Abstract
In postmenopausal women the mechanisms responsible for hypertension have not been completely elucidated, and there are no gender-specific guidelines for women despite studies showing that blood pressure is not as well controlled to goal in women as in men. In the present study we tested the hypotheses that the sympathetic nervous system and the renal sympathetic nerves contribute to hypertension in aging female rats, that sympathetic activation may be mediated by the melanocortin 3/4 receptor (MC3/4R), and that MC3/4R activation may be due to increases in leptin. α-1, β-1,2-Adrenergic blockade reduced blood pressure in both young (3–4 mo) and old (18–19 mo) female spontaneously hypertensive rats (SHR). Renal denervation attenuated the hypertension more in old females than young females. MC3/4R antagonism with SHU-9119 given intracerebroventricularly had no effect on blood pressure in either young or old females but significantly reduced blood pressure in old males. Plasma leptin levels were similar in old male and female SHR and in old versus young females. These data suggest that the hypertension in old female SHR is in part due to activation of the sympathetic nervous system, that the renal nerves contribute to the hypertension, and that the mechanism responsible for sympathetic activation in old females is independent of the MC3/4R.
Keywords: aging, renal denervation, leptin, melanocortin 3/4 receptor, sex differences
aging in both women and men is associated with increases in blood pressure. However, the National Health and Nutrition Examination Survey (NHANES 1999–2004) showed that after 50 years of age, the prevalence of hypertension is higher in women than in men regardless of ethnicity (6). In fact, cardiovascular disease is one of the most important causative factors in increased mortality and morbidity in women, and hypertension is a leading risk factor for cardiovascular disease (20, 23, 27, 41). The mechanisms responsible for the increase in blood pressure in postmenopausal women have not been completely determined.
Just as in many women, as they age, female spontaneously hypertensive rats (SHR) exhibit increases in blood pressure such that by the time they reach 16–18 mo of age, their blood pressure is similar to or higher than in age-matched males (20, 41). We have evaluated several systems that are known to contribute to blood pressure control in these old females, such as the renin-angiotensin system, the endothelin system, and eicosanoids such as 20-HETE pathways (21, 38–40). We found that blockade of these pathways separately and/or in combination reduces the blood pressure in old female SHR, but does not normalize the blood pressure (21, 38–40), thus suggesting additional systems may contribute to the hypertension.
While the causes of hypertension in aging humans have not been completely elucidated, activation of the sympathetic nervous system (SNS) has been implicated (1, 16). Growing evidence suggests that the SNS acts through the renal sympathetic nerves, increasing the renin release, altering the glomerular filtration rate, and increasing tubular sodium reabsorption, and there may be gender differences in these effects (1, 9, 15, 25). We have shown previously that renal sympathetic denervation reduces the blood pressure in young male and female SHR by the same percentage (17). So while renal sympathetic nerve activity contributes to hypertension in both male and female SHR while they are young, it does not explain the sex difference in blood pressure that is found in these rats. The role that the sympathetic nervous system plays and, in particular, the role that the renal sympathetic nerves may play in mediating the hypertension in old female SHR has not been determined to our knowledge.
One of the ways in which the sympathetic nervous system can be stimulated is via activation of pro-opiomelanocortin (POMC) neurons and melanocortin 3/4 receptors (MC3/4R). This pathway is thought to play a role in mediating obesity hypertension with one of the activating factors being leptin (10, 12, 14). da Silva and colleagues (8) showed previously that despite not being obese, blockade of the MC3/4R with SHU-9119 in young male SHR reduced their blood pressure, suggesting the MC3/4R pathway is a mechanism responsible for SNS activation in young males. Whether MC3/4Rs play a role in mediating the hypertension in aging female SHR is not clear. Studies have shown that leptin levels increase with aging in women (7) and are typically higher in women than men (22), since leptin is released from adipose tissue and women have more adipose tissue especially with aging, even when they are not overweight. Furthermore, leptin levels were found to be associated with hypertension in postmenopausal women in the Family Heart Study (22). Again, whether leptin levels are higher in old female SHR than in males, and whether this could contribute to their hypertension is unknown.
Based on these data and the data showing that sympathetic activation plays a role in many forms of primary essential hypertension in humans, we tested the hypotheses that 1) upregulation of the SNS indeed does contribute to hypertension in aging female SHR; 2) the renal sympathetic nerves contribute to the hypertension in old female SHR more than in young females; and 3) that the mechanism responsible for activation of the SNS in old females is mediated in part via MC3/4R and perhaps is due to higher levels of leptin that activate MC3/4R.
MATERIALS AND METHODS
Rats
Retired breeder females were obtained from the vendor (Taconic Farms, Germantown, PA) at 4–9 mo of age, aged in the Laboratory Animal Facilities (LAF) at the University of Mississippi Medical Center (UMMC), and used at 18–20 mo of age. Male SHR were obtained from the vendor at 10–12 wk of age and allowed to age to 18–20 mo in the UMMC LAF. Young female SHR were obtained from the vendor at 10 wk of age and used at 12–16 wk of age. The rats were maintained on standard rat chow (Teklad, Harlan SD, Indianapolis, IN) and tap water in an environment with 12-h:12-h light-dark cycle while aging and throughout the protocols. The protocols complied with the Guidelines for the Care and Use of Laboratory Animals by the National Institutes of Health and were reviewed and approved by the Institutional Animal Care and Use Committee at UMMC.
Experimental Design
Protocol 1.
ADRENERGIC BLOCKADE.
SHR female rats, aged 12 wk and 18 mos, were divided into two groups (n = 5 per group). Radiotelemetry transmitters (TA11PA-C40; Data Sciences International, St. Paul, MN) were implanted in the abdominal aortae using isoflurane anesthesia, as we have previously described (17, 21, 39). After 2 wk recovery from surgery, mean arterial pressure (MAP) was measured for 5 days as a baseline period. After that, both groups were treated with terazosin (10 mg·kg−1·day−1 sc; a selective α1-receptor antagonist) and propranolol (10 mg·kg−1·day−1 sc; a nonselective β1,2-receptor antagonist) via osmotic minipumps (Alzet) for 7 days with continuous MAP measurement. During the treatment period, all rats drank similar amounts of water (30–35 ml/day) and excreted similar amounts of urine (29.6–33.5 ml/day), and body weights were similar throughout the study.
α- and β-blockade adequacy was tested on day 5 of blockade via femoral vein catheters using phenylephrine (4 μg/200 μl iv) followed 5 min later with isoproterenol (0.7 μg/200 μl iv). The agonist infusion was timed exactly, and its effects on both MAP and heart rate (HR) were recorded 1 min after each bolus and compared with MAP and HR immediately before the infusion (2, 3, 5, 24, 28). Adrenergic blockade was also tested in untreated young female SHR as controls (n = 3).
Protocol 2.
RENAL DENERVATION.
Male and female SHR, aged 3 or 16–18 mo (n = 6/group), were subjected to right uninephrectomy (UNX) via dorsal incision during isoflurane anesthesia. After 2 wk recovery, rats were subjected to midline abdominal incision under isoflurane anesthesia, and left renal denervation was performed by painting the left renal nerves and the left renal artery with 10% phenol in ethanol solution and then cutting all the visible renal nerves, as we previously described (17). All sham animals also received left UNX 2 wk before, and on the day of surgery, renal nerves were identified but left undisturbed. Immediately after renal denervation, radiotelemetry transmitters (TA11PA-C40, Data Sciences International Transoma) were implanted into the abdominal aorta below the renal arteries, as previously described (21, 39).
Two weeks after renal denervation and telemetry implantation, MAP and HR were measured continuously in all animals for 5 days as previously described (21, 39). At the end of the experiment, the animals were anesthetized with isoflurane, and kidneys were removed and snap frozen in liquid nitrogen for measurement of norepinephrine content by liquid chromatography/mass spectroscopy (17, 42), at the Medical College of Wisconsin under the direction of Dr. David Mattson.
Protocol 3.
INHIBITION OF MC3/4R WITH SHU-9119.
Young (n = 16) and old female (n = 12) SHR, aged 12 wk or 18–20 mo, respectively, and old males, aged 18–20 mo (n = 6 per group), were implanted with radiotelemetry transmitters using isoflurane anesthesia. After transmitter implantation, a stainless steel cannula (26 gauge, 10 mm long) was implanted into the right lateral cerebral ventricle, as previously described (8). The guide cannula was anchored into place with two stainless steel machine screws, a metal cap, and dental acrylic, and a stylet was inserted to seal the cannula to keep it from becoming clogged. Several days after the rats recovered from surgery, accuracy of the cannula placement was tested by measuring the dipsogenic response (immediate drinking of at least 5 ml of water in 10 min) to an intracerebroventricular (icv) injection of 100 ng of angiotensin II. After 2 wk recovery, MAP and HR were measured during a 5-day baseline period, and then an osmotic minipump (Alzet) was implanted subcutaneously in the scapular region and connected to the intracerebroventricular cannula using polyethylene tubing for infusion of the MC3/4R antagonist SHU-9119 (1 nmol/h, 0.5 μl/h icv) for 10 days. The dose of SHU-9119 infusion was based on our previous studies showing that this dose effectively blocks MC3/4R and increases food intake (8). Since SHU-9119 causes an increase in food intake, body weight was measured before intracerebroventricular cannula implantation and again at the end of the study, and food intake was recorded daily. After the experiment, the animals were euthanized, and the brains were removed, sectioned, and stained with cresyl violet to confirm the placement of the cannula in the right lateral ventricle.
Plasma leptin levels.
Plasma leptin was measured using an enzyme-linked immunosorbent assay (ELISA; Crystal Chem, Downers Grove, IL) as per manufacturer instructions. For leptin measurements comparing old males and females, blood was drawn at euthanasia in control and after SHU treatment. SHU had no effect on plasma leptin levels, so the data were combined for the control and SHU-treated rats (n = 12/group). For comparison between young and old females, leptin was measured in blood taken from different untreated rats than described above for SHU study (n = 8/group).
Statistics
Data are presented as means ± SE. The significance of difference in mean values between and within groups was determined using a two-way ANOVA for repeated measures and a Dunn's test for preplanned comparisons using SigmaPlot software. The significance of absolute and/or percent change in blood pressure after adrenergic blockade and renal denervation in various valued between groups was performed using an unpaired t-test using Graphpad Prism. The significance of differences in leptin levels between groups were also analyzed using an unpaired t-test. P < 0.05 was considered to be statistically significant.
RESULTS
Protocol 1
Adrenergic blockade.
MAP was significantly higher in old females than young females during the baseline period (Fig. 1A). Blockade of α1, β1/2-adrenergic receptors significantly reduced MAP in both young and old females. In old females MAP decreased from 182 ± 4 to 104 ± 4 mmHg during the first 24 h but gradually increased to 141 ± 7 mmHg by day 7 of infusion. In young females, MAP also decreased significantly from 126 ± 2 to 91 ± 2 mmHg during the first 24 h and gradually increased back to 108 ± 2 mmHg by day 7 of infusion. Both groups increased MAP back to control levels by days 4–5 of recovery. As shown in Fig. 1B, the percentage drop in MAP on day 8 (treatment) compared with day 4 (baseline) was significantly greater in old females (−27 ± 3%) than young females (−18 ± 2%, p<0.04).
Fig. 1.
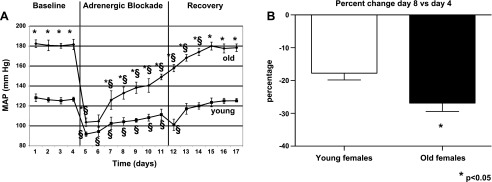
Effect of α1, β1,2-adrenergic blockade on blood pressure in young and old female spontaneously hypertensive rats (SHR). A: mean arterial pressure (MAP). MAP was measured during a 4-day baseline period and during infusion of terazosin and propranolol (10 mg·kg−1·day−1 sc each drug) in young (aged 4 mos, n = 6) and old (aged 18–20 mo, n = 6) female SHR. After infusion, rats were allowed to recover over the next 7 days, and MAP measurements were continued. Data are shown as MAP ± SE. *P < 0.05, compared with young females; §P < 0.05 compared with baseline MAP in same aged animals. B: percentage change in blood pressure comparison for day 8 vs. day 4 in old vs. young female SHR. Data are shown as percentage for either young or old females. *P < 0.05, old vs. young females.
Adequacy of blockade.
As shown in Table 1, blockade was tested as described in materials and methods. After α-adrenergic blockade, neither phenylephrine nor isoproterenol had a significant effect on MAP or HR in old and young females. These data suggest adequate blockade with α1, β1,2-adrenergic blockers in both young and old female SHR.
Table 1.
Response to adrenergic agonists in young female control or blockade-treated young and old female SHR
| Phenylephrine |
Isoproterenol |
|||
|---|---|---|---|---|
| ΔMAP, mmHg | ΔHR, beats/min | ΔMAP, mmHg | ΔHR, beats/min | |
| Young female control (n = 3) | 1 ± 8 | 36 ± 31 | −15 ± 7 | 47 ± 10 |
| Young female + blockade (n = 6/group) | 1 ± 2 | −7 ± 13 | 3 ± 3 | 6 ± 7 |
| Old female + blockade (n = 6/group) | 4 ± 2 | −6 ± 4 | 3 ± 2 | 8 ± 4 |
Values are means ± SE; n, number of rats. SHR, spontaneously hypertensive rats; MAP, mean arterial pressure; HR, heart rate.
Protocol 2
Renal denervation.
As shown in Fig. 2A, MAP was higher in old female uninephrectomized (UNX) sham SHR than in young female UNX shams. Renal denervation in UNX females reduced MAP in both groups, but had a greater effect on old females than young females both numerically (old females: −30 ± 1; young females: −17 ± 1 mmHg; P < 0.01) and by percentage (old females: −17.6 ± 0.4%; young females: −12.2 ± 0.4%, P < 0.05). Renal norepinephrine levels were similar in young and old female UNX shams and decreased to similar levels with renal denervation in both groups (Fig. 2B). HR was similar in young and old UNX sham females and was not affected by renal denervation in either group (old female UNX sham: 330 ± 11 vs. old UNX renal denervated: 336 ± 3 beats/min; and young female UNX sham: 343 ± 7 vs. young UNX denervated: 344 ± 5 beats/min; P = NS).
Fig. 2.
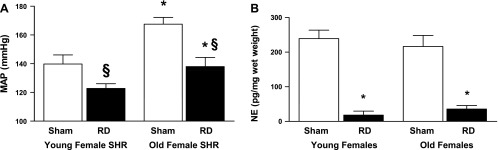
Effect of renal denervation in old and young female SHR. A: effect on MAP. MAP was measured in young (4 mo, n = 12) and old (18–20 mo, n = 12) female SHR that were either were right uninephrectomized (UNX) or UNX + renal denervated. RD, renal denervation. Data are presented as means ± SE. *P < 0.01 compared with old females of same treatment; §P < 0.05 compared with sham rats of each age. B: renal norepinephrine (NE) levels in sham and renal denervated rats. After the measurement of MAP, rats were anesthetized, and kidneys were removed for measurement of NE by liquid chromatography/mass spectroscopy. Data are presented as means ± SE. *P < 0.01 compared with sham rats.
Protocol 3
Melanocortin 3/4 receptor antagonism.
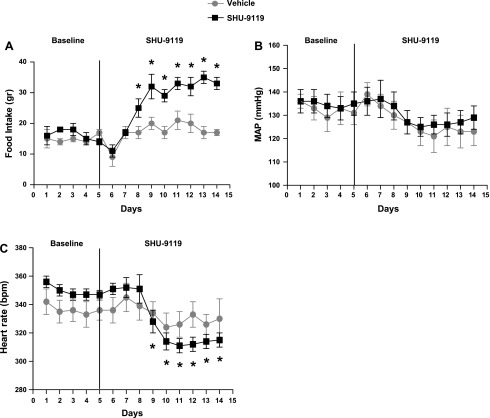
As shown in Table 2 and Fig. 3A, SHU-9119 treatment increased the food intake by 69 ± 8% in young female SHR and body weight by 29 ± 4%. Vehicle-treated young females also exhibited an increase in body weight during the experimental period of 17 ± 6% (increase in food intake of 18 ± 0.7%). Despite the effect on food intake, a hallmark of blockade, MC3/4R antagonism had no effect on MAP (Fig. 3B) but significantly reduced HR in young female SHR compared with untreated vehicle controls (Fig. 3C).
Table 2.
Changes in body weight and percentage change in body weight in rats before and after blockade with SHU-9119 (or vehicle)
| Baseline Body weight, g | After Treatment Body weight, g (% change) | |
|---|---|---|
| Young | ||
| Female control | 214.3 ± 5.9 | 232.2 ± 7.5* (+8.3 ± 1.8%)* |
| Young females + SHU-9119 | 209.3 ± 8.4 | 269.3 ± 12.5*† (+28.8 ± 3.8%)*† |
| Old | ||
| Female control | 228 ± 9 | 220 ± 10 |
| Female + SHU-9119 | 217 ± 11 | 257 ± 6*§ (18 ± 2)*†¶ |
| Male + SHU-9119 | 356 ± 27 | 438 ± 9* (23 ± 3‡ |
Values are means ± SE; n, 6 rats for all groups.
P < 0.01 compared with baseline of same sex.
P < 0.01 compared with placebo of same sex during SHU infusion;
P < 0.05 compared with young or old females in placebo or SHU infusion.
Fig. 3.
Effect of MC3/4R antagonism in young female SHR. Young female SHR (aged 4 mos) were implanted with intracerebroventricular (icv) cannulas and radiotelemeters, as described in materials and methods. Two weeks later, baseline MAP and heart rate were measured for 5 days, and rats were given SHU-9119 (1 nmol/h at 0.5 μl/h icv; n = 11) or vehicle (icv 0.9% NaCl; n = 5) for 9 days. A: food intake during the baseline period and during SHU-9119 infusion. Data are presented as grams per day and expressed as means ± SE. *P < 0.05 compared with vehicle-treated rats. B: MAP in young females during baseline and SHU-9119 (or vehicle) infusion. Data are presented as mmHg and expressed as means ± SE. C: heart rate in young females during baseline and SHU-9119 (or vehicle) infusion. Data are presented as beats per minute (bpm) and expressed as means ± SE. *P < 0.05 in SHU-9119 treated rats compared with baseline period.
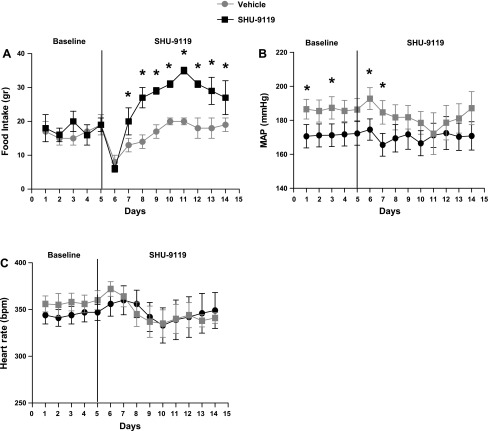
In old female SHR, chronic MC3/4R antagonism with SHU-9119 increased the food intake by 65 ± 6% (Fig. 4A) and increased body weight by 18 ± 2% (Fig. 4A, Table 2). Unlike in young females, however, vehicle-treated old females did not have an increase in body weight during the experimental period (Table 2). As noted in Fig. 4A, MAPs were different on some baseline measurement days between rats destined to get SHU-9119 treatment and controls destined to be untreated, since we randomized the rats to groups without looking at the MAP in baseline. However, as in young female SHR, SHU-9119 treatment had no effect on MAP in old female SHR compared with vehicle controls (Fig. 4B). HR was also not affected by SHU-9119 (Fig. 4C).
Fig. 4.
Effect of MC3/4R antagonism in old female SHR. Old female SHR, aged 18–20 mo, were implanted with intracerebroventricular cannulas and radiotelemeters, as described in materials and methods. Two weeks later, baseline MAP and heart rate were measured for 5 days, and then half of the rats were given SHU-9119 (1 nmol/h at 0.5 μl/h icv; n = 6) or vehicle (icv 0.9% NaCl; n = 6) for 9 days. A: food intake in old females during the baseline and SHU-9119 (or vehicle) infusion. Data are presented as grams per day and expressed as means ± SE. *P < 0.05 compared with vehicle-treated rats. B: MAP in old females during baseline and SHU-9119 (or vehicle) infusion. Data are presented as mmHg and expressed as means ± SE. *P < 0.05 SHU-treated rats compared with vehicle treated. C: heart rate in young females during baseline and SHU-9119 (or vehicle) infusion. Data are presented as bpm and expressed as means ± SE.
In old male SHR, chronic MC3/4 antagonism increased food intake by 58 ± 9% and body weight by 23 ± 3% (Fig. 5A and Table 2). However, unlike the young and old female SHR, MC3/4R antagonism reduced MAP and HR in old male SHR (Fig. 5, B and C).
Fig. 5.
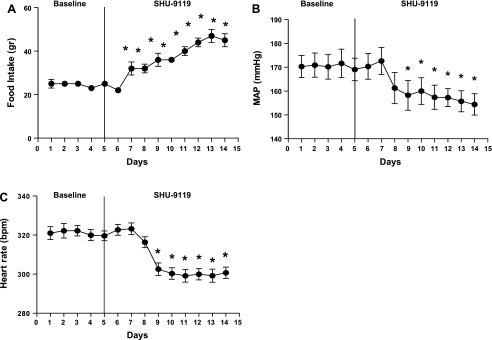
Effect of MC3/4R antagonism in old male SHR. Old male SHR, aged 18–20 mo (n = 6) were implanted with intracerebroventricular cannulas and radiotelemeters, as described in materials and methods. Two weeks later, baseline MAP and heart rate were measured for 5 days, and then SHU-9119 (1 nmol/h at 0.5 μl/h icv) was given for 9 days. A: food intake during the baseline period and during SHU-9119 infusion. Data are presented as grams per day and expressed as means ± SE. *P < 0.05 SHU-9119 treatment compared with baseline. B: MAP in old males during baseline and SHU-9119 infusion. Data are presented as mmHg and expressed as means ± SE. *P < 0.05 SHU-9119 treatment compared with baseline. C: heart rate in old males during baseline and SHU-9119 infusion. Data are presented as bpm and expressed as means ± SE. *P < 0.05 in SHU-9119-treated rats compared with baseline period.
Leptin Levels
Comparison of plasma leptin levels in old males and females showed no difference between the two groups [males (n = 12): 4.18 ± 1.12; females (n = 10): 3.87 ± 0.88 ng/ml, P = NS]. Leptin levels in other groups of age-matched young (n = 7) and old females (n = 9) (not included in the SHU study) were also not different (young: 2.21 ± 0.42; old: 2.55 ± 0.42 ng/ml, p+NS).
DISCUSSION
The main findings of these studies are the following. First, the sympathetic nervous system contributes to hypertension in young and old female SHR; however, the decrease in blood pressure with adrenergic blockade was greater in old females compared with young females, suggesting a greater contribution of the sympathetic nervous system to hypertension in old females. Second, renal denervation also reduced the blood pressure in both young and old females, and while the depressor response was slightly greater in old females, more importantly, MAP after renal denervation remained above 140 mmHg in old females, supporting our previous studies showing that mechanisms other than the renal nerves also contribute to the hypertension in old female SHR. Third, MC3/4R antagonism reduced blood pressure in old males [as previously shown in young males (5)], but had no effect in young or old females, suggesting that while sympathetic activation plays an important role in mediating the hypertension in females, the mechanisms responsible for activation of the SNS are likely independent of MC3/4R activation. Finally, leptin levels are not different in old male and female SHR, leaving the role of leptin in mediating the activation of MC3/4R in old males in question.
Aging in humans is associated with significant increases in muscle sympathetic nerve activity (MSNA), with women exhibiting higher MSNA than men after the age of 60 yr (25, 26, 30). In addition, there is evidence in premenopausal women that sympathetic nerve activity may be reduced by estradiol (35, 36). In support of this hypothesis, Narkiewicz and colleagues (25) reported that MSNA increased more with age in women than in men. Tank and colleagues (33) reported that MSNA correlated well with aging in both men and women, and but that increasing waist-to-hip ratio, body mass, and waist circumference correlated with increasing MSNA in men but not in women. Vianna and colleagues (34) also reported that the increase in MAP after MSNA burst was attenuated with aging but was attenuated more in women than men.
Our present studies support the notion that the SNS plays an essential role in mediating hypertension in aging female SHR. We have shown previously that the renin-angiotensin system (39), the endothelin system (38), and eicosanoid synthesis, particularly 20-HETE (40), also contribute to the hypertension in aging female SHR. In fact, we recently showed that blockade of all three of these systems still fails to normalize the MAP to 100 mmHg (21). The results of the present study suggest that activation of the SNS can easily account for the remaining elevation in blood pressure.
We previously showed that the renal SNS plays a role in mediating the hypertension in young male and female SHR (17). In addition, we showed that the sex difference in blood pressure in these young rats could not be explained on the basis of renal nerve activation alone since the percentage reduction in MAP with renal denervation was similar in young males and females (17). In our current study, renal denervation in old female SHR caused a reduction in blood pressure that was greater (both numerically and by percentage) than in young females. Importantly, the MAP remained at 139 ± 4 mmHg in the old females, supporting our previous studies of other nonrenal SNS mechanisms contributing to their hypertension. Renal sympathetic nerve activity decreases with age in humans, as shown by Esler and colleagues (49), but is increased in the presence of weight gain and metabolic syndrome (12, 31), common occurrences in postmenopausal women. Unlike postmenopausal women, the old female SHR do not gain weight with aging making them atypical compared with most postmenopausal women. In humans, the use of radiofrequency renal nerve ablation has been fairly successful for the treatment of resistant hypertension (29, 30). However, to our knowledge, there have been no studies in which gender differences in the efficacy of renal nerve ablation to reduce blood pressure have been evaluated. Based on our present studies, it is possible that renal nerve ablation may be more effective in older women than in younger women. That being said, preliminary studies in two young women with polycystic ovary syndrome (PCOS) showed that radiofrequency renal nerve ablation attenuated their systolic blood pressure by ∼8 mmHg in one and 27 mmHg in the other woman (19, 32), but the women remained hypertensive.
Various studies have shown that hypertension in women, particularly aging women, is not as well controlled to therapeutic goal as in men (11, 18, 27). Pharmacotherapy guidelines for treatment of hypertension are similar in men and women since there is evidence that both genders respond with similar efficacy (11, 27). The use of β-blockers for first line therapy in uncomplicated hypertension has been debated and is no longer recommended in individuals at average risk (27). However, β-blockers are considered as first-line therapy in women at increased risk of cardiovascular disease, such as in women with known coronary heart disease, peripheral vascular disease, or a 10-year Framingham risk score greater than 20% (27). Our data in old female SHR would support the concept that use of adrenergic blockade in chronically hypertensive postmenopausal women may be helpful in controlling their blood pressure.
In the present study we also evaluated the role of the MC3/4R in mediating the hypertension in female SHR. da Silva and colleagues (8) reported that blockade of the MC3/4R in young male SHR reduced their blood pressure by ∼25 mmHg. Based on these data, we were surprised when we found no depressor response to SHU-9119 in either old or young female SHR. Therefore, we determined whether hypertension in old male SHR would respond to MC3/4R antagonism and found that SHU-9119 caused ∼15 mmHg decrease in their blood pressure, similar to young males (8). Thus the fact that the MC3/4R antagonism did not significantly reduce the blood pressure in female SHR suggests that different mechanisms may be responsible for activation of the SNS in male and female SHR. These mechanisms are not likely to be due to leptin, although leptin is thought to play a major role in activation of proopiomelanocortin (POMC) neurons that release α-melanocyte-stimulating hormone (α-MSH), the primary agonist for the MC3/4R. In our study, we found that plasma leptin levels are not different in old male and female SHR. However, it is possible that leptin levels in the brain may be different between old males and females and thus still contribute to activation of the MC3/4R in males and activation of the SNS. Alternatively, differences in sex steroids may be important in regulating POMC expression and therefore α-MSH release and MC3/4R activation. For example, testosterone-treated rats have higher levels of POMC expression in the arcuate nucleus of the hypothalamus (4), and there is an age-related decrease in POMC expression in male rats (13). Whether higher testosterone levels in male SHR, especially young males, could explain the greater effect of MC3/4 blockade to lower blood pressure compared with female SHR is unclear.
Another intriguing finding of our studies is that the effect of MC3/4R inhibition on food intake is dissociated from the blood pressure in females, but not in males. These data suggest that perhaps in female SHR, the areas of the brain that control food intake are different from those controlling blood pressure, but that these areas may overlap in males. This finding may be a strain difference rather than a sex difference since we have preliminary data in our model of PCOS in Sprague-Dawley rats that MC3/4R antagonism attenuates the increase in blood pressure but increases food intake (data not shown). Alternatively, as mentioned above, androgens can upregulate the MC3/4R, and the PCOS model is made by giving the rats androgens (4, 13). To evaluate the role of MC3/4R, we will have to perform studies in other hypertensive models, such as Dahl salt-sensitive rats or nongenetic models to further evaluate any potential sex differences.
With regard to other potential mechanisms that could impact sympathetic nerve activity in females, Xue and colleagues (37) reported that activation of estrogen receptor (ER) β in the paraventricular nucleus and rostroventrolateral medulla attenuates sympathetic nerve activity and reduces blood pressure in aldosterone hypertension in female rats. Thus it is possible that a reduction in estrogens with aging could contribute to SNS-mediated hypertension in women. This is not likely to be the mechanism for sympathetic activation in our old females, since ovariectomy has little effect on blood pressure in female SHR, regardless of whether they are young or old (39).
Limitations of the Studies
One limitation of our studies is that we have not measured the expression or the ligand binding of the adrenergic receptors in our young and old female SHR. Thus it is possible that the adrenergic receptor expression or binding affinity decreases with age, such that the old females do not respond as well as young females do to adrenergic blockade. In addition, we used adrenergic blockade as an indicator that the SNS in general is activated in young and old female SHR and then evaluated whether the renal nerves contributed to the hypertension in these animals. Because we were interested in the chronic control of blood pressure, we did not perform ganglionic blockade that is an acute indicator of total body SNS activity. Thus it is possible that the nonrenal sympathetic nervous system may contribute to the hypertension in old and young female SHR, which was not the question we addressed in these studies.
Perspectives and Significance
As mentioned above, hypertension in women, particularly aging women, is not as well controlled to therapeutic goal as in men (11, 18, 27). The reasons for this problem are not clear, but the guidelines to treat hypertension in men and women are currently not different. The fact that we have shown several sex differences in the mechanisms responsible for hypertension in one of the genetic animal models of hypertension (38–40, including the present studies), suggest that there may also be gender differences in the mechanisms responsible for hypertension in humans with aging. Indeed, our data suggest that more studies are needed to evaluate whether there are any gender differences in the efficacy of both pharmacological agents and renal nerve ablation to treat hypertension. To date, despite the mandate by National Institutes of Health to include both men and women in clinical trials including those with hypertensive subjects, few studies are actually powered statistically to evaluate whether gender differences exist or not. Thus better-designed studies are necessary to determine the efficacy of antihypertensive drugs and procedures in men and women as they age.
GRANTS
These studies were supported by National Heart, Lung, and Blood Institute Grants P01 HL-05971 and R01 HL-66072.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.O.M., R.L., M.F.M., and J.M.d.C. performed experiments; R.O.M., R.L., J.M.d.C., J.E.H., R.J.R., and J.F.R. analyzed data; R.O.M., R.L., M.F.M., and J.F.R. prepared figures; R.O.M., R.L., and J.F.R. drafted manuscript; R.O.M., R.L., M.F.M., J.M.d.C., J.E.H., R.J.R., and J.F.R. edited and revised manuscript; R.O.M., R.L., M.F.M., J.M.d.C., J.E.H., R.J.R., and J.F.R. approved final version of manuscript; R.L., J.E.H., R.J.R., and J.F.R. interpreted results of experiments; J.F.R. conception and design of research.
ACKNOWLEDGMENTS
The authors thank Dr. David Mattson, Medical College of Wisconsin, for measurement of renal norepinephrine levels.
REFERENCES
- 1.Anderson EA, Sinkey CA, Lawton WJ, Mark AL. Elevated sympathetic nerve activity in borderline hypertensive humans. Evidence from direct intraneural recordings. Hypertension 14: 177–183, 1989 [DOI] [PubMed] [Google Scholar]
- 2.Bucher B, Heitz C, Stoclet JC. Age-related changes of in vivo beta-adrenergic responsiveness in normotensive an spontaneously hypertensive rats. Eur J Pharmacol 102: 31–37, 1984 [DOI] [PubMed] [Google Scholar]
- 3.Chen WQ, Cai H, Zhang C, Ji XP, Zhang Y. Is overall blockade superior to selective blockade of adrenergic receptor subtypes in suppressing left ventricular remodeling in spontaneously hypertensive rats? Hypertens Res 33: 1071–1081, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Chowen-Breed J, Fraser HM, Vician L, Damassa DA, Clifton DK, Steiner RA. Testosterone regulation of proopiomelanocortin messenger ribonucleic acid in the arcuate nucleus of the male rat. Endocrinology 124: 1697–702, 1989 [DOI] [PubMed] [Google Scholar]
- 5.Coote JH, Sato Y. Reflex regulation of sympathetic activity in the spontaneously hypertensive rat. Circ Res 40: 571–577, 1977 [DOI] [PubMed] [Google Scholar]
- 6.Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988–1994 and 1999–2004. Hypertension 52: 818–827, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Czarnecka D, Posnik-Urbanska A, Kawecka-Jaszcz K, Kolasinska-Klock W, Wojciechowska W, Redak D. Indices of autonomic nervous system activity in women with mild hypertension in the perimenopausal period. Kardiol Pol 67: 243–251, 2009 [PubMed] [Google Scholar]
- 8.da Silva AA, do Carmo JM, Kanyicska B, Dubinion J, Brandon E, Hall JE. Endogenous melanocortin system activity contributes to the elevated arterial pressure in spontaneously hypertensive rats. Hypertension 51: 884–890, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiBona GF. Sympathetic nervous system and hypertension. Hypertension 61: 556–560, 2013 [DOI] [PubMed] [Google Scholar]
- 10.do Carmo JM, da Silva AA, Dubinion J, Sessums PO, Ebaady SH, Wang Z, Hall JE. Control of metabolic and cardiovascular function by the leptin-brain melanocortin pathway. IUBMB Life 65: 692–698, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engberding N, Wenger NK. Management of hypertension in women. Hypertens Res 35: 251–60, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Esler M, Rumantir M, Wiesner G, Kaye D, Hastings J, Lambert G. Sympathetic nervous system and insulin resistance: from obesity to diabetes. Am J Hypertens 14: 304S–309S, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Gruenewald DA, Matsumoto AM. Age-related decrease in proopiomelanocortin gene expression in the arcuate nucleus of the male rat brain. Neurobiol Aging 12: 113–121, 1991 [DOI] [PubMed] [Google Scholar]
- 14.Hall JE, Da Silva AA, do Carmo JM, Dubinioon J, Hamza S, Munusamy S, Smith G, Stec DE. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem 285: 17271–17276, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH, Joyner MJ. Sex differences in sympathetic neural-hemodynamic balance: implications for human blood pressure regulation. Hypertension 53: 571–576, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hogarth AJ, Graham LN, Corrigan JH, Deuchars J, Mary DA, Greenwood JP. Sympathetic nerve hyperactivity and its effect in postmenopausal women. J Hypertens 29: 2167–2175, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Iliescu R, Yanes LL, Bell W, Dwyer T, Baltatu OC, Reckelhoff JF. Role of the renal nerves in blood pressure in male and female SHR. Am J Physiol Regul Integr Comp Physiol 290: R341–R344, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Kim JK, Alley D, Seeman T, Karlamangla A, Crimmins E. Recent changes in cardiovascular risk factors among women and men. J Womens Health 15: 734–746, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Lansdown A, Rees DA. The sympathetic nervous system in polycystic ovary syndrome: a novel therapeutic target? Clin Endocrinol (Oxf) 77: 791–801, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Lima R, Wofford M, Reckelhoff JF. Hypertension in postmenopausal women. Curr Hypertens Rep 14: 254–60, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lima R, Yanes LL, Davis DD, Reckelhoff JF. Roles played by 20-HETE, angiotensin II and endothelin in mediating the hypertension in aging female spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 304: R248–R251, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma D, Feitosa MF, Wilk JB, Laramie JM, Yu K, Leiendecker-Foster C, Myers RH, Province MA, Borecki IB. Leptin is associated with blood pressure and hypertension in women from the National Heart, Lung and Blood Institute Family Heart Study. Hypertension 53: 473–479, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maranon R, Reckelhoff JF. Sex and gender differences in control of blood pressure. Clin Sci (Lond) 125: 311–318, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mestivier D, Dabire H, Chau NP. Effects of autonomic blockers on linear and nonlinear indexes of blood pressure and heart rate in SHR. Am J Physiol Heart Circ Physiol 281: H1113–H1121, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension 45: 522–525, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Ng AV, Callister R, Johnson DG, Seals DR. Age and gender influence muscle sympathetic nerve activity at rest in healthy humans. Hypertension 21: 498–503, 1993 [DOI] [PubMed] [Google Scholar]
- 27.Reckelhoff JF, Wofford M. Hypertension in women. In: Women and Health (2nd ed.), edited by Rexrode K, Wickline M. New York: Elsevier, chapt. 70, 2012 [Google Scholar]
- 28.Saragoca M, Tarazi RC. Impaired cardiac contractile response to isoproterenol in the spontaneously hypertensive rat. Hypertension 3: 380–385, 1981 [DOI] [PubMed] [Google Scholar]
- 29.Schlaich MP, Straznicky N, Grima M, Ika-Sari C, Dawood T, Mahfoud F, Lambert E, Chopra R, Socratous F, Hennebry S, Eikelis N, Böhm M, Krum H, Lambert G, Esler MD, Sobotka PA. Renal denervation: a potential new treatment modality for polycystic ovary syndrome? J Hypertens 29: 991–996, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Schlaich MP, Krum H, Esler MD. New therapeutic approaches to resistant hypertension. Curr Hypertens Rep 12: 296–302, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Seals DR, Esler MD. Human ageing and the sympathoadrenal system. J Physiol 528: 407–417, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Symplicity HTN1 Investigators Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension 57: 911–917, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Tank J, Heusser K, Doedrich A, Hering D, Luft FC, Busjahn A, Narkiewic K, Jordan J. Infuences of gender on the interaction between sympathetic nerve traffic and central adiposity. J Clin Endocrinol Metab 93: 4974–4978, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vianna LC, Hart EC, Fairfax ST, Charkoudian N, Joyner MJ, Fadel PJ. Influence of age and sex on the pressor response following a spontaneous burst of muscle sympathetic nerve activity. Am J Physiol Heart Circ Physiol 302: H2419–H2427, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vongpatanasin W, Tuncel M, Wang Z, Arbique D, Mehrad B, Jialal I. Differential effects of oral versus transdermal estrogen replacement therapy on C-reactive protein in postmenopausal women. J Am Coll Cardiol 41: 1358–1363, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Weitz G, Elam M, Born J, Fehm HL, Dodt C. Postmenopausal estrogen administration suppresses muscle sympathetic nerve activity. J Clin Endocrinol Metab 86: 344–348, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Xue B, Zhang Z, Beltz TG, Johnson RF, Gou F, Hay M, Johnson AK. Estrogen receptor-β in the paraventricular nucleous and rostroventrolateral medulla plays an essential protective role in aldosterone/salt-induced hypertension in female rats. Hypertension 61: 1255–1262, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yanes LL, Romero D, Cucchiarelli VE, Fortepiani LA, Gomez-Sanchez CE, Santacruz F, Reckelhoff JF. Role of endothelin in a model of postmenopausal hypertension. Am J Physiol Regul Integr Comp Physiol 288: R229–R233, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Yanes LL, Romero DG, Iles JW, Iliescu R, Gomez-Sanchez CE, Reckelhoff JF. Sexual dimorphism in the renin-angiotensin system in aging spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 291: R383–R390, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Yanes LL, Lima R, Romero DG, Moulana M, Yuan K, Zhang Ryan MJ, Reckelhoff JFH. Postmenopausal hypertension: role of HETE. Am J Physiol Regul Integr Comp Physiol 300: R1543–R1548, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yanes LL, Reckelhoff JF. Postmenopausal hypertension. Am J Hypertens 24: 740–749, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoshida M, Yoshida E, Satoh S. Effect of renal nerve denervation on tissue catecholamine content in spontaneously hypertensive rats. Clin Exp Pharmacol Physiol 22: 512–517, 1995 [DOI] [PubMed] [Google Scholar]