Abstract
Despite the strong connection between angiogenesis and osteogenesis in skeletal repair conditions such as fracture and distraction osteogenesis, little is known about the vascular requirements for bone formation after repetitive mechanical loading. Here, established protocols of damaging (stress fracture) and nondamaging (physiological) forelimb loading in the adult rat were used to stimulate either woven or lamellar bone formation, respectively. Positron emission tomography was used to evaluate blood flow and fluoride kinetics at the site of bone formation. In the group that received damaging mechanical loading leading to woven bone formation (WBF), 15O water (blood) flow rate was significantly increased on day 0 and remained elevated 14 days after loading, whereas 18F fluoride uptake peaked 7 days after loading. In the group that received nondamaging mechanical loading leading to lamellar bone formation (LBF), 15O water and 18F fluoride flow rates in loaded limbs were not significantly different from nonloaded limbs at any time point. The early increase in blood flow rate after WBF loading was associated with local vasodilation. In addition, Nos2 expression in mast cells was increased in WBF-, but not LBF-, loaded limbs. The nitric oxide (NO) synthase inhibitor Nω-nitro-l-arginine methyl ester was used to suppress NO generation, resulting in significant decreases in early blood flow rate and bone formation after WBF loading. These results demonstrate that NO-mediated vasodilation is a key feature of the normal response to stress fracture and precedes woven bone formation. Therefore, patients with impaired vascular function may heal stress fractures more slowly than expected.
Keywords: stress fracture, vasodilation, woven, bone formation, l-NAME
repetitive mechanical loading of the skeleton can stimulate the production of new bone, ranging from the injury response of woven bone formation to the adaptive response of lamellar bone formation (29). Because there is a strong connection between bone formation and vascularity in scenarios such as development and fracture healing (14, 18, 19, 35), it is reasonable to postulate a role for vascular support in loading-induced bone formation. However, the vascular response that follows repetitive mechanical loading of bone and its role in subsequent bone formation, remains largely unexplored.
The mechanisms that differentially regulate loading-induced woven and lamellar bone formation are beginning to emerge, based on comparisons from forelimb compression in the rat. Woven bone formation (WBF) is stimulated after creation of a stress fracture during a single bout of damaging, cyclic loading (51). In contrast, lamellar bone formation (LBF) is induced by a single bout of nondamaging, cyclic loading at physiological strain magnitudes with fewer cycles (31). Inflammatory markers, such as IL-6, are upregulated several hundred-fold in the first few hours after WBF loading and persist for at least 3 days, but there is no such upregulation after LBF loading (24, 30). Additionally, robust increases in angiogenic gene expression and vascularity have been associated with WBF loading, whereas only a small upregulation of angiogenic genes and no increase in vascularity was detected after LBF loading (31).
Increases in inflammatory and angiogenic genes suggest that the regulation of blood flow to the site of bone formation is critical. In fact, increased blood flow after stress fracture has been previously observed (33). Vasomotor processes tightly control blood flow by altering the size of blood vessels. These processes can be regulated by inflammatory cells, including neutrophils, macrophages, and mast cells (27). Importantly, activated mast cells can degranulate and release inflammatory mediators, including nitric oxide (NO), a potent vasodilator (8, 17, 32). NO is produced by NO synthase (NOS) in a conversion of l-arginine to NO and l-citrulline (39). Because NO has a very short half-life (2–5 s) (5), the function of NOS is critical for NO signaling. A recent study reported that the expression of Nos2, also known as iNOS, is upregulated 50-fold 1 h after WBF loading (30), suggesting that NO-mediated vasodilation may be responsible for increased blood flow after stress fracture.
Blood flow rate, as well as fluoride metabolism, can be measured in vivo using positron emission tomography (PET), an imaging technique used clinically and in research for evaluating the kinetics of physiological processes. Many radioactive isotopes have been developed for use in PET because of their specific activity in the body; 15O water and 18F fluoride were used in this study. Because 15O water is not preferentially bound, it freely diffuses throughout the vasculature and has been used to determine blood flow rate in many tissues, including bone (4). 18F fluoride is a bone-seeking radioisotope that has been used to evaluate skeletal kinetics, diseases, turnover, and microdamage (13, 21, 26, 44). Previously, 18F fluoride PET was used to analyze the skeletal response to WBF loading in the ulna (42). In that study, only static analysis was performed, blood flow rate was not quantified, and nondamaging LBF loading was not considered.
The main objective of this study was to examine the inflammatory and vascular responses to the production of new bone after damaging (WBF) and nondamaging (LBF) mechanical loading. Blood flow rate and fluoride kinetics at the site of bone formation were measured using PET imaging. Local vasodilation, mast cell infiltration, and Nos2 expression were quantified at the site of bone formation using histology. Finally, the role of the nitric oxide was evaluated by chemical inhibition of NO synthesis.
MATERIALS AND METHODS
Study design.
A total of 88 male Fischer F344 rats (Harlan) was obtained at 13–14 wk of age and housed under standard conditions until 18–22 wk of age. The right forelimb of each animal was mechanically loaded using one of two loading protocols designed to induce new bone formation at the midshaft of the ulna. The contralateral (left) forelimb was used as a nonloaded control. The damaging loading protocol (WBF loading) creates fatigue damage at the mid-diaphysis of the ulna, resulting in a stress fracture that leads to an abundant woven bone response during the repair process (52). In contrast, the nondamaging loading protocol (LBF loading) stimulates an increase in the rate of lamellar bone formation at the same location without creating damage or decreasing bone strength (31). The amount of lamellar bone formed after LBF loading is modest, with a mineral apposition rate (MAR) of <2 μm/day (31). All protocols were approved by the Animal Studies Committee at Washington University in St. Louis.
Mechanical loading.
First, rats were anesthetized with isoflurane gas (1–3%). Mechanical loading of the right ulna of each animal was then performed as previously described (48). Briefly, the right forelimb was axially compressed by placing the olecranon process and the flexed carpus into specially designed fixtures. A material testing system (Instron Electropuls 1000) was used to apply force and monitor displacement. For WBF loading, a 0.3-N compressive preload was applied followed by a cyclic haversine waveform of 18 N at 2 Hz until an increase in peak displacement of 1.3 mm, relative to the 10th cycle (65% of the average total displacement to fatigue fracture) (52). For LBF loading, a 0.3-N compressive preload was applied followed by a cyclic rest-inserted trapezoidal waveform with a peak force of 15 N at 0.1 Hz for 100 cycles (31). After the procedure, rats were given an intramuscular injection of analgesic (0.05 mg/kg buprenorphine) and allowed unrestricted cage activity.
Positron emission tomography.
PET scans were completed 0, 1, 3, 7, and 14 days after loading using a microPET-Focus or microPET-Inveon (Concorde Microsystems); “0 days” indicates 2–4 h after loading. The spatial resolution of the PET scan was 1.5–1.7 mm. 15O water (30–55 MBq) was administered by tail vein injection in a short bolus, immediately followed by a 10-min scan (1 frame × 3 s, 6 × 2 s, 5 × 5 s, 11 × 10 s, 5 × 30 s, 5 × 60 s). Next, 18F fluoride (9–15 MBq) was administered in the same manner, followed by a 60-min scan (1 frame × 3 s, 6 × 2 s, 9 × 5 s, 6 × 10 s, 4 × 30 s, 2 × 60 s, 2 × 120 s, 10 × 300 s). Each animal (n = 8 per group) was scanned using both radioisotopes at all five time points.
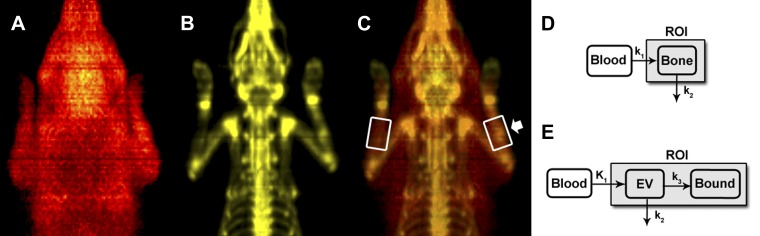
Novel methods for analyzing dynamic PET data from the rat forelimb were recently described in detail (46). Briefly, regions of interest (ROI) 1.5 times the ulnar diameter, 1/3 the ulnar length, and centered at the mid-diaphysis were defined using anatomical landmarks from 18F fluoride scans (Fig. 1, A–C). Additionally, smaller 3D ROI were drawn on the heart to obtain input functions of each injected radioisotope. These input functions were used in conjunction with compartment models of 15O water and 18F fluoride kinetics (Fig. 1, D and E) to estimate physiological parameters of flow rate and fluoride metabolism. The 15O water model is a one-compartment (bone), two-parameter model, similar to Kety (23) and others (25, 38). The two parameters are flow rate (k1) and clearance rate (k2). For 18F fluoride, a two-compartment (extravascular and bound), three-parameter model was used, similar to Hawkins et al. (20). The three parameters are flow rate (K1), clearance rate (k2), and incorporation rate (k3). Finally, total uptake of fluoride is calculated as Ki = (K1k3)/(k2+k3). The experimental data were fit to the model using nonlinear least squares optimization (MATLAB) to find values for each parameter. All data were normalized for the weight of the animal and the activity of the injected radioisotope.
Fig. 1.
PET imaging and analysis. Coronal view of 3D PET images of 15O water (A), 18F fluoride (B), and overlay of A and B (C). C: note the regions of interest in white boxes as well as increased uptake of both 15O water and 18F fluoride in the loaded limb (arrow). D: 15O water model: the input function is labeled blood, k1 is the flow rate, k2 is the clearance rate, and the bone compartment is labeled. E: 18F fluoride model: the input function is labeled blood, K1 is the flow rate into the extravascular space, k2 is the clearance from the extravascular space, k3 is the incorporation into the bound compartment, EV is the extravascular compartment, and the bound fluoride compartment is labeled. In both figures, the shaded compartment (ROI) represents the scope of the measured region of interest.
Histological analysis.
Sections (5 μm) of formalin-fixed, paraffin-embedded forelimbs were cut 1 mm distal to the midpoint for histological analysis. This is the site of maximal bone formation along the ulnar length (51). After deparaffination in xylenes and rehydration in graded ethanol solutions, antigen retrieval was performed by a 30-min incubation in a saturated sodium hydroxide methanol solution diluted 1:3 in methanol. Twenty minutes in 3% H2O2 was used to block endogenous peroxidase activity, then sections were incubated in normal goat serum (sc-2043, Santa Cruz; 1.5% in PBS) to reduce nonspecific background staining. After this, slides were incubated in rabbit polyclonal Nos2 antibody (sc-651, Santa Cruz; 1:50 dilution) or mouse monoclonal αSMA antibody (A2547, Sigma; 1:1,000 dilution) at 4°C overnight. Negative control slides were prepared by substituting normal goat serum for the primary antibody. To visualize binding, biotinylated goat anti-rabbit (sc-2018, Santa Cruz) or anti-mouse (sc-2017, Santa Cruz) secondary antibody was applied for 30 min followed by avidin-biotin-peroxidase complex for 30 min. Finally, slides were developed using diaminobenzidine for 60 s. The slides were then dehydrated, mounted, and imaged with bright field microscopy. After imaging, coverslips were removed on Nos2 slides by an overnight incubation in xylenes. Then, sections were rehydrated and stained with toluidine blue. After dehydration, the slides were mounted and imaged again to visualize mast cells, similar to others (16).
Image analysis was performed using FIJI (41), with n = 6 per group at days 1, 3, 7, and 14. To quantify vasodilation, the total area of the anterior interosseus artery (AIA) was determined using αSMA-stained sections. This artery is known to anastomose with the proximal interosseus artery near the mid-diaphysis of the ulna (22, 36, 47). In most of the sections analyzed, the AIA was not branched. In less than 20% of the sections analyzed, the area of the branched AIA was summed to obtain the total arterial area. To analyze mast cell infiltration and Nos2 expression, mast cells in the expanded periosteum were first identified by metachromatic toluidine blue staining, then classified as Nos2 positive or negative by immunohistochemistry.
NOS inhibition.
Nω-nitro-l-arginine methyl ester (l-NAME) is a potent inhibitor of nitric oxide synthase (NOS). In the final round of experimentation, drinking water was used to administer l-NAME (Sigma N5751; 1 g/l) starting 2 days before WBF loading and continuing until death. Vehicle control animals received normal drinking water. The resulting l-NAME dosage of ∼25 mg·kg−1·day−1 was well within the optimal dosing range for rats (3). Treatment was well tolerated—no animals exhibited discomfort or died during the study period. The first set of animals (n = 6 per group) was subjected to PET imaging and killed at day 3, with forelimbs harvested for histological analysis. A separate set of animals (n = 6 per group) was killed at day 7 to quantify bone formation.
Assessment of woven bone production.
Ex vivo microcomputed tomography (μCT40, Scanco Medical) was used to analyze bone formation at the ulnar mid-diaphysis 7 days after WBF loading. The central 8 mm of each ulna was scanned separately at 45 kV and 177 μA with 200-ms integration time. The scan tube diameter was 16.4 mm, and medium resolution was used to obtain a 16-μm voxel size. Scan slices were acquired in the transverse plane by placing the forelimb parallel to the z-axis of the scanner. Hand drawn contours (sigma = 1.2, support = 2, lower/upper threshold = 330/1,000) were used to manually segment bone with Scanco imaging software. Woven bone volume was calculated by subtracting the original cortical bone volume from the total bone volume in the entire scan. Woven bone BMD was calculating by analyzing only woven bone in the middle 20 slices of the woven bone extent.
Dynamic histomorphometry was used to quantify woven bone area. Rats were given two intraperitoneal injections of fluorescent bone formation markers. Calcein (5 mg/kg, Sigma C0875) was administered immediately after loading and Alizarin (30 mg/kg, Sigma A3882) was administered 5 days after loading. After microCT imaging, forelimbs were embedded in poly-(methyl methacrylate). Transverse sections (100 μm) were cut (SP 1600, Leica Microsystems) 1 mm distal to the midpoint and then polished to 30 μm and mounted on glass slides. Digital images of these sections were captured using fluorescence microscopy (Olympus IX-51) with fluorescein isothiocyanate (FITC) and tetramethylrhodamine isothiocyanate (TRITC) filters for calcein and alizarin, respectively. Image analysis was performed using Bioquant Osteo.
Statistics.
All results are given as fold changes (loaded limb/nonloaded limb) and plotted as mean ± standard deviation. Statistical evaluation was performed using Statview 5.0 (SAS Institute). For Tables 1 and 2, repeated-measures ANOVA was used to compare across loading groups and time points with significant differences detected using Fisher's protected least significant difference post hoc test. Paired Student's t-test was used to compare loaded and nonloaded limbs. For Tables 3 and 4, paired Student's t-test was used as before, with unpaired Student's t-test used to compare between WBF and LBF loaded limbs. In each case, P < 0.05 was considered significant.
Table 1.
15O water PET imaging compartment model parameters after WBF or LBF loading
| Day | k1, ml·g−1·s−1 | k2, s−1 |
|---|---|---|
| WBF loading | ||
| 0 | 1.33 ± 0.23*† | 1.00 ± 0.04 |
| 1 | 1.33 ± 0.17*† | 1.02 ± 0.03 |
| 3 | 1.32 ± 0.24*† | 1.01 ± 0.02 |
| 7 | 1.22 ± 0.20*† | 1.03 ± 0.04 |
| 14 | 1.24 ± 0.19† | 1.02 ± 0.03 |
| LBF loading | ||
| 0 | 1.02 ± 0.14 | 0.99 ± 0.01 |
| 1 | 1.01 ± 0.13 | 1.01 ± 0.04 |
| 3 | 0.97 ± 0.11 | 1.00 ± 0.01 |
| 7 | 1.02 ± 0.18 | 1.00 ± 0.03 |
| 14 | 1.13 ± 0.21‡ | 1.01 ± 0.02 |
Mean fold change (loaded/nonloaded) ± SD, n = 8. WBF, woven bone formation.
P < 0.05 vs. lamellar bone formation (LBF) loading,
P < 0.05 vs. nonloaded,
P < 0.10 vs. nonloaded.
Table 2.
18F fluoride PET imaging compartment model parameters after WBF or LBF loading
| Day | K1, ml·g−1·s−1 | k2, s−1 | k3, s−1 | Ki, ml·g−1·s−1 |
|---|---|---|---|---|
| WBF loading | ||||
| 0 | 1.47 ± 0.32*† | 0.97 ± 0.04*† | 1.05 ± 0.04*† | 1.59 ± 0.42*† |
| 1 | 1.24 ± 0.41 | 0.97 ± 0.04 | 1.02 ± 0.07 | 1.31 ± 0.49 |
| 3 | 1.72 ± 0.50*† | 0.95 ± 0.05*† | 1.10 ± 0.08*† | 1.98 ± 0.69*† |
| 7 | 2.36 ± 0.94*† | 1.03 ± 0.05 | 1.14 ± 0.11*† | 2.63 ± 1.17*† |
| 14 | 1.43 ± 0.44*† | 0.99 ± 0.03 | 1.04 ± 0.05* | 1.51 ± 0.55*† |
| LBF loading | ||||
| 0 | 1.05 ± 0.10 | 1.00 ± 0.01 | 1.01 ± 0.02 | 1.06 ± 0.12 |
| 1 | 1.13 ± 0.20 | 0.99 ± 0.01 | 1.02 ± 0.02 | 1.16 ± 0.24 |
| 3 | 1.07 ± 0.17 | 1.02 ± 0.04 | 1.01 ± 0.03 | 1.06 ± 0.20 |
| 7 | 1.06 ± 0.09 | 1.01 ± 0.03 | 1.01 ± 0.02 | 1.06 ± 0.11 |
| 14 | 1.00 ± 0.11 | 1.00 ± 0.01 | 1.00 ± 0.01 | 1.01 ± 0.12 |
Mean fold change (loaded/nonloaded) ± SD, n = 8.
P < 0.05 versus LBF loading,
P < 0.05 vs. nonloaded.
Table 3.
15O water PET imaging compartment model parameters in l-NAME- or vehicle-treated animals after WBF loading
| Day | k1, ml·g−1·s−1 | k2, s−1 |
|---|---|---|
| WBF loading—l-NAME treated | ||
| 1 | 1.10 ± 0.11* | 1.02 ± 0.04 |
| 3 | 1.11 ± 0.14* | 1.02 ± 0.02 |
| WBF loading—vehicle | ||
| 1 | 1.35 ± 0.25† | 1.01 ± 0.02 |
| 3 | 1.32 ± 0.16† | 0.99 ± 0.03 |
Mean fold change (loaded/nonloaded) ± SD, n = 6.
P < 0.05 vs. vehicle,
P < 0.05 vs. nonloaded.
Table 4.
18F fluoride PET imaging compartment model parameters in l-NAME- or vehicle-treated animals after WBF loading
| Day | K1, ml·g−1·s−1 | k2, s−1 | k3, s−1 | Ki, ml·g−1·s−1 |
|---|---|---|---|---|
| WBF loading—l-NAME treated | ||||
| 1 | 1.11 ± 0.03*† | 1.00 ± 0.01 | 1.01 ± 0.01* | 1.12 ± 0.02*† |
| 3 | 1.43 ± 0.43† | 0.96 ± 0.04† | 1.06 ± 0.02*† | 1.82 ± 0.34† |
| WBF loading—vehicle | ||||
| 1 | 1.30 ± 0.17† | 0.97 ± 0.05 | 1.05 ± 0.03† | 1.49 ± 0.15† |
| 3 | 1.63 ± 0.60† | 0.94 ± 0.04† | 1.11 ± 0.05† | 2.09 ± 0.84† |
Mean fold change (loaded/nonloaded) ± SD, n = 6.
P < 0.05 vs. vehicle,
P < 0.05. vs. nonloaded.
RESULTS
Increased blood flow rate and fluoride metabolism after WBF but not LBF loading.
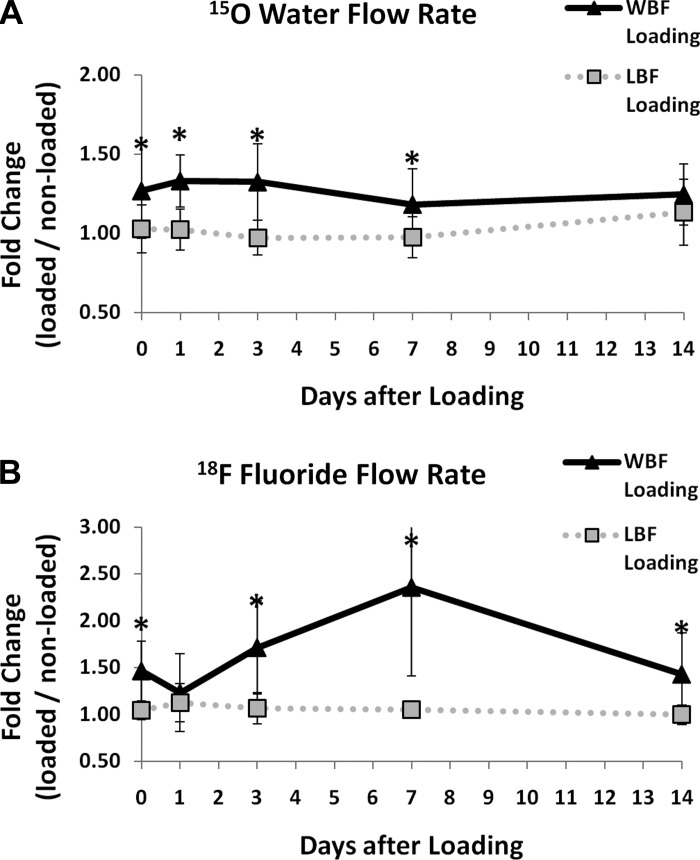
Blood flow and fluoride metabolism were assessed by PET imaging 0, 1, 3, 7, and 14 days after damaging (WBF) or nondamaging (LBF) mechanical loading. 15O water PET demonstrated that blood flow rate in loaded limbs was significantly increased after WBF loading but not LBF loading (Fig. 2A). 15O water flow rate (k1) was significantly higher (22–33%) in WBF loaded limbs compared with nonloaded control limbs 0 to 14 days after loading (Table 1). In contrast, 15O water flow rate (k1) was not significantly different in LBF loaded limbs compared with control limbs at any time point. Accordingly, from 0 to 7 days after loading, 15O water flow rate (k1) was significantly greater in WBF loaded limbs than LBF loaded limbs. At day 14, WBF and LBF loaded limbs were not significantly different because of a trend for increased flow rate after LBF loading (P = 0.06, LBF day 7 vs. day 14). Neither WBF nor LBF loading was associated with any significant differences in clearance rate (k2) at any time point.
Fig. 2.
15O water and 18F fluoride flow rates after loading. A: 15O water flow rate (k1) after woven bone formation (WBF) loading is significantly increased compared with lamellar bone formation (LBF) loading on days 0–7. B: 18F fluoride flow rate (K1) is significantly increased after WBF loading compared with LBF loading 0 to 14 days after loading (with the exception of day 1). Mean ± standard deviation, n = 8, *P < 0.05 WBF vs. LBF.
On the basis of 18F fluoride PET, loaded limbs had significantly increased fluoride kinetics after WBF loading but not LBF loading (Fig. 2B). 18F fluoride flow rate (K1) was significantly increased in WBF loaded limbs compared with nonloaded control limbs 0, 3, 7, and 14 days after loading; increases were between 43 and 136% (Table 2) and peaked 7 days after loading (P < 0.05 compared with all other days). 18F fluoride incorporation rate (k3) and total fluoride flux (Ki) were also significantly increased in WBF loaded limbs compared with control limbs, with increases on day 7 of 14 and 163%, respectively. Clearance rate (k2) changes in WBF loaded limbs were small (<5%) and not significantly different from control limbs, except day 3 (P < 0.05). For LBF loaded limbs, there were no significant differences in any parameters compared with control limbs; modest increases (13–16%) in flow rate (K1) and total flux (Ki) 1 day after loading did not reach significance. Accordingly, on days 0, 3, 7, and 14, WBF loaded limbs had significantly higher flow rate (K1), incorporation rate (k3), and total flux (Ki) than LBF loaded limbs.
Vasodilation, mast cell infiltration, and increased Nos2 expression follow WBF loading.
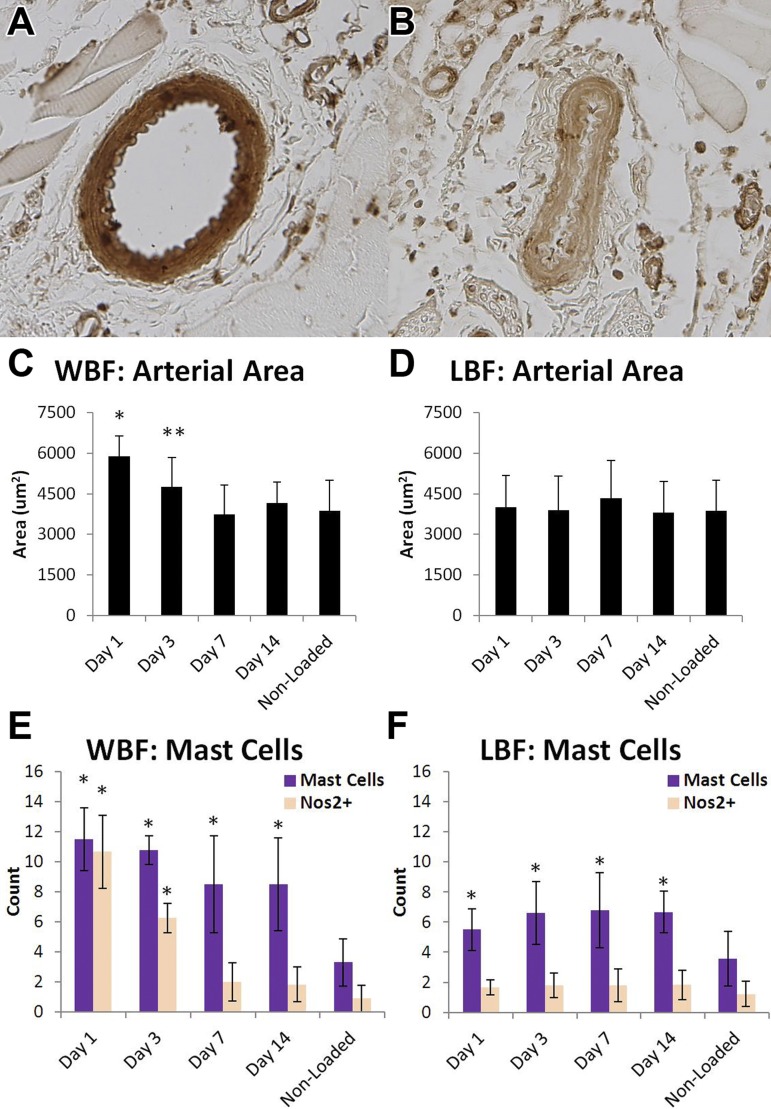
Changes in blood flow rate may be attributed to vasomotor activity or angiogenesis. To assess the contribution of vasodilation, histological analysis of WBF and LBF loaded forelimbs, as well as nonloaded control forelimbs, was performed. Total arterial area of the anterior interosseus artery was quantified using αSMA stained slides (Fig. 3, A and B). In WBF loaded limbs, total arterial area was significantly increased on day 1 (+50%) and day 3 (+29%) compared with nonloaded control limbs but returned to baseline on days 7 and 14 (Fig. 3C). These results are in contrast to LBF loading, where there were no significant differences in total arterial area in LBF loaded limbs compared with nonloaded limbs at any time point (Fig. 3D).
Fig. 3.
Local vasodilation was associated with mast cell infiltration and Nos2 expression. Arterial area was measured by analysis of αSMA-stained sections (A, dilated; B, constricted). C: WBF loaded limbs had significantly greater arterial area on days 1 and 3 compared with all other days and nonloaded limbs. D: LBF loaded limbs did not have significantly increased arterial area at any time point. These results were closely mirrored by Nos2+ mast cells in WBF (E) and LBF (F) loaded limbs. Mean ± standard deviation, n = 6, *P < 0.05, **P < 0.10 vs. nonloaded.
Because mast cells are involved in vasomotor activity, mast cells in the expanded periosteal region were quantified 1, 3, 7, and 14 days after WBF and LBF loading. Immunohistochemistry was used to quantify the number of mast cells that were expressing Nos2 (Nos2+). In WBF loaded limbs, total mast cell count was maximal 1 day after loading and decreased with time, although mast cells were present in significantly higher numbers in loaded limbs than nonloaded limbs at all time points (Fig. 3E). Importantly, Nos2+ mast cells were significantly increased by as much as 10-fold in WBF loaded limbs on days 1 and 3 compared with nonloaded control limbs, but on days 7 and 14 there were no significant increases. Outside of mast cells, there was very little staining for Nos2 in the expanded periosteal region—neither osteoblasts nor stromal cells in the nascent woven bone were Nos2+. In LBF loaded limbs, total mast cell count was significantly increased at all time points, although the magnitude of the increase was less than in WBF loaded limbs and there was no temporal trend in the 2 wk after loading. Despite the increase in mast cell count in LBF loaded limbs, Nos2+ mast cells were not significantly increased at any time point compared with nonloaded control limbs (Fig. 3F).
NOS inhibition blocked increases in blood flow rate and impaired bone formation after WBF loading.
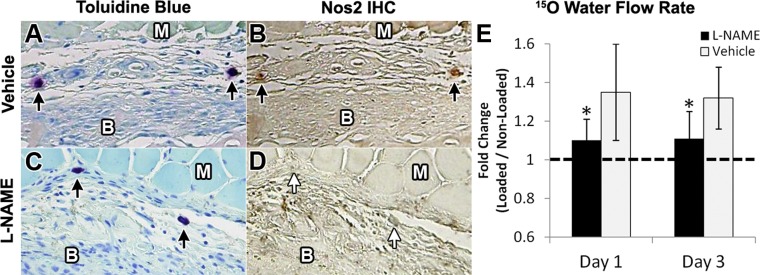
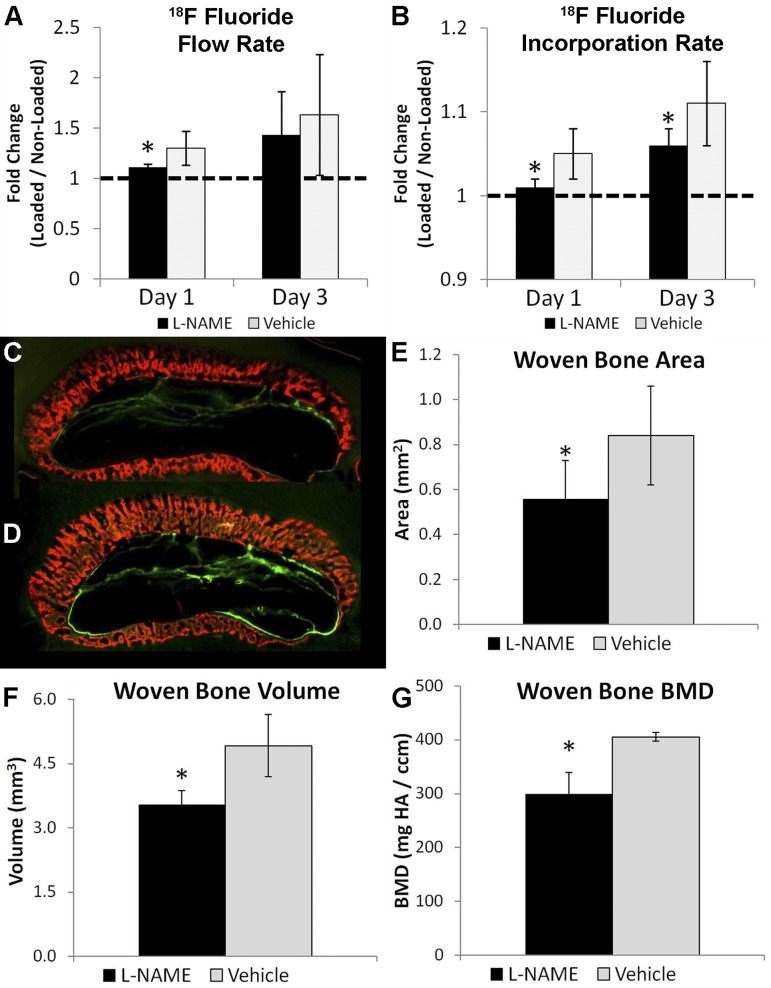
Because Nos2-associated vasodilation was observed at early time points after WBF loading, the NOS inhibitor l-NAME was administered to a group of animals (1 g/l in drinking water) while another group received normal water (vehicle). Immunohistochemistry against Nos2 confirmed that l-NAME treatment blocked the expression of Nos2 in mast cells after WBF loading (Fig. 4, A–D). 15O water PET imaging was used to determine blood flow rate in l-NAME- and vehicle-treated animals after WBF loading. l-NAME treatment blocked the early increases in blood flow rate after WBF loading, suggesting that inhibition of NOS prevented vasodilation (Fig. 4E). 15O water flow rate (k1) was significantly increased (32–35%) in vehicle loaded limbs compared with nonloaded control limbs, matching the results from the first experiment (Fig. 2). In contrast, there were no significant differences between l-NAME loaded limbs and nonloaded control limbs at day 1 or 3 (Table 3).
Fig. 4.
NG-nitro-l-arginine methyl ester (l-NAME) treatment blocked Nos2 expression and decreased blood flow rate. A: mast cells were detected in the expanded periosteum 3 days after loading in vehicle treated animals and B, only these cells stained clearly positive for Nos2. C: in contrast, mast cells were detected in l-NAME treated animals but D, these cells did not express Nos2. E: l-NAME treatment also decreased blood flow rate at days 1 and 3 relative to vehicle. Muscle (M) and bone (B) are labeled in each cross section. Mean ± standard deviation, n = 6, *P < 0.05 vs. vehicle.
In addition, l-NAME-treated animals had decreased fluoride metabolism compared with vehicle animals (Table 4). 18F fluoride flow rate (K1) and total fluoride flux (Ki) were significantly increased in both vehicle and l-NAME-treated loaded limbs at days 1 and 3 compared with nonloaded control limbs but were significantly decreased in l-NAME-treated limbs compared with vehicle at day 1 (Fig. 5A). Importantly, 18F fluoride incorporation rate (k3) was significantly decreased in l-NAME-treated animals compared with vehicle at both day 1 and 3 (Fig. 5B).
Fig. 5.
l-NAME treatment impaired bone formation after WBF loading. A: 18F fluoride flow rate (K1) was significantly decreased at day 1 in l-NAME-treated animals vs. vehicle. B: 18F fluoride incorporation rate (k3) was significantly decreased at both days 1 and 3 in l-NAME-treated animals vs. vehicle. Representative images from dynamic histomorphometry 10 days after WBF loading in l-NAME-treated (C) and vehicle-treated (D) animals. Newly mineralized woven bone is labeled red. E: woven bone area was decreased 23% in l-NAME-treated animals. By microCT, woven bone volume was decreased 27% (F) and woven bone BMD was decreased 26% vs. vehicle (G). Mean ± standard deviation, n = 6, *P < 0.05 vs. vehicle.
Because these results suggested that l-NAME treatment impaired the skeletal repair response, dynamic histomorphometry and microCT were used to quantify woven bone formation. Woven bone area in l-NAME-treated WBF loaded limbs was significantly decreased (−23%) compared with vehicle loaded limbs based on fluorochrome labeled histological sections (Fig. 5, C–E). Additionally, mineralization of the woven bone appeared delayed. In vehicle-treated limbs, the entirety of the woven bone area was labeled with the fluorochrome, but l-NAME-treated limbs had areas of woven bone that remained unlabeled. This result was corroborated using microCT, which revealed l-NAME-treated WBF loaded limbs had significantly less woven bone volume (−27%) and decreased woven bone mineral density (−26%) at day 7 compared with vehicle loaded limbs (Fig. 5, F and G).
DISCUSSION
Here, the early vascular response to injurious and adaptive mechanical loading of bone was examined in a rat model. Significant increases in blood flow and fluoride metabolism were observed as woven bone formation (WBF) occurred in response to a stress fracture generated by damaging mechanical loading. Measurement of arterial area at the site of bone formation revealed local vasodilation in the first 3 days after WBF loading. In addition, WBF loaded limbs had significant infiltration of Nos2-positive mast cells at early time points. Mast cells were the predominant cell type expressing Nos2 in the expanded periosteal region. Treatment with the NOS inhibitor l-NAME blocked Nos2 expression in mast cells after WBF loading. Importantly, l-NAME treatment of WBF loaded animals also blocked the increases in blood flow rate at early time points and decreased woven bone formation at later time points. Taken together with previous work on angiogenesis in this model (45), these results indicate that a stress fracture stimulates an immediate, nitric oxide-mediated vasodilation response that precedes angiogenesis and woven bone formation as part of normal healing. In contrast, loading known to stimulate lamellar bone formation (LBF loading) does not cause increases in blood flow or vasodilation, does not stimulate inflammation, and leads to only a small, delayed vascular response that accompanies modest bone formation.
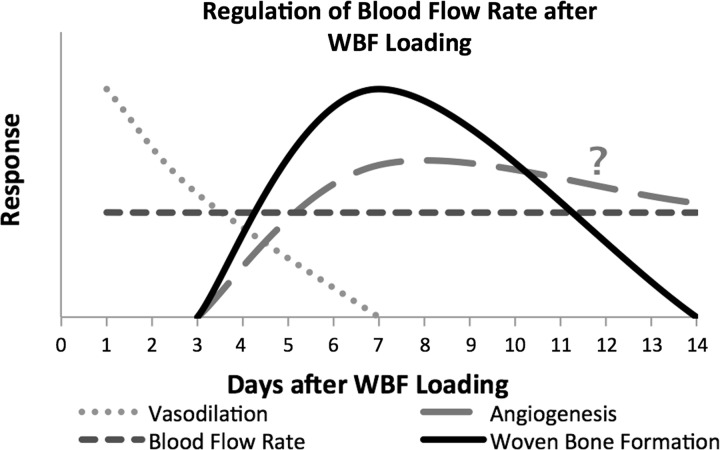
The results of this study clarify the early vascular response after stress fracture. As early as a few hours after WBF loading, the blood flow rate increased ∼30%; this significant increase was maintained for 14 days after loading. Using a microsphere injection technique, Muir and colleagues (33) also reported an increase in bone blood flow immediately after stress fracture. In contrast to this study, they reported that blood flow rate returned to baseline 14 days after stress fracture. The discrepancy at this time point may be attributed to differences in the magnitude of stress fracture as well as the region of interest. In this model, there are large increases in periosteal vascularity by day 14 (28, 31), consistent with our current finding that blood flow rate is elevated compared with the nonloaded limb. Nonetheless, the early increase in blood flow occurs prior to angiogenesis, which begins around day 3 in this model (45). Although a 20% increase in vessel volume was previously observed 3 days after WBF loading (31), this early increase in vessel volume is now demonstrated to be an adaptation of the existing vascular network. Immediately after WBF loading, mast cell infiltration and release of nitric oxide rapidly increases the blood flow rate at the site of skeletal damage through vasomotor action, similar to the increased blood flow (4) and NO-mediated vasoreactivity (11) observed after complete fracture. Importantly, this phenomenon is distinct from angiogenesis, a process that generally requires days for significant vascular expansion (9). However, many of the molecular events that regulate inflammation and vasomotor activity also regulate angiogenesis (6, 12, 34, 56). Therefore, these results support the conclusion that increased blood flow is initially facilitated by vasodilation and then maintained at the site of bone formation through angiogenesis (45) as inflammation subsides. Angiogenesis likely continues as the woven bone is remodeled, but this process is not well understood. This proposed model of the temporal regulation of bone blood flow after WBF loading is summarized in Fig. 6.
Fig. 6.
Working model for regulation of blood flow rate after WBF loading. The data presented here are summarized in this proposed model. Blood flow rate is immediately increased and maintained at the site of bone formation in the 2 wk after WBF loading. Elevated blood flow rate is initially facilitated by vasodilation and then maintained at the site of bone formation through angiogenesis as inflammation subsides. Inhibition of either vasodilation or angiogenesis impairs the woven bone formation response. The regulation of angiogenesis in the context of woven bone remodeling from days 7 to 14 and beyond is not well understood.
Because WBF loading is directly analogous to a stress fracture (51), this study demonstrates that blood flow is an important physiological parameter that is regulated during stress fracture healing. In particular, vasomotor activity is required to increase blood flow during the early stages of stress fracture repair, suggesting that patients with impaired vascular function may repair stress fractures poorly. Clinical observation supports this hypothesis—stress fractures in the fifth metatarsal have been observed to heal more slowly than stress fractures in the other metatarsals, possibly due to poor blood perfusion in this area (37, 43). In addition, poor vascular function may explain impaired bone healing caused by diabetes (53, 54) and smoking (1, 2). Therefore, assessing vascular function is an important consideration for patient care after stress fracture.
Previous studies have shown that the inhibition of NOS may have a direct effect on bone cells as well as mechanotransduction in bone. Osteocytes are known to release NO in response to fluid shear stress (7), and in vitro studies have shown that NOS inhibition impaired the proliferation and function of the osteoblast-like cell lines MG63 and ROS 17/2.8 (40). In addition, several studies have demonstrated that l-NAME treatment decreased loading-induced lamellar bone formation (10, 15, 50). In total, these results suggest that NOS inhibition decreases the osteogenic potential of mechanically stimulated bone (49). Here, the reduction in woven bone formation after l-NAME treatment was attributed to decreased blood flow due to impaired vasodilation, but other effects of l-NAME treatment may have contributed to this result. Additional experiments would be required to separate these effects.
In contrast to the relatively constant increase in blood flow rate, fluoride metabolism peaked 7 days after WBF loading and declined sharply between days 7 and 14. This time course is in agreement with previous studies: the strongest upregulation of bone matrix proteins (bone sialoprotein, osteocalcin) occurs between days 3 and 7 (55), whereas woven bone area does not increase between 7 and 14 days after WBF loading (51). Thus increases in fluoride uptake on days 3 and 7 are indicative of woven bone formation. In contrast, increased fluoride uptake at early time points is attributed primarily to crack formation, bolstered in part by increased overall blood flow. Skeletal damage that occurs during WBF loading opens additional bone surface for ion exchange, increasing 18F fluoride uptake beyond blood flow and bone formation alone (26).
The PET imaging in this study was strengthened by the use of simultaneous 15O water and 18F fluoride PET. In previous work, 18F fluoride alone was used to compare limbs with different levels of fatigue damage (42). Although increased fluoride uptake was associated with increased fatigue damage and increased woven bone formation, the relative contribution of blood flow to fluoride uptake was not clear. Here, the coordination between blood flow and fluoride uptake is clarified by using two radiopharmaceuticals. In particular, l-NAME treatment was shown to significantly decrease 15O water flow rate after WBF loading, but only had a limited effect on 18F fluoride flow rate, confirming that 18F fluoride flow rate primarily corresponds to mineralization, rather than blood flow.
Conclusion.
In this study, generation of a stress fracture by WBF loading was shown to induce a significant increase in blood flow rate that was maintained for 2 weeks. In contrast, LBF loading did not induce any significant differences in blood flow rate. The early increases in blood flow rate after WBF loading were found to be associated with increased local vasodilation, mast cell infiltration, and Nos2 expression. Inhibition of NOS by l-NAME treatment abolished the expression of Nos2 in mast cells, blocked the increase in blood flow rate at early time points, and impaired woven bone formation. In conclusion, these results demonstrate that NO-mediated vasodilation is an important feature of normal stress fracture healing that increases blood flow during the early stages of repair. In contrast, lamellar bone formation after normal physiological strain does not stimulate a significant vascular response.
GRANTS
This study was funded by a grant from the National Institutes of Health National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01 AR050211) and was performed in facilities supported by the Washington University Center for Musculoskeletal Research (P30 AR057235).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Nikki Fettig, Lori Strong, Dr. Richard Laforest, and J.R. Rutlin for assistance with PET scanning and analysis.
REFERENCES
- 1.Adams CI, Keating JF, Court-Brown CM. Cigarette smoking and open tibial fractures. Injury 32: 61–65, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol 43: 1731–1737, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Arnal JF, Warin L, Michel JB. Determinants of aortic cyclic guanosine monophosphate in hypertension induced by chronic inhibition of nitric oxide synthase. J Clin Invest 90: 647–652, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashcroft GP, Evans NT, Roeda D, Dodd M, Mallard JR, Porter RW, Smith FW. Measurement of blood flow in tibial fracture patients using positron emission tomography. J Bone Joint Surg Br 74: 673–677, 1992 [DOI] [PubMed] [Google Scholar]
- 5.Balbatun A, Louka FR, Malinski T. Dynamics of nitric oxide release in the cardiovascular system. Acta Biochim Pol 50: 61–68, 2003 [PubMed] [Google Scholar]
- 6.Brandi ML, Collin-Osdoby P. Vascular biology and the skeleton. J Bone Miner Res 21: 183–192, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Burger EH, Klein-Nulend J. Mechanotransduction in bone–role of the lacuno-canalicular network. FASEB J 13, Suppl: S101–S112, 1999 [PubMed] [Google Scholar]
- 8.Cerri PS, Pereira-Junior JA, Biselli NB, Sasso-Cerri E. Mast cells and MMP-9 in the lamina propria during eruption of rat molars: quantitative and immunohistochemical evaluation. J Anat 217: 116–125, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chappell JC, Wiley DM, Bautch VL. How blood vessel networks are made and measured. Cells Tissues Organs 195: 94–107, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chow JW, Fox SW, Lean JM, Chambers TJ. Role of nitric oxide and prostaglandins in mechanically induced bone formation. J Bone Miner Res 13: 1039–1044, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Corbett SA, McCarthy ID, Batten J, Hukkanen M, Polak JM, Hughes SP. Nitric oxide mediated vasoreactivity during fracture repair. Clin Orthop Relat Res 365: 247–253, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Costa C, Incio J, Soares R. Angiogenesis and chronic inflammation: cause or consequence? Angiogenesis 10: 149–166, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Doot RK, Muzi M, Peterson LM, Schubert EK, Gralow JR, Specht JM, Mankoff DA. Kinetic analysis of 18F-fluoride PET images of breast cancer bone metastases. J Nucl Med 51: 521–527, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang TD, Salim A, Xia W, Nacamuli RP, Guccione S, Song HM, Carano RA, Filvaroff EH, Bednarski MD, Giaccia AJ, Longaker MT. Angiogenesis is required for successful bone induction during distraction osteogenesis. J Bone Miner Res 20: 1114–1124, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Fox SW, Chambers TJ, Chow JW. Nitric oxide is an early mediator of the increase in bone formation by mechanical stimulation. Am J Physiol Endocrinol Metab 270: E955–E960, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Furuta S, Vadiveloo P, Romeo-Meeuw R, Morrison W, Stewart A, Mitchell G. Early inducible nitric oxide synthase 2 (NOS 2) activity enhances ischaemic skin flap survival. Angiogenesis 7: 33–43, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Giles TD, Sander GE, Nossaman BD, Kadowitz PJ. Impaired vasodilation in the pathogenesis of hypertension: focus on nitric oxide, endothelial-derived hyperpolarizing factors, and prostaglandins. J Clin Hypertens (Greenwich) 14: 198–205, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glowacki J. Angiogenesis in fracture repair. Clin Orthop Relat Res Oct: S82–S89, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Hausman MR, Schaffler MB, Majeska RJ. Prevention of fracture healing in rats by an inhibitor of angiogenesis. Bone 29: 560–564, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Hawkins RA, Choi Y, Huang SC, Hoh CK, Dahlbom M, Schiepers C, Satyamurthy N, Barrio JR, Phelps ME. Evaluation of the skeletal kinetics of fluorine-18-fluoride ion with PET. J Nucl Med 33: 633–642, 1992 [PubMed] [Google Scholar]
- 21.Hsu WK, Feeley BT, Krenek L, Stout DB, Chatziioannou AF, Lieberman JR. The use of 18F-fluoride and 18F-FDG PET scans to assess fracture healing in a rat femur model. Eur J Nucl Med Mol Imaging 34: 1291–1301, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hubmer MG, Fasching T, Haas F, Koch H, Schwarzl F, Weiglein A, Scharnagl E. The posterior interosseous artery in the distal part of the forearm. Is the term “recurrent branch of the anterior interosseous artery” justified? Br J Plast Surg 57: 638–644, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Kety SS. Theory of blood-tissue exchange and its application to measurement of blood flow. Methods Med Res 223–227, 1960 [Google Scholar]
- 24.Kidd LJ, Stephens AS, Kuliwaba JS, Fazzalari NL, Wu AC, Forwood MR. Temporal pattern of gene expression and histology of stress fracture healing. Bone 46: 369–378, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Kubo S, Yamamoto K, Magata Y, Iwasaki Y, Tamaki N, Yonekura Y, Konishi J. Assessment of pancreatic blood flow with positron emission tomography and oxygen-15 water. Ann Nucl Med 5: 133–138, 1991 [DOI] [PubMed] [Google Scholar]
- 26.Li J, Miller MA, Hutchins GD, Burr DB. Imaging bone microdamage in vivo with positron emission tomography. Bone 37: 819–824, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Martin P, Leibovich SJ. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol 15: 599–607, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Matsuzaki H, Wohl GR, Novack DV, Lynch JA, Silva MJ. Damaging fatigue loading stimulates increases in periosteal vascularity at sites of bone formation in the rat ulna. Calcif Tissue Int 80: 391–399, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McBride SH, Silva MJ. Adaptive and injury response of bone to mechanical loading. Bonekey Osteovision 1: 192, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKenzie JA, Bixby EC, Silva MJ. Differential gene expression from microarray analysis distinguishes woven and lamellar bone formation in the rat ulna following mechanical loading. PLos One 6: e29328, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKenzie JA, Silva MJ. Comparing histological, vascular and molecular responses associated with woven and lamellar bone formation induced by mechanical loading in the rat ulna. Bone 48: 250–258, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morales JK, Falanga YT, Depcrynski A, Fernando J, Ryan JJ. Mast cell homeostasis and the JAK-STAT pathway. Genes Immun 11: 599–608, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muir P, Sample SJ, Barrett JG, McCarthy J, Vanderby R, Jr, Markel MD, Prokuski LJ, Kalscheur VL. Effect of fatigue loading and associated matrix microdamage on bone blood flow and interstitial fluid flow. Bone 40: 948–956, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Nathan C. Points of control in inflammation. Nature 420: 846–852, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Pechak DG, Kujawa MJ, Caplan AI. Morphological and histochemical events during first bone formation in embryonic chick limbs. Bone 7: 441–458, 1986 [DOI] [PubMed] [Google Scholar]
- 36.Penteado CV, Masquelet AC, Chevrel JP. The anatomic basis of the fascio-cutaneous flap of the posterior interosseous artery. Surg Radiol Anat 8: 209–215, 1986 [DOI] [PubMed] [Google Scholar]
- 37.Raghavan P, Christofides E. Role of teriparatide in accelerating metatarsal stress fracture healing: a case series and review of literature. Clinical medicine insights. Endocrinol Diabetes 5: 39–45, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raichle ME, Martin WR, Herscovitch P, Mintun MA, Markham J. Brain blood flow measured with intravenous H2(15)O. II. Implementation and validation. J Nucl Med 24: 790–798, 1983 [PubMed] [Google Scholar]
- 39.Rengasamy A, Johns RA. Regulation of nitric oxide synthase by nitric oxide. Mol Pharmacol 44: 124–128, 1993 [PubMed] [Google Scholar]
- 40.Riancho JA, Salas E, Zarrabeitia MT, Olmos JM, Amado JA, Fernandez-Luna JL, Gonzalez-Macias J. Expression and functional role of nitric oxide synthase in osteoblast-like cells. J Bone Miner Res 10: 439–446, 1995 [DOI] [PubMed] [Google Scholar]
- 41.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods 9: 676–682, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silva MJ, Uthgenannt BA, Rutlin JR, Wohl GR, Lewis JS, Welch MJ. In vivo skeletal imaging of 18F-fluoride with positron emission tomography reveals damage- and time-dependent responses to fatigue loading in the rat ulna. Bone 39: 229–236, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Smith JW, Arnoczky SP, Hersh A. The intraosseous blood supply of the fifth metatarsal: implications for proximal fracture healing. Foot Ankle 13: 143–152, 1992 [DOI] [PubMed] [Google Scholar]
- 44.Temmerman OP, Raijmakers PG, Heyligers IC, Comans EF, Lubberink M, Teule GJ, Lammertsma AA. Bone metabolism after total hip revision surgery with impacted grafting: evaluation using H2 15O and [18F]fluoride PET; a pilot study. Mol Imaging Biol 10: 288–293, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomlinson RE, McKenzie JA, Schmieder AH, Wohl GR, Lanza GM, Silva MJ. Angiogenesis is required for stress fracture healing in rats. Bone 52: 212–219, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tomlinson RE, Silva MJ, Shoghi KI. Quantification of skeletal blood flow and fluoride metabolism in rats using PET in a pre-clinical stress fracture model. Mol Imaging Biol 14: 348–354, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tonkin MA, Stern H. The posterior interosseous artery free flap. J Hand Surg Br 14: 215–217, 1989 [DOI] [PubMed] [Google Scholar]
- 48.Torrance AG, Mosley JR, Suswillo RF, Lanyon LE. Noninvasive loading of the rat ulna in vivo induces a strain-related modeling response uncomplicated by trauma or periostal pressure. Calcif Tissue Int 54: 241–247, 1994 [DOI] [PubMed] [Google Scholar]
- 49.Turner CH, Pavalko FM. Mechanotransduction and functional response of the skeleton to physical stress: the mechanisms and mechanics of bone adaptation. J Orthop Sci 3: 346–355, 1998 [DOI] [PubMed] [Google Scholar]
- 50.Turner CH, Takano Y, Owan I, Murrell GA. Nitric oxide inhibitor l-NAME suppresses mechanically induced bone formation in rats. Am J Physiol Endocrinol Metab 270: E634–E639, 1996 [DOI] [PubMed] [Google Scholar]
- 51.Uthgenannt BA, Kramer MH, Hwu JA, Wopenka B, Silva MJ. Skeletal self-repair: stress fracture healing by rapid formation and densification of woven bone. J Bone Miner Res 22: 1548–1556, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uthgenannt BA, Silva MJ. Use of the rat forelimb compression model to create discrete levels of bone damage in vivo. J Biomech 40: 317–324, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—a meta-analysis. Osteoporos Int 18: 427–444, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Williams SB, Cusco JA, Roddy MA, Johnstone MT, Creager MA. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J Am Coll Cardiol 27: 567–574, 1996 [DOI] [PubMed] [Google Scholar]
- 55.Wohl GR, Towler DA, Silva MJ. Stress fracture healing: fatigue loading of the rat ulna induces upregulation in expression of osteogenic and angiogenic genes that mimic the intramembranous portion of fracture repair. Bone 44: 320–330, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ziche M, Morbidelli L, Choudhuri R, Zhang HT, Donnini S, Granger HJ, Bicknell R. Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J Clin Invest 99: 2625–2634, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]