Abstract
First evidence of cases of haemophilia dates from ancient Egypt, but it was when Queen Victoria from England in the 19th century transmitted this illness to her descendants, when it became known as the “royal disease”. Last decades of the 20th century account for major discoveries that improved the life expectancy and quality of life of these patients. The history and evolution of haemophilia healthcare counts ups and downs. The introduction of prophylactic schemes during the 1970s have proved to be more effective that the classic on-demand replacement of clotting factors, nevertheless many patients managed with frequent plasma transfusions or derived products became infected with the Human Immunodeficiency Virus (HIV) and Hepatitis C virus during the 1980s and 1990s. Recombinant factor VIII inception has decreased the risk of blood borne infections and restored back longer life expectancies. Main concerns for haemophilia healthcare are shifting from the pure clinical aspects to the economic considerations of long-term replacement therapy. Nowadays researchers’ attention has been placed on the future costs and cost-effectiveness of costly long-term treatment. Equity considerations are relevant as well, and alternative options for less affluent countries are under the scope of further research. The aim of this review was to assess the evidence of different treatment options for haemophilia type A over the past four decades, focusing on the most important technological advances that have influenced the natural course of this “royal disease”.
Electronic supplementary material
The online version of this article (doi:10.1007/s12288-012-0209-0) contains supplementary material, which is available to authorized users.
Keywords: Hemophilia A, Coagulation disorder, Clotting factor disorder
Introduction
Haemophilia accounts for a long historic pathway; some authors may argue the first case dates from ancient Egypt [1], others state the first registered reference comes from Hebrew texts from the II century A.D, these writings explicitly banned circumcision for those children with a previous family history of at least two deceased brothers due to haemorrhage after this procedure. In the 19th Century Haemophilia became popular when Queen Victoria from England “transmitted” the haemophilia A genetic inheritance to several royal houses in Europe, including her latest son Leopold who died at the age of 30, after a bleeding episode due to a mild knee trauma; by that time haemophilia became known as the “royal disease” [2].
It is estimated that one per 5000–10,000 male births is going to suffer haemophilia A [3, 4]. Debuting age, location and severity of bleeding depend on the activity level of clotting factor VIII (FVIII). Clinical categories of haemophilia are: patients with mild deficiency (5–40 % activity of FVIII), usually tend to bleed only after major surgical procedures, patients with moderate deficiency (1–5 % activity of FVIII) and severe (<1 % activity) usually become symptomatic after minor surgical procedures or spontaneously. Around 70–80 % of bleeding episodes affect the joints leading to haemarthrosis and progressive haemophilic arthropathy as the most important long time complication [3, 4].
The clinical spectrum of severe haemophilia has evolved throughout history from being a catastrophic and highly fatal condition in the early 20th century to a chronic and “manageable” disorder in recent decades. In 1940 the first successful medical treatment for haemophilia was published in the Lancet, an 11-year-old boy that experienced a major bleeding episode after a squint surgery was experimentally treated with a whole-blood transfusion and survived [2]. Further advances in transfusion technology achieved during World War II, eased access of haemophiliac patients to blood or plasma transfusions, as a result life expectancy reached in average 39.7 years [5].
Judith G. Pool in 1964 discovered that the cryoprecipitate fraction of plasma contained proportionally greater quantities of FVIII [4]. The new product could be transported and administered by the patient himself reducing barriers to prompt therapy. Mortality rates, scholar and work abstention significantly dropped and the haemophilic patients reached an average life expectancy of 60 years [1]. By 1970s medium purity concentrates were authorized for commercialization. At the same time Nilsson and Ahlberg in Sweden pioneered the regular administration of FVIII in a prophylactic scheme, these new circumstances raised life expectancy again, this time to up to 68 years of age [1, 4]. Enhanced by the availability of new therapeutic options, the easier methods of administration and the raising life expectancy, plasma demand steadily grew throughout the following years. Solely in the U.S. there was a pool of approximately 20,000 donors, most of them poor people getting paid for this activity. Even though there was a boom in the rates of blood donations, the screening process was far from systematic and some donors were considered to be at greater risk of transmissible diseases than the general population. Infusion and transfusion of plasma derivatives were not safe and complications started to appear soon after [5].
In 1982 the first US haemophilic patient was reported as being infected with the Human Immunodeficiency Virus (HIV) [6]; this new discovery raised concern and led to further report of additional cases; HIV reached incidence rates of 60 cases per million in 1990 in the US and one case in every seven people in the UK among haemophilic population [6, 7]. HIV accounted for a quarter of all causes of death in this population during the 1990s in Netherlands [8, 9]. It has been estimated that 80 % of all deaths from the Acquired Immunodeficiency Syndrome (AIDS) occurred before 1995. It was only after the mid 1990s with the introduction of antiretroviral therapy (ARVT) that HIV related mortality in the haemophilic patients dropped [10], a Canadian study that monitored all causes of death in haemophilic patients between 1982 and 2003 (n = 2427) showed a reduction in mortality rates due to HIV from 74.6 % during 1982–1997 to 42.9 % after ARVT introduction [6, 11]. By 1992 an estimated of 60 % of the US haemophilic patients, and 80 % of all patients ever treated with clotting factor concentrates were already infected with hepatitis C [12, 13].
During the mid-1980s the genetic sequence of FVIII gene was achieved to produce recombinant factor VIII (rFVIII)[14]. The new rFVIII did not require any type of plasma for its production, first patient treated was reported in 1987. Since 1985 there have been no reports of viral transmission linked to the use of rFVIII in the developed world; virally safe products for hepatitis C are also available from 1992 onwards [8, 13]. With the development of this new replacement alternative the prognosis of haemophilia has dramatically changed; for instance in UK life expectancy for a mild to moderate haemophiliac rounds 70 years of age, very close to that for general local population, whereas for severe cases of haemophilia it is estimated to be at least 15 years less, similar findings have been reported for the Dutch haemophilic population [15].
From the 1990s onwards risk of blood borne infections has been controlled with the extended use of recombinant replacement therapy, as well as with the introduction of more sensitive immunoassays for the serological markers associated with transfusion-transmitted viruses (TTVs). Main concerns in the haemophiliac community have changed, the development of inhibitors (a neutralizing immunoglobulin directly acting against FVIII) is a frequent and serious complication that has captured researchers’ attention; hence there is academic interest on comparing different treatment options and their association with the emergence of these antibodies. From 2000 onwards the main concerns of researchers have been placed around the future costs and cost-effectiveness of long-term treatment. Equity considerations are relevant as well, and alternative options for less affluent countries are under the scope of further research.
Most of the scientific evidence on haemophilia A treatment comes from High Income Countries (HIC) [16], and although all the promising findings from new technological developments, it is not yet clear if risks or complications will raise in the near future for rFVIII users, or if the incremental costs derived from higher survival rates and costly treatment options will lead to unsustainable health systems. The question if there are any other therapeutic alternatives for less affluent countries becomes relevant.
This systematic review was undertaken to assess the evidence of different treatment options for haemophilia A, with special interest on how the major improvements over the last four decades have influenced the natural course of disease. Special attention was placed on the type and quality of published data, and results are presented decade by decade throughout these 40 years.
Methods
Publications considering the clinical effectiveness of different treatment options for haemophilia A (including at least one of these dimensions: bleeding episodes, frequency and importance of adverse effects, potential complications, quality of life, cost-effectiveness, and cost-utility and the development of inhibitors) were sought. The following search terms were used throughout the search: [hemophilia A], [coagulation disorder], [clotting factor disorder], [clotting factor deficiency], [clotting factor disease], [treat*], [therap*], [manage*], [current], [updat*], [novel], [classic*], [traditional], [conventional], [health outcome], [Impact], [effect*], [quality of life] taking into account headings and sub-headings.
Possible studies of interest were sought from Medline, Embase, health economics and health technology assessment database, Ovid, ACP Journal Club, Cochrane Controlled Trials Register, The Cochrane Database of Systematic Reviews and Econlit. The Medline strategy is listed in Appendix 1 in Supplementary Material. Three independent researchers ran the search, a fourth researcher acted as peer reviewer and provided additional sources of data from relevant published and grey literature sources.
Inclusion criteria were: Systematic reviews of literature and meta-analysis, randomized controlled trials, cohort studies, case and control studies, case studies, economic evaluations and review articles. Complete articles published in English from 1970 onwards were sought. 1970 was set as relevant date since it was the time of starting use of plasma-derived clotting factors; studies of patients at all ages were included, only those publications assessing treatment for haemophilia A (regardless of severity, complications, type of treatment, age of diagnosis or treatment initiation) were considered. Publications that described and/or compared the classic/conventional versus current/updated therapeutic strategies for haemophilia A were included.
Exclusion criteria were: publications considering patients with coagulation disorders different than haemophilia A, publications addressing patients with acquired haemophilia A (due to the clinical differences of presentation, natural course of disease, co-morbidities, and therapeutic response to usual treatment).
Quality control and assessment of data included, extraction and synthesis by three different reviewers with the aim to reduce potential bias, the PRISMA workflow was used to systematically assess papers retrieved and to control for duplication and eligibility criteria. Given the heterogeneity of studies and evidence, a detailed qualitative quality assessment matrix was constructed, considering date of publication, period of analysis, type of study. Studies were ranked based on quality. RCTs, Meta-analyses, Systematic reviews, Cohort studies fulfilling all criteria for internal validity according to the type of study were scored as ++; when findings came from case and control studies, case studies, observational studies fulfilling all criteria for internal validity according to the type of study, or from RCTs, Meta-analyses, Systematic reviews, Cohort studies partially fulfilling criteria for internal validity, they were ranked as +; evidence from the grey literature or case–control studies, case studies, observational studies that did not fulfil any criterion for internal validity were scored 0. The table of evidence and the grading criteria is depicted in Appendix 2 in Supplementary Material.
Results
Stock of Available Evidence
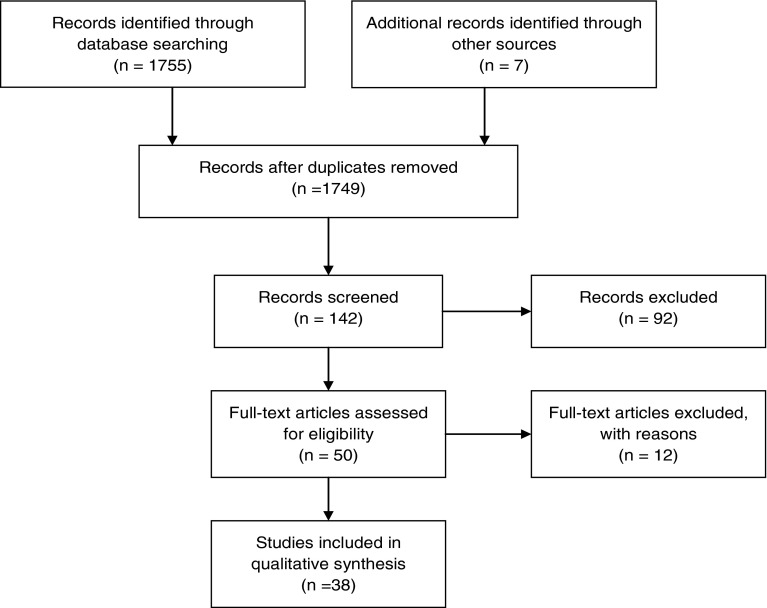
A total of 1,755 articles were retrieved from the search as potentially relevant references; seven records were suggested by an expert in haemophilia to be added to the total number of possible articles to be analysed; 1,749 publications were recognized after controlling for duplicates; after abstract scanning a total of 1,607 articles were excluded because they did not specifically addressed the main topic of research (only subjects with haemophilia A); after scoping for references that described and/or compared different treatment options for haemophilia A, 142 relevant references we obtained; 50 full text articles were screened for eligibility criteria and 12 full text were excluded with reasons, a total of 38 publications were finally included in this qualitative analysis (See Fig. 1). Available evidence by decade, by treatment focus, and by type and quality of publication are presented below. A summary of evidence is provided at the last part of the results section.
Fig. 1.
Prisma flow diagram systematic review on hemophilia A
Major Findings by Decade
The 1970’s
No reference published during the period between 1970 and 1979 was retrieved from our search, notwithstanding we found five publications that included in their analyses the changes, improvements and concerns related with the different options of treatment for haemophilia A during this period of time. Four narrative reviews and one cohort study referred to this decade, the main subjects of these articles included: the evolution of treatment of haemophilia throughout history, discussion of therapeutic options at that time, the perceived risks associated with the use of blood products during the 1970s, the availability and possible benefits of home-based blood transfusions, and the improvement of quality of life derived from treatment. The cohort study reported the risk of inhibitors development in a population of UK patients followed between 1977 and 1999, suggesting an inverse association between early exposure to exogenous clotting factor and the appearance of inhibitors in these patients during the 1970s.
The 1980’s
Despite no articles were found as published during this; four references account for data from this period of time, they are all narrative reviews of the treatment options for haemophilia during this decade. The major emerging concern was the onset of communicable diseases transmitted via transfusion products used for the treatment of haemophilia A, all the publications depicted the association between standard treatment (cryoprecipitate) and the development of contagious diseases like AIDS, Hepatitis C and Hepatitis non A non C, claimed for the need of safer screening in the manufacturing process of products for haemophilia care, and urged for research and development for a new and safer blood-derived products. The first case report of the clinical efficacy (case report with two patients) of rFVIII, fostered the industrial production of rFVIII becoming publicly available in 1989.
The 1990’s
We found three publications from this decade. One cohort study described the relationship between FVIII replacement characteristics and inhibitor development in previously untreated patients between 1990 and 2000; this study concluded that regular prophylaxis was associated with a 60 % lower risk than on-demand treatment (RR, 0.4; CI, 0.2–0.8) for developing inhibitors, and that inhibitor occurrence appeared to be associated with the age of first exposure to treatment, decreasing from 41 % for those treated within the first month of age to 18 % in those treated after 18 months of age; one narrative review described two different clinical approaches on minimizing or delaying inhibitor development, and a report from the grey literature that presented rFVIII, as new therapeutic alternative for children with severe haemophilia A. The development of recombinant products and the appearance of inhibitors became major topics of research that led to academic publications comparing the different available options.
The 2000’s
The stock of knowledge about haemophilia exponentially increased during this decade, 32 publications were found in our search (80 % of all references retrieved), some of these articles accounted in their period of analysis for previous decades. We sought two systematic reviews from this period of time about the cost-effectiveness of treatment options in patient with inhibitors, and on the efficacy of the immune tolerance treatment to control for it.
Three cohort studies were also found comparing different treatment options, the type of factor VIII used (FVIII vs. rFVIII), and the timeframe exposure and the development of inhibitors; additionally twelve review articles, two randomized clinical trials (RCTs), one case and control study, one case study and one economic evaluation were retrieved and appraised from this period, the dominant research topic was the identification of risk factors for inhibitors development, and also alternative comparison of different treatment options in patients with inhibitors, the advantages of prophylaxis (primary and secondary) over on-demand therapy, the comparison between alternative primary prophylaxis regimes in terms of haemophilic arthritis prevention, the association between the number of bleeding episodes and quality of life, the cost-effectiveness and the availability of alternative regimes in developed and developing countries.
2010 Onwards
Five review articles and one systematic review have been published since 2010 up to date (23rd September 2011), this publications discussed the role of prophylaxis in the prevention of haemarthrosis in children with haemophilia, the sensed need to establish a gold standard for primary prophylaxis, the alternative current options to treat acute bleeding events in patients with inhibitors, and the available options to treat mild haemophilia A.
The Report of Evolving Treatment Strategies
None of the publications retrieved through the search was dedicated to on-demand therapy entirely, nevertheless three articles (narrative reviews), described the use of this approach during the 1970s and early 1980s, right before the inception of recombinant technology, and before prophylaxis became current practice, access barriers to prompt treatment in hospital settings are mentioned as main disadvantages of this therapeutic scheme, delays between the initiation treatment and the subsequent repercussions on the quality of life of patients are discussed in a few of these articles; one case and control study unveiled the differences in the quality of life between patients treated with on-demand versus those treated with primary prophylaxis in Europe favouring prophylaxis in terms of physical functioning, less role limitations, bodily pain, general health, vitality, social functioning, emotional role limitations, and overall mental health.
Three quarter of our reviewed articles focused on primary prophylaxis (a total of 13 articles were retrieved, seven narrative reviews, one cohort study, one case and control study, one case study, one economic evaluation and two reports from international conferences from the World Federation of Haemophilia (WFH) classified as grey literature). The main topics discussed by these papers were, the need to decide on an international standard for primary and secondary prophylaxis, the optimal age for initiation and duration of prophylactic treatment, the advantages of prophylaxis in terms of prevention of haemarthrosis and disability in the long-term, and subsequently the positive impact of this approach on the overall quality of life when compared with on-demand therapy; the determinants and barriers to comply with treatment as well as the comparative cost-effectiveness of prophylaxis and the possible financial limitations for its instauration in developing countries.
Just a limited number of articles were related to secondary prophylaxis, we found two RCTs and one review article linked with this scheme of treatment. One RCT enrolled 38 male patients with a high baseline bleeding frequency (mean = four bleeds per month) in a pre-prophylaxis stage for a period of 3 months. Twenty-two patients were randomized to receive daily rFVIIa prophylaxis with either 90 or 270 IU/Kg during a period of 3 months, followed by a 3-month period post prophylaxis. Bleeding frequency was reduced by 45 % and 59 % during prophylaxis with 90 and 270 IU/Kg, respectively (P < 0.0001). Patients reported significantly fewer hospital admissions and days absent from work/school during prophylaxis compared to the pre-prophylaxis period. The second RCT examined the role of secondary prophylaxis with rFVII in quality of life improvement; rFVIIa prophylaxis significantly reduced bleeding frequency versus prior on-demand therapy (P < 0.0001). Hospital admissions (5.9 vs. 13.5 %; P = 0.0026) and school/work absenteeism (16.7 vs. 38.7 %; P = 0.0127) were reduced during prophylaxis, and tended to remain during post-prophylaxis. The review article referred to the benefits of secondary prophylaxis on joint damage prevention, functional capacity and quality of life, suggesting it to be considered as an alternative therapeutic option for patients that cannot or are not willing to receive primary prophylaxis.
Eight publications were focused on haemophilia type A with inhibitors treatment. Two were systematic reviews; five were reviews articles and one a case study. One of the systematic reviews and one review article summarised the best available evidence on the clinical effectiveness to treat acute bleeding events in haemophilia A patients with inhibitors, comparing high-doses of FVIII, Porcine FVIII and activated prothrombin complex concentrates (APCC) with the final outcome (control of spontaneous bleeding episodes and haemorrhages secondary to surgery); one systematic review and one narrative review discussed the efficacy, safety and effectiveness of the products currently available for immune tolerance induction; one review article described the efficacy and effectiveness of rFVII as a new option to treat patients with inhibitors in terms reducing inhibitor levels in the short and long-term; one review article compared the efficacy of different drugs emphasising on the haemostatic effect of rFVIII and FEIBA, and the use of prophylaxis with rFVIIa (surgical- and non-surgical settings) and the associated reduction in the number of bleeding events and the improved quality of life. Finally one case study described the use of Rituximab in a single dose for three patients with high and low titter inhibitors, showing clinical improvement in terms of bleeding frequency and inhibitor levels.
Type of Publications and Quality of Evidence Level
A total of 38 articles were qualified, three of them were classified as systematic reviews (one ranked as middle quality and two as high quality of evidence). Only two RCTs were retrieved in our search (one classified as high level of evidence and the second one combined with an economic study middle level). More than a half of the publications were review articles (total number of 21), and all of them were classified as middle quality of evidence. Five cohort studies were obtained (all of them were ranked as high quality). Two case studies (ranked as middle level of quality of evidence). Three economic evaluations retrieved (one was ranked as middle level and combined with a RCT, and the other two were ranked as high quality of evidence). Three reports were retrieved from the grey literature, all of them scored as low quality of evidence.
It was remarkable finding the lack of RCTs comparing different treatment options for severe haemophilia A, perhaps derived from the ethical limitations of randomization after prophylaxis proved to be clinically effective. During the 1970s and 1980s although very limited, narrative reviews and grey literature dominated the stock of knowledge with their subsequent prompt to bias. After the 1990s the number of publications for haemophilia A have exponentially increased via reports from cohort studies, systematic reviews and a few number of randomized controlled trials. From the 2000 onwards quality of evidence has improved as well as the number of cost-effectiveness and cost-utility analyses regarding this condition.
Summary of Findings on Treatment
Over the last four decades management of patients with haemophilia A has experienced dramatic improvements, notwithstanding its ups and downs throughout history. A wide range of therapeutic strategies have been developed since the 1970 decade. On the one hand the conventional on-demand treatment, consisting of missing factor supply after the onset of a bleeding episode, is an early approach that despite an incredible improvement in life expectancy, accounted for poor quality of life outcomes; access barriers to hospital provision of clotting factor, and the subsequent joint damage from lack of prevention were pitfalls faced by clinicians and patients [4].
In contrast, prophylaxis defined as a form of prevention has proven to be superior in preventing bleeding events, and their subsequent sequelae. Although there are several prophylactic schemes a consensus meeting of experts held in London in 2002, helped to define “primary prophylaxis” as a long-term continuous treatment (intent of treating 52 weeks/year up to adulthood receiving treatment at a minimum of 46 weeks/year), started before the age of 2 years and prior to any clinically evident joint bleeding or before the onset of joint damage irrespective of age (defined as having had no more than one joint bleed) [17]. This prophylactic replacement of clotting factor has been recommended as the gold standard of care by the WFH and the World Health Organization (WHO).
Several studies retrieved from our search have demonstrated superior effectiveness of primary prophylaxis in the reduction of bleeding frequency, hence on preventing and reverting of haemophilic arthropathy versus on-demand therapy. The US Joint Outcome Study (JOS), the first RCT that compared prophylaxis and on-demand therapy, included 65 young children (<than 30 months of age), who were randomized to receive prophylaxis versus on-demand treatment (infusions of 25 IU/Kg of FVIII every 2 days for prophylaxis versus on-demand treatment three or more infusions of FVIII, using at least 80 UI/Kg to treat articular bleeds), the annual mean incidence of bleeding episodes was much less in the prophylaxis group compared to the on-demand group (0.63 ± 1.35 vs. 4.89 ± 3.57 respectively P < 0.001), further findings showed that 93 % of patients allocated in the prophylaxis group had normal joint indexes assessed by Magnetic Resonance Imaging (MRI), in contrast to 55 % of patients treated on-demand (P = 0.006) [18]. These results were backed by a prospective 10 year Italian trial (ESPRIT), which enrolled 40 patients younger than 7 years of age with negative clinical and radiological scores for joint damage; patients were randomized to be treated with rFVIII 25 IU Kg three times a week or on-demand (25 IU Kg) until complete bleed control, results indicated that prophylaxis is associated with significantly fewer breakthrough bleeds than on-demand treatment (0.24 vs. 1.30 bleeds per month, respectively; P < 0.001) [18–20]. A retrospective cohort analysis involving 156 Norwegian and Swedish patients suggested that patients who received prophylaxis required fewer total invasive procedures than those who received on-demand treatment [21]. Less number of bleeding episodes, and life-threatening haemorrhages under prophylaxis, should be associated with a much better joint status and a better quality of life. A European study assessing health-related quality of life (HR-QoL) applied the short-form 36 (SF-36) which accounts for eight dimensions of HR-QoL to 1,033 haemophiliac patients of 12 years of age with moderate to severe haemophilia, five of these eight dimensions were significantly higher in HIV negative patients receiving prophylactic therapy when compared to on-demand, these dimensions included, physical functioning, bodily pain and mental health [19, 22].
According to the literature, besides the benefit of haemophilic arthropathy prevention from prophylaxis, a marked reduction of intracranial haemorrhages, lower muscular-skeletal pain, lower rates of inpatient admissions and average of stay, improved school and work attendance and improved academic achievement have been reported [17, 23]. A starting age of treatment between 1 and 2 years of age could be associated with no risk at all of developing haemarthrosis under sustained treatment [17], a Dutch cohort study that evaluated the optimal age to start prophylaxis demonstrated that an early start resulted in complete prevention of joint damage for 70 % of boys compared with 31 % for boys who started prophylaxis after three or more bleeds.
Approximately 10 % of severe haemophiliacs do not bleed as frequently as would be expected from their circulating factor levels [23]. The JOS study suggests that the occurrence of the first joint bleed rather than a specific age may represent a reasonable criterion for starting prophylaxis [20]. After two decades of follow-up, the radiological Pettersson joint score (a scoring system that increases based on radiological evidence of haemophilic joint damage) was 8 % higher for every year prophylaxis was postponed after the first joint bleed, this data suggests that primary prophylaxis should be started at an early age but can be individualized based on the bleeding pattern of each individual [24].
A consensus about the best prophylaxis protocol is still undetermined. Primary prophylaxis based on the Swedish protocol (also known as the high-dose Malmö protocol) involves the administration of 20–40 FVIII UI/Kg three times a week, and is currently considered the gold standard of care. This protocol is recommended by the WFH, WHO, the UK Haemophilia Centre Doctors Organization and the Medical and Scientific Advisory Council of the US National Haemophilia Foundation as the optimal treatment until a cure is available [21, 24]. Nevertheless there are several protocols available and still being used. The Dutch intermediate-dose prophylaxis protocol supplies 15–25 FVIII IU/Kg infused two or three times a week and the subsequent prophylactic dose is adjusted based on spontaneous breakthrough bleeding into joints and not according to the subject body weight or trough levels of FVIII [24].
The Canadian approach was a dose-escalation scheme of primary prophylaxis started in 1997. In this prospective study, boys at ages 1 to 2, 5 years with severe haemophilia A, started on a once-weekly infusion of FVIII (50 IU Kg). If clinically significant bleeding into muscles and/or joints occurred, the frequency of FVIII infusion was increased to twice weekly (dose 30 IU Kg); continuation of bleeding resulted in escalation of the prophylaxis regimen up to 25 IU Kg) on alternate days. Criteria for escalation included: ‡ three clinically determined bleeds into any one joint over a consecutive 3-month period; ‡ four significant soft tissue/joint bleeds over a consecutive 3-month period and ‡ five bleeds into any one joint while on the same dosage (step) of factor therapy over any period of time [24–26].
Nonetheless all the possible options, the Swedish high-dose prophylaxis regimen is associated with a significantly lower rate of joint bleeding in comparison with the Dutch intermediate-dose regime; FVIII consumption and costs were approximately twofold higher for the former scheme. After at least 20 years of follow-up, the extent of haemophilic arthropathy measured by a radiologic scale is similar for these two prophylaxis regimens. In the Canadian study one-third of patients appeared to be successfully maintained on a once per week prophylaxis regimen for a considerable period of time without the need for escalation. This suggests that rapid progression in treatment, as employed in the Swedish regimen may be unnecessary in a small proportion of patients. Yet in the Canadian study several patients developed target joints prior to the escalation of therapy. Furthermore, despite the absence of life-threatening bleeds seen in the Canadian study, there is a concern that with once-weekly prophylaxis these patients remain at risk of serious and even life-threatening bleeds for most of the time [27]. Nevertheless this approach might be less costly while infusing less factor concentrate than with a traditional prophylaxis regimen, and also may reduce the need for a central venous catheter (CVC), and hence its complications (infection or thrombosis). A problem with this approach is that there are currently no standard criteria for determining unacceptable bleeds. Moreover, the long-term joint outcome of this approach and its protective effect against other serious bleeds is not known, because of the infrequent once-weekly dosing in many patients [21].
There was no literature found in our search that supported the interruption of prophylaxis in the adulthood, institutions like the WFH, The US national foundation of haemophilia and the WHO recommend continuing prophylaxis in the adulthood, because adults remain at risk of developing joint or other kind of bleedings. A follow-up Dutch and Danish study showed that 28 out of 80 (35 %) severe haemophiliacs permanently discontinued prophylaxis in early adulthood. These patients experienced on average 3.2 joint bleeds annually over the 3 years after discontinuing prophylaxis. Patients who remained on prophylaxis experienced on average 1.8 joint bleeds annually during the same time period [18, 23]. Paradoxically, limited evidence suggests that those patients who permanently discontinued prophylaxis tended to have a milder bleeding pattern than those who continued prophylaxis [25]. According to one reference counselling for adolescents and young adults about the consequences of abandoning primary prophylaxis is essential to prevent complications [28].
Secondary prophylaxis is defined as a long-term continuous treatment not fulfilling the criteria for primary prophylaxis [4, 17], it has the primary aim to reduce and arrest joint bleeding and to halt the progression of joint destruction. It is intended to reduce the risk of other serious haemorrhage, such as intracranial bleeds [29]. In a study of 21 patients receiving secondary prophylaxis at three different ages (1–2, 3–6 and >6 years), Kreuz et al. found that although the number of joint bleeds decreased significantly during prophylaxis in the two older groups, radiologic and orthopaedic scores still deteriorated for those who reported more than five joint bleeds before the initiation of prophylaxis, suggesting that once joint damage had started, further joint deterioration could not be prevented by the initiation of prophylactic therapy [21], even though secondary prophylaxis cannot reverse the changes of chronic arthropathy, it may be beneficial by reducing frequency of bleeding, hospital admissions and lost days from school or work, and by decreasing damage progression. Patients treated with secondary prophylaxis had a decreased number of joint bleeding episodes at the expense of higher clotting factor concentrate consumption [4]. A growing consensus among haemophilia specialists is that an individualized protocol for each patient based on the bleeding pattern and manifestations of the disease should guide decisions regarding the prophylaxis regimen. Recent studies examining delayed initiation of secondary prophylaxis are encouraging because they demonstrated that even delayed prophylaxis can reduce the frequency of joint haemorrhages, lessen chronic joint pain, enhance quality of life, and when combined with aggressive physiotherapy, may improve physical function and the radiographic appearance of target joints [29].
There are several barriers for the use and the adherence to prophylaxis, a study of patients at the Louisiana Comprehensive Haemophilia Care Centre found lower rates of adherence among patients receiving high-intensity treatment regimens [30], of the 18 patients in the study’s high-intensity group, only three (17 %) had high adherence [19]. Some of the barriers that influence the adoption of and adherence to prophylaxis are the cost and availability of clotting factors (accounting for about 80–90 % of the total cost of treatment), prohibitive particularly for less affluent communities [18]. Six additional identified barriers to prophylaxis were (as indicated by 30 % of patients families in a study of 52 patients in the Mountain States Regional Hemophilia and Thrombosis Center, Aurora, CO, USA): the greater amount of venous access devices (required for long-term treatments—three folding on-demand regimens); the complications associated with those devices (including infections and thrombosis); the need for therapy as perceived by the patient; immediate social and family needs; parents inability to gain cooperation from their young children, and specially the time required for prophylactic infusions. These data suggest that products with higher dosages, longer half-lives or more convenient mechanisms of infusion could improve adherence in patients with haemophilia [19].
Notwithstanding the clear clinical effectiveness of prophylaxis, it also results more costly with respect to on-demand treatment, Miners et al. [31] calculated an Incremental Cost Effectiveness Ratio (ICER) in the UK well above the £30,000 per QALY gained threshold, when compared to on-demand therapy. A recent review of calculations (adjusting clotting factor price, and long-term effect of treatment) by the same author estimated it is getting closer to being considered cost-effective (according to NICE thresholds in the UK).
Despite the consistent evidence about the benefits of prophylactic therapy, a recent global survey of 147 haemophilia treatment centres (HTCs) throughout the world showed that about a half of all patients with severe haemophilia A (54 %) still receive on-demand treatment, and only 19 % are provided primary prophylaxis [19]. Even in HIC, prophylaxis coverage is not universal, in the US only 50 % of severe haemophiliacs type A are treated under the gold standard approach, this compared with 77 % of patients in Canada (Universal Data Collection (UDC)) [29]. The situation is much worse in emerging countries in which the development of effective healthcare programs for haemophiliacs is still limited, and where limited resources, the short availability of VIII factor concentrates means and an important barrier to provide prophylactic regimens.
After the improvement in safety of human derived blood products, the development of recombinant factor concentrates and the subsequent reduction in morbidity and mortality by blood-transmitted diseases, the development of inhibitors (neutralizing antibody direct against FVIII), became the major concern of in haemophilia care. In patients who develop inhibitors, the location and frequency of bleeding episodes is usually similar to those who did not develop alloantibodies, however, the prophylactic treatment is unfeasible and the treatment of acute episodes is complicated, since inhibitors increase the rate of FVIII neutralization, by partial or total reduction of its clinical activity [32, 33]. Most centres consider >0.6 Bethesda units (BU) as a positive result for having an inhibitor.
Several factors have been related to the development of inhibitors, the most strongly associated is a type of genetic mutation of FVIII, as well as the type of Human Leucocitary Antigen (HLA) and the polymorphisms in the genes codified for cytokines (African or Hispanic background) of each individual [34], as well as the type of replacement therapy used and the age of starting up. In a study by Santagostino and Mancuso [20], 25 out of 108 children with haemophilia received prophylaxis and had a lower inhibitor risk than those treated on-demand (adjusted OR 0.2; CI: 0.06–0.9), suggesting a protective effect of prophylaxis from inhibitor development. A second study (Concerted Action on Neutralizing Antibodies in severe haemophilia A—CANAL), 87 out of 386 (24 %) previously untreated patients receiving treatment for at least 50 consecutive days developed clinically relevant inhibitors. Regular prophylaxis was associated with a 60 % lower risk than on-demand treatment (RR 0.4; CI, 0.2–0.8). Factors associated with an increased risk of developing inhibitors included a high-intensity treatment at first exposure to FVIII and high cumulative dose of FVIII during five consecutive treatment days. The incidence of inhibitors appeared to be associated with age at first treatment, decreasing from 41 % for those treated within the first month of age to 18 % in those treated after 18 months [33, 35].
The CANAL study also reported the association of FVIII product type (i.e. plasma-derived versus recombinant) and switching between FVIII products with the risk of developing inhibitor and concluded that neither plasma-derived FVIII products were associated with a lower inhibitor risk than rFVIII products, nor the switching between FVIII product brands increased the inhibitor risk. In contrast a cohort that evaluated 62 patients treated with the same brand of high-purity plasma-derived FVIII (pFVIII) containing Von Willebrand factor (VWF) and 86 patients treated with full-length rFVIII concluded that the risk of inhibitor development was higher in patients treated with rFVIII than in patients treated with pFVIII, regardless of other risk factors (F8 genotype; non-white origin; history of inhibitors in patients with a family history of haemophilia; age at first FVIII infusion). The adjusted relative risk (RR) for inhibitor development with rFVIII versus pFVIII was 2.4 [36, 37].
In a retrospective cohort study by Chalmers et al. assessed the incidence of inhibitors in 348 children with severe haemophilia A. They found that 68 out of 348 (20 %) developed inhibitors (10 % corresponding to high titter inhibitors). The incidence with regards to the age of initial FVIII exposure was: 26 % in patients younger than 1 month, 25 % in patient between 1 and 6 months, 21 % between 6 and 12 months, 20 % in 12–18 months and 9 % in patients older than 18 months of age. A significant difference in inhibitor development and age at first exposure across all age groups was found (P = 0.018), but no significant difference was observed in children treated at different time points during the first year of life (P = 0.44). In this study, exposure to FVIII during the neonatal period was not associated with a higher incidence of inhibitors compared with those treated later during the first year of life. Mortality associated with the development of inhibitors has change through years, in severe haemophiliacs without HIV, inhibitor development doubled mortality during 1977–1992 in the UK, but during 1993–1999 mortality was identical with and without inhibitors. In severe haemophiliacs without HIV but with inhibitors, mortality from causes involving bleeding decreased during 1977–1999 (P = 0.001) as did mortality involving intracranial haemorrhage (P = 0.007), these results do not appear to be related with the type of treatment for haemophilia or the treatment for the inhibitors [38].
Currently there is an increasing range of options to treat haemophilic patients with inhibitors, these include: high-doses of human FVIII, high purity factor VIII (pFVIII): by passing agents: (Prothrombin Complex Concentrates—PCCs), Activated Prothrombin Complex concentrate (aPCCs)], FVIII bypassing agent—FEIBA, Recombinant Factor VIIa (rFVIIa), and Immune tolerance therapy, these are further described in Appendix 3 in Supplementary Material [40–45].
Conclusions
Notwithstanding the ups and downs in the haemophilia care history, a sustained success has emerged from the larger availability of safer plasma-derived and recombinant replacement products from the late 1980s onwards, especially in the developed world. Improvement in administration techniques and dosing regimens, the introduction of home treatment, a progressive shift from on-demand treatment to prophylaxis, the onset of antibodies inactivating the infused clotting factor (inhibitors), and the further development of options to treat and possibly eradicate them, account for this recent successful story.
The development of rFVIII potentially eliminates the risk of infectious disease transmission, nevertheless a theoretical risk of transmitting emerging non-viral pathogens such as the prion responsible for variant of the Creutzfeldt Jakob disease (vCJD) still remains. With each new challenge a new successful solution has emerged, all these developments have resulted in increased life expectancy and HR-QoL for haemophilic patients, henceforth an illness with a different spectrum has emerged.
Literature has largely demonstrated the superior clinical effectiveness of prophylaxis when compared with on-demand therapy. Data from the WFH and WHO proved that prophylaxis is still distant to become universal, and for those countries with the lowest per capita gross national product (GNP) haemophilia healthcare is either inadequate, or there is no care at all. The impact of these deficiencies in haemophilic patients’ life expectancy and quality of life are expected to be substantial [39]. Currently, in emerging countries with lower incomes, the implementation of prophylactic treatment programs seems unachievable, especially with rFVIII. In this order of ideas, it remains as an important challenge to improve access to prophylaxis in emerging communities.
Further data and long-term studies are needed to determine whether a group of patients who can safely discontinue prophylaxis can be identified, hence procuring long-term financial sustainability of health systems; additional robust cost-effectiveness studies comparing the current on-demand practice in developing countries with alternative prophylaxis regimes are also needed. Finally studies comparing different immune tolerance protocols will serve to determinate the best options in terms of efficacy, safety and cost-effectiveness for those societies able to afford it.
Electronic supplementary material
Acknowledgments
The authors want to acknowledge the logistic support of the school of medicine at Pontificia Universidad Javeriana, as well as, Hospital San Ignacio and the Department of Clinical Epidemiology and Biostatistics at Pontificia Universidad Javeriana, as well as.
Conflict of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest with the subject matter.
References
- 1.Aledort LM. History of haemophilia. Haemophilia. 2007;13(Suppl 5):1–2. doi: 10.1111/j.1365-2516.2007.01576.x. [DOI] [PubMed] [Google Scholar]
- 2.Oldenburg J, Dolan G, Lemm G. Haemophilia care then, now and in the future. Haemophilia. 2009;15(Suppl 1):2–7. doi: 10.1111/j.1365-2516.2008.01946.x. [DOI] [PubMed] [Google Scholar]
- 3.Stachnik J. Hemophilia: etiology, complications, and current options in management. Formul J. 2010;45:218–227. [Google Scholar]
- 4.Wong T, Recht M. Current options and new developments in the treatment of haemophilia. Drugs. 2011;71(3):305–320. doi: 10.2165/11585340-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Lee CA. The best of times, the worst of times: a story of haemophilia. Clin Med. 2009;9(5):453–458. doi: 10.7861/clinmedicine.9-5-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evatt BL. The tragic history of AIDS in the hemophilia population, 1982–1984. J Thromb Haemost. 2006;4(11):2295–2301. doi: 10.1111/j.1538-7836.2006.02213.x. [DOI] [PubMed] [Google Scholar]
- 7.Mannucci PM. AIDS, hepatitis and hemophilia in the 1980s: memoirs from an insider. J Thromb Haemost. 2003;1(10):2065–2069. doi: 10.1046/j.1538-7836.2003.00483.x. [DOI] [PubMed] [Google Scholar]
- 8.Chorba TL, Holman RC, Strine TW, Clarke MJ, Evatt BL. Changes in longevity and causes of death among persons with hemophilia A. Am J Hematol. 1994;45(2):112–121. doi: 10.1002/ajh.2830450204. [DOI] [PubMed] [Google Scholar]
- 9.Ragni MV, Tegtmeier GE, Levy JA, Kaminsky LS, Lewis JH, Spero JA, et al. AIDS retrovirus antibodies in hemophiliacs treated with factor VIII or factor IX concentrates, cryoprecipitate, or fresh frozen plasma: prevalence, seroconversion rate, and clinical correlations. Blood. 1986;67(3):592–595. [PubMed] [Google Scholar]
- 10.Mocroft A, Ledergerber B, Katlama C, Kirk O, Reiss P, d’Arminio MA, et al. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet. 2003;362(9377):22–29. doi: 10.1016/S0140-6736(03)13802-0. [DOI] [PubMed] [Google Scholar]
- 11.Porter K, Babiker A, Bhaskaran K, Darbyshire J, Pezzotti P, Porter K, et al. Determinants of survival following HIV-1 seroconversion after the introduction of HAART. Lancet. 2003;362(9392):1267–1274. doi: 10.1016/S0140-6736(03)14570-9. [DOI] [PubMed] [Google Scholar]
- 12.Makris M, Garson JA, Ring CJ, Tuke PW, Tedder RS, Preston FE. Hepatitis C viral RNA in clotting factor concentrates and the development of hepatitis in recipients. Blood. 1993;81(7):1898–1902. [PubMed] [Google Scholar]
- 13.Van der Poel CL, Reesink HW, Mauser-Bunschoten EP, Kaufmann RH, Leentvaar-Kuypers A, Chamuleau RA, et al. Prevalence of anti-HCV antibodies confirmed by recombinant immunoblot in different population subsets in The Netherlands. Vox Sang. 1991;61(1):30–36. doi: 10.1111/j.1423-0410.1991.tb00923.x. [DOI] [PubMed] [Google Scholar]
- 14.Mannucci PM, Tuddenham EG. The hemophilias–from royal genes to gene therapy. N Engl J Med. 2001;344(23):1773–1779. doi: 10.1056/NEJM200106073442307. [DOI] [PubMed] [Google Scholar]
- 15.Larsson SA. Life expectancy of Swedish haemophiliacs, 1831–1980. Br J Haematol. 1985;59(4):593–602. doi: 10.1111/j.1365-2141.1985.tb07353.x. [DOI] [PubMed] [Google Scholar]
- 16.Prabhu R. Novel strategies to improve recombinant factor VIII production and its in vivo recovery. Indian J Hematol Blood Transfus. 2010;26(3):124–125. doi: 10.1007/s12288-010-0036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berntorp E, Astermark J, Bjormarkman VS, Blanchette K, Fisher K. Consensus perspectives on prophylactic therapy for haemophilia: summary statement. Haemophilia. 2003;9(Suppl 1):1–4. doi: 10.1046/j.1365-2516.9.s1.17.x. [DOI] [PubMed] [Google Scholar]
- 18.Franchini M, Coppola A, Molinari AC, Santoro S, Schinco V, Speciale V, Tagliaferri A. Forum on: the role of recombinant factor VIII in children with severe haemophilia A. Haemophilia. 2009;15(2):578–586. doi: 10.1111/j.1365-2516.2008.01975.x. [DOI] [PubMed] [Google Scholar]
- 19.Berntorp E. Joint outcomes in patients with haemophilia: the importance of adherence to preventive regimens. Haemophilia. 2009;15(6):1219–1227. doi: 10.1111/j.1365-2516.2009.02077.x. [DOI] [PubMed] [Google Scholar]
- 20.Santagostino E, Mancuso ME. Prevention of arthropathy in haemophilia: prophylaxis. Haemophilia. 2008;14(Suppl 6):16–19. doi: 10.1111/j.1365-2516.2008.01884.x. [DOI] [PubMed] [Google Scholar]
- 21.Petrini P. Identifying and overcoming barriers to prophylaxis in the management of haemophilia. Haemophilia. 2007;13(Suppl 2):16–22. doi: 10.1111/j.1365-2516.2007.01501.x. [DOI] [PubMed] [Google Scholar]
- 22.Royal S, Schramm W, Bertntorp E, Giagrande A, Gringeri A, Ludlam C, Kronker B, Szucs T. Quality-of-life differences between prophylactic and on-demand factor replacement therapy in European haemophilia patients. Haemophilia. 2002;8(1):44–50. doi: 10.1046/j.1365-2516.2002.00581.x. [DOI] [PubMed] [Google Scholar]
- 23.Carcao MD, Aledort LM. The round table group “Prophylaxis in the haemophilia population-optimizing therapy”. Haemophilia. 2007;13(3):227–232. doi: 10.1111/j.1365-2516.2007.01457.x. [DOI] [PubMed] [Google Scholar]
- 24.Blanchette VS. Prophylaxis in the haemophilia population. Haemophilia. 2010;16(Suppl 5):181–188. doi: 10.1111/j.1365-2516.2010.02318.x. [DOI] [PubMed] [Google Scholar]
- 25.Dunn AL. Management and prevention of recurrent hemarthrosis in patients with hemophilia. Curr Opin Hematol. 2005;12(5):390–394. doi: 10.1097/01.moh.0000169285.66841.c8. [DOI] [PubMed] [Google Scholar]
- 26.Feldman BM. Tailored prophylaxis in severe hemophilia A: interim results from the first 5 years of the Canadian Hemophilia Primary Prophylaxis Study. J Thromb Haemost. 2006;4(6):1228–1236. doi: 10.1111/j.1538-7836.2006.01953.x. [DOI] [PubMed] [Google Scholar]
- 27.Carcao M, Chambost H, Ljung R. Devising a best practice approach to prophylaxis in boys with severe haemophilia: evaluation of current treatment strategies. Haemophilia. 2010;16(Suppl 2):4–9. doi: 10.1111/j.1365-2516.2009.02196.x. [DOI] [PubMed] [Google Scholar]
- 28.Valentino L. Controversies regarding the prophylactic management of adults with severe haemophilia A. Haemophilia. 2009;15(Suppl 2):5–22. doi: 10.1111/j.1365-2516.2009.02159.x. [DOI] [PubMed] [Google Scholar]
- 29.Valentino L. Secondary prophylaxis therapy: what are the benefits, limitations and unknowns? Haemophilia. 2004;10(2):147–157. doi: 10.1111/j.1365-2516.2003.00870.x. [DOI] [PubMed] [Google Scholar]
- 30.Dimichele D, Rivard G, Hay A, Antunes S. Inhibitors in haemophilia: clinical aspects. Haemophilia. 2004;10(Suppl 4):140–145. doi: 10.1111/j.1365-2516.2004.00993.x. [DOI] [PubMed] [Google Scholar]
- 31.Miners A. Revisiting the cost-effectiveness of primary prophylaxis with clotting factor for the treatment of severe haemophilia A. Haemophilia. 2009;15(4):881–887. doi: 10.1111/j.1365-2516.2009.02019.x. [DOI] [PubMed] [Google Scholar]
- 32.Carcao M, Lambert T. Prophylaxis in haemophilia with inhibitors: update from international experience. Haemophilia. 2010;16(Suppl 2):16–23. doi: 10.1111/j.1365-2516.2009.02198.x. [DOI] [PubMed] [Google Scholar]
- 33.Lloyd M, Wight JS, Paisley S, Night C. Control of bleeding in patients with haemophilia A with inhibitors: a systematic review. Haemophilia. 2003;9(4):464–520. doi: 10.1046/j.1365-2516.2003.00782.x. [DOI] [PubMed] [Google Scholar]
- 34.Dimichele D. Immune tolerance therapy for factor VIII inhibitors: moving from empiricism to an evidence-based approach. J Thromb Haemost. 2007;5(Suppl 1):143–150. doi: 10.1111/j.1538-7836.2007.02474.x. [DOI] [PubMed] [Google Scholar]
- 35.Gouw SC, van der Bom JG, Marijke van den Berg H. Treatment-related risk factors of inhibitor development in previously untreated patients with hemophilia A: the CANAL cohort study. Blood. 2007;109(11):4648–4654. doi: 10.1182/blood-2006-11-056291. [DOI] [PubMed] [Google Scholar]
- 36.Farrugia A. Evolving perspectives in product safety for haemophilia. Haemophilia. 2002;8(3):236–243. doi: 10.1046/j.1365-2516.2002.00596.x. [DOI] [PubMed] [Google Scholar]
- 37.Goudemand J, Rothschild C, Demiguel V, Vinciguerrat C, Lambert T, Chambost H, Borel-Derlon A, Claeyssens S, Laurian T, Calvez T. Influence of the type of factor VIII concentrate on the incidence of factor VIII inhibitors in previously untreated patients with severe hemophilia A. Blood. 2006;107(1):46–51. doi: 10.1182/blood-2005-04-1371. [DOI] [PubMed] [Google Scholar]
- 38.Darby SC, Keeling DM, Spooner RJ, Wan KS, Giangrande PL, Collins PW, Hill FG, Hay CR. The incidence of factor VIII and factor IX inhibitors in the hemophilia population of the UK and their effect on subsequent mortality, 1977–1999. J Thromb Haemost. 2004;2(7):1047–1054. doi: 10.1046/j.1538-7836.2004.00710.x. [DOI] [PubMed] [Google Scholar]
- 39.Tusell J, Perez-Bianco R. Prophylaxis in developed and in emerging countries. Haemophilia. 2002;8(3):183–188. doi: 10.1046/j.1365-2516.2002.00619.x. [DOI] [PubMed] [Google Scholar]
- 40.Von Depka M. Managing acute bleeds in the patient with haemophilia and inhibitors: options, efficacy and safety. Haemophilia. 2005;9(Suppl 1):18–23. doi: 10.1111/j.1365-2516.2005.01157.x. [DOI] [PubMed] [Google Scholar]
- 41.Astermark J, Santagostino E, Wk Hoots. Clinical issues in inhibitors. Haemophilia. 2010;16(Suppl 5):54–60. doi: 10.1111/j.1365-2516.2010.02294.x. [DOI] [PubMed] [Google Scholar]
- 42.Konkle BA, Ebbesen LS, Erhardtsen E, Bianco RP, Lssitchkov T, Rusen L, Serban Randomized, prospective clinical trial of recombinant factor VIIa for secondary prophylaxis in hemophilia patients with inhibitors. J Thromb Haemost. 2007;5(9):1904–1913. doi: 10.1111/j.1538-7836.2007.02663.x. [DOI] [PubMed] [Google Scholar]
- 43.Hoots WK, Ebbesen LS, Konkle BA, Auerswald GK, Roberts HR, Weatherall J, et al. Secondary prophylaxis with recombinant activated factor VII improves health-related quality of life of haemophilia patients with inhibitors. Haemophilia. 2008;14(3):466–475. doi: 10.1111/j.1365-2516.2008.01654.x. [DOI] [PubMed] [Google Scholar]
- 44.Wight J, Paisley S, Knight C. Immune tolerance induction in patients with haemophilia A with inhibitors: a systematic review. Haemophilia. 2003;9(4):436–463. doi: 10.1046/j.1365-2516.2003.00781.x. [DOI] [PubMed] [Google Scholar]
- 45.Aleem A, Saidu A, Abdulkarim H, Al-Diab AR, Al-Sagheer A, Qayum A, et al. Rituximab as a single agent in the management of adult patients with haemophilia A and inhibitors: marked reduction in inhibitor level and clinical improvement in bleeding but failure to eradicate the inhibitor. Haemophilia. 2009;15(1):210–216. doi: 10.1111/j.1365-2516.2008.01865.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.