Abstract
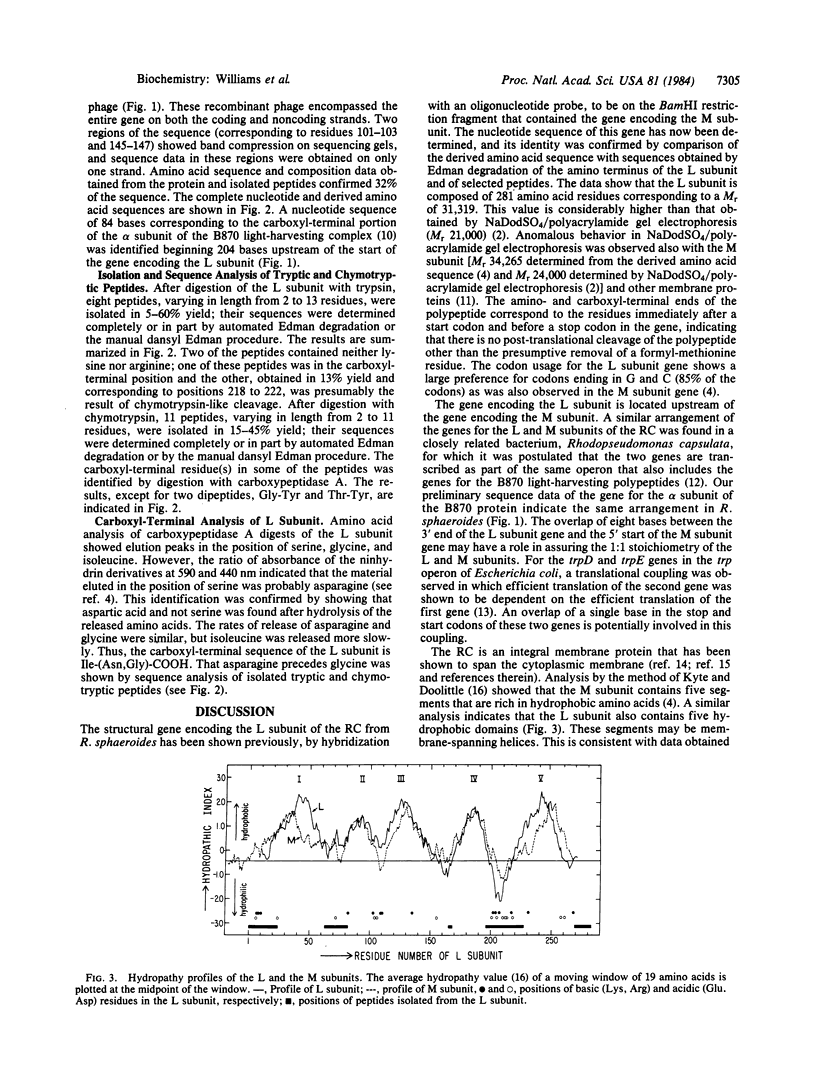
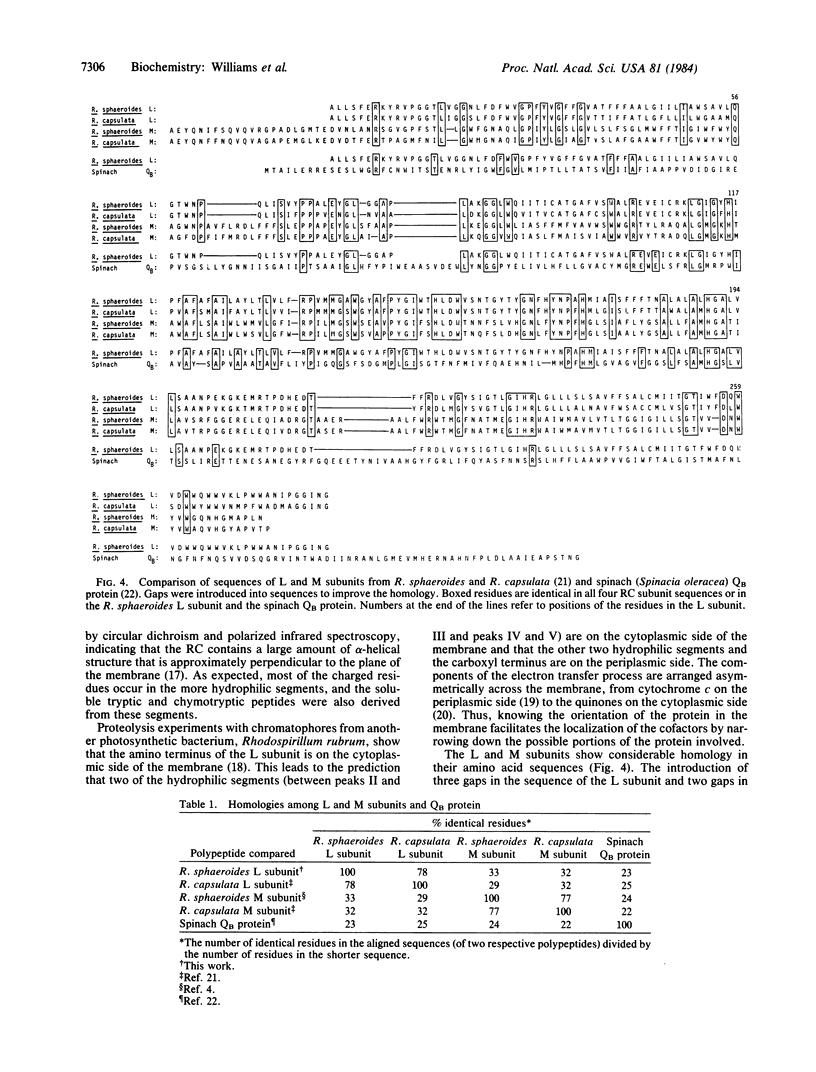
The reaction center is an integral membrane protein that, together with several cofactors, mediates the primary photochemical events in bacterial photosynthesis. The amino-terminal sequences of the three subunits, L, M, and H, of the reaction center protein and the sequence of the structural gene encoding the M subunit have been reported previously. In the present study, we found that the 3' end of the structural gene encoding the L subunit overlaps by eight bases the 5' end of the gene encoding the M subunit. The primary structure of the L subunit has been determined from the nucleotide sequence of the gene and from analyses of the amino and carboxyl termini of the protein. The sequences of a number of tryptic and chymotryptic peptides were used to corroborate the nucleotide sequence. The L subunit was found to be composed of 281 amino acids (Mr 31,319) and to contain five hydrophobic segments. It is homologous to the M subunit and to a plant thylakoid protein referred to as the QB or Mr 32,000 protein.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunisholz R. A., Wiemken V., Suter F., Bachofen R., Zuber H. The light-harvesting polypeptides of Rhodospirillum rubrum. II. Localisation of the amino-terminal regions of the light-harvesting polypeptides B 870-alpha and B 870-beta and the reaction-centre subunit L at the cytoplasmic side of the photosynthetic membrane of Rhodospirillum rubrum G-9+. Hoppe Seylers Z Physiol Chem. 1984 Jul;365(7):689–701. doi: 10.1515/bchm2.1984.365.2.689. [DOI] [PubMed] [Google Scholar]
- Bunker G., Stern E. A., Blankenship R. E., Parson W. W. An x-ray absorption study of the iron site in bacterial photosynthetic reaction centers. Biophys J. 1982 Feb;37(2):539–551. doi: 10.1016/S0006-3495(82)84699-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAYTON R. K., SMITH C. Rhodopseudomonas spheroides: high catalase and blue-green double mutants. Biochem Biophys Res Commun. 1960 Aug;3:143–145. doi: 10.1016/0006-291x(60)90210-2. [DOI] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F. Similar amino acid sequences: chance or common ancestry? Science. 1981 Oct 9;214(4517):149–159. doi: 10.1126/science.7280687. [DOI] [PubMed] [Google Scholar]
- Eisenberger P., Okamura M. Y., Feher G. The electronic structure of Fe2+ in reaction centers from Rhodopseudomonas sphaeroides. II. Extended x-ray fine structure studies. Biophys J. 1982 Feb;37(2):523–538. doi: 10.1016/S0006-3495(82)84698-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson J. M., Rahire M., Bennoun P., Delepelaire P., Diner B., Rochaix J. D. Herbicide resistance in Chlamydomonas reinhardtii results from a mutation in the chloroplast gene for the 32-kilodalton protein of photosystem II. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3617–3621. doi: 10.1073/pnas.81.12.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg J., McIntosh L. Molecular Basis of Herbicide Resistance in Amaranthus hybridus. Science. 1983 Dec 23;222(4630):1346–1349. doi: 10.1126/science.222.4630.1346. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Matthews B. W., Fenna R. E., Bolognesi M. C., Schmid M. F., Olson J. M. Structure of a bacteriochlorophyll a-protein from the green photosynthetic bacterium Prosthecochloris aestuarii. J Mol Biol. 1979 Jun 25;131(2):259–285. doi: 10.1016/0022-2836(79)90076-7. [DOI] [PubMed] [Google Scholar]
- Okamura M. Y., Steiner L. A., Feher G. Characterization of reaction centers from photosynthetic bacteria. I. Subunit structure of the protein mediating the primary photochemistry in Rhodopseudomonas spheroides R-26. Biochemistry. 1974 Mar 26;13(7):1394–1403. doi: 10.1021/bi00704a013. [DOI] [PubMed] [Google Scholar]
- Oppenheim D. S., Yanofsky C. Translational coupling during expression of the tryptophan operon of Escherichia coli. Genetics. 1980 Aug;95(4):785–795. doi: 10.1093/genetics/95.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty K. M., Dutton P. L. Properties of the flash-induced proton binding encountered in membranes of Rhodopseudomonas sphaeroides: a functional pK on the ubisemiquinone? Arch Biochem Biophys. 1976 Feb;172(2):335–345. doi: 10.1016/0003-9861(76)90085-0. [DOI] [PubMed] [Google Scholar]
- Prince R. C., Baccarini-Melandri A., Hauska G. A., Melandri B. A., Crofts A. R. Asymmetry of an energy transducing membrane the location of cytochrome c2 in Rhodopseudomonas spheroides and Rhodopseudomonas capsulata. Biochim Biophys Acta. 1975 May 15;387(2):212–227. doi: 10.1016/0005-2728(75)90104-8. [DOI] [PubMed] [Google Scholar]
- Spielmann A., Stutz E. Nucleotide sequence of soybean chloroplast DNA regions which contain the psb A and trn H genes and cover the ends of the large single copy region and one end of the inverted repeats. Nucleic Acids Res. 1983 Oct 25;11(20):7157–7167. doi: 10.1093/nar/11.20.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner L. A., Pardo A. G., Margolies M. N. Amino acid sequence of the heavy-chain variable region of the crystallizable human myeloma protein Dob. Biochemistry. 1979 Sep 18;18(19):4068–4080. doi: 10.1021/bi00586a003. [DOI] [PubMed] [Google Scholar]
- Sutton M. R., Rosen D., Feher G., Steiner L. A. Amino-terminal sequences of the L, M, and H subunits of reaction centers from the photosynthetic bacterium Rhodopseudomonas sphaeroides R-26. Biochemistry. 1982 Aug 3;21(16):3842–3849. doi: 10.1021/bi00259a019. [DOI] [PubMed] [Google Scholar]
- Tanford C., Reynolds J. A. Characterization of membrane proteins in detergent solutions. Biochim Biophys Acta. 1976 Oct 26;457(2):133–170. doi: 10.1016/0304-4157(76)90009-5. [DOI] [PubMed] [Google Scholar]
- Tarr G. E., Beecher J. F., Bell M., McKean D. J. Polyquarternary amines prevent peptide loss from sequenators. Anal Biochem. 1978 Feb;84(2):622–7?0=ENG. doi: 10.1016/0003-2697(78)90086-6. [DOI] [PubMed] [Google Scholar]
- Theiler R., Suter F., Wiemken V., Zuber H. The light-harvesting polypeptides of Rhodopseudomonas sphaeroides R-26.1. I. Isolation, purification and sequence analyses. Hoppe Seylers Z Physiol Chem. 1984 Jul;365(7):703–719. doi: 10.1515/bchm2.1984.365.2.703. [DOI] [PubMed] [Google Scholar]
- Theiler R., Zuber H. The light-harvesting polypeptides of Rhodopseudomonas sphaeroides R-26.1. II. Conformational analyses by attenuated total reflection infrared spectroscopy and the possible molecular structure of the hydrophobic domain of the B 850 complex. Hoppe Seylers Z Physiol Chem. 1984 Jul;365(7):721–729. [PubMed] [Google Scholar]
- Valkirs G. E., Feher G. Topography of reaction center subunits in the membrane of the photosynthetic bacterium, rhodopseudomonas sphaeroides. J Cell Biol. 1982 Oct;95(1):179–188. doi: 10.1083/jcb.95.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Steiner L. A., Ogden R. C., Simon M. I., Feher G. Primary structure of the M subunit of the reaction center from Rhodopseudomonas sphaeroides. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6505–6509. doi: 10.1073/pnas.80.21.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youvan D. C., Alberti M., Begusch H., Bylina E. J., Hearst J. E. Reaction center and light-harvesting I genes from Rhodopseudomonas capsulata. Proc Natl Acad Sci U S A. 1984 Jan;81(1):189–192. doi: 10.1073/pnas.81.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youvan D. C., Bylina E. J., Alberti M., Begusch H., Hearst J. E. Nucleotide and deduced polypeptide sequences of the photosynthetic reaction-center, B870 antenna, and flanking polypeptides from R. capsulata. Cell. 1984 Jul;37(3):949–957. doi: 10.1016/0092-8674(84)90429-x. [DOI] [PubMed] [Google Scholar]
- Zurawski G., Bohnert H. J., Whitfeld P. R., Bottomley W. Nucleotide sequence of the gene for the M(r) 32,000 thylakoid membrane protein from Spinacia oleracea and Nicotiana debneyi predicts a totally conserved primary translation product of M(r) 38,950. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7699–7703. doi: 10.1073/pnas.79.24.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zürrer H., Snozzi M., Hanselmann K., Bachofen R. Localisation of the subunits of the photosynthetic reaction centers in the chromatophore membrane of Rhodospirillum rubrum. Biochim Biophys Acta. 1977 May 11;460(2):273–279. doi: 10.1016/0005-2728(77)90213-4. [DOI] [PubMed] [Google Scholar]



