Abstract
The involvement of the calcium-sensing receptor (CaSR) in Ca2+ homeostasis was investigated in larval zebrafish, Danio rerio. The expression of CaSR mRNA was first observed at 3 h posfertilization (hpf) and increased with development until plateauing at ∼48 hpf. At 4 dpf, CaSR mRNA was increased in fish acclimated to low Ca2+ water (25 μM vs. 250 μM in normal water). Using immunohistochemistry and confocal microscopy, we demonstrated that the CaSR is expressed in the olfactory epithelium, neuromasts, ionocytes on the yolk sac epithelium, and corpuscles of Stannius. Results of double immunohistochemistry and/or in situ hybridization indicated that the CaSR is localized to a subset of mitochondrion-rich ionocytes enriched with Na+/K+-ATPase and epithelial Ca2+ channel (ecac). Translational knockdown of the CaSR prevented 4 dpf larvae from regulating whole body Ca2+ levels when exposed to a low Ca2+ environment. Further, the increases in ecac mRNA expression and Ca2+ influx, normally associated with exposure to low-Ca2+ water, were prevented by CaSR knockdown. These findings demonstrate that larval zebrafish lacking the CaSR lose their ability to regulate Ca2+ when confronted with a low-Ca2+ environment. Results from real-time PCR suggested that the mRNA expression of the hypocalcemic hormone stanniocalcin (stc-1) remained elevated in the CaSR morphants following acclimation to low-Ca2+ water. Overall, the results suggest that the CaSR is critical for Ca2+ homeostasis in larval zebrafish exposed to low environmental Ca2+ levels, possibly owing to its modulation of stanniocalcin mRNA expression.
Keywords: calcium-sensing receptor, calcium homeostasis, ecac, stanniocalcin, zebrafish
calcium is an essential element for growth and survival, and its levels are tightly regulated in vertebrates, including fish. Unlike mammals, which rely on diet as their main source of Ca2+, fish acquire the majority of their Ca2+ from the external environment and regulate internal Ca2+ levels by adjusting its absorption across the gill (adults) or integument (larvae). For example, fish acclimated to a low-Ca2+ environment maintain whole body Ca2+ balance by increasing their capacity to absorb Ca2+ from the water (7, 32, 35). The increase in Ca2+-transporting capacity is associated with the proliferation of specific ion-transporting cells (ionocytes) on the gill (6, 35) or integument (32). In zebrafish (Danio rerio), Ca2+ uptake is accomplished by a subset of mitochondrion-rich ionocytes enriched with Na+/K+-ATPase (NaR cells), which express epithelial Ca2+ channels (ECaC) at the apical membrane, and plasma membrane Ca2+-ATPase and Na+/Ca2+ exchanger (NCX) at the basolateral membrane (23, 32). The rate-limiting step in the uptake of Ca2+ across epithelia is generally considered to be its movement across the apical membrane via ECaC (34). In keeping with its pivotal role in Ca2+ uptake, ecac expression and the density of ecac-expressing cells were significantly increased in larval zebrafish acclimated to low-Ca2+ water (32). However, the mechanism by which fish detect changes in the environmental Ca2+ availability and, thereby, modulate their Ca2+-transporting pathways, is poorly understood. Previous studies have suggested that fish may respond to changes in environmental Ca2+ levels by activation/inactivation of the extracellular Ca2+-sensing receptor (CaSR) (for review, see Ref. 28).
The CaSR is a member of the G protein-coupled seven transmembrane-domain receptor superfamily (for review, see Ref. 19); binding of Ca2+ to the extracellular domain of the CaSR elicits a downstream signaling cascade, involving various protein kinases and phospholipases (3). It is well established that in mammals, where the CaSR is expressed in various tissues, including the kidney, intestine, bone, and parathyroid gland (4, 19), that it is involved in regulating Ca2+ absorption and systemic Ca2+ handling (8). It was also demonstrated that mice fed a Ca2+-enriched diet exhibited a reduction in Ca2+ uptake owing to reduced intestinal expression of Ca2+ transport proteins (CaT), whereas CaT expression remained elevated in the CaSR-deficient mice fed the same diet (24). Furthermore, inactivation of the CaSR has been shown to reduce the capacity to increase urinary Ca2+ excretion in response to hypercalcemia (15).
In teleost fish, the CaSR is expressed in numerous tissues, including the gill, olfactory organ, kidney, intestine, and the corpuscles of Stannius (11, 16, 26, 29, 31, 36). In some tissues, the expression level of CaSR is dependent on external salinity; for example, acclimation to freshwater (FW) resulted in an increase in the mRNA expression of CaSR in the kidney of Mozambique tilapia (Oreochromis mossambicus) (30). A recent study in developing zebrafish has also shown that the CaSR is essential for normal skeletal development (17) and regulation of Ca2+ balance (26). Functional characterization of the piscine CaSR in a human embryonic kidney cell line demonstrated its sensitivity to extracellular Ca2+ levels, and thus, it is equipped to detect alterations in Ca2+ levels in the external environment, as well as within the body (30, 31). Electrophysiological studies in the FW goldfish (Carassius auratus) revealed that the olfactory nerve increases its signaling activity with increasing environmental Ca2+ levels over a naturally occurring range (0.05–3 mM), presumably via activation of the CaSR (22). In contrast, exposure to increased water Ca2+ concentrations resulted in decreased firing frequency in the olfactory organ of the marine sea bream, Sparus aurata (21). Although these different results may simply reflect species differences, it is conceivable that the functional properties of the CaSR vary between marine and FW fish.
In mammals, the CaSR exerts its Ca2+-regulatory functions by modulating the secretion of calciotropic hormones [e.g., parathyroid hormone (PTH)] and by its direct action in tissues involved in Ca2+ transport (e.g., intestine and kidney) (2). PTH is also suggested to regulate Ca2+ homeostasis in fish (13, 14, 26); however, the potential involvement of the piscine CaSR in the regulation of PTH remains unclear. A few previous studies on fish have demonstrated that the CaSR may regulate Ca2+ homeostasis through its action on stanniocalcin (STC), which is a hypocalcemic hormone that is synthesized and secreted primarily from the corpuscles of Stannius. For example, pharmacological treatment of CaSR mimetics (increase the sensitivity of CaSR) was found to stimulate the secretion of STC and decrease Ca2+ uptake in FW rainbow trout Oncorhynchus mykiss (36). Similarly, calcimimetic administration was found to increase plasma STC levels and reduced plasma concentrations of Ca2+ in the European flounder Platichthys flesus (12). It has also been suggested that the CaSR regulates whole body Ca2+ balance by modulating mRNA expression of pth-1 and stc-1 in larval zebrafish (26).
In the gill epithelium, the CaSR is expressed in Na+/K+-ATPase (NKA)-rich cells (11, 29, 31), which are thought to be important, though possibly not exclusive, sites of Ca2+ transport (33, 37). The expression of CaSR in Ca2+-transporting cells suggest that the CaSR may play a role in modulating Ca2+ transport function in response to changing levels of environmental Ca2+. However, the physiological role of the CaSR in homeostatic regulation of Ca2+ has not been fully elucidated in fish (26).
With the above background, the potential involvement of the CaSR in Ca2+ homeostasis was examined in zebrafish Danio rerio. We hypothesized that the known ability of zebrafish to respond to low environmental Ca2+ levels by increasing Ca2+ uptake (32) is linked to the sensing ability of the CaSR. In the present study, the tissue distribution of CaSR in adult zebrafish and the cellular localization of CaSR in larval zebrafish were examined. Combined whole-mount in situ hybridization and immunohistochemistry were performed to evaluate the possible expression of the CaSR in ecac-expressing ionocytes. The involvement of the CaSR in Ca2+ homeostasis was investigated using a morpholino gene knockdown approach; Ca2+ fluxes and whole body Ca2+ content in fish acclimated to normal and low-Ca2+ water were examined in fish experiencing CaSR knockdown. Additionally, real-time PCR was conducted to evaluate the interactive effects of low-Ca2+ water exposure and CaSR knockdown on the mRNA expression levels of ecac, pth-1, and stc-1.
MATERIALS AND METHODS
Fish.
Adult zebrafish (Danio rerio; Hamilton-Buchanan 1822) were purchased from Big Al's Aquarium Services (Ottawa, ON, Canada) and kept in the University of Ottawa Aquatic Care Facility, where they were maintained in plastic tanks supplied with aerated, dechloraminated City of Ottawa tap water at 28°C. The ionic composition of the water was Ca2+ = 0.25 mM; Na+ = 0.78 mM; Cl− = 0.4 mM; K+ = 0.025 mM; pH 7.6. Fish were subjected to a constant 14:10-h light-dark photoperiod and fed daily until satiation with no. 1 crumble-Zeigler (Aquatic Habitats). Morpholino and sham-injected embryos were reared in 50-ml petri dishes supplemented with either dechloraminated City of Ottawa tap water or with treatment water, as detailed below. The petri dishes were kept in incubators set at 28.5°C. All experiments were performed on fish at 4 days postfertilization (dpf), except where mentioned otherwise. The experiments were conducted in compliance with guidelines of the Canadian Council of Animal Care and after the approval of the University of Ottawa Animal Care Committee (protocol BL-226).
Acclimation experiments.
Control (normal) and low-Ca2+ water were prepared with double deionized water supplemented with CaSO4·2H2O, MgSO4·7H2O, NaCl, K2HPO4, and KH2PO4. The Ca2+ concentrations of the normal and low-Ca2+ water were 250 μM and 25 μM, respectively. All other ion concentrations were kept constant (in mM): 0.8 Na+, 0.16 Mg2+, and 0.3 K+. Fish were transferred to control or low-Ca2+ water at 1 dpf and were sampled for subsequent experiments at 4 dpf (detailed below).
PCR analysis.
The mRNA expression of CaSR in different tissues of adults and during early developmental stages in embryos and larvae (post-hatch) was evaluated using RT-PCR. Total RNA was extracted using TRIzol (Invitrogen), and genomic DNA was removed with DNase I (Invitrogen), following the manufacturer's guidelines. First-strand cDNA was synthesized using RevertAid H Minus reverse transcriptase (Fermentas, CA) and random hexamer primers. PCR was conducted in 25-μl reaction volumes using 200-ng cDNA template with the following program; initial denaturation at 94°C for 30 s, followed by 30 or 40 cycles of 94°C for 30 s, 58°C for 60 s, and 72°C for 45 s, with final extension for 10 min at 72°C. Primer sets used in the present study are summarized in Table 1, and all amplicons were sequenced to ensure the correct PCR products were amplified. The housekeeping gene 18S was used as an internal control (25). The mRNA levels of CaSR in 4 dpf zebrafish following their acclimation to low-Ca2+ water were evaluated using real-time PCR, as described previously (25). The interactive effects of low Ca2+-water exposure and CaSR knockdown on the mRNA expression of Ca2+ transport-related genes [epithelial Ca2+ channels (ecac), NCX (ncx1b), and plasma membrane Ca2+-ATPase (pmca2)], and calciotropic hormones [parathyroid hormone-1 (pth-1) and staniocalcin-1 (stc-1)] were also examined using real-time PCR. Quantitative RT-PCR (RT-qPCR) assays were performed using a Bio-Rad CFX96 qPCR system with Brilliant III SYBR Green Master Mix (Agilent Technologies). All RT-qPCR was performed using the following conditions: 95°C for 3 min, 40 cycles of 95°C for 20 s, and 58°C for 20 s, with final extension for 5 min at 72°C. Data were normalized to the expression of 18S and were presented relative to the control group (sham-injected fish or fish acclimated to “normal” water).
Table 1.
Primer sets used for real-time PCR analysis
| Sequence | Reference | |
|---|---|---|
| casr | Forward: 5′-AAA TGC CCA AAC AAC TCC TG-′ | N/A* |
| Reverse: 5′-GGT TTG ATG CCT TCA CGA TT-′ | ||
| ecac | Forward: 5′-TCC TTT CCC ATC ACC CTC T-′ | (27) |
| Reverse: 5′-GCA CTG TGG CAA CTT TCG T-′ | ||
| ncx1b | Forward: 5′-TAA AGT GGC AGC GAT ACA GGT-′ | (27) |
| Reverse: 5′-CAG ATC AAG GCG AAG ATG G-′ | ||
| pmca2 | Forward: 5′-AAG CAG TTC AGG GGT TTA C-′ | (27) |
| Reverse: 5′-CAG ATC ATT GCC TTG TAT CA-′ | ||
| pth-1 | Forward: 5′-TCA TAA GCA TGT GGA GCT GAG GCA-′ | N/A* |
| Reverse: 5′-ACG ATG GGT TCA TGA GCT TCT CCA-′ | ||
| stc-1 | Forward: 5′-CCA GCT GCT TCA AAA CAA ACC-′ | (39) |
| Reverse: 5′-ATG GAG CGT TTT CTG GCG A-′ |
Primer sets for casr and pth-1 were designed in the present study. casr, Ca2+-sensing receptor; ecac, epithelial Ca2+ channels; ncx1b, Na+/Ca2+ exchanger isoform 1b; pmca2, plasma membrane Ca2+-ATPase isoform 2; pth-1, parathyroid hormone isoform 1; stc-1, stanniocalcin isoform 1; N/A, not applicable.
Western blot analysis.
For Western blot analysis, proteins from 10 larvae (n = 1) were extracted using an RIPA buffer (150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris·HCl, 1 mM EDTA, and 1 mM phenylmethanesulfonyl fluoride) plus protease inhibitor cocktail (Roche). Samples (50 μg of protein) were loaded on a 10% SDS-PAGE and transferred to PVDF membrane (Bio-Rad). After transfer, the membrane was blocked with 5% skim milk in Tris buffer plus 0.05% Tween 20 (TBST) for 2 h at room temperature. The membrane was then probed with tilapia CaSR antibody (1:1,000 dilution; CHIKKMVGDYDRRA) in TBST with 2% skimmed milk at 4°C overnight. The epitope of tilapia CaSR antibody is 93% identical to the zebrafish CaSR at the NH3 terminus (SKDQDLAARPESTQC), and the use of this antibody with zebrafish was validated in a previous study (17). After washing with TBST (three times and 5 min each; 3×5 min), the membrane was probed with 1:5,000 goat anti-rabbit antibodies (Invitrogen) for 2 h at room temperature. The membrane was then washed (5×5 min), and the bands were detected using enhanced chemiluminescence (SuperSignal West femto chemiluminescent substrate; Pierce) with a ChemiDoc system (Bio-Rad). Subsequently, the membrane was reprobed with β-actin antibodies (1:4,000; Sigma) after stripping with Re-Blot Plus solution (Millipore).
Whole-mount immunohistochemistry.
For immunostaining of CaSR, 4-dpf larvae were first fixed with 4% paraformaldehyde in a PBS for 1 h at room temperature. After fixation, the fish were briefly rinsed with PBS with 0.1% Tween (PBST), and then gradually dehydrated with 100% methanol. Following rehydration with PBST, the fish were blocked with 3% BSA for 1 h and then incubated with 1:500 dilution of CaSR antibody in PBST (plus 3% BSA and 0.8% Triton-X) at 4°C overnight. Subsequently, the fish were incubated in an Alexa Fluor 488-coupled goat anti-rabbit IgG at 1:500 dilution (Invitrogen) for 2 h in the dark at room temperature. The images were acquired using a confocal laser scanning microscopy (A1R+; Nikon Instruments).
To determine whether CaSR was expressed in mitochondrion-rich cells, 4 dpf larvae were incubated with 1 μM Mitotracker Red (Invitrogen, Burlington, ON, Canada) for 30 min prior to fixation. The potential expression of CaSR in Na+/K+-ATPase-rich cells (NaR) was also examined by staining the fish with both CaSR and NKA (α5, diluted 1:250 in PBST; Developmental Studies Hybridoma Bank, University of Iowa) antibodies after fixation. The CaSR and Na+/K+-ATPase were then labeled with rabbit Alexa Fluor 488- and mouse Alexa Fluor 546-conjugated secondary antibodies, respectively, and images were acquired as described above.
Whole-mount in situ hybridization.
A fragment of zebrafish ecac mRNA from 4 dpf larval zebrafish cDNA was PCR-amplified (forward; 5′-TGG CTC AGG ATG CAG AAC AG-3′, reverse; 5′-TAG GGT CCC AGC ATC TCG AA-3′; size = 772 bp), cloned into a pDrive cloning vector (Qiagen) and sequenced. After plasmid purification and linearization, an ecac RNA probe was synthesized by in vitro transcription in the presence of digoxigenin (dig)-UTP (Roche). Whole-mount in situ hybridization of larval zebrafish was performed as described previously (38), with minor modifications. In brief, 1-phenyl-2-thiourea-treated fish at 4 dpf were fixed with 4% paraformaldehyde overnight at 4°C and were washed several times with PBST before gradual dehydration using methanol. After rehydration with PBST, the fish were permeabilized in acetone for 20 min at −20°C and then washed with PBST. The fish were first prehybridized in a hybridization buffer supplemented with 500 μg/ml yeast tRNA and 50 μg/ml heparin (Sigma) for 2 h at 65°C and then incubated with 100 ng of ecac RNA probe overnight at 65°C. After serial washing with hybridization buffer and PBST, the fish were incubated in a blocking solution containing 10% calf serum in PBST for 2 h before incubating with an alkaline phosphatase-conjugated anti-dig antibody (1:2,000 dilutions at 4°C overnight). Subsequently, the fish were washed with PBST and incubated in a nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate staining buffer until the desired coloration intensity was obtained.
To evaluate whether CaSR and NKA were expressed in ecac-expressing ionocytes, in situ hybridization, and immunohistochemistry were performed in combination. Following in situ hybridization, fish were prefixed in 4% PFA at room temperature for 20 min. The fish were then washed several times with PBST and then incubated overnight at 4°C with the CaSR (1:500 dilution) or Na+/K+-ATPase (1:250 dilution) antibody. The CaSR and NKA were then labeled with rabbit Alexa Fluor 488- and mouse Alexa Fluor 546-conjugated secondary antibodies, respectively, and images were visualized as described above.
Microinjection of antisense morpholino oligonucleotide.
A morpholino oligonucleotide (5′-ACT TCA GAT GAA ACC TCA TTG CTT C-3′; Genetools) was designed to bind to the translation start site of zebrafish CaSR (based on the sequence information obtained from GenBank ID XM_684005.2 and Ensembl Gene ID ENSDART00000013649). The morpholino was diluted in a Danieau buffer [58 mM NaCl, 0.7 mM KCl, 0.4 mM MgSO4, 0.6 mM Ca(NO3)2, 5.0 mM HEPES (pH 7.6)] plus 0.05% phenol red before injection. A “sham” group was injected with a standard control morpholino (5′-CCT CTT ACC TCA GTT ACA ATT TAT A-3′; GeneTools) prepared as the CaSR morpholino. In preliminary trials, 2 to 6 ng of morpholino (1 nl constant volume) was injected into one-cell stage embryos using a microinjector system (model IM 300; Narishige). Because we observed that injection of 4 ng morpholino was the optimum dose to effectively knock down the CaSR expression (see results) without inducing developmental abnormalities, this dose was used in all subsequent experiments. All experiments were performed at 4 dpf, except where otherwise mentioned, and only fish that had emerged from the chorion were used for experiments.
Measurement of Ca2+ fluxes.
Following acclimation to either low- or normal-Ca2+ water, fish were transferred to a 2.0-ml microfuge tube and exposed to 0.2 μCi/ml 45Ca2+ (as CaCl2; American Radiolabeled Chemicals) for 2 h; water samples were collected at 0 and 2 h. All fluxes were performed in the normal-Ca2+ water. At the end of the flux period, fish were killed with an overdose of MS-222 and rinsed in isotope-free water; three fish were pooled as one sample (n = 1). Fish were digested with a tissue solubilizer (Solvable; Perkin Elmer) and later neutralized using glacial acetic acid. The radioactivity of the digest and the water samples was measured using a liquid scintillation counter (LS-6500; Beckman Coulter, Canada) following the addition of a scintillation cocktail (BioSafe-II; Research Products International). The Ca2+ influx (pmol·fish−1·h−1) was determined using the formula:
where F is the total radioactivity counted in the fish (counts/min), SA is the specific activity of the water (cpm/nmol), n is the number of fish, and t is the duration of the experiment in hours.
To measure the whole body Ca2+ content, fish were killed with an overdose of MS-222, briefly rinsed in double-deionized water, and 10 fish were pooled as one sample (n = 1). The fish were digested with 1 N HNO3 at 70°C for 48 h, and diluted appropriately with deionized water. The total Ca2+ concentration was measured by flame emission spectrophotometry (Spectra AA 220FS; Varian), and verified using certified Ca2+ standards (Fisher Scientific).
Statistical analysis.
All statistical analyses were performed using Sigmaplot (version 11.2; Systat Software). Data were either analyzed using Student's t-test, or two-way ANOVA (morpholino knockdown and Ca2+ treatment as two independent variables) followed by a post hoc Holm-Sidak test. Data were either log or square-root transformed when the assumptions of equal variance or normal distribution were violated (determined automatically by the statistical software). Data are reported as the means ± SE, and P ≤ 0.05 was taken as the level of significance.
RESULTS
CaSR mRNA expression in embryos and adult zebrafish.
The results of RT-PCR analysis (following 40 cycles of amplification) suggested that the CaSR mRNA was expressed ubiquitously in all tissues examined (Fig. 1A). At 30 cycles, the CaSR was detected only in the gill, intestine, liver, ovary, testis, and muscle, presumably owing to its relatively higher expression levels in these tissues (data not shown). In developing zebrafish, CaSR mRNA was first detected at 3 h postfertilization (hpf) (Fig. 1B). Real-time PCR data demonstrated that CaSR expression in 4 dpf fish was significantly increased after acclimation to low-Ca2+ water (Fig. 1C).
Fig. 1.
A: mRNA expression of calcium-sensing receptor (CaSR) in various tissues of adult zebrafish and at different developmental stages of embryos and larvae (B); 18S was used as an internal control. C: effects of acclimation to normal (250 μM) or low (25 μM) Ca2+ water on the mRNA expression levels of CaSR in larval zebrafish at 4 days postfertilization (dpf). Data were normalized with 18S and were expressed relative to the fish maintained in normal Ca2+ water. *Statistical difference using Student's t-test, P < 0.05. Data are expressed as means ± SE; n = 6.
Immunolocalization of CaSR protein in larval zebrafish.
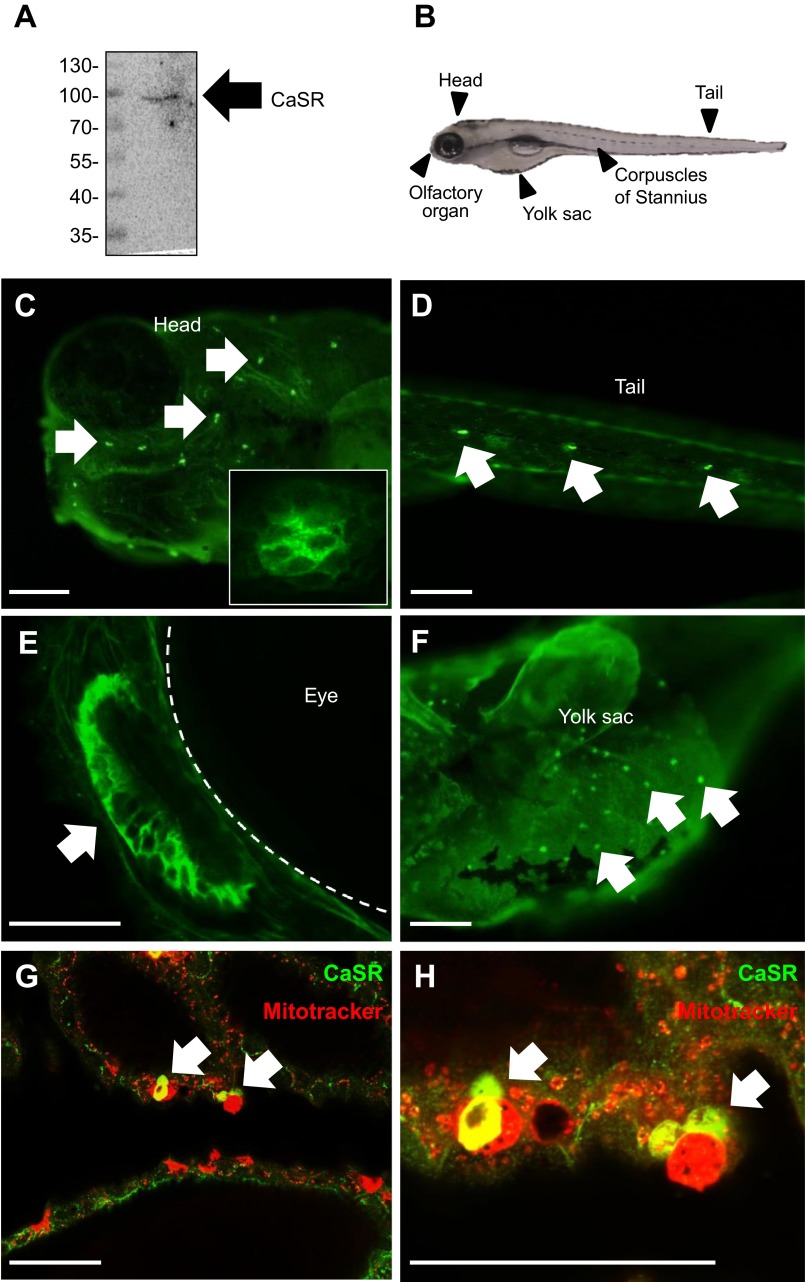
Western blot analysis showed that the tilapia CaSR antibody detected a single band at ∼100 kDa in the lysates of 4 dpf zebrafish (Fig. 2A). Immunohistochemistry and confocal microscopy were used to examine the cellular localization of CaSR in 4 dpf zebrafish larvae. Figure 2B shows an image of a larval zebrafish and regions where immunostaining of the CaSR was observed. It was found that the CaSR protein was expressed in neuromasts on the head (Fig. 2C) and tail (Fig. 2D), the olfactory epithelium (Fig. 2E), epithelial cells on the yolk sac (Fig. 2F), and the corpuscles of Stannius (Fig. 2, G and H).
Fig. 2.
A: representative Western blot showing that the CaSR antibody yielded a single immunoreactive band at ∼100 kDa in the lysates of zebrafish larvae at 4 days postfertilization (dpf). B: a picture of a 4-dpf larval zebrafish with arrows, indicating the regions where CaSR immunostaining was observed. Fluorescent immunohistochemistry and confocal microscopy of the CaSR, showing the CaSR protein expression (green) in neuromasts on the head region (C) as well as on the tail (D). The inset image in C is a neuromast under higher magnification. CaSR is also expressed in the olfactory epithelium (D), ionocytes on the skin of the yolk sac (E), and corpuscles of Stannius (F, G). F and G: double immunostaining of CaSR (green) and Mitotracker (red) was performed. Scale bars = 50 μm.
CaSR is localized in ecac-expressing ionocytes.
To determine whether CaSR protein was expressed in ionocytes on the yolk sac epithelium, double immunohistochemistry and/or in situ hybridization were performed. The results showed that CaSR protein was expressed in a subset of NKA- (Fig. 3, A–C) or Mitotracker- (Fig. 3, D–F) positive cells. Expression of ecac mRNA was confined to a subpopulation of NKA-positive cells (Fig. 4, A–C); CaSR protein was also observed in a subset of ecac-expressing cells (Fig. 4, D–F).
Fig. 3.
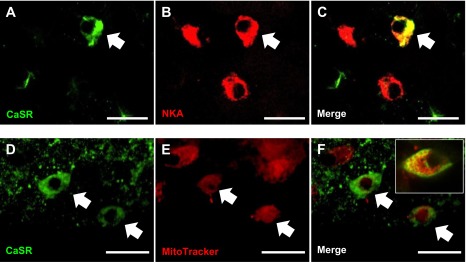
Double fluorescence immunohistochemical confocal images of zebrafish larvae at 4 dpf, illustrating the presence of the calcium-sensing receptor (CaSR; green) in ionocytes on the skin of the yolk sac. Some CaSR-positive cells were enriched with Na+/K+-ATPase (NKA; red) (A–C) or mitochondria (Mitotracker staining, red) (D–F). The inset image in F shows membranous CaSR expression in a mitochondrion-rich cell under higher magnification. Cells labeled with arrows represent areas of colocalization. Scale bar = 20 μm.
Fig. 4.
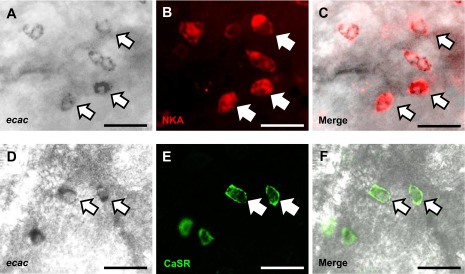
Double immunohistochemistry and in situ hybridization images of zebrafish larvae at 4 dpf, illustrating the presence of the calcium-sensing receptor (CaSR) in ecac-expressing ionocytes on the skin of the yolk sac. Cells labeled with arrows represent areas of colocalization. A portion of ecac mRNA was colocalized with NKA (A–C)and CaSR-positive cells (D–F). Scale bar = 20 μm.
Morpholino knockdown reduces the protein expression of the CaSR.
Figure 5A shows a representative Western blot of the CaSR in the lysates of sham fish and CaSR morphants at 4 dpf. Subsequent quantitative analysis revealed that the protein expression of the CaSR was significantly reduced in CaSR morphants compared with sham fish (Fig. 5B). Additionally, no immunostaining of the CaSR protein in the ionocytes (Fig. 5C) and in other CaSR-expressing tissues was observed following the knockdown (data not shown).
Fig. 5.
A: representative Western blot showing the protein expression of the calcium-sensing receptor (CaSR) in the lysates of sham fish and CaSR morphants (CaSR MO) at 4 dpf. β-actin was used as an internal control. B: subsequent quantitative analysis revealed that the protein expression level of the CaSR in the morphants was significantly reduced following morpholino knockdown (Student's t-test; P < 0.05). Data are expressed as means ± SE; n = 3. C: immunohistochemistry and confocal microscopy revealed that CaSR protein was expressed in ionocytes of sham fish (cells labeled with arrows), whereas its expression was virtually absent in CaSR MO.
CaSR morphants acclimated to a low Ca2+ environment exhibit a reduced ability to increase Ca2+ uptake and a reduction in whole body Ca2+ levels.
Two-way ANOVA analysis revealed that there were significant overall effects of Ca2+ treatment and morpholino knockdown on Ca2+ influx (both at P < 0.001). Acclimation to low-Ca2+ water significantly increased Ca2+ influx in sham fish (Fig. 6A); the impact of acclimation was markedly attenuated in the CaSR morphants. No significant interactions between water Ca2+ treatment and morpholino knockdown on Ca2+ influx were observed (P = 0.2).
Fig. 6.
Influx of Ca2+ (A) and whole body Ca2+ levels (B) in sham fish and calcium-sensing receptor morphants (CaSR MO) after acclimation to normal (250 μM) or low (25 μM) Ca2+ water. a,b,c,dBars labeled with different letters represent a statistical difference (two-way ANOVA, followed by a post hoc Holm-Sidak test; P < 0.05). Data are expressed means ± SE; n = 6.
Two-way ANOVA analysis suggested that Ca2+ treatment (P < 0.005), but not morpholino knockdown (P = 0.8), significantly affected the whole body Ca2+ content in fish. Sham fish acclimated to low Ca2+ water were able to defend whole body Ca2+ content (Fig. 6B). However, the CaSR morphants acclimated to low-Ca2+ water exhibited a significant reduction in whole body Ca2+ content (Fig. 6B). No significant interactions between water Ca2+ treatment and morpholino knockdown on whole body Ca2+ content were observed (P = 0.3).
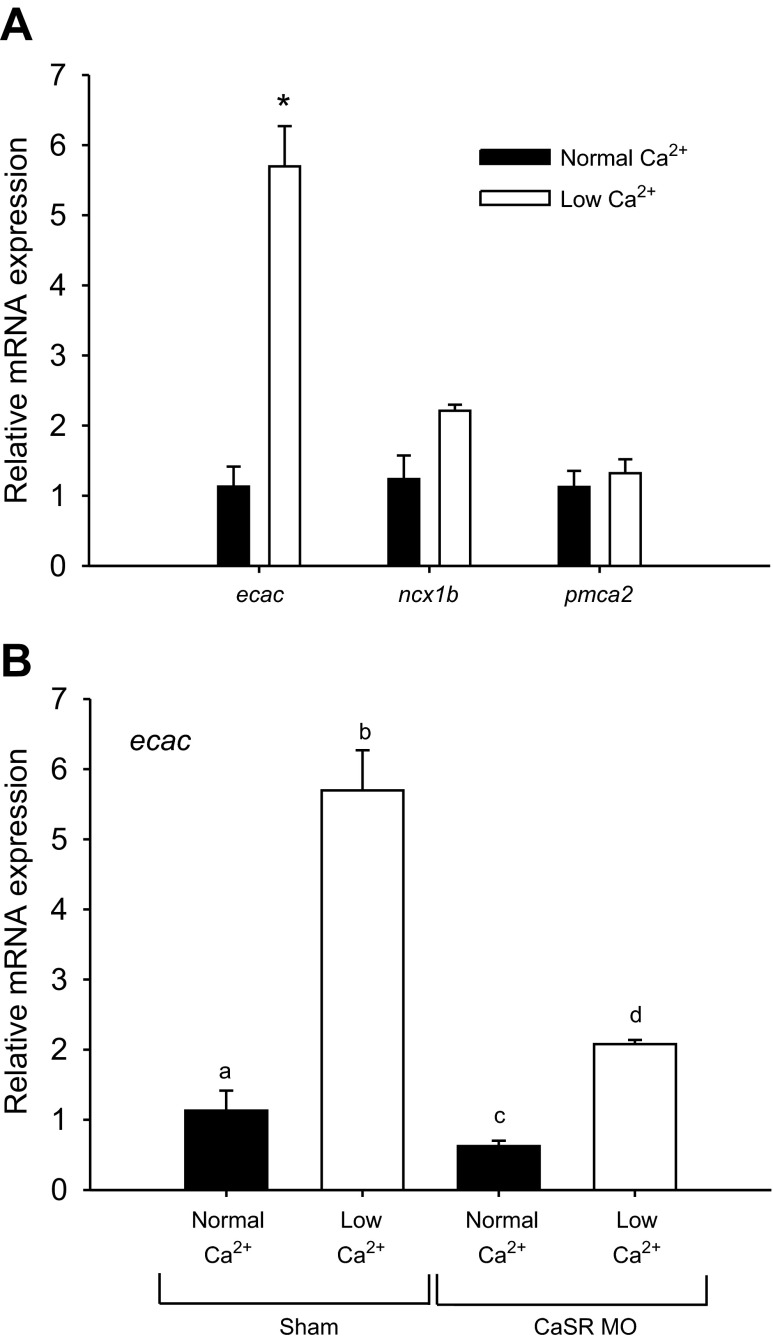
CaSR morphants acclimated to a low-Ca2+ environment exhibit a reduced ability to increase ecac mRNA expression.
The effects of acclimation to low-Ca2+ water on Ca2+ transport-related genes was examined using real-time PCR. The mRNA expression level of ecac was significantly increased in fish acclimated to low-Ca2+ water (Fig. 7A), whereas ncx1b and pmca2 were unaffected. Two-way ANOVA analysis suggested that there was a significant overall effect of both Ca2+ treatment and morpholino knockdown on ecac mRNA expression (both at P < 0.005). The increase in ecac mRNA expression in low-Ca2+ water was significantly reduced in the CaSR morphants (Fig. 7B). A statistically significant interaction between Ca2+ treatment and mopholino knockdown on ecac mRNA expression was observed (P < 0.05).
Fig. 7.
A: mRNA expression of epithelial Ca2+ channel (ecac), Na+/Ca2+ exchanger (ncx1b) and plasma membrane Ca2+-ATPase (pmca2) in 4 dpf larval zebrafish after acclimation to normal (250 μM) or low (25 μM) Ca2+ water. *Significant difference between normal and low-Ca2+-treated fish (Student's t-test; P < 0.05). Values are expressed as means ± SE; n = 6. B: mRNA expression of ecac in sham fish and calcium-sensing receptor morphants (CaSR MO) after acclimation to normal or low Ca2+ water. a,b,c,dBars labeled with different letters represent a statistical difference (two-way ANOVA, followed by a post hoc Holm-Sidak test; P < 0.05). Data are expressed as means ± SE; n = 6.
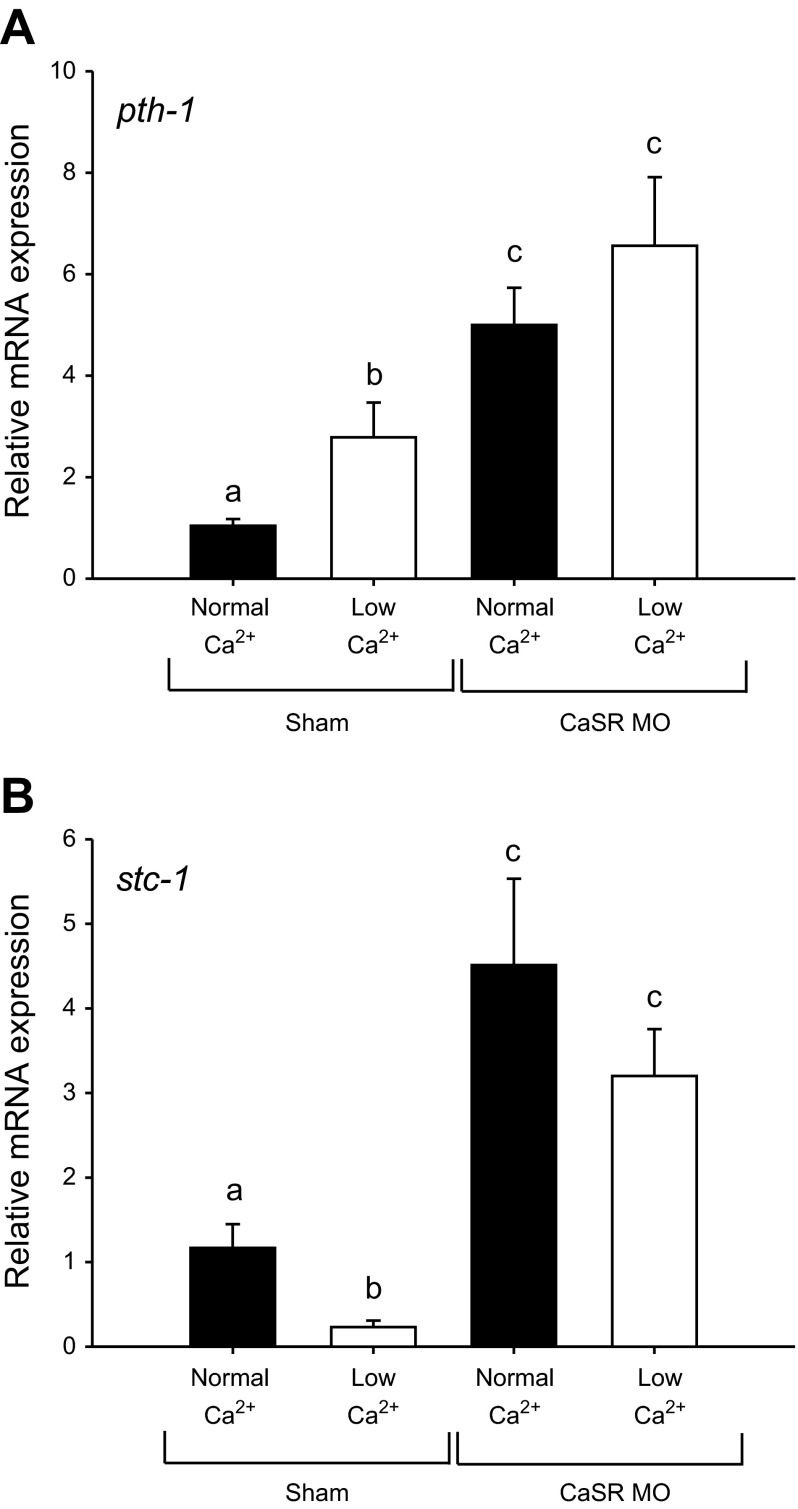
CaSR modulates the mRNA expression of parathyroid hormone pth-1 and stc-1.
A two-way ANOVA revealed that both Ca2+ treatment and morpholino knockdown resulted in an overall significant effect on pth-1 mRNA expression (both at P < 0.05). Acclimation to low-Ca2+ water significantly increased the mRNA expression of pth-1 in 4 dpf larvae (Fig. 8A). The expression of pth-1 was markedly elevated in fish experiencing CaSR knockdown, and acclimation to low-Ca2+ water did not further increase the pth-1 expression in the CaSR morphants. There was no significant interactions between Ca2+ treatment and morpholino knockdown on pth-1 mRNA expression (P = 0.2).
Fig. 8.
The mRNA expression of parathyroid hormone-1 (pth-1) (A) and stanniocalcin-1 (stc-1) (B) in sham fish and calcium-sensing receptor morphants (CaSR MO) after acclimation to normal (250 μM) or low (25 μM) Ca2+ water. a,b,cBars labeled with different letters represent a statistical difference (two-way ANOVA, followed by a post hoc Holm-Sidak test; P < 0.05). Data are expressed as means ± SE; n = 6.
A two-way ANOVA suggested that both Ca2+ treatment and morpholino knockdown resulted in an overall significant effect on stc-1 mRNA expression (both at P < 0.05). Fish acclimated to low-Ca2+ water exhibited a significant decrease in the mRNA expression level of stc-1 (Fig. 8B). Expression of stc-1 was significantly elevated in the CaSR morphants and was not reduced by exposure to low-Ca2+ water. No significant interactions between Ca2+ treatment and morpholino knockdown on stc-1 expression were recorded (P = 0.1).
DISCUSSION
Overview.
The CaSR is essential for maintaining Ca2+ balance in mammals (8, 18, 24) and is proposed to serve as a salinity sensor in fish (31). However, the physiological significance of the CaSR in Ca2+ homeostasis in fish has remained poorly understood. The results of the present study demonstrated that the CaSR is expressed in various tissues, including the olfactory epithelium, the corpuscles of Stannius, and ecac-expressing ionocytes. These observations suggest that the CaSR may be involved in environmental Ca2+ sensing, as well as regulation of Ca2+ transport function. Using a gene knockdown strategy, we showed that the capacity to regulate whole body Ca2+ levels and increase ecac expression and Ca2+ uptake was reduced in CaSR morphants acclimated to low-Ca2+ water. The reduced regulatory ability of the CaSR morphants was associated with elevated mRNA expression of the hypocalcemic hormone, stanniocalcin. Overall, the findings of the present study suggest that in larval zebrafish, the CaSR responds to the reduced water Ca2+ content by increasing ecac expression and Ca2+ influx, ultimately allowing the fish to maintain whole body Ca2+ levels.
Expression patterns of CaSR mRNA and localization of CaSR protein in ionocytes.
In the present study, we observed that the CaSR was expressed ubiquitously in tissues of adult zebrafish, including the gill, kidney, and intestine. These findings are consistent with recent studies in adult zebrafish (17, 26), as well as in many other teleosts (for a review, see Ref. 28). In larval zebrafish, we also observed that the CaSR was expressed in the olfactory epithelium. The precise role of the CaSR in the olfactory organ is unclear, but it was proposed to serve as environmental salinity sensor (31). Interestingly, we also observed that CaSR was expressed in the mechanosensitive neuromasts in larval zebrafish. A previous study showed that the plasma membrane Ca2+-ATPase (pmca2) also is expressed in neuromasts, where it may play a role in sensory organ development (9). Furthermore, PTH protein was observed in neuromasts in developing zebrafish (20). In mammals, the CaSR is known to regulate the secretion of PTH from the parathyroid cells (3); whether CaSR in zebrafish neuromasts is involved in environmental Ca2+ sensing and regulates the secretion of PTH requires further investigation.
Several previous studies have shown that the CaSR is abundantly expressed in the corpuscles of Stannius in fish (11, 12, 26, 29, 36). Similarly, we demonstrated that the CaSR protein is expressed in the presumed location of the corpuscles of Stannius in larval zebrafish, potentially to detect changes in internal Ca2+ levels. On the other hand, we observed that the mRNA expression of CaSR was significantly increased in larval zebrafish after acclimation to low-Ca2+ water. Loretz at al. (29, 30) also reported that acclimation to FW increased the mRNA expression of CaSR in the kidney of Mozambique tilapia. The authors suggested that the increased renal CaSR expression could increase tissue responsiveness to internal Ca2+ levels during acclimation to FW.
The results of previous studies indicated that the CaSR is expressed in mitochondrion- and NKA-rich cells of the gill epithelium (11, 29, 31). In the present study, we demonstrated that the CaSR is expressed in a portion of NKA- and Mitotracker-positive cells on the skin of yolk sac in larval zebrafish. In agreement with the findings of Pan et al. (32), we observed that ecac mRNA was expressed in a subset of NKA-positive cells (also known as NaR cells) on the skin of yolk sac in larval zebrafish. Results from double in situ hybridization and immunohistochemistry further revealed that CaSR is expressed in a subset of ecac-expressing ionocytes (i.e., NaR cells). In larval zebrafish, Ca2+ uptake is thought to be mediated by NaR cells on the skin of the yolk sac; the entry of Ca2+ across the apical membrane of these cells is facilitated by ECaC, and Ca2+ is subsequently extruded into the extracellular fluids via PMCA and/or NCX at the basolateral membrane (for a recent review, see Ref. 23). Therefore, the expression of CaSR in NaR cells suggests a role for the CaSR in regulating Ca2+ transport across these cells.
CaSR-deficient fish have a reduced ability to maintain Ca2+ homeostasis in a low-Ca2+ environment.
It is well documented that fish acclimated to a low-Ca2+ environment maintain whole body Ca2+ homeostasis by increasing Ca2+ uptake (7, 32, 35). Pan et al. (32) reported that larval zebrafish exposed to low-Ca2+ water increase Ca2+ uptake by increasing the expression of ecac. Similarly, we observed that the mRNA expression of ecac was increased in zebrafish acclimated to low-Ca2+ water. Acclimation to low-Ca2+ water did not significantly affect the mRNA expression of pmca2 and ncx1b, supporting previous assertions that apical influx of Ca2+ via ECaC is the rate-limiting step (32, 34). In fish experiencing CaSR knockdown, uptake of Ca2+ and ecac mRNA were both increased following acclimation to low-Ca2+ water; however, the increases were markedly lower compared with control fish. Additionally, a significant reduction in the whole body Ca2+ content was observed in the CaSR morphants acclimated to low-Ca2+ water. These results suggested that fish lacking CaSR exhibit a reduced ability to increase ecac expression and Ca2+ uptake, thereby reducing their ability to maintain whole body Ca2+ balance during acclimation to a low-Ca2+ environment. Although the present study did not measure Ca2+ efflux, it is to be noted here that Ca2+ efflux is an important component for overall Ca2+ balance. In mammals, it has been suggested that the CaSR modulates paracellular Ca2+ permeability in the kidney through its regulation on a tight junction protein claudin-14 (10). It remains to be examined whether the piscine CaSR is involved in the regulation of paracellular Ca2+ movement.
In mammals, inactivation of the CaSR was reported to increase the secretion of PTH, which can ultimately increase renal reabsorption of Ca2+ and increase plasma Ca2+ levels (18, 40). Knockdown of the CaSR was also found to increase mRNA expression of pth-1 in 3 dpf larval zebrafish (26). Similarly, the present study also demonstrated that mRNA expression of pth-1 was significantly increased in 4 dpf CaSR morphants, potentially serving to promote Ca2+ retention. Interestingly, however, we observed that the overall Ca2+ influx was decreased in the CaSR morphant, presumably owing to the elevated stc-1 mRNA expression (discussed below). Presently, the precise role of PTH in Ca2+ homeostasis in fish is not clear (14). In larval zebrafish, overexpression of PTH1 by injecting pth-1 cRNA results in an increase in whole body Ca2+ content (26). Similarly, exposure to PTH-related peptide (PTHrP; structurally similar to PTH) increases Ca2+ uptake and reduces Ca2+ efflux in larval sea bream Sparus aurata L. (13). In contrast, injections of bovine PTH reduces plasma Ca2+ concentration in the FW tilapia Oreochromis mossambicus and killifish Fundulus heteroclitus acclimated to a low-Ca2+ environment (1). Clearly, the involvement of CaSR in regulating PTH secretion and its subsequent action on Ca2+ homeostasis in zebrafish, particularly in a low-Ca2+ environment, warrants further investigation.
In the present study, the precise mechanisms by which CaSR regulates Ca2+ transport function remain unclear, but appear to be associated with the hypocalcemic hormone STC. Elevation of plasma STC levels is known to reduce Ca2+ uptake and/or plasma Ca2+ concentrations in fish, including European flounder and rainbow trout (12, 36). Tseng et al. (39) reported that larval zebrafish exposed to low-Ca2+ water exhibit a reduction in stc-1 mRNA expression and that stc-1 knockdown increases ecac expression and Ca2+ influx. Consistently, we also observed that mRNA expression of stc-1 was significantly decreased in larval zebrafish acclimated to low-Ca2+ water, presumably to increase ecac expression and Ca2+ uptake. Lin et al. (26) have recently suggested that the CaSR may have time-dependent effects on the mRNA expressions of pth-1 and stc-1 in developing zebrafish. Specifically, they demonstrated that stc-1 mRNA expression in the CaSR morphants was first decreased at 30 hpf but then returned to the control levels at 3 dpf (26). Additionally, mRNA level of stc-1 in 3 dpf CaSR morphants was significantly increased following acclimation to either low- or high-Ca2+ water (26). Similarly, the present study demonstrated that mRNA level of stc-1 was significantly increased in the CaSR morphants after acclimation to normal-Ca2+ or low-Ca2+ water. In teleosts, it has been proposed that the CaSR in the corpuscles of Stannius detect changes in internal Ca2+ levels and regulate the secretion of STC (12, 36). Because the CaSR also is expressed in the corpuscles of Stannius in larval zebrafish, it is possible that knockdown of the CaSR impaired the Ca2+-sensing ability in the corpuscles of Stannius, leading to the increase in stc-1 expression. In support of this idea, we observed that mRNA expression of stc-1 in the CaSR morphants, unlike in the sham fish, was not decreased by low Ca2+-water exposure. Overall, it is likely that the inability to reduce stc-1 mRNA expression in the CaSR morphants leads to their inability to increase ecac expression and thereby Ca2+ influx in the absence of appropriate sensory input from the CaSR.
Concluding remarks and perspectives.
In vertebrates, including fish, the maintenance of Ca2+ at appropriate levels is vital for normal physiological functions, and therefore, Ca2+ balance must be tightly regulated. FW fish living in naturally soft (low Ca2+) water face additional challenges for maintaining Ca2+ balance. Although an increase in the Ca2+-transporting capacity of fish exposed to low-Ca2+ environments is well documented (7, 32, 35), the mechanism by which fish can “detect” changes in the environmental Ca2+ availability and thereby modulate the Ca2+ transport function have remained poorly understood. Here, we report that the CaSR is expressed in the olfactory epithelium, corpuscles of Stannius, and ecac-expressing ionocytes in larval zebrafish, implying involvement of the CaSR in environmental and internal Ca2+ sensing, and possible direct regulation of Ca2+ transport function. Additionally, knockdown of the CaSR reduced the ability of fish to increase ecac expression and Ca2+ uptake, and, hence, maintain whole body Ca2+ levels, during acclimation to soft water. These results indicate that in zebrafish larvae the modulation of Ca2+ transport function accompanying reduced water Ca2+ levels, is, at least in part, mediated by the CaSR. Overall, results from the present study, as well as from the recent study by Lin et al. (26), suggest that the CaSR is essential for maintaining Ca2+ balance in developing zebrafish. In mammals, the CaSR is thought to be involved in many biological processes, including phosphate homeostasis and acid secretion (4, 5); it remains to be determined whether the CaSR has similar physiological functions in fish.
GRANTS
This study was supported by Natural Sciences and Engineering Research Council (NSERC) Discovery and NSERC Research Tools and Innovation Grants to S. F. Perry.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: R.W.K. and S.F.P. conception and design of research; R.W.K. and D.A. performed experiments; R.W.K. and D.A. analyzed data; R.W.K. and S.F.P. interpreted results of experiments; R.W.K. prepared figures; R.W.K. drafted manuscript; R.W.K. and S.F.P. edited and revised manuscript; R.W.K. and S.F.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We are extremely grateful to Dr. Christopher A. Loretz (University at Buffalo, Buffalo, NY) for providing us with the CaSR antibodies. We also thank A. Ochalski (University of Ottawa) for his excellent technical assistance on confocal imaging.
REFERENCES
- 1.Bonga SEW, Pang RK, Pang PKT. Hypocalcemic effects of bovine parathyroid hormone (1–34) and stannius corpuscle homogenates in teleost fish adapted to low-calcium water. J Exp Zool 240: 363–367, 1986 [DOI] [PubMed] [Google Scholar]
- 2.Brown EM. The extracellular Ca2+-sensing receptor: central mediator of systemic calcium homeostasis. Annu Rev Nutr 20: 507–533, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC. Cloning and characterization of an extracellular Ca2+-sensing receptor from bovine parathyroid. Nature 366: 575–580, 1993 [DOI] [PubMed] [Google Scholar]
- 4.Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev 81: 239–297, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Capasso G, Geibel PJ, Damiano S, Jaeger P, Richards WG, Geibel JP. The calcium-sensing receptor modulates fluid reabsorption and acid secretion in the proximal tubule. Kidney Int 84, 277–284, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Chang IC, Lee TH, Yang CH, Wei YY, Chou FI, Hwang PP. Morphology and function of gill mitochondria-rich cells in fish acclimated to different environments. Physiol Biochem Zool 74: 111–119, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Chen Yy, Lu Fi, Hwang Pp. Comparisons of calcium regulation in fish larvae. J Exp Zool 295A: 127–135, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Chou YHW, Pollak MR, Brandi ML, Toss G, Arnqvist H, Atkinson AB, Papapoulos SE, Marx S, Brown EM, Seidman JG, Seidman CE. Mutations in the human Ca2+-sensing-receptor gene that cause familial hypocalciuric hypercalcemia. Am J Human Genet 56: 1075–1079, 1995 [PMC free article] [PubMed] [Google Scholar]
- 9.Cruz S, Shiao JC, Liao BK, Huang CJ, Hwang PP. Plasma membrane calcium ATPase required for semicircular canal formation and otolith growth in the zebrafish inner ear. J Exp Biol 212: 639–647, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Dimke H, Desai P, Borovac J, Lau A, Pan W, Alexander RT. Activation of the Ca2+-sensing receptor increases renal claudin-14 expression and urinary Ca2+ excretion. Am J Physiol Renal Physiol 304: F761–F769, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flanagan JA, Bendell LA, Guerreiro PM, Clark MS, Power DM, Canario AVM, Brown BL, Ingleton PM. Cloning of the cDNA for the putative calcium-sensing receptor and its tissue distribution in sea bream (Sparus aurata). Gen Comp Endocrinol 127: 117–127, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Greenwood MP, Flik G, Wagner GF, Balment RJ. The corpuscles of stannius, calcium-sensing receptor, and stanniocalcin: responses to calcimimetics and physiological challenges. Endocrinology 150: 3002–3010, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guerreiro PM, Fuentes J, Power DM, Ingleton PM, Flik G, Canario AVM. Parathyroid hormone-related protein: a calcium regulatory factor in sea bream (Sparus aurata L.) larvae. Am J Physiol Regul Integr Comp Physiol 281: R855–R860, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Guerreiro PM, Renfro JL, Power DM, Canario AVM. The parathyroid hormone family of peptides: structure, tissue distribution, regulation, and potential functional roles in calcium and phosphate balance in fish. Am J Physiol Regul Integr Comp Physiol 292: R679–R696, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Hebert SC. Extracellular calcium-sensing receptor: implications for calcium and magnesium handling in the kidney. Kidney Int 50: 2129–2139, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Hentschel H, Nearing J, Harris HW, Betka M, Baum M, Hebert SC, Elger M. Localization of Mg2+-sensing shark kidney calcium receptor SKCaR in kidney of spiny dogfish, Squalus acanthias. Am J Physiol Renal Physiol 285: F430–F439, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Herberger AL, Loretz CA. Morpholino oligonucleotide knockdown of the extracellular calcium-sensing receptor impairs early skeletal development in zebrafish. Comp Biochem Physiol A 166: 470–481, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Ho C, Conner DA, Pollak MR, Ladd DJ, Kifor O, Warren HB, Brown EM, Seidman JG, Seidman CE. A mouse model of human familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Nat Genet 11: 389–394, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Hofer AM, Brown EM. Extracellular calcium sensing and signalling. Nat Rev Mol Cell Biol 4: 530–538, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Hogan BM, Danks JA, Layton JE, Hall NE, Heath JK, Lieschke GJ. Duplicate zebrafish pth genes are expressed along the lateral line and in the central nervous system during embryogenesis. Endocrinology 146: 547–551, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Hubbard PC, Barata EN, Canario AVM. Olfactory sensitivity to changes in environmental [Ca2+] in the marine teleost Sparus aurata. J Exp Biol 203: 3821–3829, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Hubbard PC, Ingleton PM, Bendell LA, Barata EN, Canário AVM. Olfactory sensitivity to changes in environmental [Ca2+] in the freshwater teleost Carassius auratus. An olfactory role for the Ca2+-sensing receptor? J Exp Biol 205: 2755–2764, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Hwang PP, Lee TH, Lin LY. Ion regulation in fish gills: recent progress in the cellular and molecular mechanisms. Am J Physiol Regul Integr Comp Physiol 301: R28–R47, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Kos CH, Karaplis AC, Peng JB, Hediger MA, Goltzman D, Mohammad KS, Guise TA, Pollak MR. The calcium-sensing receptor is required for normal calcium homeostasis independent of parathyroid hormone. J Clin Invest 111: 1021–1028, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwong RWM, Perry SF. The tight junction protein claudin-b regulates epithelial permeability and sodium handling in larval zebrafish, Danio rerio. Am J Physiol Regul Integr Comp Physiol 304: R504–R513, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin CH, Su CH, Hwang PP. Calcium-sensing receptor mediates Ca2+ homeostasis by modulating expression of PTH and stanniocalcin. Endocrinology 155: 56–67, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Lin CH, Tsai IL, Su CH, Tseng DY, Hwang PP. Reverse effect of mammalian hypocalcemic cortisol in fish: Cortisol stimulates Ca2+ uptake via glucocorticoid receptor-mediated vitamin D3 metabolism. PLoS One 6: e23689, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loretz CA. Extracellular calcium-sensing receptors in fishes. Comp Biochem Physiol A 149: 225–245, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Loretz CA, Pollina C, Hyodo S, Takei Y. Extracellular calcium-sensing receptor distribution in osmoregulatory and endocrine tissues of the tilapia. Gen Comp Endocrinol 161: 216–228, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Loretz CA, Pollina C, Hyodo S, Takei Y, Chang W, Shoback D. cDNA cloning and functional expression of a Ca2+-sensing receptor with truncated C-terminal tail from the Mozambique tilapia (Oreochromis mossambicus). J Biol Chem 279: 53288–53297, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Nearing J, Betka M, Quinn S, Hentschel H, Elger M, Baum M, Bai M, Chattopadyhay N, Brown EM, Hebert SC, Harris HW. Polyvalent cation receptor proteins (CaRs) are salinity sensors in fish. Proc Natl Acad Sci USA 99: 9231–9236, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pan TC, Liao BK, Huang CJ, Lin LY, Hwang PP. Epithelial Ca2+ channel expression and Ca2+ uptake in developing zebrafish. Am J Physiol Regul Integr Comp Physiol 289: R1202–R1211, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Perry SF. The chloride cell: structure and function in the gills of freshwater fishes. Ann Rev Physiol 59: 325–347, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Perry SF, Flik G. Characterization of branchial transepithelial calcium fluxes in freshwater trout, Salmo gairdneri. Am J Physiol Regul Integr Comp Physiol 254: R491–R498, 1988 [DOI] [PubMed] [Google Scholar]
- 35.Perry SF, Wood CM. Kinetics of branchial calcium uptake in the rainbow trout: effects of acclimation to various external calcium levels. J Exp Biol 116: 411–433, 1985 [Google Scholar]
- 36.Radman DP, McCudden C, James K, Nemeth EM, Wagner GF. Evidence for calcium-sensing receptor mediated stanniocalcin secretion in fish. Mol Cell Endocrinol 186: 111–119, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Shahsavarani A, McNeill B, Galvez F, Wood CM, Goss GG, Hwang PP, Perry SF. Characterization of a branchial epithelial calcium channel (ECaC) in freshwater rainbow trout (Oncorhynchus mykiss). J Exp Biol 209: 1928–1943, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Thisse C, Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc 3: 59–69, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Tseng DY, Chou MY, Tseng YC, Hsiao CD, Huang CJ, Kaneko T, Hwang PP. Effects of stanniocalcin 1 on calcium uptake in zebrafish (Danio rerio) embryo. Am J Physiol Regul Integr Comp Physiol 296: R549–R557, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Tu Q, Pi M, Karsenty G, Simpson L, Liu S, Quarles LD. Rescue of the skeletal phenotype in CasR-deficient mice by transfer onto the Gcm2-null background. J Clin Invest 111: 1029–1037, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]