Abstract
The study was conducted to investigate the role for dopamine in the centrally mediated sympathoexcitatory response in rats with Type 2 diabetes (T2D). T2D was induced by a combination of high-fat diet (HFD) and low-dose streptozotocin (STZ). HFD/STZ treatment for 12–14 wk resulted in significant increase in the number of FosB-positive cells in the paraventricular nucleus (PVN) and rostral ventrolateral medulla (RVLM). In anesthetized rats, administration of exogenous dopamine (dopamine hydrochloride, 20 mM) in the PVN, but not in the RVLM, elicited decreases in renal sympathetic nerve activity (RSNA) and mean arterial pressure (MAP) in control rats and but not in the T2D rats. Blocking the endogenous dopamine with dopamine D1/D5 receptor antagonist SCH39166 (2 mM) in the PVN and RVLM, resulted in increases in RSNA, MAP, and heart rate (HR) in both control and T2D rats. These responses were significantly attenuated in T2D rats compared with control rats (PVN − ΔRSNA: 21 ± 10 vs. 44 ± 2%; ΔMAP: 7 ± 3 vs. 19 ± 6 mmHg, ΔHR: 17 ± 5 vs. 32 ± 4 bpm, P < 0.05). There were no significant increases in response to dopamine D2/D3 receptor antagonist raclopride application in the PVN and RVLM of both control and T2D rats. Furthermore, there were decreased dopamine D1 receptor and D2 receptor expressions in the PVN of T2D rats. Taken together, these data suggest that reduced endogenous dopaminergic tone within the PVN may contribute to the sympathoexcitation in T2D.
Keywords: central nervous system, dopaminergic, sympathetic nerve activity
type 2 diabetes (t2d) is known to be closely linked with insulin resistance and elevated sympathetic activation (11, 23). The elevated activation of the sympathetic nervous system contributes to the onset and maintenance of cardiovascular complications, such as hypertension, cardiac arrhythmias, and atherosclerosis in T2D (41). The evidence for an increased activation of the sympathetic nervous system in diabetes is the observation of an increased heart rate (HR) and blood pressure (BP), diminished HR variability, baroreceptor dysfunction, and finally, high concentrations of catecholamine in the plasma and urine (16, 18). The underlying mechanisms linking diabetes with sympathetic activation are complex and not yet clearly understood. Previous studies suggest that the central nervous system is critical in regulating sympathetic activation and thus contributing to the altered neurohumoral drive during diabetes (1, 39, 42, 53).
Dopamine is involved in the regulation of a broad range of biological functions, including locomotor activity, cognition, food intake, and hormone secretion (38). Dopamine signaling is mediated by at least five distinct G protein-coupled receptor subtypes, classified as D1-like (D1 and D5) and D2-like (D2, D3, and D4) receptors (26). The D1 receptor is the most widespread dopamine receptor in the brain, which has been found in the striatum, olfactory tubercle, limbic system, hypothalamus, and thalamus (5). The D2 receptor is also abundant in the mammalian brain. It has been detected in many areas, including the paraventricular nucleus (PVN) of the hypothalamus (26, 40) and the dorsal vagal complex of the human medulla (12). Dopamine neurons are found in the periventricular, the anterior, medial, and lateral parvocellular regions of the PVN (49). Tyrosine hydroxylase- and dopamine-β-hydroxylase-like immunoreactive terminals were found distributed throughout the rat hypothalamus and were abundant in all parts of the PVN (9). Dopamine innervation of the PVN may be responsible for the neuroendocrine, behavioral response and sympathetic regulation (21, 24, 54).
A number of investigations have attempted to elucidate the role of dopamine in the pathogenesis and treatment of high BP (26, 30). The D1 receptor in smooth muscle cells induces vasodilation in the vasculature of the systemic circulation, whereas in the kidney, they modulate sodium excretion, induce natriuresis and diuresis, and improve renal blood flow and glomerular filtration in renal tubules (30). The D2 receptors are located in the sympathetic endings, and their stimulation reduces norepinephrine release and, thus, induces vasodilation, decreasing HR and BP (26). In the central nervous system, dopaminergic effects on the cardiovascular system include inhibitory or excitatory actions. A low dose of dopamine increases renal sympathetic nerve activity (RSNA), and a high dose of dopamine decreases RSNA, suggesting that dopamine may mediate some of the central sympathoinhibitory effects upon hyperactivation of the dopaminergic system (54).
Previously, our laboratory has carried out a series of experiments documenting the central mechanisms involved in sympathetic abnormalities contributing to the altered neurohumoral drive during streptozotocin (STZ)-induced Type 1 diabetes (36, 55, 56). Evidence indicates that the PVN of the hypothalamus is involved in the blunted renal sympathoinhibition in response to acute volume expansion in the diabetic rat. We also demonstrated significant increases in the neural activity in the PVN of rats with diabetes (17). These data suggest that the neurons in the PVN are activated, and this may contribute to the autonomic dysfunction observed during diabetes. In T2D, reduced dopaminergic tone in hypothalamic neural circuits have been observed by the others (38). Reduced dopaminergic neurotransmission in the suprachiasmatic nucleus of obese animals appears to drive norepinephrine and neuropeptide Y-mediated transmissions in other nuclei to induce the obesity syndrome. Treatment with D2 receptor agonists can reverse the metabolic syndrome in these animals (38). Despite this evidence, the central mechanisms by which dopamine contributes to altered neurohumoral drive during T2D remain unclear. In the present study, we hypothesized that reduced dopaminergic activity in the PVN neurons may contribute to the enhanced sympathoexcitation commonly observed in T2D.
MATERIALS AND METHODS
Induction of type 2 diabetes.
This study was approved by the University of Nebraska Medical Center Institutional Animal Care and Use Committee and conforms to the guidelines for the care and use of laboratory animals of the National Institutes of Health and the American Physiological Society. Male Sprague-Dawley rats (150–180 g; Sasco, Omaha, NE) were maintained in a vivarium with a 12:12-h light-dark cycle and placed on the high-fat diet (HFD; Harlan, Indianapolis, IN), in which 42% of calories are from fat. After 4 wk of HFD feeding, the rats were injected once with low-dose STZ (30 mg/kg ip) to induce partial insulin deficiency. These rats were then placed on the HFD for another 8 wk. The normal diet-fed rats were used as nondiabetic controls.
Postprandial plasma glucose, body weight, and food consumption were monitored weekly. The levels of plasma insulin and leptin were measured by immunoassay (ALPCO, Salem, NH), while plasma triglyceride level was measured by triglyceride quantification kit (BioVision, Milpitas, CA). Rats were fasted for 4 h before undergoing glucose tolerance tests. Oral glucose load was administered at 2 g/kg body wt. Glucose levels were measured from tail bleeds with a glucometer at specified time points after glucose administration.
Urinary norepinephrine excretion measurements.
Urinary norepinephrine excretion was measured as an index of overall sympathetic activation. Twelve weeks after induction, rats from all groups were placed in metabolic cages, and urine was collected for 24 h. The 50-ml collecting tubes contained mineral oil to prevent evaporation losses. After collection, urine was centrifuged and transferred to Eppendorf tubes containing 1 N HCl to prevent autooxidation of catecholamines and then stored at −80°C. Urinary norepinephrine concentration of thawed samples was measured using a commercially available ELISA kit (Labor Diagnostika Nord, Nordhorn, Germany), following the manufacturer's instructions. The limit of detection of the assay is 1.5 ng/ml norepinephrine in urine. Urinary excretion of norepinephrine was calculated using urine flow rate.
FosB and dopamine receptor immunohistochemistry.
The rats were anesthetized with pentobarbital sodium (65 mg/kg) and perfused transcardially with 150 ml of heparinized saline followed by 250 ml of 4% paraformaldehyde in 0.1 M sodium phosphate buffer. The brain was removed and postfixed at 4°C for 4 h in 4% paraformaldehyde solution and then placed in 30% sucrose. The brain was blocked in the coronal plane, and sections 30 μm in thickness were cut with a cryostat.
One set of floating sections was rinsed 4 times in 0.1 M PBS and then incubated in 0.3% hydrogen peroxide for 30 min at room temperature. After rinsing, sections were incubated in PBS containing 3% normal horse serum and 0.25% Triton X-100 for 2 h. Sections were then incubated with a polyclonal rabbit anti-FosB antibody (1:5,000; Santa Cruz Biotechnology, Santa Cruz, CA) for 48 h at 4°C. Sections were rinsed and incubated with biotinylated goat anti-rabbit IgG for 2 h at room temperature (1:100), then incubated with avidin-biotin complex (1:200; Vectastain Elite ABC kit; Vector Laboratories, Burlingame, CA) for 1 h, and rinsed in PBS for 30 min. Immunostaining was visualized by incubation in diaminobenzidine kit (DAB kit; Vector Laboratories, Burlingame, CA). Finally, they were mounted on gelatin-coated glass slides, dried, and cover-slipped.
The presence of FosB-positive cells in discrete regions of the PVN, supraoptic nucleus (SON), media preoptic nucleus (MnPO), subfornical organ (SFO), and rostral ventrolateral medulla (RVLM) was examined with a Leica microscope under bright field from all four groups of rats. Images were captured with a Qimaging digital camera (Qimaging, Surrey, Canada). The staining was evaluated by counting the number of nuclei positively stained for FosB by ImageJ software (National Institutes of Health, Bethesda, MD). Three adjacent sections were considered to represent one coronal level. The average number of cells in the sections (PVN: 1.80 ± 0.1 mm; SON: 1.80 ± 0.1 mm; MnPO: 0.4 ± 0.1 mm; SFO: 0.9 ± 0.1 mm, RVLM: 12.0 ± 0.1 mm posterior to bregma) was taken to represent the number of cells unilaterally. An independent observer blind to the two experimental groups counted cells within the identified boundaries of the PVN, SON, MnPO, SFO, and RVLM, according to the atlas of Pellegrino and Cushman (37).
Another two groups of sections were incubated with 10% normal donkey serum in PBS for 1 h at room temperature and then incubated with primary antibody against D1 receptor (anti-rabbit, 1:200; Santa Cruz Biotechnology, Santa Cruz, CA), D2 receptor (anti-goat, 1:200; Santa Cruz Biotechnology) or a neuronal marker microtubule-associated protein 2 (MAP2; anti-mouse, 1:200, Abcam, Cambridge, MA) overnight at 4°C. After washing with PBS, the sections were incubated with Cy3-conjugated donkey anti-rabbit/anti-goat secondary antibody (1:200) and Cy2-conjugated donkey anti-mouse secondary antibody (1:400; Jackson ImmunoResearch, West Grove, PA) for 2 h at room temperature. After washing with PBS and drying, the sections were cover-slipped with fluoromounting-G (SouthernBiotech, Birmingham, AL). Distribution of dopamine receptor immunofluorescence within the PVN was viewed using an Olympus (Tokyo, Japan) fluorescence microscope equipped with a digital camera (Qimaging, Surrey, British Columbia, Canada). The images were taken under the same conditions (exposure time and resolution) in both groups and not adjusted prior to quantification. ImageJ software (National Institutes of Health) was used to identify the total intensity of positive staining with Cy3. Three alternate sections (1.80 ± 0.1 mm posterior to bregma) representing the PVN were analyzed in this way, and then the mean data were calculated. Although the images showed some overlap of D1/D2 receptor with the neuronal marker MAP2, still, there is the lack of phenotypic characterization of the D1- and D2-positive cells (i.e., RVLM/spinally projecting).
General surgery for recording of renal sympathetic nerve activity and arterial pressure.
Rats were anesthetized with α-chloralose (70 mg/kg ip) and urethane (0.75 g/kg ip). The femoral vein was cannulated for administration of supplemental anesthesia and 0.9% saline. The femoral artery was cannulated for recording mean arterial pressure (MAP) and HR. The left renal nerve was isolated and cut. The central end of the nerve was used to record the electrical signal with the PowerLab (ADInstruments, Colorado Springs, CO) to monitor RSNA, as described before (14, 56). Basal nerve activity was determined at the beginning of the experiment, and background noise was determined by nerve activity recorded at the end of the experiment (after the rat was euthanized). The nerve activity during the experiment was calculated by subtracting the background noise from the recorded value. The changes in integration and frequency of the nerve discharge were expressed as a percentage from basal value. Responses of MAP and HR were expressed as the absolute difference between the basal value and the value after each dose of a drug.
Microinjection of dopamine, D1/D5 antagonist and D2/D3 antagonist into the PVN.
Rats were placed in a stereotaxic apparatus. An incision was made on the midline of the scalp to expose bregma. The coordinates of the right PVN with reference to bregma were calculated as being 1.5 mm posterior, 0.4 mm lateral, and 7.8 mm ventral to the dura (14). Thirty to forty-five minutes after the surgery, a needle (0.2 mm OD) that was connected to a microsyringe (0.5 μl) was lowered into the PVN. Stable baseline parameters were monitored for 10–20 min before each microinjection. At the completion of the experiment, monastral blue dye (2% Chicago blue, 30 nl) was injected into the brain for histological verification. As a comparison, microinjection was also performed in the RVLM within the brain stem. The coordinates of the right RVLM with reference to bregma were calculated as being 12.5 mm caudal to the bregma, 2.1 mm lateral to the midline, and 9.2 mm ventral to the surface of cerebellum (52). The RVLM was chemically identified by a transient pressor response (at least 20 mmHg) to injection of l-glutamate (50 mM in 100 nl).
Experiment 1.
In a set of six control rats and six T2D rats, dopamine hydrochloride (Tocris, Ellisville, MO) was microinjected (20 mM in 100 nl) into the PVN and RVLM. The responses in RSNA, MAP, and HR over the 30 min were recorded.
Experiment 2.
In another set of control (n = 8) and T2D rats (n = 8), RSNA, MAP, and HR were measured, while D1/D5 antagonist SCH39166 (Tocris, Ellisville, MO) or D2/D3 antagonist raclopride (Tocris) was microinjected into the PVN and RVLM (2 mM in 100 nl), respectively. The changes in RSNA, MAP, and HR over the 30 min were recorded.
Micropunch of PVN for Western blot measurements.
In a separate group of control (n = 6) and T2D (n = 6) rats, the brains were removed and frozen on dry ice after the death of the rats with an overdose of pentobarbital sodium (150 mg/kg ip). The location for the punching of the PVN sections (between 1.60 and 2.20 mm posterior to bregma) was determined relative to the fornix and the optic tract. Six frozen serial coronal sections (100 μm each) of the PVN were cut with a cryostat, according to a stereotaxic atlas of the rat brain by Pellegrino and Cushman (37) and bilaterally punched with an 18-gauge needle using the Palkovits and Brownstein technique (31), such that there were 12 total punches per brain. The punches for each brain were combined and placed in 100 μl of protein extraction buffer (10 mM Tris, 1 mM EDTA, 1% SDS, 0.1% Triton-X-100, and 1 mM phenylmethylsulfonyl fluoride) to extract the protein. As a comparison, 10 serial sections of RVLM (100 μm/section, 1.5 to 2.5 mm rostral to the obex) were also cut and bilaterally punched (total 20 punches) with an 18-gauge needle using the Palkovits and Brownstein technique (31) and then processed for protein extraction.
Western blot measurement of D1 receptor and D2 receptor protein.
The total protein concentration from the extracted protein described above was measured with a bicinchoninic acid assay kit (Pierce, Rockford, IL). Samples were adjusted to contain the same concentration of total protein, and then equal volumes of 2 × 4% SDS sample buffer were added. The protein samples were loaded onto a SDS PAGE gel and subjected to electrophoresis. The fractionated proteins on the gel were transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, MA). The membrane was probed with primary antibody [rabbit anti-D1 receptor, goat anti-D2 receptor (1:500; Santa Cruz, CA), or rabbit anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH 1:2,000; Santa Cruz, CA)] overnight, and then probed with secondary antibody (peroxidase conjugated anti-rabbit or goat IgG, 1:5,000; Pierce, Rockford, IL). An enhanced chemiluminescence substrate (Pierce) was applied to the membrane, followed by an exposure within a UVP system (UVP BioImaging, Upland, CA) for visualization. Kodak 1D software (Kodak, Rochester, NY) was used to quantify the signal. The expression of protein was calculated as the ratio of intensity of the D1 receptor and D2 receptor, respectively, relative to the intensity of GAPDH band.
Statistical analysis.
Data are presented as means ± SE. The data were subjected to two-way ANOVA followed by comparison for individual group differences with the Newman-Keuls test. Statistical significance was indicated by a value of P < 0.05.
RESULTS
General data.
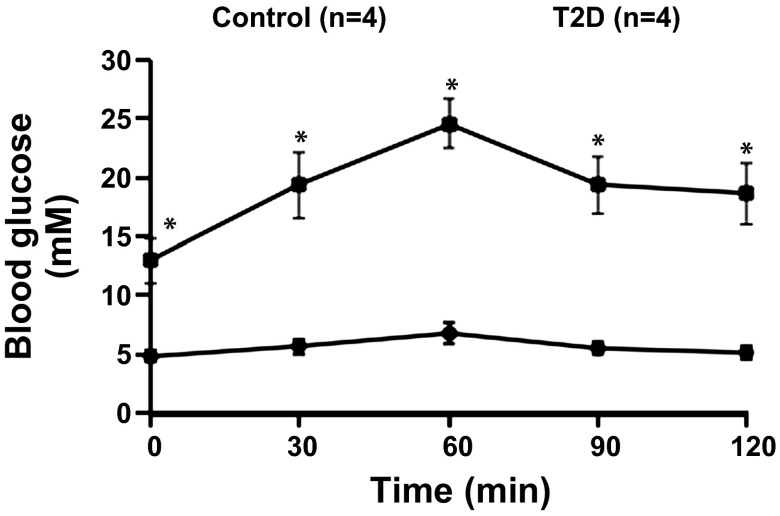
T2D was induced by a combination of both HFD and injection of low-dose STZ. Table 1 illustrates the general characteristics of control and T2D rats used in these experiments. After 12–14 wk of the treatments (HFD and STZ injection), the body weight and weight of the retroperitoneal fat pad were significantly higher in T2D rats. The T2D rats also show increased plasma triglyceride and decreased brown adipose tissue. The fasting blood glucose, 2 h plasma glucose level, and plasma leptin were significantly higher in T2D rats than those in control rats. Blood glucose tolerance (Fig. 1) and the insulin sensitivity index (Table 1) were significantly decreased in the T2D rats compared with that in control rats. These data indicated that HFD and low-dose STZ induced hyperglycemia, hyperleptinemia, hyperlipidemia, and insulin resistance (lower insulin sensitivity index and blood glucose tolerance) in T2D rats.
Table 1.
Characteristics of control and T2D rats in the experiments
| Control (n = 15) | T2D (n = 16) | |
|---|---|---|
| Body weight, g | 423 ± 21 | 485 ± 22* |
| Retroperitoneal fat pad, g | 5.2 ± 0.4 | 7.7 ± 2.1* |
| Epydidimal fat pad, g | 8.6 ± 1.6 | 9.8 ± 1.5 |
| Brown adipose tissiues, g | 1.1 ± 0.3 | 0.3 ± 0.1* |
| Kidney weight, g | 1.8 ± 0.3 | 3.6 ± 0.8* |
| Plasma triglyceride, mM | 1.1 ± 0.3 | 3.6 ± 0.4* |
| Basal MAP, mmHg | 94 ± 7 | 100 ± 5 |
| Urine NE, μg/ml | 12.9 ± 2.9 | 20.9 ± 2.5* |
| 24 h urine volume, ml | 15 ± 3 | 33 ± 5* |
| Fasting plasma glucose, mM | 5.3 ± 0.6 | 13.4 ± 2.2* |
| 2 h plasma glucose, mM | 6.5 ± 0.4 | 17.3 ± 1.8* |
| Fasting plasma insulin, mU/l | 12.0 ± 1.6 | 14.6 ± 1.3 |
| 2 h plasma insulin, mU/l | 14.3 ± 2.7 | 18.9 ± 3.9 |
| Insulin Sensitivity Index | −4.0 ± 0.2 | −5.3 ± 0.3 |
| Plasma leptin, ng/ml | 328 ± 37 | 482 ± 52* |
| Basal heart rate, bpm | 362 ± 23 | 372 ± 36 |
| Basal Int.RSNA, μV·s | 3.3 ± 0.3 | 4.3 ± 0.3* |
| 24 h urine NE, μg | 194 ± 54 | 689 ± 79* |
Values are presented as means ± SE. MAP, mean arterial pressure; NE, norepinephrine; bpm, beats/min; Int.RSNA, integrated renal sympathetic nerve activity; T2D, Type 2 diabetes.
P < 0.05 vs. control rats.
Fig. 1.
Plasma glucose during intraperitoneal glucose tolerance test in control and Type 2 diabetic (T2D) rats. Data are expressed as means ± SE. *P < 0.05 vs. control rats.
Meanwhile, the basal RSNA and 24-h urinary norepinephrine levels were significantly increased in T2D rats, suggesting that there was an increased overall sympathetic tone in the T2D rats (Table 1). However, there were no significant differences in basal MAP and HR between control and T2D rats.
Increased FosB-positive cells in rats with T2D.
Significant increases in the numbers of FosB-positive cells in the PVN and other nuclei (SON and SFO) of the brain were observed after 12–14 wk HFD/STZ treatment. The number of FosB-positive cells was significantly increased in the PVN (207 ± 31 vs. 139 ± 22, P < 0.05), SON (32 ± 5 vs. 19 ± 4, P < 0.05), SFO (114 ± 10 vs. 67 ± 15, P < 0.05), and RVLM (50 ± 10 vs. 23 ± 9, P < 0.05) of T2D rats compared with the control rats (Fig. 2). In T2D rats, there was no significant alteration of FosB-positive cells in the MnPO (29 ± 5 vs. 23 ± 5, P > 0.05) and the adjacent areas of the PVN in T2D rats. Increased FosB may indicate chronic neuronal activation within the hypothalamus in T2D rats (50, 57). Figure 2A shows the representative images of FosB staining from the PVN, SON, MnPO, and SFO.
Fig. 2.
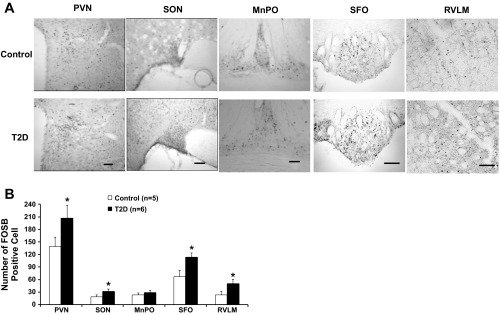
A: representative images of FosB staining in the paraventricular nucleus (PVN) (−1.8 mm from bregma), supraoptic nucleus (SON) (−1.8 mm from bregma), medial preoptic nucleus (MnPO) (−0.4 mm from bregma), subfornical organ (SFO) (−1.1 mm from bregma), and rostral ventrolateral medulla (RVLM) (−12.0 mm from bregma) from one rat/group (control and T2D). Scale bar = 50 μm. B: number of FosB-positive cells in the PVN, SON, MnPO, SFO, and RVLM in the two groups of rats. Data are expressed as means ± SE. *P < 0.05 compared with control.
Decreased responses to microinjection of dopamine into the PVN in T2D rats.
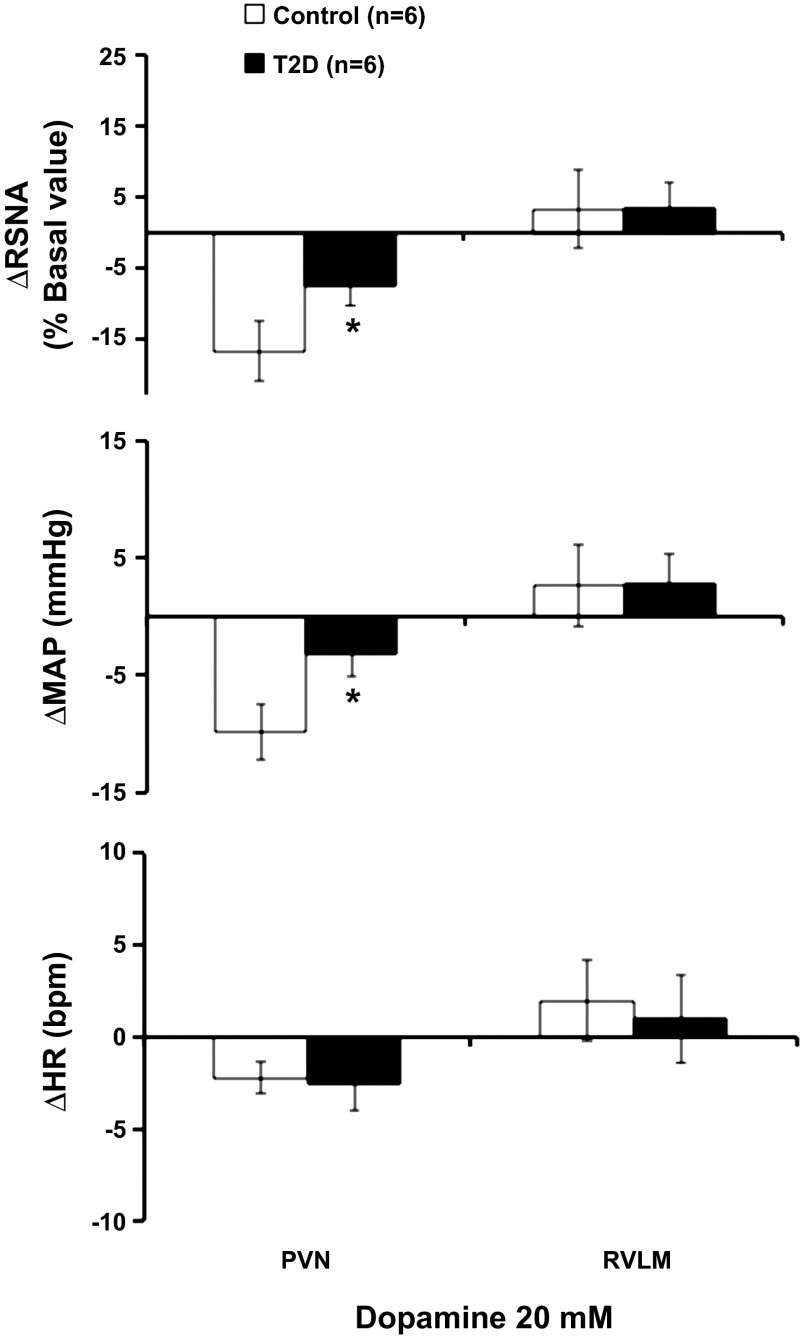
Administration of dopamine hydrochloride (20 mM) in the PVN elicited decreases in RSNA and MAP in control rats but barely showed any change in the T2D rats. Microinjection of dopamine elicited decreases in RSNA and MAP, reaching −17 ± 4% and −10 ± 2 mmHg, in control rats. The RSNA and MAP responses were significantly attenuated in T2D rats compared with the control rats, reaching −7 ± 3% and −3 ± 2 mmHg, respectively (P < 0.05) (Fig. 3). Microinjection of dopamine did not change the HR in both control and T2D groups. As a comparison, administration of dopamine hydrochloride (20 mM) in the RVLM elicited no decreases in RSNA, MAP, and HR in both groups (Fig. 3).
Fig. 3.
The mean data of changes in renal sympathetic nerve activity (RSNA), mean arterial pressure (MAP), and heart rate (HR) after microinjections of dopamine hydrochloride (20 mM) into the PVN and RVLM in control and T2D rats. *P < 0.05 vs. control group.
Decreased responses to microinjection of dopamine antagonists into the PVN in T2D rats.
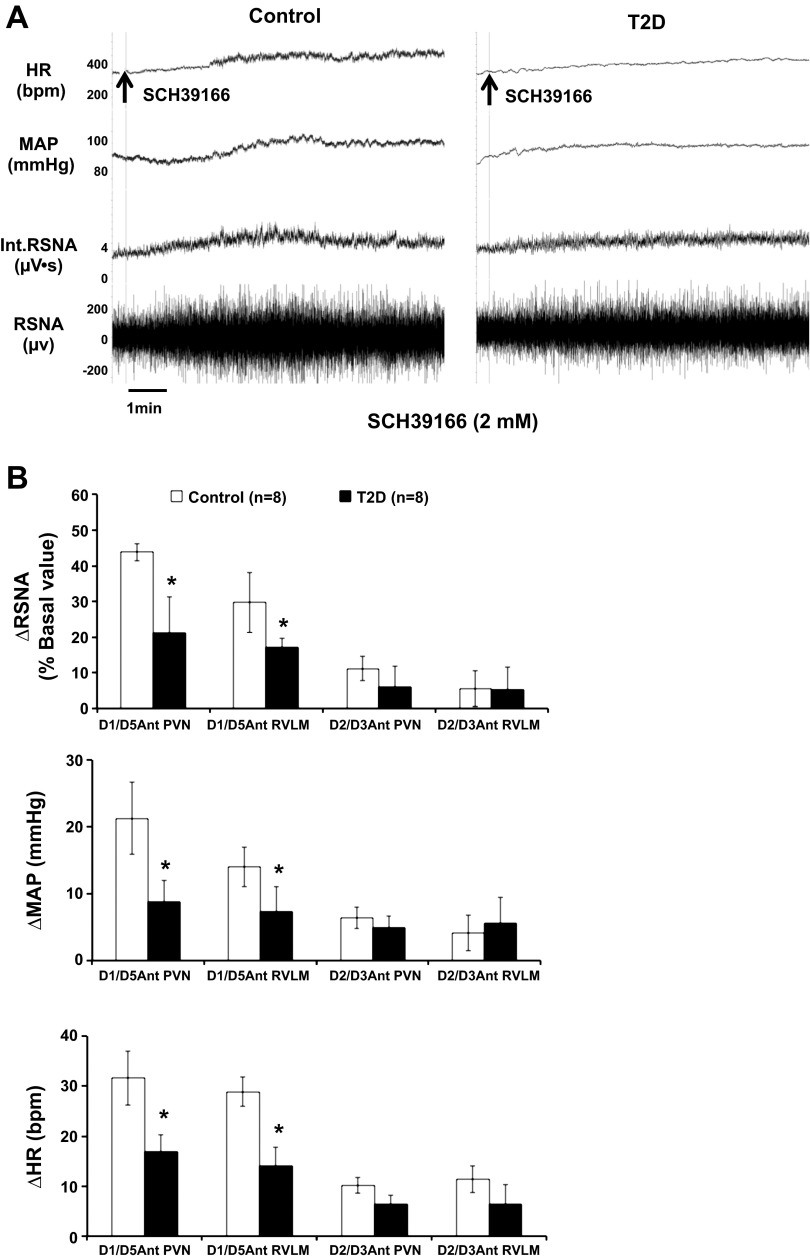
Administration of dopamine D1/D5 antagonist SCH39166 (2 mM) in the PVN and RVLM elicited increases in RSNA, MAP, and HR in both groups (Fig. 4, A and B). Microinjection of SCH39166 elicited significant increases in RSNA, MAP, and HR, reaching 44 ± 2%, 19 ± 6 mmHg, and 32 ± 4 bpm in the PVN in control rats. The RSNA, MAP, and HR responses were significantly attenuated in T2D rats compared with the control rats, reaching 21 ± 10%, 7 ± 3 mmHg, and 17 ± 5 bpm in the PVN (P < 0.05). Microinjection of SCH39166 also elicited significant increases in RSNA, MAP, and HR in the RVLM (reaching 30 ± 8%, 14 ± 3 mmHg, and 29 ± 5 bpm) in control rats. In the RVLM, the responses were also significantly blunted in T2D rats compared with the control rats (RSNA: 17 ± 3%, MAP: 7 ± 4 mmHg, and HR: 14 ± 4 bpm, P < 0.05) (Fig. 4B).
Fig. 4.
RSNA, MAP, and HR responses to dopamine antagonists microinjected into the PVN and RVLM. A: segments of original recordings from individual rats from each experimental group showing responses of HR, MAP, integrated RSNA (Int.RSNA), and RSNA to D1/D5 antagonist SCH39166 microinjected into the PVN. B: mean data of changes in RSNA, MAP, and HR after microinjections of dopamine antagonists (D1/D5 antagonist SCH39166 and D2/D3 antagonist raclopride) into the PVN and RVLM in control and T2D rats. *P < 0.05 vs. control group. Ant: antagonist.
Administration of dopamine D2/D3 antagonist raclopride (2 mM) in the PVN and RVLM did not elicit significant increases in RSNA (11 ± 3% vs. 4 ± 5%), in the PVN (6 ± 3% vs. 5 ± 6% in the RVLM, P > 0.05), MAP (7 ± 2 mmHg vs. 4 ± 2 mmHg in the PVN, 3 ± 3 mmHg vs. 6 ± 4 mmHg in the RVLM, P > 0.05), and HR (10 ± 4 bpm vs. 6 ± 1 bpm in the PVN, 11 ± 2 bpm vs. 6 ± 3 bpm in the RVLM, P > 0.05) in both control and T2D groups (Fig. 4B).
Decreased dopamine receptor protein expression in the PVN and RVLM in T2D rats.
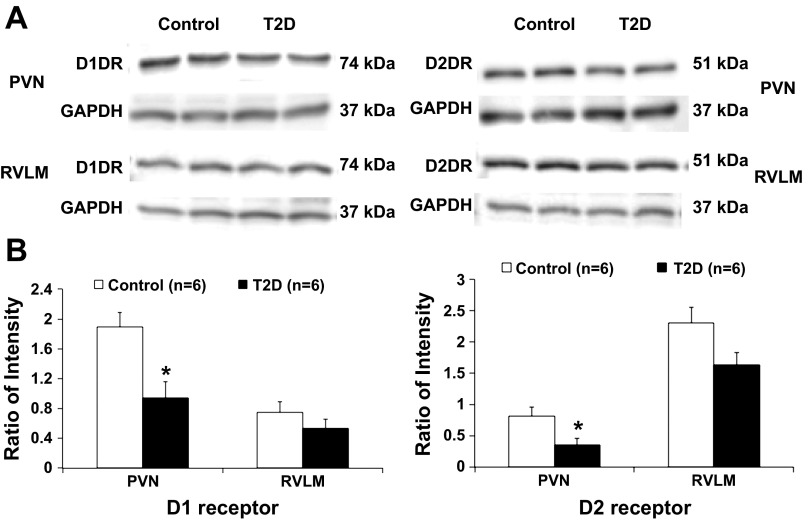
Western blot analysis showed 74-kDa bands representing D1 receptor and 51-kDa bands representing D2 receptor in the PVN and RVLM of control and T2D rats. T2D rats had a significantly lower protein level of D1 receptor (ratio of intensity: 0.9 ± 0.2 vs. 1.9 ± 0.2, P < 0.05) and D2 receptor (ratio of intensity: 0.4 ± 0.1 vs. 0.8 ± 0.1, P < 0.05) in the PVN (Fig. 5). In the RVLM, both D1 receptor and D2 receptor protein expression were not significantly changed in the T2D compared with the control rats (ratio of intensity: D1 receptor 0.8 ± 0.2 vs. 0.6 ± 0.1; D2 receptor 1.6 ± 0.3 vs. 2.3 ± 0.3, P > 0.05).
Fig. 5.
A: example of visualized bands of dopamine receptor (D1 receptor and D2 receptor) and GAPDH in the PVN and RVLM in control and T2D rats. B: mean data of band densities normalized by GAPDH. *P < 0.05 vs. control group.
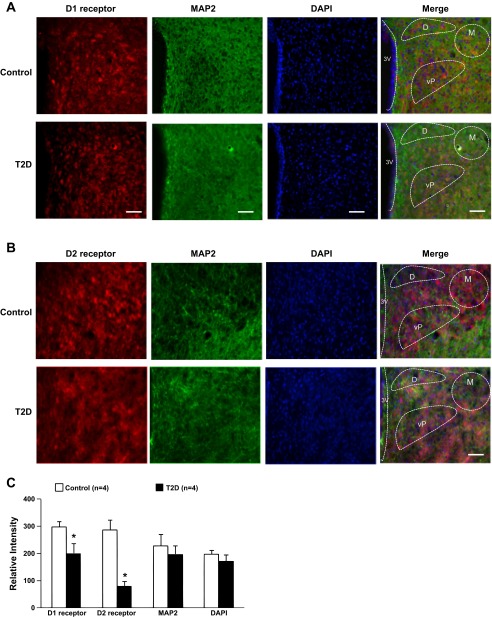
As an in situ confirmation of the alteration in D1 receptors and D2 receptors within the PVN, the immunofluorescence for D1 receptors and D2 receptors were found to be decreased in the PVN from rats with T2D compared with control rats (Fig. 6). Both D1 receptor and D2 receptor immunofluorescent signals were colocalized with neuronal marker MAP2 within the PVN. There were no significant differences of MAP2 and DAPI immunofluorescence between the groups.
Fig. 6.
A: immunofluorescent photomicrographs from the sections of the PVN region (bregma −1.8 mm) stained for D1 receptor (red), microtubule associated protein 2 (MAP2 in green) and 4′,6-diamidino-2-phenylindole (DAPI in blue) in a control and a T2D rat. Scale bar = 100 μm. B: immunofluorescent photomicrographs from the sections of the PVN region (bregma −1.8 mm) stained for D2 receptor (red), MAP2 (green), and DAPI (blue) in a control and a T2D rat. Scale bar = 100 μm. C: mean data of D1 receptor and D2 receptor, MAP2, and DAPI immunofluorescent signal in the PVN in control and T2D rats. M, magnocellular; D, dorsal cap; vP, ventrolateral parvocellular; 3V, third ventricle. *P < 0.05 vs. control group.
Brain histology.
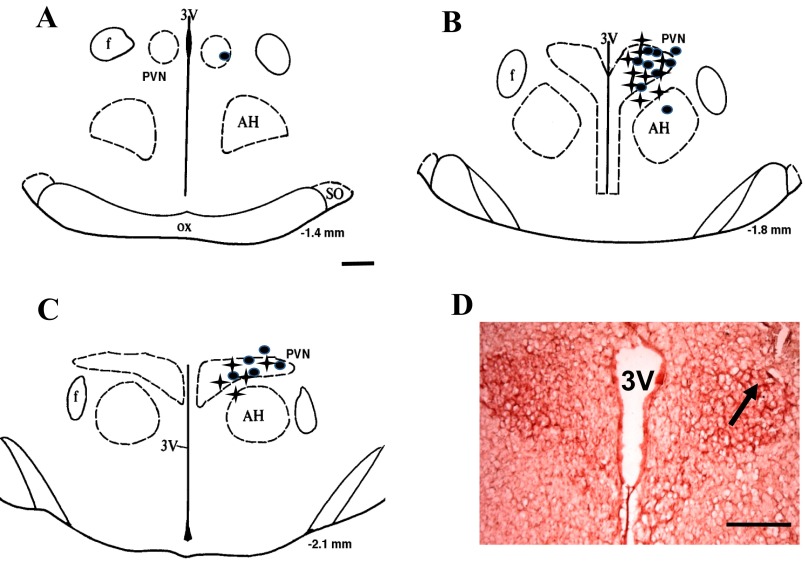
Figure 7 illustrates the brain histological data. Twenty-eight injections were in the PVN area, while four injections missed the PVN.
Fig. 7.
A–C: schematic representations of serial sections from the rostral (−1.4) to the caudal (−2.1) extent of the region of the PVN. The distance (in mm) posterior to bregma is shown for each section. Solid circles represent the sites of termination of an injection that is within the PVN region in control group. Plus signs represent that in T2D group. D: histological photo showing the injection site (arrow) in the PVN of one rat. AH, anterior hypothalamic nucleus; f, fornix; 3V, third ventricle; ox, optic tract; PVN, paraventricular nucleus; SO, supraoptic nucleus.
DISCUSSION
In the present study, we have found significant increases in the numbers of FosB-positive cells in the PVN of HFD/STZ-induced T2D rats. Second, exogenous dopamine administration in the PVN elicited decreases in RSNA and MAP in the control group and not in the T2D rats. Third, blocking the endogenous actions of dopamine with D1/D5 receptor antagonist in the PVN resulted in increases in RSNA, MAP, and HR in both control and T2D rats. These responses were significantly attenuated in T2D rats compared with control rats. Fourth, there were no significant increases in responses to dopamine receptor D2/D3 antagonist application in the PVN of both control and T2D rats. Consistent with these observations, we also found decreased D1 receptor and D2 receptor expression in the PVN of T2D rats. Taken together, these results show that reduced endogenous dopaminergic tone within the PVN may contribute to the sympathoexcitation in T2D.
There are a number of different animal models that are used for studying T2D (46). Rats fed a HFD develop obesity, hyperinsulinemia, and insulin resistance, but not frank hyperglycemia (45, 47). In our studies, T2D has been induced by HFD combined with single low-dose injection of STZ. Our study shows that HFD and low-dose STZ induces hyperglycemia, hyperleptinemia, hyperlipidemia, and insulin resistance (lower insulin sensitivity index and blood glucose tolerance). Clinical T2D is characterized by a progressive increase of the insulin resistance and is followed by inadequate compensatory insulin secretion from pancreatic β-cells. In this T2D rat model, a HFD initiates the insulin resistance, which is one of the key characteristics of the T2D patients. Low-dose STZ induces a mild impairment of insulin release, one feature of the late stage of the human T2D, which closely mimics the natural history of the disease events of human T2D (from insulin resistance to β-cell dysfunction) (19, 22, 29, 33). Elevated 24-h urinary level of norepinephrine and increased basal integrated RSNA, observed in this study (Table 1), are indications of increased overall sympathoexcitation in T2D rats. It has been recognized that this model of T2D would produce a spectrum of dysfunction (a range of blood glucose: 12.5–17.5 mM mild hyperglycemia, ∼80% of the rats; >17.5 mM severe hyperglycemia, ∼20% of the rats). We took rats that had mild hyperglycemic (blood glucose: 12.5–17.5 mM) to get a fairly consistent model of T2D.
FosB has been extensively used as an indicator of synaptic activation in the central nervous system (10, 15), although FosB activation may not distinguish between excitatory and inhibitory neuronal activation. The present study identified and mapped central structures that demonstrated long-term neuronal activation (12–14 wk after HFD/STZ induction) in rats using immunohistochemical detection of FosB. The results showed an increased number of FosB-positive cells in the PVN, SON, SFO, and RVLM in rats with T2D. This suggests that activation of neurons, including autonomic and neuroendocrine neurons in the hypothalamus and brain stem may play a major role in the processes leading to sympathetic hyperactivity in rats with T2D. This is consistent with the observation that global sympathoexcitation is higher in T2D condition, confirmed in our studies by elevated levels of catecholamine (24-h urinary excretion of norepinephrine).
In the HFD/STZ-induced diabetic rats, we did not see an increase in the baseline blood pressure and heart rate. Blood pressure is a consequence of a number of factors, including RSNA-mediated changes in renovasoconstriction. There may have been some differential changes in sympathetic outflow such that there may be vasodilation in other vascular beds. Such differential outflow has been demonstrated previously in other studies (34). The lack of an increase in blood pressure with a concomitant increase in RSNA in diabetic animals may be attributed to a number of possible contributing factors, including but not limited to 1) decreased cardiac output due to a dysfunction in the myocardium, 2) differential sympathetic outflow, 3) hypovolemia due to osmotic diuresis, and 4) impairment of sympathetic innervation of heart and blood vessels (2).
There is accumulating evidence for a neurotransmitter-like role for dopamine in the regulation of sympathetic activation (7, 40). The PVN of the hypothalamus is an important site for autonomic and endocrine homeostasis. Dopamine neurons have been found in various areas of PVN, including dorsal parvocellular PVN and medial and periventricular subdivisions of the PVN (13, 48), areas where preautonomic neurons reside. The PVN-mediated inhibitory response in RSNA can be mimicked by dopamine, and the effect can be reversed by application of the D1 receptor antagonists SCH23390 (54). In our study, exogenous administration of dopamine within the PVN caused inhibitory effect of RSNA and BP. On the contrary, D1 receptor antagonist injections, not D2 receptor antagonist injections, in the PVN significantly increased RSNA, BP, and HR. These responses were significantly attenuated in T2D rats. These results suggest that central dopamine (within the PVN) may exert an inhibitory influence on the sympathetic activity in descending pathways via D1 receptor. This combined with the data showing decreased expression of dopamine receptors in the PVN, it is possible that any disturbance of dopaminergic regulation within the PVN would change the tonic inhibitory influence on the overall sympathetic nervous outflow, leading to the sympathoexcitation commonly observed in T2D.
Our study showed that application of dopamine receptor D1/D5 antagonist specifically within the PVN resulted in increases of RSNA, MAP, and HR in both control and T2D rats. However, there were no significant increases in responses to dopamine receptor D2/D3 antagonist application in the PVN of both control and T2D rats. This would indicate that the dopaminergic sympathoinhibitory effect in the PVN may not be mediated by D2 receptor because they were unaffected by blocking the D2 receptor with raclopride. A previous study (54) indicated that stimulation of the PVN in the presence of intrathecally administered D2 receptor antagonist does not prevent the sympathoexcitatory response in renal nerve activity. However, the inhibitory responses on renal nerve activity after PVN stimulation can be blocked by intrathecal application of D1 receptor antagonist (54). This would indicate dopamine via D1 receptor might mediate some of the PVN-mediated inhibitory effects. Thus, dopamine may have a mixed response on sympathetic nerve activity and cardiovascular regulation depending on receptor subtype activation.
Our results also showed differential sympathetic and BP responses to dopamine administration in the PVN and RVLM. Exogenous administration of dopamine within the PVN caused inhibitory effect on RSNA and MAP but not from the RVLM. This would suggest a tonic inhibitory dopaminergic influence on the sympathetic outflow dictated from the PVN. It is recognized that the RVLM is also critical for maintaining basal sympathetic vasomotor tone and is an essential component of many sympathetic reflexes (4). The dopaminergic pathway has shown to be involved in RVLM C1 neuron-mediated cardiovascular and autonomic function (3, 43). In our study, application of dopamine receptor D1/D5 antagonist to the RVLM resulted in increases of RSNA, MAP, and HR. This would support the notion that dopaminergic pathway within the RVLM is also involved in the regulation of sympathetic activity. Furthermore, this dopaminergic regulation by RVLM is also blunted in the diabetic condition.
SCH39166, has been shown to be a potent, specific, D1 receptor antagonist with several features, which are advantageous over its predecessor, such as SCH23390 (51). SCH39166 has been shown to be more selective for binding to D1 receptors than SCH23390 with higher affinity, greater saturability, and greater specificity (51). SCH23390 can inhibit G protein-coupled inward rectifier K+ (GIRK) channel currents, causing depolarization of neurons (20). As a consequence, blockade of the GIRK channel in neurons may decrease spontaneous action potential formation and increase the release of excitatory neurotransmitters. However, there is no report about the similar effects to SCH39166, to date. Since SCH39166 is an analog of SCH23390, we cannot exclude the possibility that SCH39166 has the similar GIRK inhibitory effect contributing to the sympathoexcitation. This remains to be explored further in the future.
Altered neurohormonal drive plays an important pathophysiological role in diabetes (32, 44). In our previous study, we have found an enhanced excitatory effect through an ANG II-superoxide mechanism (35) and attenuated inhibitory effect through a nitric oxide mechanism (56), contributing to sympathoexcitation in Type 1 diabetes. In T2D, insulin resistance leads to activation of neurohormones, and this, in turn, leads to further insulin resistance (25). Insulin resistance of muscle and adipose tissue hampers glucose uptake in the tissues. As the brain appears to be a key regulator of energy metabolism, this may cause alterations of some key candidate neurotransmitters in relevant brain areas, such as the PVN. Reduced dopaminergic neurotransmission in hypothalamic nuclei has been implicated in the pathogenesis of T2D (38). For example, selective destruction of dopaminergic neurons in the area of the suprachiasmatic nucleus severely impairs insulin sensitivity and promotes body fat accretion. Diminished dopaminergic tone in the suprachiasmatic area may affect neural circuits in these hypothalamic nuclei to impact metabolism and is associated with markedly enhanced noradrenergic transmission in these same hypothalamic nuclei. The results from our study showed an attenuated dopaminergic inhibitory influence on the RSNA and MAP, suggesting that the impaired dopaminergic tone within the PVN may lead to the elevated sympathoexcitation, commonly observed in T2D.
Other evidence has indicated that insulin resistance may impair dopamine function in the central nervous system (27). In one study, 12 wk HFD resulted in peripheral insulin resistance and oxidative stress. These changes were accompanied by impaired dopamine function in the dorsal striatum (27). Specifically, in vivo electrochemical measures of dopamine neuronal function revealed decreased potassium-evoked dopamine release, which correlated with the degree of insulin resistance. In our T2D rats, HFD/STZ induction resulted in impaired glucose tolerance and peripheral hyperglycemia, hyperleptinemia, hyperlipidemia, and insulin resistance. It would be interesting for future studies to directly measure the effects of insulin, leptin, and high lipid on the function of dopamine neurons in the PVN. HFD feeding also increases markers of oxidative stress in multiple brain regions (6, 28). Oxidative damage decreases dopamine transporter expression and dopamine metabolite levels (8). The contribution of oxidative stress to the observed deficits in dopamine function cannot be ruled out and remain a likely possibility.
In conclusion, we postulate that the reduced dopaminergic activity within the PVN of HFD/STZ-induced T2D rats leads to an altered renal sympathoinhibition in diabetes. This altered dopaminergic mechanism within the PVN may contribute to the increased renal sympathetic neural activity observed in T2D. These results provide a potential target for the treatment of enhanced sympathoactivation commonly observed in T2D.
GRANTS
This work was supported by National Institutes of Health Grant RO1 DK-082956-03 and American Heart Association National Scientist Development Grant.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: H.Z., X.L., Y.L., P.K.M., and K.P.P. conception and design of research; H.Z. and X.L. performed experiments; H.Z. and X.L. analyzed data; H.Z., X.L., Y.L., and K.P.P. interpreted results of experiments; H.Z. and X.L. prepared figures; H.Z. and K.P.P. drafted manuscript; H.Z., Y.L., P.K.M., and K.P.P. edited and revised manuscript; H.Z., X.L., Y.L., P.K.M., and K.P.P. approved final version of manuscript.
ACKNOWLEDGMENTS
Technical support from Dr. Lirong Xu is greatly appreciated.
REFERENCES
- 1.Ao Y, Ko M, Chen A, Marvizon JC, Adelson D, Song MK, Go VL, Liu YY, Yang H. Potent hyperglycemic and hyperinsulinemic effects of thyrotropin-releasing hormone microinjected into the rostroventrolateral medulla and abnormal responses in type 2 diabetic rats. Neuroscience 169: 706–719, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borges GR, de Oliveira M, Salgado HC, Fazan R., Jr Myocardial performance in conscious streptozotocin diabetic rats. Cardiovasc Diabetol 5: 26, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Card JP, Sved JC, Craig B, Raizada M, Vazquez J, Sved AF. Efferent projections of rat rostroventrolateral medulla C1 catecholamine neurons: Implications for the central control of cardiovascular regulation. J Comp Neurol 499: 840–859, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Dampney RA. The subretrofacial vasomotor nucleus: Anatomical, chemical and pharmacological properties and role in cardiovascular regulation. Prog Neurobiol 42: 197–227, 1994 [DOI] [PubMed] [Google Scholar]
- 5.Dearry A, Gingrich JA, Palardeai P, Fremeau RT, Jr, Bates MD, Caron MG. Molecular cloning and expression of the gene for a human D1 dopamine receptor. Nature 347: 72–76, 1990 [DOI] [PubMed] [Google Scholar]
- 6.Fachinetto R, Burger ME, Wagner C, Wondracek DC, Brito VB, Nogueira CW, Ferreira J, Rocha JB. High fat diet increases the incidence of orofacial dyskinesia and oxidative stress in specific brain regions of rats. Phamacol Biochem Behav 81: 585–592, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Gladwell SJ, Pyner S, Barnes NM, Coote JH. D1-like dopamine receptors on retrogradely labelled sympathoadrenal neurones in the thoracic spinal cord of the rat. Exp Brain Res 128: 377–382, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Hatcher JM, Richardson JR, Guillot TS, OcCormack AL, Di Monte DA, Jones DP, Pennell KD, Miller GW. Dieldrin exposure induces oxidative damage in the mouse nigrostriatal dopamine system. Exp Neurol 204: 619–630, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horie S, Shioda S, Nakai Y. Catecholaminergic innervation of oxytocin neurons in the paraventricular nucleus of the rat hypothalamus as revealed by double-labeling immunoelectron microscopy. Acta Anat (Basel) 147: 184–192, 1993 [DOI] [PubMed] [Google Scholar]
- 10.Howe BM, Bruno SB, Higgs KA, Stigers RL, Cunningham JT. FosB expression in the central nervous system following isotonic volume expansion in unanesthetized rats. Exp Neurol 187: 190–198, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Huggett RJ, Scott EM, Gilbey SG, Stocker JB, Mackintosh AF, Mary DA. Impact of type 2 diabetes mellitus on sympathetic neural mechanisms in hypertension. Circulation 108: 3097–3101, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Hyde TM, Knable MB, Murray AM. Distribution of dopamine D1-D4 receptor subtypes in human dorsal vagal complex. Synapse 24: 224–232, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Jansen ASP, Wessendorf MW, Loewy AD. Transneuronal labeling of CNS neuropeptide and monoamine neurons after pseudorabies virus injections into the stellate ganglion. Brain Res 683: 1–24, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Kleiber AC, Zheng H, Schultz HD, Peuler JD, Patel KP. Exercise training normalizes enhanced glutamate-mediated sympathetic activation from the PVN in heart failure. Am J Physiol Regul Integr Comp Physiol 294: R1863–R1872, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knight WD, Little JT, Carreno FR, Toney GM, Mifflin SW, Cunningham JT. Chronic intermittent hypoxia increases blood pressure and expression of FosB/DeltaFosB in central autonomic regions. Am J Physiol Regul Integr Comp Physiol 301: R131–R139, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kondo K, Matsubara T, Nakamura J, Hotta N. Characteristic patterns of circadian variation in plasma catecholamine levels, blood pressure and heart rate variability in Type 2 diabetic patients. Diabet Med 19: 359–365, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Krukoff TL, Patel KP. Alterations in brain hexokinase activity associated with streptozotocin-induced diabetic mellitus in the rat. Brain Res 522: 157–160, 1990 [DOI] [PubMed] [Google Scholar]
- 18.Kuehl M, Stevens MJ. Cardiovascular autonomic neuropathies as complications of diabetes mellitus. Nat Rev Endocrinol 8: 405–416, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Kusakabe T, Tanioka H, Ebihara K, Kirata M, Miyamoto L, Miyanaga F, Hige H, Aotani D, Fujisawa T, Masuzaki H, Hosoda K, Nakao K. Beneficial effects of leptin on glycaemic and lipid control in a mouse model of type 2 diabetes with increased adiposity induced by streptozotocin and a high-fat diet. Diabetologia 52: 675–683, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Kuzhikandathil EV, Oxford GS. Classic D1 dopamine receptor antagonist R-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride (SCH23390) directly inhibits G protein-coupled inwardly rectifying potassium channels. Mol Pharmacol 62: 119–126, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Liposits Z, Paull WK. Association of dopaminergic fibers with corticotropin releasing hormone (CRH)-synthesizing neurons in the paraventricular nucleus of the rat hypothalamus. Histochemistry 93: 119–127, 1989 [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Tu H, Zheng H, Zhang L, Tran TP, Muelleman RL, Li YL. Alterations of calcium channels and cell excitability in intracardiac ganglion neurons from type 2 diabetic rats. Am J Physiol Cell Physiol 302: C1119–C1127, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masuo K, Rakugi H, Ogihara T, Esler MD, Lambert GW. Cardiovascular and renal complications of type 2 diabetes in obesity: role of sympathetic nerve activity and insulin resistance. Curr Diabetes Rev 6: 58–67, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Melis MR, Argiolas A, Gessa GL. Apomorphine-induced penile erection and yawning: site of action in brain. Brain Res 415: 98–104, 1987 [DOI] [PubMed] [Google Scholar]
- 25.Miller JA, Floras JS, Zinman B, Skorecki KL, Logan AG. Effect of hyperglycaemia on arterial pressure, plasma renin activity and renal function in early diabetes. Clin Sci 90: 189–195, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev 78: 189–225, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Morris JK, Bomhoff GL, Gorres BK, Davis VA, Kim J, Lee PP, Brooks WM, Gerhardt GA, Geiger PC, Stanford JA. Insulin resistance impairs nigrostriatal dopamine function. Exp Neurol 231: 171–180, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrison CD, Pistell PJ, Ingram DK, Johnson WD, Liu Y, Fernandez-Kim SO, White CL, Purpera MN, Uranga RM, Bruce-Keller AJ. High fat diet increases hippocampal oxidative stress and cognitive impairment in aged mice: implications for decreased Nrf2 signaling. J Neurochem 114: 1581–1589, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mu J, Woods J, Zhou YP, Roy RS, Li Z, Zycband E, Feng Y, Zhu L, Li C, Howard AD, Moller DE, Thornberry NA, Zhang BB. Chronic inhibition of dipeptidyl peptidase-4 with a sitagliptin analog preserves pancreatic beta-cell mass and function in a rodent model of type 2 diabetes. Diabetes 55: 1695–1704, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Murphy MB. Dopamine: a role in the pathogenesis and treatment of hypertension. J Hum Hypertens 14 Suppl: S47–S50, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Palkovits M, Brownstein M. Brain microdissection techniques. In: Brain Microdissection Techniques, edited by Cuello AE. Chichester, UK: John Wiley, 1983 [Google Scholar]
- 32.Parker M. The neurohormonal hypothesis: a theory to explain the mechanism of disease progression in heart failure. J Am Coll Cardiol 20: 248–254, 1992 [DOI] [PubMed] [Google Scholar]
- 33.Pascoe WS, Storlien LH. Inducement by fat feeding of basal hyperglycemia in rats with abnormal β-cell function. Model for study of etiology and pathogenesis of NIDDM. Diabetes 39: 226–233, 1990 [DOI] [PubMed] [Google Scholar]
- 34.Patel KP, Knuepfer M. Effect of afferent renal nerve stimulation on blood pressure and heart rate and noradrenergic activity in conscious rats. J Auton Nerv Syst 17: 121–130, 1986 [DOI] [PubMed] [Google Scholar]
- 35.Patel KP, Mayhan WG, Bidasee KR, Zheng H. Enhanced angiotensin II-mediated central sympathoexcitation in streptozotocin-induced diabetes: role of superoxide anion. Am J Physiol Regul Integr Comp Physiol 300: R311–R320, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel KP, Zhang PL. Baroreflex function in streptozotocin (STZ)-induced diabetic rats. Diabetes Res Clin Pract 27: 1–9, 1995 [DOI] [PubMed] [Google Scholar]
- 37.Pellegrino LJ, Cushman AJ. A Stereotaxic Atlas of the Rat Brain. New York: Appleton-Century-Crofts, 1967 [Google Scholar]
- 38.Pijl H. Reduced dopaminergic tone in hypothalamic neural circuits: expression of a “thrifty” genotype underlying the metabolic syndrome? Eur J Pharmacol 480: 125–131, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Prior LJ, Elkelis N, Armitage JA, Davern PJ, Burke SL, Montani JP, Barzel B, Head GA. Exposure to a high-fat diet alters leptin sensitivity and elevates renal sympathetic nerve activity and arterial pressure in rabbits. Hypertension 55: 862–868, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Pyner S. Neurochemistry of the paraventricular nucleus of the hypothalamus: Implications for cardiovascular regulation. J Chem Neuroanat 38: 197–208, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Rahmouni K, Haynes WG. Leptin and the central neural mechanisms of obesity hypertension. Drugs Today (Barc) 38: 807–817, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Rahmouni K, Sigmund CD, Haynes WG, Mark AL. Hypothalamic ERK mediates the anorectic and thermogenic sympathetic effects of leptin. Diabetes 58: 536–542, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schreihofer AM, Stornetta RL, Guyenet PG. Regulation of sympathetic tone and arterial pressure by rostral ventrolateral medulla after depletion of C1 cells in rat. J Physiol 529: 221–236, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med 341: 577–585, 1999 [DOI] [PubMed] [Google Scholar]
- 45.Srinivasan K, Patole PS, Kaul CL, Ramarao P. Reversal of glucose intolerance by pioglitazone in high fat diet-fed rats. Methods Find Exp Clin Pharmacol 26: 327–333, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Srinivasan K, Ramarao P. Animal models in type 2 diabetes research: an overview. Indian J Med Res 125: 451–472, 2007 [PubMed] [Google Scholar]
- 47.Storlien LH, James DE, Burleigh KM, Chisholm DJ, Kraegen EW. Fat feeding causes widespread in vivo insulin resistance, decreased energy expenditure, and obesity in rats. Am J Physiol Endocrinol Metab 251: E576–E583, 1986 [DOI] [PubMed] [Google Scholar]
- 48.Strack AM, Sawyer WB, Platt KB, Loewy AD. CNS cell groups regulating the sympathetic outflow to the adrenal gland as revealed by transneuronal cell body labeling with pseudorabies virus. Brain Res 491: 274–296, 1989 [DOI] [PubMed] [Google Scholar]
- 49.Swanson LW, Sawchenko PE, Berod A, Hartman BK, Helle KB, Vanorden DE. An immunohistochemical study of the organization of catecholaminergic cells and terminal fields in the paraventricular and supraoptic nuclei of the hypothalamus. J Comp Neurol 196: 271–285, 1981 [DOI] [PubMed] [Google Scholar]
- 50.Vahid-Ansari F, Leenen FHH. Pattern of neuronal activation in rats with CHF after myocardial infarction. Am J Physiol Heart Circ Physiol 275: H2140–H2146, 1998 [DOI] [PubMed] [Google Scholar]
- 51.Wamsley JK, Hunt ME, McQuade RD, Alburges ME. [3H]SCH39166, a D1 dopamine receptor antagonist: binding characteristics and localization. Exp Neurol 111: 145–151, 1991 [DOI] [PubMed] [Google Scholar]
- 52.Wang Y, Patel KP, Cornish KG, Channon KM, Zucker IH. nNOS gene transfer to RVLM improves baroreflex function in rats with chronic heart failure. Am J Physiol Heart Circ Physiol 285: H1660–H1667, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Ward KR, Bardgett JF, Wolfgang L, Stocker SD. Sympathetic response to insulin is mediated by melanocortin 3/4 receptors in the hypothalamic paraventricular nucleus. Hypertension 57: 435–441, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang Z, Wheatley M, Coote JH. Neuropeptides, amines and amino acids as mediators of the sympathetic effects of paraventricular nucleus activation in the rat. Exp Physiol 87: 663–674, 2002 [DOI] [PubMed] [Google Scholar]
- 55.Zhang PL, Patel KP. Blunted diuretic and natriuretic responses to central administration of clonidine in streptozocin-induced diabetic rats. Diabetes 40: 338–343, 1991 [DOI] [PubMed] [Google Scholar]
- 56.Zheng H, Mayhan WG, Bidasee KR, Patel KP. Blunted nitric oxide-mediated inhibition of sympathetic nerve activity within the paraventricular nucleus in diabetic rats. Am J Physiol Regul Integr Comp Physiol 290: R992–R1002, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Zheng H, Sharma NM, Liu X, Patel KP. Exercise training normalizes enhanced sympathetic activation from the paraventricular nucleus in chronic heart failure: role of angiotensin II. Am J Physiol Regul Integr Comp Physiol 303: R387–R394, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]