Abstract
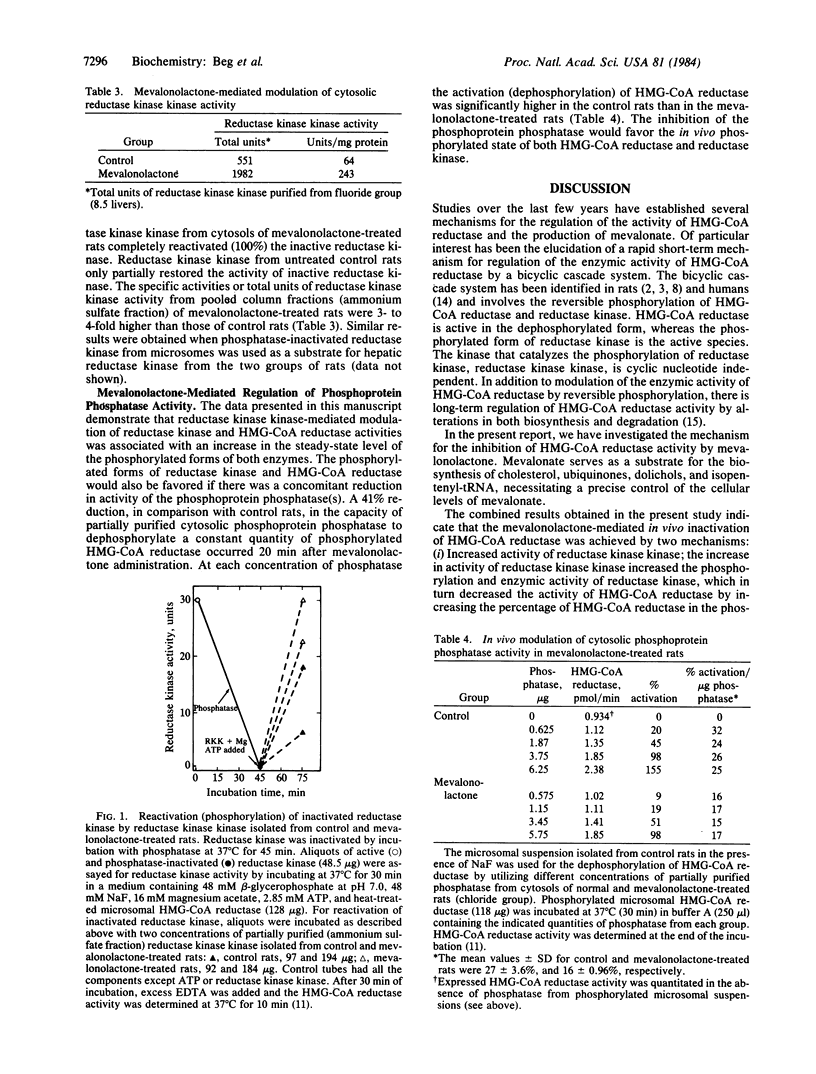
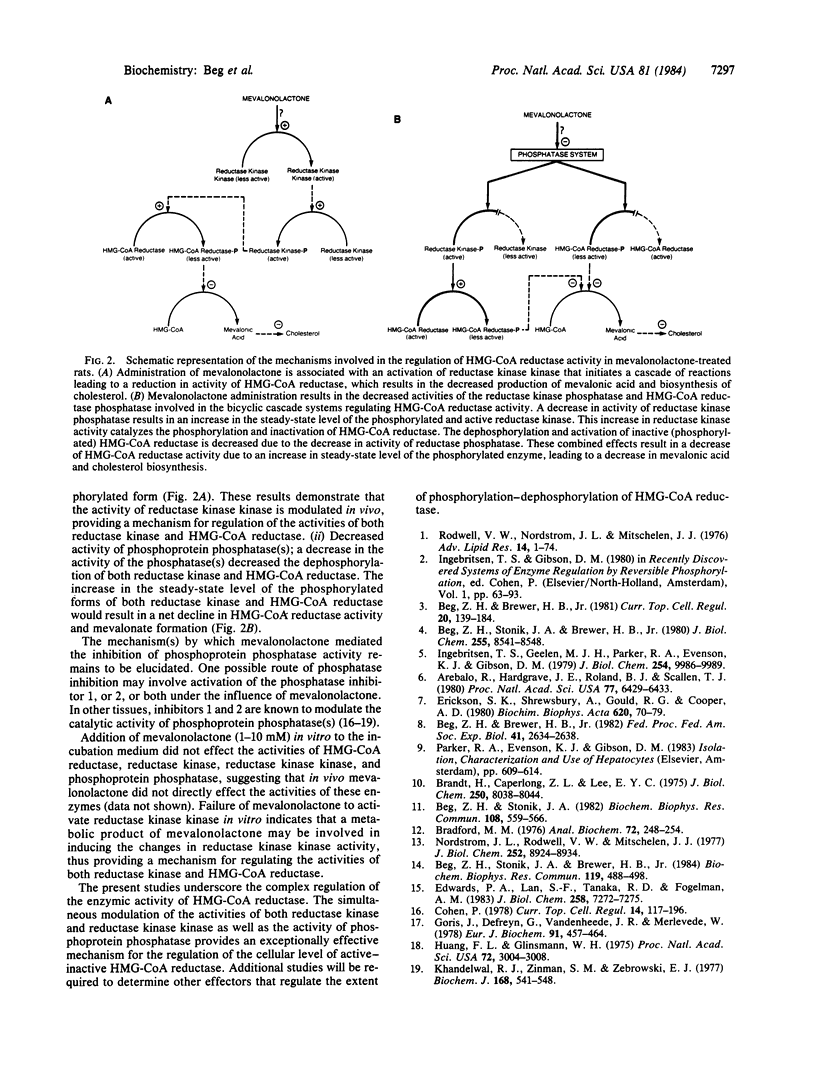
It has been previously demonstrated that the enzymic activity of rat liver 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA reductase; EC 1.1.1.34) is modulated in vitro and in vivo by a bicyclic cascade system involving reversible phosphorylation of HMG-CoA reductase and reductase kinase. In the present study, administration of mevalonolactone to rats caused a rapid inhibition of HMG-CoA reductase activity. The initial short-term (20-min) reversible inhibition (38%) of enzyme activity was due to increased phosphorylation of HMG-CoA reductase. The inhibition of HMG-CoA reductase activity by increased phosphorylation was associated with an increased activity and phosphorylation (2- to 3-fold) of reductase kinase. The increased phosphorylation of reductase kinase was catalyzed by reductase kinase kinase, which was significantly elevated (3- to 4-fold) after the administration of mevalonolactone to rats. The mechanism for the in vivo activation of reductase kinase kinase is as yet unknown. Mevalonolactone administration was also associated with a significant inhibition of phosphoprotein phosphatase activity, which dephosphorylates both HMG-CoA reductase (activation) and reductase kinase (inactivation). These results indicate that mevalonolactone administration to rats in vivo was associated with an inhibition of HMG-CoA reductase activity by two mechanisms: (i) an increase in the degree of phosphorylation of both HMG-CoA reductase and reductase kinase due to increased activity of reductase kinase kinase; (ii) a decrease in the dephosphorylation of both HMG-CoA reductase and reductase kinase secondary to inhibition of phosphoprotein phosphatase activity. These combined effects favor an increase in the steady-state level of the phosphorylated forms of both HMG-CoA reductase and reductase kinase, resulting in a net reduction in the enzymic activity of HMG-CoA reductase and mevalonate formation. These results demonstrate that the activity of reductase kinase kinase is modulated in vivo, providing a mechanism for the regulation of the activities of both reductase kinase and HMG-CoA reductase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arebalo R. E., Hardgrave J. E., Noland B. J., Scallen T. J. In vivo regulation of rat liver 3-hydroxy-3-methylglutaryl-coenzyme A reductase: enzyme phosphorylation as an early regulatory response after intragastric administration of mevalonolactone. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6429–6433. doi: 10.1073/pnas.77.11.6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beg Z. H., Brewer H. B., Jr Modulation of rat liver 3-hydroxy-3-methylglutaryl-CoA reductase activity by reversible phosphorylation. Fed Proc. 1982 Aug;41(10):2634–2638. [PubMed] [Google Scholar]
- Beg Z. H., Brewer H. B., Jr Regulation of liver 3-hydroxy-3-methylglutaryl-CoA reductase. Curr Top Cell Regul. 1981;20:139–184. doi: 10.1016/b978-0-12-152820-1.50008-0. [DOI] [PubMed] [Google Scholar]
- Beg Z. H., Stonik J. A., Brewer H. B., Jr Human hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase: evidence for the regulation of enzymic activity by a bicyclic phosphorylation cascade. Biochem Biophys Res Commun. 1984 Mar 15;119(2):488–498. doi: 10.1016/s0006-291x(84)80275-2. [DOI] [PubMed] [Google Scholar]
- Beg Z. H., Stonik J. A., Brewer H. B., Jr In vitro and in vivo phosphorylation of rat liver 3-hydroxy-3-methylglutaryl coenzyme A reductase and its modulation by glucagon. J Biol Chem. 1980 Sep 25;255(18):8541–8545. [PubMed] [Google Scholar]
- Beg Z. H., Stonik J. A. Reversible inactivation of 3-hydroxy-3-methylglutaryl coenzyme A reductase: reductase kinase and mevalonate kinase are separate enzymes. Biochem Biophys Res Commun. 1982 Sep 30;108(2):559–566. doi: 10.1016/0006-291x(82)90865-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brandt H., Capulong Z. L., Lee E. Y. Purification and properties of rabbit liver phosphorylase phosphatase. J Biol Chem. 1975 Oct 25;250(20):8038–8044. [PubMed] [Google Scholar]
- Cohen P. The role of cyclic-AMP-dependent protein kinase in the regulation of glycogen metabolism in mammalian skeletal muscle. Curr Top Cell Regul. 1978;14:117–196. doi: 10.1016/b978-0-12-152814-0.50008-3. [DOI] [PubMed] [Google Scholar]
- Edwards P. A., Lan S. F., Tanaka R. D., Fogelman A. M. Mevalonolactone inhibits the rate of synthesis and enhances the rate of degradation of 3-hydroxy-3-methylglutaryl coenzyme A reductase in rat hepatocytes. J Biol Chem. 1983 Jun 25;258(12):7272–7275. [PubMed] [Google Scholar]
- Erickson S. K., Shrewsbury M. A., Gould R. G., Cooper A. D. Studies on the mechanisms of the rapid modulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase in intact liver by mevalonolactone and 25-hydroxycholesterol. Biochim Biophys Acta. 1980 Oct 6;620(1):70–79. doi: 10.1016/0005-2760(80)90186-1. [DOI] [PubMed] [Google Scholar]
- Goris J., Defreyn G., Vandenheede J. R., Merlevede W. Protein inhibitors of dog-liver phosphorylase phosphatase dependent on and independent of protein kinase. Eur J Biochem. 1978 Nov 15;91(2):457–464. doi: 10.1111/j.1432-1033.1978.tb12698.x. [DOI] [PubMed] [Google Scholar]
- Huang F. L., Glinsmann W. H. Inactivation of rabbit muscle phosphorylase phosphatase by cyclic AMP-dependent kinas. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3004–3008. doi: 10.1073/pnas.72.8.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingebritsen T. S., Geelen M. J., Parker R. A., Evenson K. J., Gibson D. M. Modulation of hydroxymethylglutaryl-CoA reductase activity, reductase kinase activity, and cholesterol synthesis in rat hepatocytes in response to insulin and glucagon. J Biol Chem. 1979 Oct 25;254(20):9986–9989. [PubMed] [Google Scholar]
- Khandelwal R. L., Zinman S. M., Zebrowski E. J. The effect of streptozotocin-induced diabetes and of insulin supplementation on glycogen metabolism in rat liver. Biochem J. 1977 Dec 15;168(3):541–548. doi: 10.1042/bj1680541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom J. L., Rodwell V. W., Mitschelen J. J. Interconversion of active and inactive forms of rat liver hydroxymethylglutaryl-CoA reductase. J Biol Chem. 1977 Dec 25;252(24):8924–8934. [PubMed] [Google Scholar]
- Rodwell V. W., Nordstrom J. L., Mitschelen J. J. Regulation of HMG-CoA reductase. Adv Lipid Res. 1976;14:1–74. doi: 10.1016/b978-0-12-024914-5.50008-5. [DOI] [PubMed] [Google Scholar]


