surgical controls (SCon), and the remaining three were nonsurgical controls (NSCon). Postoperative retention was tested on 13 LHx, 3 SCon, and 3 NSCon rats. These animals were subsequently tested for acquisition of a new CTA using another CS. In addition, 16 more experimentally naïve rats were included in the protocol to investigate postlesion CTA acquisition. Ten had LH lesions, while 3 were SCon, and 3 were NSCon. Thus, the final count in the postlesion acquisition trials was 23 LHx, 6 SCon, and 6 NSCon.
Preoperative CTA acquisition.
Water was removed at 1800 to begin deprivation training. The next morning, the rats received dH2O for 15 min at 0830 and then for an additional 2 h at 1400. For both periods, water intake was recorded to the nearest 0.5 ml. The rats adapted to this schedule for 7 days before CTA training began. The conditioned stimulus (CS) was alanine (0.3 M), an amino acid that is preferred by rats and tastes sweet to humans. The unconditioned stimulus (US) was 0.15 M LiCl (6, 35).
After deprivation training, on days 1, 4, and 7, the rats received 15 min access to the CS instead of their morning water, and intake was recorded as usual. Fifteen minutes following CS removal, each rat was injected with LiCl (1.33 ml per 100 g body wt ip). Afternoon access to dH2O occurred as usual. Between CS trials (days 2, 3, 5, and 6), the rats had access to dH2O morning and afternoon to maintain the deprivation regimen. Upon completion of three acquisition trials, 14 rats underwent lesion surgery, 3 rats underwent sham surgery.
Surgery.
LH LESIONS.
The rats previously assigned to receive surgery were deprived of water overnight. Approximately 15 min prior to surgery, they were weighed and then injected with atropine (0.1 mg/kg ip) and gentamicin (6.0 mg ip). Subsequently, they were anesthetized with pentobarbital sodium (50 mg/kg ip) and mounted in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA). Supplemental doses of barbiturate (5.0 mg, approximately every 45 min) were given to maintain surgical levels of anesthesia throughout the procedure. Body temperature was monitored and maintained at 36 ± 1°C. With the scalp exposed and leveled, bilateral holes (∼1.0 mm diameter) were drilled in the skull centered at 3.0 mm behind β and 2.0 mm lateral to the midline. The skull surface and dura were moistened frequently with physiological saline.
The lesions were made using a 1.0 μl Hamilton syringe filled with freshly prepared ibotenic acid (20 μg/μl, 0.5 μl) in PBS (pH 7.4; Research Biochemicals International, Natick, MA). The tip of the blunt syringe needle was positioned 8.8 mm deep to the skull surface, and each injection occurred over 25 min. Following both infusions, the skull holes were packed with Gelfoam and the scalp closed with wound clips.
Postoperative recovery.
After surgery, all animals were returned to their individual cages with ad libitum access to standard lab chow and dH2O for at least 2 wk. Analgesia (rimadyl, 5.0 mg/kg sc) and an antibiotic (gentamicin, 4 mg/kg im) were provided daily for a minimum of a week. One LHx rat died following surgery. As previously observed (2, 18, 22, 39, 40), the LHx rats demonstrated aphagia and adipsia to varying degrees.
Retention testing.
Following recovery from surgery, the rats with preoperative training (n = 19) were tested for retention of the aversion to 0.3 M alanine acquired prior to surgery. The rats were dehydrated again for this protocol. As previously, the rats had access to dH2O for 15 min each morning at 0830 and again for 2 h each afternoon at 1400. After 8 days of water training, the rats were presented with two bottles during the morning session on day 9. One bottle contained dH2O, and the other contained 0.3 M alanine (two-bottle test); the right/left position of the stimulus bottles was alternated and counterbalanced across lesion conditions. No LiCl was administered. Intake was recorded to the nearest 0.5 ml. There were three alanine vs. dH2O tests on days 9, 12, and 15. On the fourth test day, day 18, the rats received only 1 bottle containing 0.3 M alanine also in extinction. Only a single dH2O bottle was available on test day afternoons and for both the morning and afternoon sessions on the intervening days.
Postoperative CTA acquisition.
Following the retention tests, the rats (n = 19) were maintained on the water deprivation schedule described above for an additional week. These rats then had access to 0.1 M sucrose in the morning. Normally, they would receive LiCl following the CS exposure, but at least half of them failed to drink, perhaps because of generalization to the prior alanine aversion. Because of this possible confound, following two more AM water days, the rats were switched 0.01 M malic acid as a CS (32). Although sour to humans, at this concentration, water-deprived rats consume considerable amounts of malic acid.
The other set of LHx rats (n = 10) and control rats (SCon 3, NSCon 3), which did not receive a preoperative CTA, were trained postoperatively with 0.3 M alanine as the CS. The postoperative CTA protocol for all of these rats (n = 35) was the same as for the preoperative training, three CS-US pairings each separated by 2 days with AM water. The only difference was an added fourth trial that consisted of a 15-min AM CS presentation but no subsequent LiCl injection. Following the final test day, the rats were rehydrated, and their 24-h water intake was monitored.
Data analysis.
The data were subjected to ANOVA with repeated measures using a generalized linear model (Statistica version 8.0, Statsoft). Post hoc assessments were conducted with the Duncan test (Dun). The criterion for statistical significance was set at P ≤ 0.05.
Histology.
Six LHx and three surgical controls were anesthetized as previously described and injected with wheat germ agglutinin-horseradish peroxidase into the pontine PBN. These brains were used to demonstrate that PBN axons are preserved after ibotenic acid lesion of the LH (see Refs. 2 and 30 for details). The remaining rats (17 LHx and 3 SCON) were cut in two alternating series. One series was stained with the cresyl violet, while in the other, a Weil procedure was used (3, 7, 14, 15). All anatomical measurements were determined using the Paxinos and Watson (1992) atlas (26).
Results
Histology.
The cresyl violet sections revealed differential placement of the LH lesions along the anterior-posterior axis. Two groups were distinguished. The anterior lesions (aLHx; n = 6) were centered at −2.9 ± 0.06 mm posterior to β (range −2.6 mm to −3.1 mm). In aggregate, the aLHx lesions extended about 0.9 mm rostral and 1.1 mm caudal to the level of the injection or from the rostral edge of the ventromedial nucleus to just rostral of the mammillary bodies. The posterior lesion (pLHx, n = 7) track marks were at −3.8 ± 0.08 mm posterior to β (range −3.5 mm to −4.4 mm), and extended 1.2 mm rostral and 0.8 mm caudal or from the midventromedial nucleus to the rostral half of the mammillary bodies. At their fullest, these lesions extended from the medial border of the internal capsule to the fornix and dorsally into the zona incerta and the fields of Forel. Typically, the aLHx and pLHx damage overlapped at the level of the posterior half of the ventromedial nucleus. See Fig. 6B for a photomicrograph of a representative LH lesion.
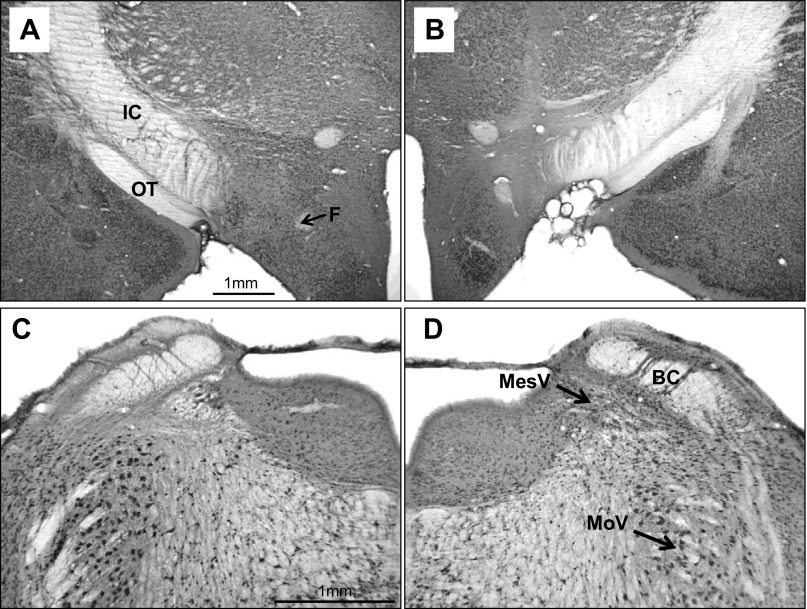
Fig. 6.
Experiment 2. Photomicrographs of coronal sections through the lateral hypothalamus (LH; top) and the parabrachial nuclei (PBN; bottom) in a rat (no. 06–17) with asymmetric lesions (Neu-N stain). A: intact left LH. B: lesioned right LH. C: lesioned left PBN. D: intact right PBN. C: acellular area extends into the supratrigeminal area above the motor trigeminal nucleus (MoV) and the locus coeruleus medial to mesencephalic trigeminal nucleus (MesV). BC, brachium conjunctivum; F, fornix; IC, internal capsule; OT, optic tract. Note that the magnification in C and D is double that of A and B.
After processing, the WPA-HRP revealed considerable reaction product in the forebrains of both the control and LHx rats. This demonstrated that even in the LHx brains, PBN axons reached their normal distributions in the amygdala and BNST (2, 24).
Preoperative CTA learning.
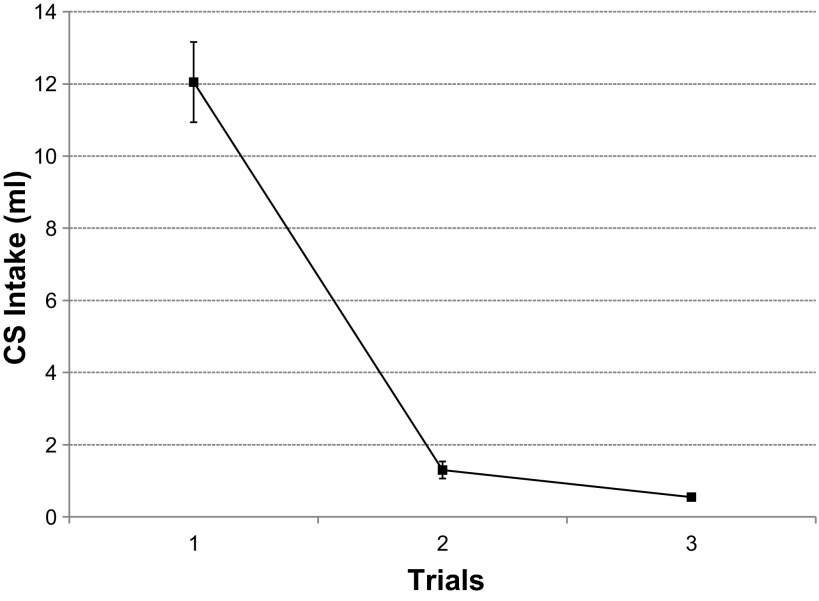
All rats (n = 19) learned the preoperative CTA after a single pairing with LiCl, F(2,36) = 94.3, P ≤ 0.001 (Fig. 1). On average, the rats consumed 12.1 ± 1.1 ml of alanine during the first conditioning trial, i.e., before receiving LiCl, and only 1.3 ± 0.2 ml on the second (Dun, P ≤ 0.001) The intake of alanine was negligible (0.55 ml) on the third and the last conditioning trial and was similar to the second (Dun, P = 0.41). The rats assigned to become the LHx cohort and the controls did not vary in their alanine intake across groups [F(1, 18) = 0.39, P = 0.54].
Fig. 1.
Experiment 1A. Prelesion conditioned taste aversion (CTA) acquisition. Conditioned stimulus (CS) = 0.3 M alanine. Values are expressed as means ± SE (n = 19) across three pairings with the unconditioned stimulus (US), LiCl. The group consumed less CS during trial 2 than trial 1 (P < 0.001).
Retention of the preoperatively learned CTA.
During the retention trials, the surgical (SCon) and nonsurgical (NSCon) controls did not differ in their alanine intake [F(1,4) = 0.09, P = 0.78], so their data were combined, (Con). During the two-bottle retention tests, alanine intake did not differ between the LHx and Con rats, F(1,17) = 1.02, P = 0.33 (Fig. 2A). Total fluid intake (water + alanine) exhibited a similar pattern but was marginally different, F(1,17) = 3.93, P = 0.06. Again, during the final, one-bottle test, alanine intake only approached a significant difference between the LHx and CON rats [F(1,17) = 3.36, P = 0.07].
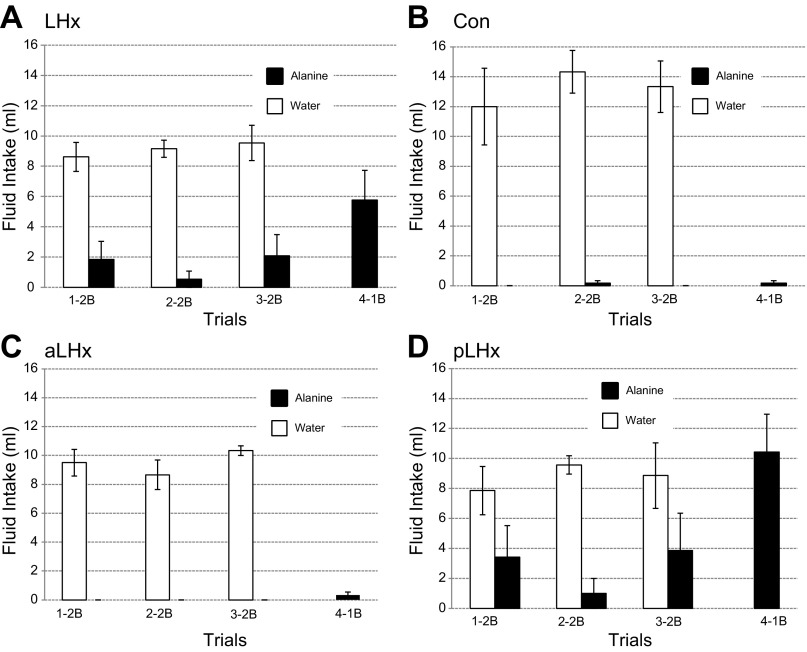
Fig. 2.
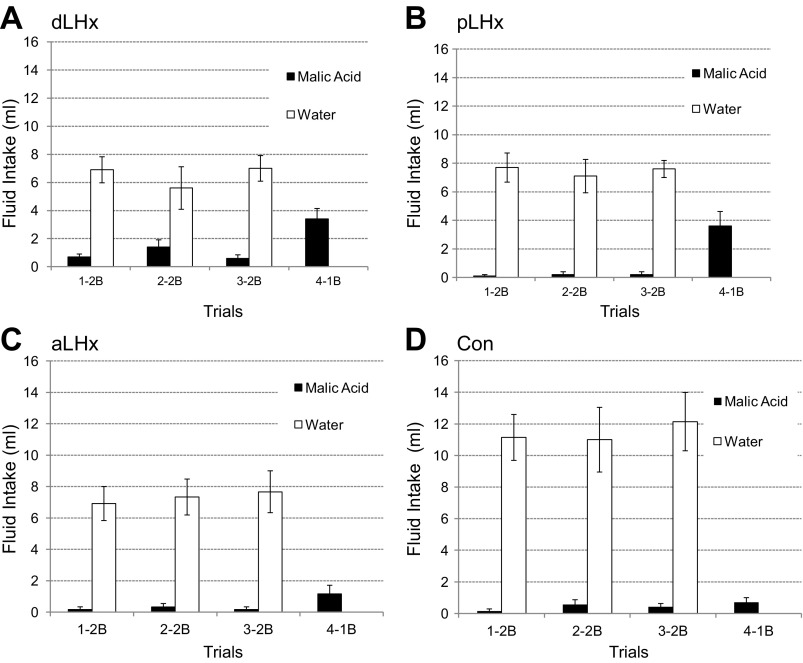
Experiment 1A. Postlesion CTA retention tests. The first three 15-min exposures were two-bottle (2B), water and the CS, alanine. The fourth test was one-bottle (1B), alanine. Lateral hypothalamic lesions (LHx); n = 13 (A), controls; n = 6 (B), anterior LHx (aLHLx); n = 6 (C), posterior LHx (pLHx), n = 7 (D). Data are expressed as means ± SE. In each 2B test, some rats with pLHx ingested alanine. Both the controls (Con) and the aLHx group drank only water, but the differences between the three groups were not significant, F(2,16) = 2.28, P = 0.13. In the 4th 1B test, the latter two groups also failed to ingest the CS. In the same test, however, the pLHx group ingested more than 10 ml of alanine, F(2,16) = 13.6, P < 0.001.
When aLHx and pLHx groups were compared with the Con during the three two-bottle tests, neither alanine nor total fluid intake differed significantly [alanine: F(2,16) = 2.28, P = 0.13; total: F(2,16) = 2.98, P = 0.08]. During the one-bottle test, however, the pLHx rats ingested more alanine than either the aLHx group or the controls [F(2,16) = 13.6, P < 0.001; Dun pLHx vs. aLHx, P < 0.001; pLHx vs. Con, P < 0.001]. The aLHx and Con rats were similar (Dun P = 0.94).
Postoperative CTA acquisition.
The rats with prelesion CTA experience had 0.01 M malic acid as the CS; the naïve group (4 aLHx, 6 pLHx, 3 SCon, and 3 NSCon) had 0.3 M alanine. Both CSs were paired with 0.15 M LiCl. Regardless of the CS, after the first trial, the intake of all four control groups (n = 12) did not differ [F(1,10) = 0.06, P = 0.81]. They were combined in to a single Con group.
On the first trial, prior to the LiCl injections, alanine intake was greater than malic acid for both LHX and Con groups [F(1,31) = 11.42, P < 0.001]. In this trial, the LHx rats ingested 15.5 ± 1.05 ml of alanine and 9.8 ± 0.83 ml of malic acid. Con intake was 14 ± 1.5 ml and 8.2 ± 1.08 ml, respectively. By the fourth trial, both LHx and Con intake was near zero regardless of the CS. To control for the initial intake differences, each rat's CS intake on trials 2–4 was divided by its intake on the first day and expressed as a ratio. With this conversion, the differences in alanine and malic acid intake within the LHx and Con groups disappeared [F(1,31) = 0.41, P = 0.52]. Thus, the LHx cohorts with and without prelesion CTA experience were combined, as were all four control groups. On the 2nd and 3rd trial, the intake ratios of the combined LHx and Con groups were significantly different [F(1,33) = 11.0, P ≤ 0.002].
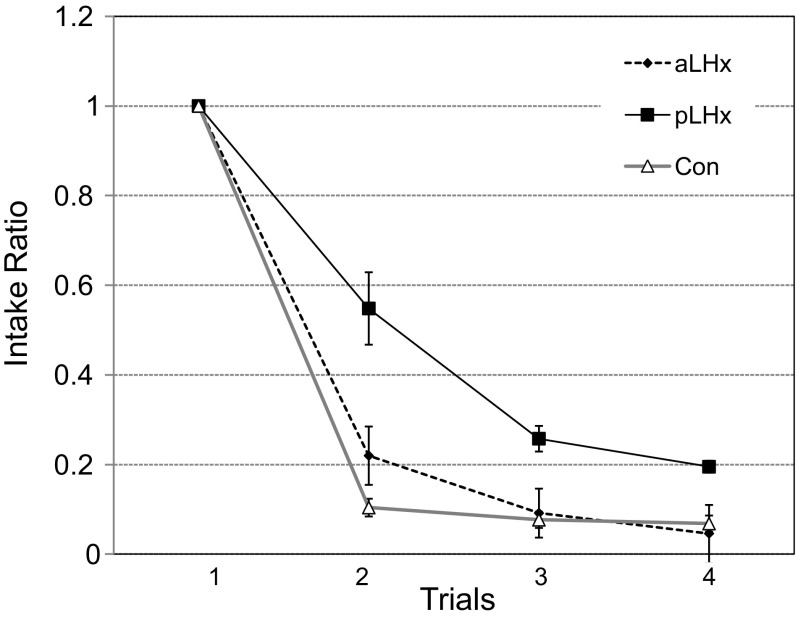
When the LHx rats were separated by lesion placement in the A-P plane (Fig. 3), again regardless of the CS, those with more posterior damage (pLHx intake ratio = 0.4 ± 0.07) learned the CTA more slowly than either the aLHx or the Con rats: F(2,32) = 16.7, P < 0.001 (aLHx intake ratio = 0.2 ± 0.06, Dun P = 0.003; Con ratio = 0.1 ± 0.02, P = 0.001). The aLHx and Con groups were similar to each other (Dun P = 0.28).
Fig. 3.
Experiment 1A. Postlesion CTA acquisition by groups with aLHx and pLHx LH lesions and by controls (Con). The data are expressed as ratios of trial 1 consumption to permit comparison across the two CSs, malic acid, and alanine, which produce differential initial intake. The control and aLHx groups learned the CTA in one pairing and virtually ceased ingestion thereafter. After a single pairing with LiCl, the pLHx group ingested half as much CS as on trial 1 and then leveled off at a ratio of 0.2 in trials 3 and 4.
Although the overall difference between the LHx and Con rats disappeared on the fourth and final trial [F(1,33) = 1.23, P = 0.28], when the aLHx and pLHx groups were compared separately with the controls, there was a significant difference, F(2,32) = 3.61, P < 0.05. This difference was carried by the pLHx vs. Con comparison (Dun P = 0.04) and by the pLHx vs. aLHx contrast (Dun P = 0.03). As in trials 2 and 3, the aLHx group did not differ from the Con (Dun P = 0.72).
Postoperative body weight and food and water consumption.
The body weight of the LHx rats (467 ± 8.5 g) was lower than the controls [514 ± 9.1 g; F(1,9) = 5.6, P = 0.04]. Mean food consumption of the LHx rats (25.8 ± 2.3 g) was also marginally lower than the controls [30.0 ± 2.5 g; F(1,9) = 5.2, P = 0.05]. Water consumption volumes were similar between the two groups [F(1,8) = 0.67, P = 0.44]. The differences between the LHx and control groups ameliorated somewhat over the 20-day postoperative period, and no interventions were necessary. The details of their ingestive behavior are discussed further elsewhere (2).
Discussion
The CTA retention and reacquisition experiments demonstrated that rats with more anterior LH lesions were able to remember a preoperatively acquired aversion and to learn a new CTA as well as controls. As with the aLHx and CON groups, most pLHx rats (5 of 7) avoided the CS during the three two-bottle postlesion retention tests. On the fourth, one-bottle trial, however, all seven pLHx rats ingested considerable amounts of alanine (average 10.3 ml, range 4–21 ml, Fig. 2D). In the same trial, none of the aLHx or control animals ingested more than 1.0 ml (Fig. 2, B and C). Nevertheless, when tested with a new CS, malic acid, the same pLHx rats learned this CTA, albeit more slowly than the aLHx or CON cohorts. Similarly, with an alanine CS, the naïve rats with anterior LH damage learned a postlesion CTA normally, i.e., the time course and degree were similar to their own controls and to the experienced aLHx and CON groups. With the same CS, 4 of 6 naïve pLHx rats failed to learn a CTA after three pairings with LiCl.
Thus, the rats with more posterior LH damage consistently exhibited CTA deficits, but they were never as severe as in animals with electrolytic lesions of the LH (36) or bilateral damage to the pontine PBN (35, 37). Previous studies with either LH or PBN lesions completely disrupted CTA learning but permitted retention of a prelesion learned aversion (36, 44). The differences between the current data and those reported by Schwartz and Teitelbaum (36) could reflect the lesion procedures, less damage to fibers of passage, the volume of the tissue damage, or its placement. Given the rostrocaudal spread of our lesions, placement seems the least likely explanation. Hence, we decided to replicate this experiment using the same testing protocol but adding a group of rats with double LH lesions on each side.
EXPERIMENT 1B
Materials and Methods
Animals and maintenance.
Thirty seven adult male Sprague-Dawley rats (Charles River, Wilmington, MA) weighing 440–490 g at the time of surgery were maintained as in experiment 1A.
Preoperative CTA acquisition.
Malic acid (0.01 M) was the conditioned stimulus (CS), and 0.15 M LiCl served as the unconditioned stimulus (US) (31). Upon completion of CTA training, the rats underwent lesion surgery.
Surgery.
The rats were dehydrated, and 30 received ibotenic acid lesions, as described under experiment 1A with the following modifications. One set of rats received anterior lesions (aLHx; n = 11) and another posterior lesions (pLHx rats; n = 8) using ibotenic acid (0.5 μl, 20 μg/μl) at −2.5 mm and −3.1 mm posterior to β, respectively, 2.0 mm right and left of the sagittal suture, and 8.8 mm deep to the skull surface. The third group with double lesions (dLHx, n = 11) received bilateral injections (0.4 μl) at both sets of coordinates. Of these animals, six with double, five with anterior, and three with posterior lesions died during or within days following surgery. Four of the remaining rats underwent double surgeries as described above but with saline injected instead of ibotenic acid and served as surgical controls. The remaining three rats were NSCon. During the behavioral tasks, there was no statistical difference between these controls so their data were collapsed [(Con), F(1,6) = 2.1, P = 0.2]. The 26 surviving rats consisted of 6 dLHx, 6 aLHx, 6 pLHx, and 7 Con.
Retention testing.
Following 3 wk of recovery, these rats were again dehydrated and tested for retention of the preoperatively learned aversion. As done previously, each rat was given a two-bottle test with distilled water and the CS, 0.01 M malic acid, in 3 AM sessions lasting 15 min each. On the fourth and final day, the rats had access to only malic acid in a one-bottle test. Two days intervened between tests when only water was available. On all days the rats had access to water for 2 h in the afternoon.
Postoperative CTA acquisition.
The postoperative CTA acquisition was the same as the preoperative procedure except that 0.3 M alanine was the CS and after four trials (trials 1–3 with LiCl, trial 4 without), the rats had a single two-bottle choice test—water vs. alanine—also in extinction.
Data analysis.
The data were analyzed as in experiment 1A.
Histology.
One animal from each of the experimental groups had the neuronal tracer WGA-HRP injected into the PBN to check that axons arising in these nuclei reached their normal forebrain targets. The procedures were identical to those used in experiment 1A and Ref. 2. The brains of the remaining 15 rats with LH lesions (5 aLHx, 5 dLHx, and 5 pLHx) and 2 controls were fixed, sectioned, and mounted as previously. The extent of lesions was determined using two series of sections that included the hypothalamus stained with the cresyl violet and Weil methods (7, 14, 15).
Results
Histology.
Cresyl violet-stained sections through the preoptic-hypothalamic continuum revealed differential placement of the lesions along the anterior-posterior axis (26). The area of common damage extended from −2.6 to −3.7 mm posterior to β, and included the hypothalamus lateral to the fornix and the mammillothalamic tracts from the level of the midventromedial nucleus to its posterior boundary. The neuronal loss often extended dorsally into the subthalamic nucleus and the zona incerta. Anterior lesions extended from −1.4 mm to −3.8 mm, posterior lesions from −2.0 mm to −4.6 mm. Thus, both anterior and posterior lesions were ∼2.5 mm in length. The double lesions extended from −1.7 mm to −4.8 mm posterior to β, with an average length of 3.1 mm.
Prelesion CTA acquisition.
All rats (n = 37) acquired the prelesion CTA to malic acid in one trial and were essentially at zero intake by trial 3 [F(1,38) = 139.4, P < 0.001]. When graphed, these data look essentially identical to those seen in Fig. 1, and thus, they are not shown. The rats designated to receive LH lesions differed at best marginally from controls on the three acquisition trials [F(3,22) = 2.6, P = 0.08]. On the basis of subsequent lesion location, the groups did not differ significantly in initial CS intake or in their rate of learning [interaction F(6,44) = 1.96, P > 0.09].
Postlesion CTA retention.
During the three two-bottle tests, the dLHx rats consumed negligible amounts of CS (0.9 ± 0.33 ml), but this was still more than either the aLHx, pLHx, or Con rats [0.22 ± 0.17 ml, 0.16 ± 0.14 ml, and 0.33 ± 0.2 ml, respectively, F(3,29) = 4.5, P = 0.01 (Fig. 4, A–D)]. The latter three groups did not differ from one another (Dun P > 0.6). During the final, one-bottle test, the lesion groups increased CS intake somewhat. The dLHx rats ingested more than the controls [3.40 ± 0.68 ml vs. 0.75 ± 0.25 ml, F(3,21) = 4.11, P ≤ 0.02, Dun P = 0.01]. The pLHx group increased similarly, Dun P = 0.03. The aLHx animals did not differ from the controls, Dun P = 0.66.
Fig. 4.
Experiment 1B. Postlesion CTA retention tests. The first three 15-min exposures were 2B tests with water and the CS, malic acid. The fourth test was 1B, malic acid. Double LHx (dLHx; A), posterior LHx (pLHx; B), anterior LHx (aLHx; C), and controls (D). Data are expressed as means ± SE. The pattern of intake matched the retention in experiment 1A although, on trial 4, the increased CS intake of the pLHx group was less dramatic.
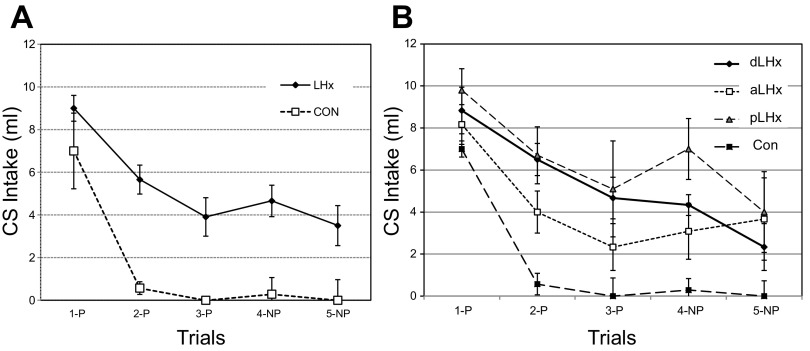
Postlesion CTA acquisition.
The three lesion groups and the controls all learned the alanine CTA but at different rates and to different degrees, F(3,21) = 7.7, P < 0.002 (Fig. 5). The initial CS intake was similar across all four groups [F(3,22) = 0.41, P = 0.75], but in the remaining three trials, the controls ingested near zero alanine, while the rats with LH lesions on average continued to ingest half as much CS (54.6%) as on their initial exposure, F(3,22) ≥ 4.65, P < 0.02. When given a choice in trial 5, however, the pLHx, aLHx, and dLHx groups decreased CS intake enough that the comparison with the controls, still at zero, was no longer significant, F(3,20) = 1.84, P = 0.17. Nevertheless, the parallel comparisons for water and total fluid intake remained significantly different [Data not shown; F(3,20) = 12.17, P < 0.001; F(3,20) = 4.29, P < 0.02, respectively].
Fig. 5.
Experiment 1B. Postlesion CTA acquisition is shown, with the CS being 0.3 M alanine. A: controls (n = 8) and all LHx, n = 18. B: performance by lesions subgroups: double (dLHx; n = 6), aLHx (n = 6), pLHx (n = 6), and Con. After the initial pairing with LiCl, the Con intake dropped to near zero (trial 2) and remained there in subsequent trials. On trial 2, the combined LHx intake (A) also dropped significantly but only by about a third (63%). In the remaining two 1B trials (trials 3 and 4), the LHx rats maintained their intake above 40% of their initial CS consumption with the pLHx subgroup highest and the aLHx group lowest (B) Even in trial 5, when they had a choice between the CS and water, the LHx animals ingested 39% as much CS as on trial 1. CS, conditioned stimulus, 0.3 M alanine; P, CS intake paired with LiCl; NP, no LiCl administered.
Weight, food consumption, and water consumption.
After the lesions, both food and water intake dropped dramatically. Despite access to a mash of powdered chow and diluted sweetened condensed milk and, in some cases, the milk by gavage, 14 rats died. The surviving LHx rats lost weight equally across groups. Compared with the controls, collectively, they lost more than 80 g at 4 days and 123 g at 20 days, the end of the observation period (see Ref. 2 for statistics and details).
Discussion
Regardless of the lesion location, the LHx rats remembered a prelesion CTA. Animals with double or posterior lesions did consume more of the prelesion CS than the controls or the aLHx group, but the differences were trivial. Overall, the LHx rats also acquired a new CTA, but most never ceased ingesting the CS completely. If we define “ceasing ingestion” as intake of 1.0 ml or less, then seven of eight controls met this criterion on their first post-LiCl test (trial 2), and all of them did so on each of the subsequent one-bottle trials. The aLHx rats (n = 6) were evenly divided between those that learned the aversion by trial 4 and those that did not. Only one rat in one trial in the pLHx and none in the dLHx group ever reached the “ceased ingestion” criterion.
The differences between the rats in similar groups in experiments 1A and 1B were a matter of degree. The aLHx rats in experiment 1A learned a new CTA as did the controls; both groups ingested less than 10% of their initial CS intake by trial 3. The experiment 1A pLHx group learned more slowly than controls, and their final intake on trial 4 was about 20% of that on trial 1. On trial 4 of experiment 1B, however, the aLHx and pLHx groups ingested 37% and 69% of their initial intake, respectively. These differences paralleled the differences in food intake and weight loss in the two groups (2).
To various degrees, the CTA results for experiment 1B also mimicked those reported by Schwartz and Teitelbaum (36) using electrolytic lesions. In this earlier study, after 4 LiCl pairings, 7 of 10 LHx animals failed to learn altogether. Although the methods and the measures differed between the two studies, in the present experiment, after the same number of CS-US trials, 60% (11/18) of the LHx rats failed to reduce their CS intake by more than 50%. Most other studies of CTA acquisition after LH damage report a similar range of deficits (41, 42, 45), but see Roman et al. (32) for an exception.
The histology revealed that, on average, both the anterior and posterior lesions in experiment 1B were 0.5 mm longer in the A-P plane than those in experiment 1A. The experiment 1B lesions also extended further dorsally and, less uniformly, more medially. This increased volume of destruction presumably accounts for the greater severity of the deficits in the second experiment. The histological procedures were not designed to quantify the volume of the lesions, much less the degree of overlap or the precise areas destroyed. We did correlate the degree of body weight change and the deficits in CTA learning, two objective measures that varied as a function of lesion placement and relative size. Weight change was the difference between the largest gain, set at zero, and the gain or loss for every other rat on day 15, the last trial for which complete data were available. The taste aversion score was the percentage of CS intake on trial 4 as a function of trial 1. In experiment 1A, the overall correlation between body weight change and CTA performance for both LHx and the controls was −0.41, P = 0.11. This effect was carried by the aLHx group, Pearson's r = −0.42, P = 0.35. The correlations for the pLHX rats and the controls were essentially zero, −0.04 and −0.09, respectively. In experiment 1B, however, the overall weight difference-CTA performance is r = 0.72, P < 0.001. Again, this appeared to be carried by the aLHx group but, because of the sample size, the correlation was not significant (r = 0.73, P = 0.16). For the pLHx rats, r = 0.05. The r for the double-lesion rats, dLHx, was 0.73, P = 0.16, presumably because their damage extended more rostrally. Although hardly definitive, these correlations imply that the effects of LH lesions on body weight and CTA learning are to some degree independent.
Nevertheless, the two experiments failed to separate the feeding (and drinking) deficits from the reduced ability to suppress ingestion of a normally preferred fluid when it was associated with malaise. The second experiment attempted to do this by using lesions of the LH in one hemisphere and the parabrachial nuclei in the other (2).
EXPERIMENT 2
As analyzed above, bilateral damage to the pontine PBN interferes with taste aversion learning in a manner similar to lateral hypothalamic lesions. Rats with either bilateral LHx or bilateral parabrachial lesions (PBNx) remember a prelesion CTA but either fail to acquire a new, postlesion aversion or do so to a lesser extent (2–4, 6, 28). Both anatomical and electrophysiological evidence link the PBN and the LH via reciprocal axonal projections (12, 14, 21, 24, 33). Because the PBN projections are substantially ipsilateral (15, 24), we hypothesized that damage to the PBN on one side of the brain accompanied by an LH lesion on the other side would interfere with the postlesion acquisition of a CTA in a manner similar to bilateral LH or bilateral PBN lesions.
Methods
Twenty-eight Sprague-Dawley rats were housed and maintained as described previously. Half of these animals were given prelesion Na-appetite experience, and half were not. All had postlesion experience with Na appetite. Three weeks elapsed between the end of the Na appetite tests and the beginning of the CTA trials.
Surgery.
Following acclimatization, 24 rats received LH and PBN ibotenic acid lesions. Out of these, one set received PBN damage to one side and LH damage to the other side (asymmetrical or Asym, n = 13). The second group had LH and PBN lesions on the same side (ipsilateral or Ipsi, n = 11). In both groups, lesion placement was counterbalanced. The LH lesions were positioned stereotaxically at −3.0 mm from β, 2.0 mm lateral, and 8.8 mm deep into the skull surface, and the lesion procedure was identical to that described in experiment 1, except that it was unilateral. The parabrachial lesions were placed under electrophysiological guidance using procedures detailed elsewhere (2, 24, 28, 35). Once a PBN gustatory response was located, the recording electrode was replaced with a micropipette glued to the shaft of a 1.0-μl syringe and filled with ibotenic acid (20 μg/μl in PBS, pH = 7.4). The pipette was then positioned at the same coordinates used to record taste responses, and a 0.2-μl infusion was made manually over 10 min. The pipette remained in place for an additional 10 min before being withdrawn. One Ipsi rat died following surgery.
Postoperative CTA acquisition and extinction.
Twelve Asym, 10 Ipsi, and 4 NSCon were tested. The acquisition training was similar to experiments 1A and 1B, but 0.3 M sucrose was the CS. There were three CS pairings with LiCl (US), then one CS-only trial, and one two-bottle trial both without LiCl. Except for the final two-bottle exposure, each acquisition trial was followed by 2 days during which water was offered in the morning. The day following the two-bottle trial, a formal extinction series began without intervening water days. Each extinction block consisted of two one-bottle trials followed by a single two-bottle test (CS vs. water). Five such blocks were conducted over 15 days. The same water restriction paradigm was maintained throughout.
Weight, food, and water.
Weight and food and water intake were measured daily as in experiment 1A and 1B.
Data analysis.
The data were analyzed as described for experiment 1A.
Histology.
After data collection was complete, the rats were reanesthetized and perfused, and the brains were extracted as in experiment 1. The brains were frozen and cut at 50 μm in three series. One series was stained with cresyl violet, as done previously, another was stained with Neu-N (2, 10), and the third was held in reserve.
Results
Histology.
The LH lesions of both the Asym and Ipsi rats were centered at −2.9 ± 0.36 mm posterior to β; the PBN lesions averaged −9.5 ± 0.24 mm caudal to the same landmark. Ten rats had lesions confined to the medial half of the PBN. The remaining 12 animals had complete medial PBN damage that extended into the lateral half of the nuclei to varying degrees (Fig. 6). One Asym rat was excluded from analysis due to an inadequate PBN lesion.
The PBN and LH damage was almost equally distributed between the Asym and Ipsi groups. The trigeminal motor nucleus was not involved in the lesions, but the supratrigeminal area, the locus coeruleus, or both were encroached upon in some rats. With the one exception, the LH and PBN lesions were adequate in the rats in both groups, so all of their data were included in the analysis.
Conditioned taste aversion.
ACQUISITION.
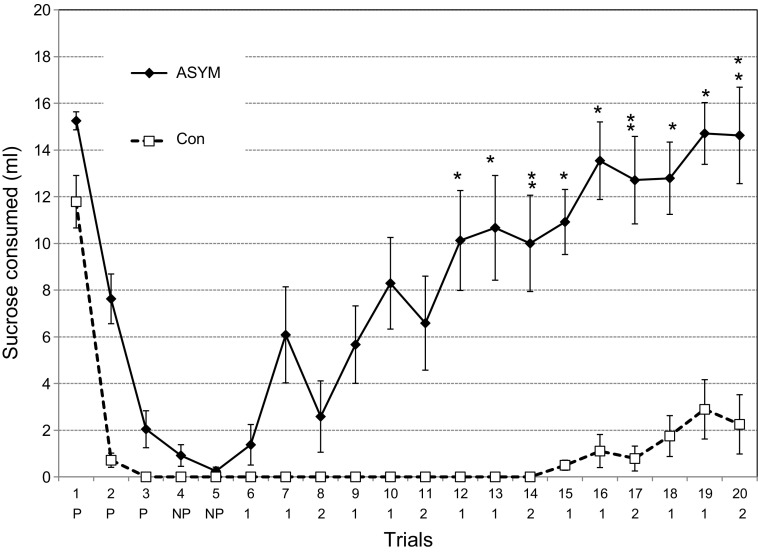
During the first three pairings, the CS intake of the Ipsi group and the NSCon did not differ [F(1,12) = 0.03, P = 0.86], so their data were collapsed into a single control group (Con, n = 14). During the first four trials, the CS intake of the Asym group was significantly different from that of controls [F(3,72) = 9.58, P < 0.001; Fig. 7]. On trial 1, prior to any LiCl injections, the Asym group ingested more CS than the controls (15.3 ± 0.4 ml vs. 11.8 ± 1.1 ml, Dun P < 0.01). On trial 2, after one CS-US pairing, this group difference was exaggerated because the controls ingested less than 1.0 ml of CS, while the Asym rats still drank 7.6 ± 1.1 ml (Dun P < 0.001). By trial 3, however, the difference had dissipated with the Asym intake down to 2.0 ± 0.8 ml compared with 0.8 ± 0.3 ml for the controls (Dun P = 0.12). The groups also did not differ on trial 4 (one-bottle, no LiCl, Dun P = 0.45) or trial 5 [two-bottle, no LiCl, F(1,24) = 36.5, P < 0.001, Dun P = 0.93].
Fig. 7.
Experiment 2. Acquisition and extinction of a sucrose CTA in the Asym group (n = 12; asymmetrical PBN and LH lesions) and Con (n = 14; nonsurgical controls and animals with ipsilateral PBN and LH lesions). Initial intake differed for the two groups (15.3 vs. 11.8 ml). After one CS-US pairing (trial 2), Con intake dropped to near zero (0.7 ml) and remained there in the remaining planned acquisition trials (trials 3–5). The Asym group also learned the CTA, but their intake only dropped by half (7.6 ml) in trial 2 and did not reach zero until the last acquisition trial (trial 5). Trials 1–4 were CS only, trial 5 was 2B, CS, and water. The formal extinction series began with trial 6 and consisted of repeated daily sequences of two 1B CS and one 2B access (CS and water) until trial 20. The second line below the abscissa indicates, for trials 1–5, those paired with LiCl (P) and those not paired with LiCl (NP), and beginning with trial 6, the 1B (1) and 2B (2) sequence of extinction. Asterisks indicate trials in which Asym intake was greater than that of the controls, ANOVAs with post hoc tests. Acquisition (trials 1–4) F(1,24) = 34.9, P < 0.001; extinction: one-bottle test, F(1,24) = 45.0, P < 0.001; two-bottle test: F(1,24) = 36.5, P < 0.001; post hoc tests, *P ≤ 0.05, **P ≤ 0.01.
Within groups, the Asym rats drank more CS during the first pairing (15.1 ± 0.4 ml) than in the second (7.6 ± 1.1 ml, Dun P < 0.001) and more in the second session than the third (3.1 ± 0.2 ml, Dun P < 0.001). The control rats also drank less CS during trials 2 and 3 (0.7 ± 0.3 ml, 0.0 ml) than in the first session (11.8 ± 1.1 ml, Dun P < 0.001). Their consumption in trials 2 and 3 was similar (Dun P = 0.43). In short, both the Asym and Con groups learned the CTA but at different rates.
Extinction.
The formal extinction series began with trial 6 and continued with repeated cycles of two one-bottle tests and one two-bottle test for a total of 15 days (Fig. 7). The one- and two-bottle tests were analyzed as separate series. Regardless of the exposure, the Asym rats extinguished their aversion to the CS more rapidly than the controls [one-bottle test, F(1,24) = 47.0, P < 0.001; two-bottle test, F(1,24) = 36.7, P < 0.001]. The two groups separated by trial 13 (Dun P = 0.04) and, regardless of the test (one or two bottle), remained so until the end of the extinction trials.
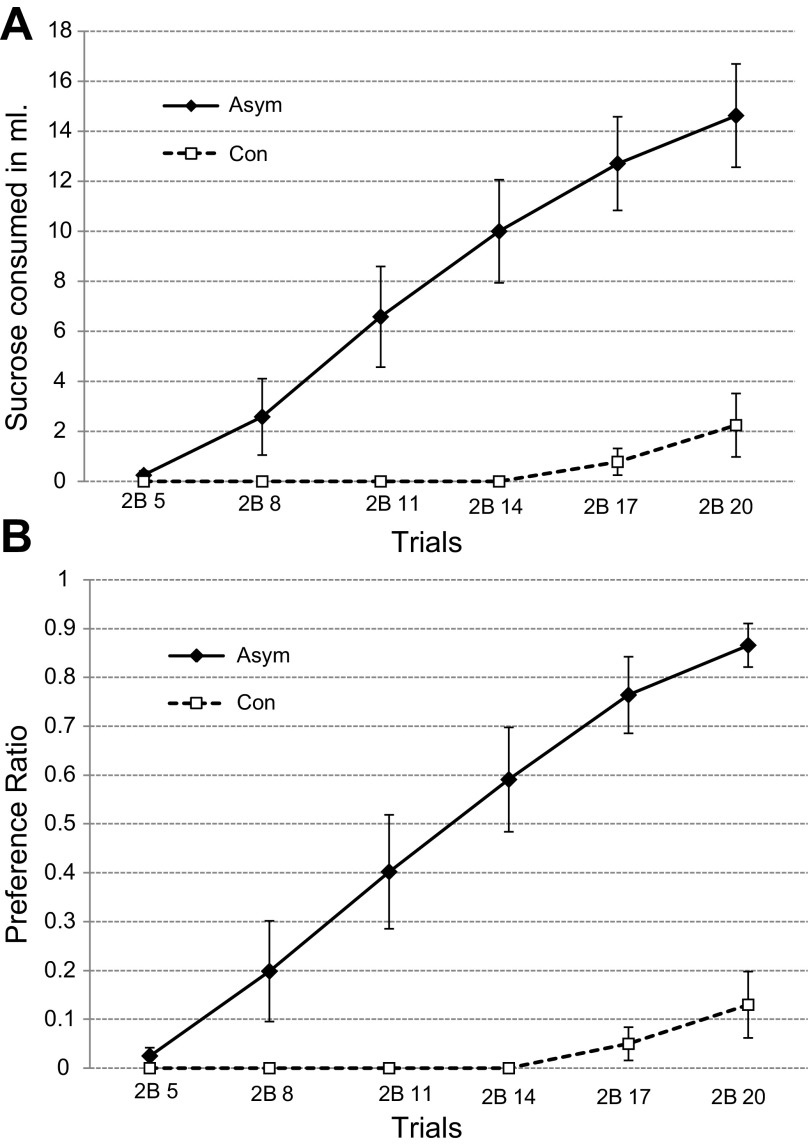
When compared separately with the preference ratios, the data from the two-bottle tests demonstrate that the Asym group's rapid CTA extinction derives from a regular substitution of CS instead of water intake (Fig. 8). During each of the two-bottle tests, the Asym rats consumed less water than the controls [6.4 ± 0.6 ml vs. 14.2 ± 0.4 ml, F(1,24) = 54.85, P < 0.001]. If water and sucrose intake are combined during these trials, however, the total intake of the two groups did not differ [Asym = 14.2 ± 0.65 ml; Con = 14.7 ± 0.32 ml, F(1,24) = 0.09, P = 0.77].
Fig. 8.
Experiment 2. A: intake during the six 2B extinction trials (means ± SE). The group with asymmetric PBN and LH lesions (Asym) consumed more of the sucrose CS than the controls (Con). B: preference ratios for the 2B extinction trials are defined as the CS intake divided by the sum of the CS and water intake for each trial. Total fluid intake was equivalent, Asym = 14.2 ± 0.65 ml; controls = 14.7. ± 0.32 ml.
Pre-CTA weight, food, and water.
Unlike the bilateral LHx rats in experiment 1B, following surgery, the Asym group did not require any intervention to maintain weight or hydration. Food intake and weight also did not differ between the Asym rats and their controls [F(1,24) = 0.06, P = 0.8, F(1,24) = 0.87, P = 0.36, respectively]. Although adequate to maintain hydration, the Asym rats spontaneously drank 25% less water than the CON group [22.7 ml vs. 30.3 ml, F(1,24) = 16.6, P < 0.001].
DISCUSSION
The rats with PBN and LH lesions on the same side of the brain, the ipsilateral group, did not differ from the normal controls. This demonstrates that unilateral damage of these two structures per se does not interfere with learning a CTA. In contrast, rats with asymmetric lesions—PBN on one side, LH on the other—did differ from the combined controls in both the acquisition and retention of a CTA learned after the central damage. They learned the aversion more slowly, and they extinguished it much more rapidly. Rapid learning, often in one trial, and slow extinction are both cardinal features that distinguish CTA from most other experimental learning paradigms. Therefore, for normal acquisition and maintenance of this learned aversion, the neurons in the PBN and LH must communicate with one another.
Unlike bilateral PBN or LH lesions, however, the asymmetric damage did not prevent learning, just slowed it down. We used the same criterion as in experiment 1B, i.e., ≤1.0 ml of intake, and found that 11 of the 14 controls ceased CS intake after a single pairing with LiCl. On all subsequent acquisition trials, all 14 failed to ingest measurable quantities of CS. Of the 12 Asym rats, only one ceased ingestion after one CS-US pairing. After three pairings, three Asym animals (25%) continued to drink some 0.3 M sucrose. Intake continued to decline during trials 4 and 5, one-bottle and two-bottle tests, respectively, both without LiCl. For half of the Asym group, however, CS consumption increased dramatically beginning with trial 7, the second one-bottle test of the formal extinction rounds. By trial 13, all but three Asym rats were drinking 7.0 ml or more of the CS when all of the controls remained at zero. By the final one-bottle trial (trial 19), the Asym rats consumed the same volume of CS as on their first exposure (trial 1), F(1,22) = 0.16, P = 0.70, while the controls had barely budged from complete avoidance, F(1,26) = 27.5, P < 0.001.
Thus, the asymmetrical PBN-LH lesions produce a relatively minor slowing in acquisition but a major deficit in extinction. The difficulty with this distinction is that the two processes are not independent. In this instance, the controls reached zero CS intake by trial 3, the Asym rats by trial 5. The ingestive behaviors reached equivalence but, because of the floor effect, the underlying associations may not have been of the same magnitude. If the Asym PBN-LH lesions reduced the strength of the learning sufficiently, then rapid extinction could be a normal expression of the inefficient associative process.
In experiment 1A, however, the initial CTA was similar across all of the animals because it was acquired before the LH lesions were made. After the lesions, there were three two-bottle retention trials. All of the rats, except two with posterior LH damage, remembered the learned aversion on those three trials, and the groups were statistically equivalent [F(2,16) = 2.28, P = 0.13]. The fourth trial was a single CS bottle. The controls and the rats with more anterior LH lesions maintained their aversion, ingesting not more than 1.0 ml of CS even without a water alternative. Without the two animals that failed to retain the CTA, the remaining pLHx rats averaged 7.0 ± 1.6 ml in the one-bottle trial. All together, the pLHx rats ingested almost as much CS, as they did in their first pre-LiCl exposure [10.4 vs. 13.3 ml, F(1,10) = 2.53; P = 0.14]. Although the circumstances of the two experiments differ, they each implicate LH damage in rapid extinction of a CTA and, in one case, the strength of the original association was not an issue.
Regardless, the primary deficit underlying the rapid extinction is not evident. The asymmetric lesions could inhibit transfer to long-term memory, enhance the extinction process, or both. In this scenario, the delayed acquisition could reflect this active forgetting, and the rapid extinction would be the primary deficit. The current data cannot distinguish between these interpretations or other, intermediate processes. Nevertheless, other behavioral effects of either the PBN or LH lesions make some interpretations more likely than others.
Reduced taste intensity after PBN damage is an obvious, but not a likely, explanation for the rapid extinction. Bilateral PBN damage eliminates the acquisition of a gustatory CTA but does not necessarily prevent the same rats from discriminating low concentrations of sapid stimuli from water (38). Similar tests have yet to be conducted after bilateral LH lesions or asymmetric PBN-LH damage. Regardless, in experiment 2, the PBN and, thus, both the thalamocortical and limbic gustatory projections are intact unilaterally.
The case against PBN damage decreasing visceral afferent activity is less straightforward. In experiment 2, the rate of CTA learning was delayed by one trial, but then paralleled the controls. This argues against a substantial decrease in the effectiveness of the LiCl US. During the postlesion CTA acquisition in experiment 1B, more than half of the rats with bilateral LH damage decreased their CS intake after the first LiCl pairing but then subsequently failed to reduce it further even after 3 CS-US pairings (Fig. 6). This might be attributed to reduced effectiveness of the LiCl. In normal rats, however, lowering the dose of LiCl, slows the rate of CTA acquisition, but the subjects eventually cease CS intake (6). Therefore, if the asymmetric PBN/LH damage alters the central visceral afferent activity, the change is not equivalent to just reducing the dose of LiCl.
The best evidence that compromised visceral afferent activity contributes to the CTA deficit that follows bilateral PBN damage comes from experiments with restricted lesions and different CS sensory modalities. Although there is substantial anatomical overlap, visceral afferent axons from the nucleus of the solitary tract terminate primarily in the lateral PBN subnuclei and those conveying gustatory afferent activity end more medially (8, 25). In fact, damage centered more medially in PBN eliminates the acquisition of a CTA using a gustatory but not a trigeminal CS (5,13). Damage centered more laterally eliminates the acquisition of a CTA with a CS of either modality and, thus, implicates blocking the vagal visceral afferent system (29).
Similar experiments are lacking for bilateral LH lesions, so the effects of parabrachial and hypothalamic damage cannot be compared directly on this issue. Indirect evidence, however, demonstrates a strong relationship between hypothalamic feeding syndromes and the vagus nerve. Bilateral section of the subdiaphragmatic vagus prior to lesions of the ventromedial hypothalamus prevents the dramatic overeating and obesity that attends such damage. After the establishment of hypothalamic obesity, the same peripheral nerve cuts rescue the effect (27). At the time, the rescue was ascribed to cutting vagal efferents, but a role for vagal afferent activity was not excluded. Lateral hypothalamic damage causes profound sensory neglect of visual, olfactory, or somatosensory stimuli (19, 20). Although gustatory or visceral afferent activity was not assessed in these experiments, the large, electrolytic lesions used in the original CTA-LH paper almost certainly interrupted both gustatory and visceral afferent PBN axons destined for the hypothalamus and the rest of the ventral forebrain (15, 24, 33, 36).
In short, we cannot exclude reduced visceral afferent activity from contributing to the deficits in CTA learning produced by bilateral damage to the PBN or the LH. Therefore, to determine whether visceral afferent activity contributed to the CTA effects of PBN/LH asymmetric lesions, such animals should be tested with both a gustatory and an oral trigeminal CS. If these rats exhibited slowed acquisition and rapid extinction with a taste CS but not with a trigeminal stimulus, then we could assume that the consequences of the LiCl injections were not compromised. The reverse outcome, however, would not be definitive. If both the gustatory and trigeminal CS produced the same effects on CTA acquisition, this would not exclude a visceral afferent deficit but neither would it demand one.
Perspectives and Significance
Asymmetrical lesions have at least three advantages. They permit a near perfect control—ipsilateral damage of the same two areas. They can dissociate the multiple effects of bilateral lesions into their components. They can implicate a neural system rather than a center in the control of a function. The present series of experiments (Ref. 2 and this article) illustrate each of these advantages. In both sets of experiments, the ipsilateral controls behaved as did the normal rats even though their PBN and LH damage matched that of the asymmetric group except for the laterality of the lesions. Bilateral LH lesions interfered with sodium appetite and the acquisition of a CTA. The same damage also reduced food intake and produced sustained weight loss, the classic symptoms of the lateral hypothalamic syndrome (39, 40). Asymmetric PBN/LH lesions had no effect on food intake or body weight, but disrupted CTA and eliminated sodium appetite.
The effect of asymmetric PBN/LH lesions on sodium appetite was identical to that of bilateral damage to either the PBN or the LH. This permits the inference that, in order for an animal to exhibit sodium appetite, these two areas must be able to interact (2). The same asymmetric damage also altered a CTA but the effects differed from those produced by bilateral lesions of either the PBN or the LH, which, in turn, differed from one another. Bilateral lesions of the PBN eliminate the acquisition of a CTA even after multiple CS-US pairings (35). In most cases, bilateral electrolytic lesions of the LH also blocked acquisition of a CTA (36). After similar ibotenic acid damage, however, the rats in experiments 1A and 1B initially reduced their CS intake but, despite more CS-US pairings, some intake persisted. The Asym PBN/LH group learned the CTA but, on average, after a one-trial delay. Then they extinguished their aversion rapidly, even when given a choice of the CS or water. In this case, the inference is the same, normal acquisition of a CTA requires interaction between cells in the PBN and in the LH.
The different CTA deficits after asymmetric lesions highlight both the strengths and the limitations of the technique. In fact, they are one and the same. The strength is the ability to draw inferences about the functional interaction between two structures using a simple variant of a standard technique. The limitation is that, given this functional interaction, this technique says nothing about the neural mechanisms involved. Because PBN neurons project to the LH and vice-a-versa, the parsimonious interpretation of the asymmetric lesion deficits is the interruption of this reciprocal connection. Both the PBN and the LH, however, have multiple other possible pathways, some of which are known to influence CTAs. This limitation, however, is inherent in all experiments on the brain that involve functional deficits. The asymmetric lesion approach simply makes it more obvious.
GRANTS
This research was supported by National Institute of Deafness and Other Communication Disorders Grants DC-000240, DC-05435, and DC-008937.
Present adresses: S. Dayawansa, Department of Pathology, University of Buffalo, Buffalo, NY, 14214; S. Ruch, Division of General Education, Pennsylvania College of Health Sciences, Lancaster, PA 17602.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.D. and S.R. performed experiments; S.D., S.R., and R.N. analyzed data; S.D. prepared figures; S.D. and R.N. drafted manuscript; S.D., S.R., and R.N. approved final version of manuscript; S.R. and R.N. conception and design of research; S.R. and R.N. interpreted results of experiments; R.N. edited and revised manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Han Li for assistance with the lesions, S. Peckins for technical assistance, and K. Matyas and N. Horvarth for histology.
REFERENCES
- 1.Bielavska E, Bures J. Universality of parabrachial mediation of conditioned taste aversion. Behav Brain Res 60: 35–42, 1994. [DOI] [PubMed] [Google Scholar]
- 2.Dayawansa S, Peckins S, Ruch S, Norgren R. Parabrachial and hypothalamic interaction in sodium appetite. Am J Physiol Regul Integr Comp Physiol 300: R1091–R1099, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Lorenzo PM. Long-delay learning in rats with parabrachial pontine lesions. Chem Senses 13: 219–229, 1988. [Google Scholar]
- 4.Flynn FW, Grill HJ, Schulkin J, Norgren R. Central gustatory lesions. II. Effects on sodium appetite, taste aversion learning, and feeding behaviors. Behav Neurosci 105: 944–954, 1991. [DOI] [PubMed] [Google Scholar]
- 5.Grigson PS, Reilly S, Shimura T, Norgren R. Ibotenic acid lesions of the parabrachial nucleus and conditioned taste aversion: Further evidence for an associative deficit. Behav Neurosci 112: 160–171, 1998. [PubMed] [Google Scholar]
- 6.Grigson PS, Shimura T, Norgren R. Brainstem lesions and gustatory function: III. The role of the nucleus of the solitary tract and the parabrachial nucleus in retention of a conditioned taste aversion in rats. Behav Neurosci 111: 180–187, 1997. [PubMed] [Google Scholar]
- 7.Hajnal A, Norgren R. Taste pathways that mediate accumbens dopamine release by sapid sucrose. Physiol Behav 84: 363–369, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Herbert H, Moga M, Saper C. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol 293: 540–580, 1990. [DOI] [PubMed] [Google Scholar]
- 9.Ivanova SF, Bures J. Acquisition of conditioned taste aversion in rats is prevented by tetrodotoxin blockade of a small midbrain region centered around the parabrachial nuclei. Physiol Behav 48: 543–549, 1990. [DOI] [PubMed] [Google Scholar]
- 10.Jongen-Relo A. L Specific neuronal protein: a new tool for histological evaluation of excitotoxic lesions. Physiol Behav 76: 449–56, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Li CS, Cho YK. Efferent projection from the bed nucleus of the stria terminalis suppresses activity of taste-responsive neurons in the hamster parabrachial nuclei. Am J Physiol Regul Integr Comp Physiol 291: R914–R926, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Li CS, Cho YK, Smith DV. Modulation of parabrachial taste neurons by electrical and chemical stimulation of the lateral hypothalamus and amygdala. J Neurophysiol 93: 1183–1196, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Liang NC, Grigson PS, Norgren R. Pontine and thalamic influences on fluid rewards: II. Sucrose and corn oil conditioned aversions. Physiol Behav 105: 589–594, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lundy RF, Jr, Norgren R. Activity in the hypothalamus, amygdala, and cortex generates bilateral and convergent modulation of pontine gustatory neurons. J Neurophysiol 91: 1143–1157, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Lundy RF, Jr, Norgren R. Gustatory system. In: The Rat Nervous System (3rd ed.), edited by Paxinos G. San Diego, CA: Elsevier Academic Press, 2004, p. 891–921. [Google Scholar]
- 16.Lundy RF, Jr, Norgren R. Pontine gustatory activity is altered by electrical stimulation in the central nucleus of the amygdala. J Neurophysiol 85: 770–783, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Mao L, Cho YK, Li CS. Modulation of activity of gustatory neurons in the hamster parabrachial nuclei by electrical stimulation of the ventroposteromedial nucleus of the thalamus. Am J Physiol Regul Integr Comp Physiol 294: R1461–R1473, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Markowska A, Bakke HK, Walther B, Ursin H. Comparison of electrolytic and ibotenic acid lesions in the lateral hypothalamus. Brain Res 328: 313–323, 1985. [DOI] [PubMed] [Google Scholar]
- 19.Marshall P, Teitelbaum J. Further analysis of sensory inattention following lateral hypothalamic damage in rats. J Comp Physiol Psych 86: 375–395, 1974. [DOI] [PubMed] [Google Scholar]
- 20.Marshall J, Turner B, Teitelbaum P. Sensory neglect produced by lateral hypothalamic lesions. Science 174: 523–525, 1971. [DOI] [PubMed] [Google Scholar]
- 21.Moga MM, Herbert H, Hurley KM, Yasui Y, Gray TS, Saper CB. Organization of cortical, basal forebrain, and hypothalamic afferents to the parabrachial nucleus in the rat. J Comp Neurol 295: 624–661, 1990. [DOI] [PubMed] [Google Scholar]
- 22.Morrison SD, Mayer J. Adipsia and aphagia in rats after lateral subthalamic lesions. Am J Physiol 191: 248–254, 1957. [DOI] [PubMed] [Google Scholar]
- 23.Norgren R. Gustatory afferents to ventral forebrain. Brain Res 81: 285–295, 1974. [DOI] [PubMed] [Google Scholar]
- 24.Norgren R. Taste pathways to hypothalamus and amygdala. J Comp Neurol 166: 17–30, 1976. [DOI] [PubMed] [Google Scholar]
- 25.Norgren R. Projections from the nucleus of the solitary tract in the rat. Neuroscience 3: 207–218, 1978. [DOI] [PubMed] [Google Scholar]
- 26.Paxinos G, Watson C. The Rat Brain in Sterotaxic Coordinates. London: Elsevier, 1992. [Google Scholar]
- 27.Powley T. The ventromedial hypothalamic syndrome, satiety, and a cephalic phase hypothesis. Psychol Rev 84: 89–126, 1977. [PubMed] [Google Scholar]
- 28.Reilly S, Grigson PS, Norgren R. Parabrachial nucleus lesions and conditioned taste aversion: evidence supporting an associative deficit. Behav Neurosci 107: 1005–1017, 1993. [DOI] [PubMed] [Google Scholar]
- 29.Reilly S, Trifunovic R. Lateral parabrachial nucleus lesions in the rat: Aversive and appetitive gustatory conditioning. Brain Res Bull 52: 269–278, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Ricardo JA, Koh ET. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res 153: 1–26, 1978. [DOI] [PubMed] [Google Scholar]
- 31.Rolls BJ, Rolls ET. Effects of lesions in the basolateral amygdala on fluid intake in the rat. J Comp Physiol Psychol 83: 240–247, 1973. [DOI] [PubMed] [Google Scholar]
- 32.Roman C, Nebieridze N, Sastre A, Reilly S. Effects of lesion of the bed nucleus of the stria terminalis, lateral hypothalamus, or insular cortex on conditioned taste aversion and conditioned odor aversion. Behav Neurosci 120: 1257–1267, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Saper CB, Loewy AD. Efferent connections of the parabrachial nucleus in the rat. Brain Res 197: 291–317, 1980. [DOI] [PubMed] [Google Scholar]
- 34.Scalera G, Norgren R. Parabrachial nucleus (PBN) lesions fail to disrupt sodium appetite in experienced rats (Abstract). Appetite 21: 204, 1993. [Google Scholar]
- 35.Scalera G, Spector AC, Norgren R. Excitotoxic lesions of the parabrachial nuclei prevent conditioned taste aversions and sodium appetite in rats. Behav Neurosci 109: 997–1008, 1995. [PubMed] [Google Scholar]
- 36.Schwartz M, Teitelbaum P. Dissociation between learning and remembering in rats with lesions in the lateral hypothalamus. J Comp Physiol Psychol 87: 384–398, 1974. [DOI] [PubMed] [Google Scholar]
- 37.Spector AC, Norgren R, Grill HJ. Parabrachial gustatory lesions impair taste aversion learning in rats. Behav Neurosci 106: 147–161, 1992. [DOI] [PubMed] [Google Scholar]
- 38.Spector A, Scalera G, Grill H, Norgren R. Gustatory detection thresholds after parabrachial nuclei lesions in rats. Behav Neurosci 109: 939–954, 1995. [PubMed] [Google Scholar]
- 39.Teitelbaum P, Epstein AN. The lateral hypothalamic syndrome: recovery of feeding and drinking after lateral hypothalamic lesions. Psychol Rev 69: 74–90, 1962. [DOI] [PubMed] [Google Scholar]
- 40.Teitelbaum P, Stellar E. Recovery from the failure to eat produced by hypothalamic lesions. Science 120: 894–895, 1954. [DOI] [PubMed] [Google Scholar]
- 41.Touzani K, Sclafani A. Conditioned flavor preference and aversion: Role of the lateral hypothalamus. Behav Neurosci 115: 84–93, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Touzani K, Sclafani A. Lateral hypothalamic lesions impair flavour-nutrient and flavour-toxin trace learning. Eur J Neurosci 16: 2425–2433, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Veening JG, Swanson LW, Sawchenko PE. The organization of projections from the central nucleus of the amygdala to brainstem sites involved in central autonomic regulation: a combined retrograde transport-immunohistochemical study. Brain Res 303: 337–357, 1984. [DOI] [PubMed] [Google Scholar]
- 44.Yamamoto T, Fujimoto Y. Brain mechanisms of taste aversion learning in the rat. Brain Res Bull 27: 403–406, 1991. [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto T, Fugimoto Y, Shimura T, Sakai N. Conditioned taste aversion in the rat with excitoxic brain lesions. Neurosci Res 22: 31–49, 1995. [DOI] [PubMed] [Google Scholar]