Abstract
The root cause of the most common and serious of the sleep disorders is impairment of breathing, and a number of factors predispose a particular individual to hypoventilation during sleep. In turn, obstructive hypopneas and apneas are the most common of the sleep-related respiratory problems and are caused by dysfunction of the upper airway as a conduit for airflow. The overarching principle that underpins the full spectrum of clinical sleep-related breathing disorders is that the sleeping brain modifies respiratory muscle activity and control mechanisms and diminishes the ability to respond to respiratory distress. Depression of upper airway muscle activity and reflex responses, and suppression of arousal (i.e., “waking-up”) responses to respiratory disturbance, can also occur with commonly used sedating agents (e.g., hypnotics and anesthetics). Growing evidence indicates that the sometimes critical problems of sleep and sedation-induced depression of breathing and arousal responses may be working through common brain pathways acting on common cellular mechanisms. To identify these state-dependent pathways and reflex mechanisms, as they affect the upper airway, is the focus of this paper. Major emphasis is on the synthesis of established and recent findings. In particular, we specifically focus on 1) the recently defined mechanism of genioglossus muscle inhibition in rapid-eye-movement sleep; 2) convergence of diverse neurotransmitters and signaling pathways onto one root mechanism that may explain pharyngeal motor suppression in sleep and drug-induced brain sedation; 3) the lateral reticular formation as a key hub of respiratory and reflex drives to the upper airway.
Keywords: sleep, pharyngeal muscles, genioglossus muscle, obstructive sleep apnea, lung
this paper focuses on the physiological mechanisms underpinning state-dependent and reflex drives to the upper airway. In this context, the term “state” is used to encompass conditions of wakefulness and natural sleep, as well as drug-induced brain sedation. The further aim is to integrate, synthesize, and advance current concepts as they relate to the pathogenesis of obstructive sleep apnea (OSA). Other papers in this Highlighted Topics Series focus on different physiological and clinical aspects of upper airway control and function and the mechanical properties of the upper airway (7, 25a, 32, 93). For the reasons outlined below, we have chosen to focus on three specific areas that are not fully covered in the other papers in this series.
In the first section we summarize the recently identified mechanism underpinning the strong inhibition of genioglossus muscle during rapid-eye-movement (REM) sleep. The mechanisms mediating pharyngeal motor suppression in REM sleep had previously been a subject of debate because the powerful inhibitory mechanism operating in vivo had not been identified. Here we summarize a novel motor inhibitory signaling pathway that is operative in REM sleep. In the second section, we present evidence for the emerging principle that the common, serious, and at times life-threatening problems of sleep and sedation-induced depression of breathing and arousal responses may be working through common brain pathways acting on common cellular mechanisms. In particular, we discuss a major convergence of diverse neurotransmitters and signaling pathways onto one root mechanism that may explain pharyngeal motor suppression in states of both natural sleep and drug-induced brain sedation. We finally identify the lateral reticular formation as a key hub, or neural interface, for respiratory and reflex drives to the upper airway. The “negative pressure reflex” is used as a specific example. In this context, the involvement of the lateral reticular formation in the state-dependent modulation of respiratory and reflex drives is emphasized. Finally, for each of the three areas of focus, the applied physiology and clinical implications are highlighted throughout.
THE MECHANISM OF GENIOGLOSSUS MUSCLE INHIBITION IN REM SLEEP
REM Sleep and Motor Inhibition
The mechanisms underlying REM sleep generation are still debated, but will be briefly discussed since they relate to the potentially different inhibitory processes operating to depress spinal vs. upper airway motor activity in REM sleep.
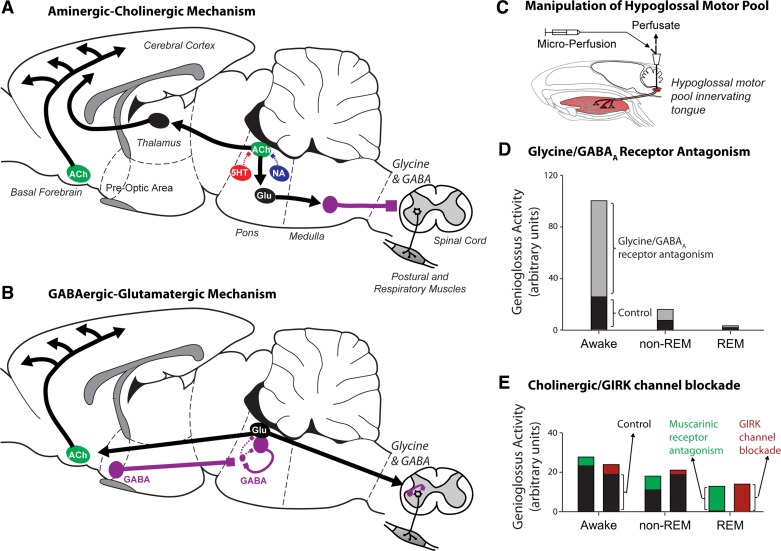
The aminergic-cholinergic mechanism of REM sleep generation is the more long-standing of the two explanations for this brain state (60, 63, 79), and the essential elements are illustrated in Fig. 1A. In this schema, a progressive withdrawal of monoaminergic neuronal activity in non-REM sleep, primarily via serotonin [5-hydroxytryptamine (5-HT)] and noradrenaline acting on 5-HT1A and α2-receptors respectively, leads to progressive disinhibition of mesopontine cholinergic neurons of the laterodorsal and pedunculopontine tegmental nuclei. This effect leads to increased cholinergic activity and increased acetylcholine release into the pontine reticular formation that triggers circuits mediating ascending thalamocortical arousal and descending motor inhibition. For the latter, cholinergic-induced activation of glutamatergic neurons of the pontine reticular formation recruits inhibitory relay neurons in the medullary reticular formation. These inhibitory interneurons then project, via the lateral reticulospinal tract, to the medial and ventral horn of the spinal cord to inhibit motor activity via glycine (predominantly) and γ-aminobutyric acid (GABA) (Fig. 1A) (95).
Fig. 1.
The rapid eye movement (REM) sleep-generating neuronal machinery according to the aminergic-cholinergic (A) and GABAergic-glutamatergic (B) explanations. Note that, in both explanations of REM sleep generation, descending inhibitory pathways involving glycine (predominantly) and γ-aminobutyric acid (GABA) cause suppression of spinal motor activity. Experimental manipulations of the hypoglossal motor pool (C), however, show that blockade of glycine and/or GABAA receptors activate genioglossus activity across all sleep-wake states, but least of all in REM sleep [D; replotted from original data (71)]. Note the initial level of genioglossus activity with artificial cerebrospinal fluid at the hypoglossal motor pool (i.e., baseline control condition), with progressive decreases in activity from wakefulness to non-REM and REM sleep. Note also the consistent increase in genioglossal activity across sleep-wake states with glycine and GABAA receptor antagonism. This pattern of response is in keeping with release of a tonic, state-independent inhibitory tone. A similar pattern of response has been observed in other studies, including at the trigeminal motor pool (10, 11, 70). The pattern of response with muscarinic receptor and G protein-coupled inwardly rectifying potassium (GIRK) channel blockade is also shown [E; replotted from original data (37)]. This pattern is consistent with blockade of a REM sleep inhibitory pathway. In the anatomical drawings from rodent brain, the solid lines indicate active neuronal groups and projections, respectively, whereas dashed lines indicate suppressed activity. Arrows indicates excitatory projections, whereas solid squares with solid lines indicate inhibitory projections. The relative position and sizes of neuronal groups are shown for visual clarity and are not meant to represent their strict anatomical positions. See text for further details. ACh, acetylcholine; 5HT, 5-hydroxytryptamine (serotonin); Glu, glutamate; NA, noradrenaline. This is an original figure modified and adapted from several sources (43, 44).
The GABAergic-glutamatergic mechanism of REM sleep generation involves different circuits for the generation of the electrocortical arousal and inhibition of motor activity in REM sleep (Fig. 1B) (8, 33, 58, 59). In this scheme, activation of GABAergic neurons in the extended region of the ventrolateral preoptic region of the hypothalamus preceding and during REM sleep leads to inhibition of GABAergic neurons of the ventrolateral periaqueductal gray and the lateral pontine tegmentum. This effect then disinhibits glutamatergic and GABAergic neurons in the vicinity of the sublaterodorsal tegmental nucleus (also known as “subcoeruleus”), which become active in REM sleep. The “REM-On” activity profile of the glutamatergic neurons in the subcoeruleus region essentially leads to electrocortical activation via ascending pathways and recruitment of cholinergic neurons in the basal forebrain. These glutamatergic neurons also lead to inhibition of spinal motor activity via long descending pathways and recruitment of short inhibitory relay neurons in the spinal cord (Fig. 1B) (8, 33, 58, 59).
Together, these brain stem circuits generate and sustain REM sleep. Despite the fundamental difference in the neuromodulators involved in generating REM sleep in the brain stem (Fig. 1, A and B), note that for each mechanism there are inhibitory glycine (predominantly) and GABA processes involved in the inhibition of spinal motor activity.
The “Problem” and Its Resolution
Episodes of major suppression of tongue muscle activity also occur during periods of REM sleep (17, 22, 42). In vivo data from naturally sleeping rodents, however, showed that, although glycine and GABAA receptor mechanisms exert strong inhibitory effects when manipulated at the hypoglossal motor nucleus in vivo (55, 72), and across sleep-wake states (70, 71), they contribute minimally to the major suppression of tongue muscle activity in REM sleep (70, 71). Such data were obtained via experimental manipulation of the hypoglossal motor pool using in vivo microperfusion in freely behaving rodents across sleep-wake states (Fig. 1C) and have since been confirmed at the trigeminal motor pool (10, 11). Most notably, individual and combined glycine and GABA receptor antagonism at the hypoglossal and trigeminal motor pools releases an inhibitory motor tone in wakefulness, non-REM sleep, and REM sleep, i.e., across all sleep-wake states (10, 11, 70, 71).
This pattern of response is depicted in Fig. 1D using data from one of the original studies at the hypoglossal motor pool (71) and has also been consistently observed in other studies, including at the trigeminal motor pool (10, 11, 70). This pattern of response is more in keeping with a gain-setting, tonic inhibitory tone that is independent of the prevailing brain state. Moreover, any motor activating effects observed in REM sleep with glycine and GABAA receptor blockade at the hypoglossal motor pool were trivial and smallest in magnitude in REM sleep compared with wakefulness and non-REM sleep (70, 71). This pattern is not expected, a priori, if there is recruitment of a glycine and/or GABAA receptor-mediated pathway that is responsible for inhibition of hypoglossal motor activity in REM sleep (i.e., as depicted for spinal motor activity in Fig. 1, A and B). Likewise, application of glycine and GABAA and GABAB receptor antagonists to the trigeminal motor pool either alone or in combination, also releases a tonic inhibition that activates trigeminal motor activity across all sleep-wake states, but again least of all in REM sleep (10, 11). In summary, these data suggest that inhibition by glycine and GABA cannot be viewed as a genuine and significant mediator of pharyngeal motor inhibition in REM sleep because the inhibitory tone is present in all states and weakest in REM sleep.
By contrast, the data in Fig. 1E show the pattern of response that is in keeping with recruitment of a motor inhibitory pathway responsible for inhibition of hypoglossal motor activity in REM sleep. Here the response is largest in magnitude in REM sleep compared with wakefulness and non-REM sleep, and the neurotransmitters, receptors, and channels that produced such an effect and pattern of response have been identified (37). Those data indicated that a muscarinic receptor mechanism that is functionally linked to G protein-coupled inwardly rectifying potassium (GIRK) channels operates at the hypoglossal motor pool, with this pathway exerting its largest inhibitory influence in REM sleep and lesser or no effects in other sleep-wake states (37). There is a strong precedent for such a signaling pathway in other physiological systems. It is well established, for example, that a cholinergic M2 receptor-mediated inhibitory signaling pathway links to GIRK channel activation, causing efflux of potassium ions, cellular hyperpolarization, and reduced neuronal excitability (26, 76). Such a mechanism is behind the effects of the classical “vagusstoff” on the heart (76). It is a new finding to sleep and respiratory neurobiology, however, that such a mechanism functions in a motor circuit to inhibit tongue muscle activity and with a pattern of response that is expected of a mechanism that is recruited in REM sleep.
Further work is needed to identify the source of these cholinergic inhibitory inputs to the hypoglossal motor pool in REM sleep. Hypoglossal premotor neurons of the intermediate medullary reticular formation, however, may be the most likely origin of the cholinergic-mediated inhibition of the tongue musculature (37). This assertion is because these neurons express cholinergic markers and innervate the hypoglossal motor pool (104), and many neurons in this region remain active during REM sleep (78, 108). see the lateral reticular formation as a key state-dependent hub of respiratory and reflex drives to the upper airway below for further discussion. The cholinergic-induced suppression of hypoglossal motor activity may be caused by presynaptic inhibition of either excitatory glutamatergic transmission (3), or inhibitory transmission (80), and/or postsynaptic effects (12).
Applied Physiology and Clinical Implications
Progress in the neurobiology of upper airway motor control notwithstanding, questions remain regarding the clinical and translational aspects of REM sleep as it pertains to OSA. First, some have questioned the clinical relevance of the intrinsically variable breathing during REM sleep (15). However, some patients experience profound desaturations during REM sleep that seem unlikely to be normal physiological variants. Rather, these are more likely the product periods of hypoventilation plus depressed ventilatory and arousal responses to asphyxia in REM sleep compared with non-REM sleep (19).
For completeness, although explaining the periods of pharyngeal motor inhibition in REM sleep have been the focus of the preceding sections, we note that REM sleep is also typically accompanied by transient excitatory drives to the cranial and spinal motor pools. These transient excitatory drives manifest as brief flurries of muscle “twitches” that emerge from a background of atonia. Excitatory glutamatergic inputs mediate these excitatory events at spinal motoneurons (97), but this finding remains to be confirmed for pharyngeal motoneurons. Such sporadic bursts of pharyngeal motor activity may have functional impacts on upper airway mechanics and may even be responsible for the sporadic improvements in airway patency in REM sleep and REM-related obstructive hypopneas in animals (35) and humans (111). Thus there are likely different manifestations of breathing variability during REM sleep with varying consequences relating to changes in ventilatory control, arousal threshold, and upper airway mechanics.
Data are also emerging that different patients develop OSA based on varying mechanisms (20, 22, 25, 110). Whereas some patients likely have unstable ventilatory control (17, 51, 105, 115), others have anatomical deficiencies and/or upper airway motor control abnormalities. Other patients likely have combinations of underlying mechanisms that predispose them to obstructive apneas in non-REM sleep and/or REM sleep. This realization has led to the concept of individualized therapies directed at the key underlying mechanisms within a patient (20, 90, 110). Nevertheless, both animal and human data also support the concept of upper airway hypotonia as a key pathological mechanism in REM sleep (23).
Appropriate therapeutic targets for REM-related apnea also remain to be defined. Manipulation of the neurochemistry controlling respiratory neurons and motoneurons, via either neurotransmitter systems or (more likely) their downstream signaling mechanisms (upper airway motor control: a common mechanism underpinning the effects of sleep, sedation and anesthesia below), may be a viable approach, particularly for selected patients (36). This subject is introduced in upper airway motor control: a common mechanism underpinning the effects of sleep, sedation and anesthesia below and discussed at length in a recent article, with a view to targeted manipulation of certain K+ (non-GIRK) channels that are almost exclusively expressed in the brain at cranial motor pools, such as those innervating the pharyngeal musculature (38). Another approach is the pharmacological suppression of REM sleep (e.g., using selective serotonin re-uptake inhibitors), which is well tolerated, although efficacy data are lacking (6). Finally, electrical stimulation of upper airway muscles may be a viable approach (92, 93), especially for patients with REM-predominant OSA, given the reliance of upper airway motor activity to maintain pharyngeal patency in certain patients (92).
Debate continues as to whether REM-predominant sleep apnea is a unique disorder or simply a mild form of OSA, commonly seen in women (16). Prevalence estimates of REM-predominant sleep apnea vary widely from roughly 10–50%, depending on the definitions and criteria used (28, 50). The recent trend toward home sleep testing, in which sleep stages are not typically assessed, may amplify further the importance of determining how various patient groups should be identified and treated. For example, if conclusive data show that patients with OSA isolated to REM sleep do not require therapy, such patients may inappropriately be given continuous positive airway pressure therapy, unless sleep stages were adequately assessed. A recent editorial has emphasized the imperfect nature of the current definitions in REM-predominant OSA and suggested alternative definitions that may bring some clarity in this area (66). Such rigor will be required to define fully the impact and appropriate management of sleep apnea during REM sleep.
UPPER AIRWAY MOTOR CONTROL: A COMMON MECHANISM UNDERPINNING THE EFFECTS OF SLEEP, SEDATION, AND ANESTHESIA
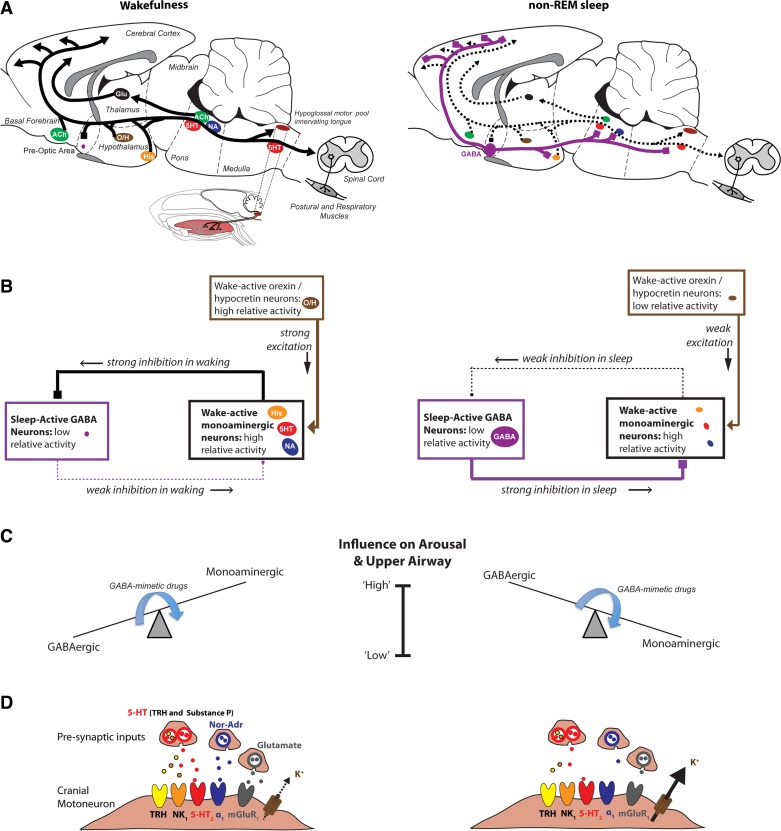
OSA is a disorder that, by definition occurs only in sleep, emphasizing the critical importance of state-dependent mechanisms on motor outflow to the pharyngeal muscles and their reflex control. Figure 2 shows some of the main neuronal groups involved in sleep-wake regulation and their potential for modulating respiratory activity. Serotonin, histamine, noradrenaline, orexin, acetylcholine, and glutamate-containing neuronal groups collectively contribute to the brain activation of wakefulness (Fig. 2A) (64, 79, 91). Activity of this arousal system is opposed by an inhibitory GABA system that originates principally (but not exclusively) from the ventrolateral preoptic area of the hypothalamus to promote sleep. Mutual inhibitory interactions between the wake-promoting and sleep-promoting neuronal systems leads to wakefulness being accompanied by relatively low activity in the GABAergic sleep-promoting system, combined with relatively high activity in the arousal-related/wake-promoting system; the situation is reversed in sleep (Fig. 2, B and C) (64, 79, 91).
Fig. 2.
State-dependent modulation of brain arousal and upper airway muscle function in wakefulness (left) and sleep (right). A: shown are the main neuronal groups involved in sleep-wake regulation and their potential for modulating spinal and upper airway motor activity. B: the reciprocally opposing wakefulness-promoting and sleep-promoting neural systems are organized such that consolidated periods of wakefulness or sleep are produced when either system predominates. C: states of brain arousal and sleep are thus the product of relatively high activity on one side of the switch plus lower activity on the other (e.g., high GABAergic inhibitory tone plus low excitatory monoaminergic tone). Many commonly used sedative and anesthetic drugs augment the GABAergic inhibitory tone and correspondingly reduce the excitatory monoaminergic tone (see arrows in C), therefore tipping the balance toward low brain arousability plus suppression of upper airway muscle tone and reflex responses. D: also shown is a model for K+ channel modulation to explain reduced hypoglossal motoneuron activity in sleep. This schema shows how reduced NA, Glu, serotonin, thyrotropin-releasing hormone (TRH), and substance P inputs to the hypoglossal motor pool in sleep can lead to augmented K+ leak at hypoglossal motoneurons and, therefore, reduced hypoglossal motor activity. As for Fig. 1, in the anatomical drawings from rodent brain, the solid lines indicate active neuronal groups and projections, respectively, whereas dashed lines indicate suppressed activity. The arrows indicates excitatory projections, whereas solid squares and lines indicate inhibitory projections. The relative position and sizes of neuronal groups are also shown for visual clarity and are not meant to represent their strict anatomical positions. See text for all further details. His, histamine; O/H, orexin/hypocretin; NK1, neurokinin-1; mGluR, metabotropic Glu receptor. This is an original figure, with A modified and adapted from several sources (43, 44).
These reciprocally opposing excitatory (wake) and inhibitory (sleep) systems have descending projections to neurons and motoneurons of the respiratory network. They can, therefore, markedly influence autonomic functions and breathing across sleep-wake states via alterations in neurotransmitter inputs and the pre- and/or postsynaptic receptor elements affected. The projections of wake and sleep-related cell groups to the respiratory network explains the rationale for previous studies that first identified the control of genioglossus activity by individual components of the wake/sleep inputs to the hypoglossal motor pool. This work has been reviewed in the context of sleep in general (41, 43) and also REM sleep (the mechanism of genioglossus muscle inhibition in rem sleep above). In short, noradrenergic, glutamatergic, and serotonergic influences contribute, to varying degrees, to the activation of pharyngeal motor pools in wakefulness, with these excitatory influences withdrawn in sleep (41, 43). Activation of a cholinergic mechanism is the strong source of motor inhibition in REM sleep (37). There is now emerging evidence, discussed below, supporting a common downstream mechanism impacted by the collective changes in wake/sleep inputs to the hypoglossal motor pool that may explain reduced pharyngeal motor activity in sleep.
K+ Channel Modulation and Pharyngeal Motor Activity
K+ channels are major determinants of subthreshold membrane activity and discharge properties and significantly modulate cell excitability. There is a wide diversity of K+ channels with various current-conducting properties. For potential control of upper airway muscle activity, of particular relevance are those channels that do the following.
Affect resting membrane potential and/or stabilize membrane potential around rest.
K+ “leak” channels strongly influence resting membrane potential and cell excitability (1, 86). “Inward rectifier” K+ channels (Kir) can also stabilize membrane potential around rest (75, 86). These channels are open at hyperpolarized membrane potentials, and their K+ conductance decreases with depolarization. Opening of such channels changes the membrane potential toward the K+ equilibrium potential, thereby reducing cell excitability. This effect, for example, could explain changes in genioglossus activity with sleep, including REM sleep (37).
Are expressed on cranial motoneurons.
Several Kir channels are expressed at pharyngeal motor nuclei, including hypoglossal motoneurons (e.g., Kir 2.2, 2.4, 4.1, 5.1, and GIRK 1 and 3 channels) (48, 86, 102, 109). Of potential high relevance to clinical medicine and translational sleep science is the finding that Kir 2.4 is the most restricted of all Kir subunits in the brain, being expressed mainly on cranial motoneurons such as the hypoglossal (102). K+ leak channels of the TASK [TWIK (tandem of pore domains in weak inward rectifier K+ channels)-related acid-sensitive K+ channel] family (TASK-1, -3) are also highly expressed on hypoglossal motoneurons (1, 4).
May be influenced by changes in neuromodulator inputs across sleep-wake states.
Endogenous noradrenergic and glutamatergic (and to a lesser extent serotonin) inputs to the hypoglossal motor pool constitute the essential components of the “wakefulness stimulus” that activates genioglossus muscle in wakefulness (41, 43). Withdrawal of this excitation contributes to reduced genioglossus activity in sleep. Importantly, noradrenaline, glutamate (via group I metabotropic glutamate receptors), serotonin, thyrotropin-releasing hormone, and substance P (the latter two are coreleased with serotonin) all inhibit K+ leak at pharyngeal motoneurons, leading to motor excitation (Fig. 2D) (86, 101). These neuromodulators are released from wake-active cell groups that have been identified to contribute to genioglossus and hypoglossal activity in vivo (41, 43, 53). Accordingly, there is now a mechanism that is only beginning to be explored (36), whereby certain K+ channels may constitute the common downstream mechanism impacted by the collective reductions in these neuromodulator inputs to the hypoglossal motor pool in sleep (Fig. 2D).
Although recent evidence has shown that K+ channel blockers at the hypoglossal motor pool can markedly increase genioglossus activity across sleep-wake states (36), and some specifically in REM sleep (37), as predicted a priori from the controlling machinery (Figs. 1 and 2), future work is needed to identify 1) if state-dependent K+ channel opening at the cranial motor pools constitutes the root mechanism underlying reduced pharyngeal muscle activity in sleep; and 2) which channel family and subtypes, and intermediate signaling molecules, are critically involved. Identifying these mechanisms could, in principle, be used to prevent the critical sleep-related loss of pharyngeal muscle activity and reflex responses that can impair pharyngeal mechanics. Given that some of these K+ channel targets show highly restricted expression, some predominantly or exclusively at the cranial motor pools (36, 102), modulating their activity is a promising avenue for future research.
Casting A Wider Net: Common Mechanisms Modulating Pharyngeal Motor Activity in States of Sleep, Sedation, and Anesthesia
Here we identify that the brain cells and pathways involved in generating and sustaining natural sleep are similar, in many ways, to those affected by commonly used sedative and anesthetic agents (2, 12, 31, 60). This concept has key relevance to understanding the effects on upper airway motor activity and reflex responses caused by sleep and neurodepressive drugs.
Augmentation of the endogenous sleep-promoting GABA system identified in Fig. 2 can also explain the sedative-hypnotic effects of commonly used neurodepressive drugs: benzodiazepines, imidazopyridines, barbiturates, ethanol, and everyday general anesthetics that are either delivered by inhalation (e.g., isoflurane) or intravenously (e.g., propofol or etomidate). All of these agents enhance GABA-mediated neuronal inhibition via interactions with different, and in some cases highly specific, binding sites on GABAA receptors (2, 31, 65). It is also for this reason that respiratory depression, impaired ventilatory responses to asphyxia, and reduced brain arousability can result from excessive stimulation of GABAA receptors and the circuits they control (Fig. 2). There are several identified sites within the endogenous sleep circuitry where anesthetics act to cause components of sedation and loss of consciousness (57, 68, 73, 74); for reviews see Refs. 31 and 60. These points reinforce the overarching principle that the sedative actions of many hypnotic and anesthetic agents can be mediated through similar cells and pathways that promote natural sleep and via these mechanisms can also depress breathing (Fig. 2).
It is emphasized that the depression of upper airway muscle activity, breathing, and brain arousability that can occur in both natural sleep and in the presence of neurodepressive drugs is likely the product of two mechanisms: augmentation of inhibitory GABAergic influences, and depression of arousal-related stimulatory influences (Fig. 2, A–C). Of note, these inhibitory and excitatory mechanisms cannot operate independently. The interconnectedness of these two mechanisms is by virtue of their reciprocal anatomical organization and neuronal projections (Fig. 2, A–C). The mutually inhibitory interactions generate the states of wakefulness and sleep, and by their other projections also influence upper airway motor activity (Fig. 2, A–C). Neurodepressive drugs essentially tip the balance within the sleep-wake circuitry (Fig. 2C), particularly at loss of consciousness (21, 40).
By virtue of the arrangement and reciprocal circuitry of the sleep-wake generating systems, the position and balance within the circuit can depress upper airway muscle activity and reflex responses by 1) augmenting the GABAergic component to increase the tonic inhibitory GABA tone that acts upon the pharyngeal musculature (i.e., as identified in the mechanism of genioglossus muscle inhibition in rem sleep above, Figs. 1D and 2C); and 2) inhibiting the arousal-related component that leads to an indirect withdrawal of excitatory neuromodulators that importantly modulate K+ channel function (Fig. 2, C and D). As a result of this organizational schema, therefore, the respiratory depression accompanying sleep, sedation, and anesthesia are working through common brain pathways operating on common signaling pathways. Identifying these common mechanisms, and the critical modifying factors, may, therefore, lead to new strategies that can reverse such upper airway motor depression in states of sleep and drug-induced brain sedation (20, 21). Interventions targeting a key hub in the organizational structure for state-dependent upper airway motor control and/or the common downstream targets upon which these mechanisms ultimately converge (e.g., critical K+ channels) may be particularly fruitful therapeutic approaches (Fig. 2).
Experiments in animals have also identified that there are certain circumstances in which upper airway motor activity can be raised by certain hypnotics and anesthetics; i.e., that the effects are not always inhibitory (27, 81, 87, 99, 116); for reviews see Refs. 42 and 43. Anesthetic doses of halothane and pentobarbital increase c-Fos expression in specific hypoglossal premotoneurons, and such a central effect may be the cause of the increased genioglossus activity observed with these drugs in rodents (87). The Kölliker-Fuse nucleus in the pons is a particularly notable site of neuronal activation (87), not least because this region projects to, and excites, hypoglossal motoneurons (54). These observations may at first seem paradoxical. However, upper airway motor-augmenting responses by GABAergic agents can be accommodated in the general scheme shown in Fig. 2. The net effect of systemically applied GABAA-receptor modulating sedatives on upper airway motor activity is a balance between motor suppression via effects on the endogenous sleep-wake circuitry (i.e., the aforementioned augmentation of tonic GABAergic inhibition and concomitant withdrawal of arousal-related excitation, Fig. 2) vs. augmenting effects acting via premotor inputs, such as the Kölliker-Fuse nucleus (87), among other possibilities. The latter effects are of growing interest because identifying such respiratory-beneficial sites of action of these sedating agents outside of the endogenous sleep circuitry may yield new pharmacological targets of potential clinical relevance to raising upper airway motor activity without affecting sleep regulation.
Applied Physiology and Clinical Implications
Repetitive alterations in sleep-wake state can be viewed as both a consequence and cause of respiratory disturbance because such alterations also act as a destabilizing influence on respiratory control (111, 112). For example, a period of respiratory disturbance can itself precipitate arousal from sleep, with the hyperpnea to that arousal itself then predisposing to subsequent hypoventilation and apneas upon a return to sleep. As such, a change in the predisposition to sleep-wake disruption, in particular arousal from sleep via a change in arousal threshold, can modify the expression of sleep-disordered breathing.
This concept originated from an appreciation for, and analysis of, the periods of stable breathing that occasionally occur spontaneously during stable non-REM sleep, even in patients with otherwise severe OSA (111, 112). There are elevations in genioglossus activity during those spontaneously occurring periods of stable breathing, consistent with the concept that upper airway dilator muscles are both necessary and sufficient for the stabilization of breathing during sleep (62, 85, 98). However, the elevations in genioglossus activity and the stimuli that drive it gradually build up over time (47). Based upon the position that arousals from sleep can be both a consequence and cause of respiratory disturbance, then a low arousal threshold (i.e., a predisposition to wake up easily) may be detrimental, as recurrent arousals would not allow sufficient accumulation of respiratory stimuli to activate pharyngeal dilator muscle and stabilize pharyngeal mechanics (20). Conversely, a high arousal threshold may be deleterious, since profound hypoxemia and hypercapnia may develop, leading to end-organ consequences. Thus the manipulation of arousal threshold may be a “double-edged sword”, with some patients predicted to benefit from elevating arousal threshold, whereas other patients may get worse (24, 39).
In this scenario, a respiratory-beneficial effect of sedating agents would work as follows. The hypothesis is that, if a sedative agent delayed arousal from sleep in response to a period of obstructive hypopnea, such that there was sufficient accumulation of chemoreceptor stimulation to stabilize breathing and restore airflow through the upper airway in that individual, then the repetitive and destabilizing influence of arousals would be curtailed, and stable breathing would follow (111, 112, 116). Prior papers summarize the published data on sedative hypnotics and upper airway motor activity and airway collapsibility in human subjects, with implications for sleep-disordered breathing (43, 81, 113, 116). Some early clinical data support the approach of using sedative/hypnotic medications in selected OSA patients to raise the arousal threshold and prevent repetitive respiratory events (24, 39), emphasizing the need for careful phenotyping of potentially responsive individuals (20, 25, 110).
THE LATERAL RETICULAR FORMATION AS A KEY STATE-DEPENDENT HUB OF RESPIRATORY AND REFLEX DRIVES TO THE UPPER AIRWAY
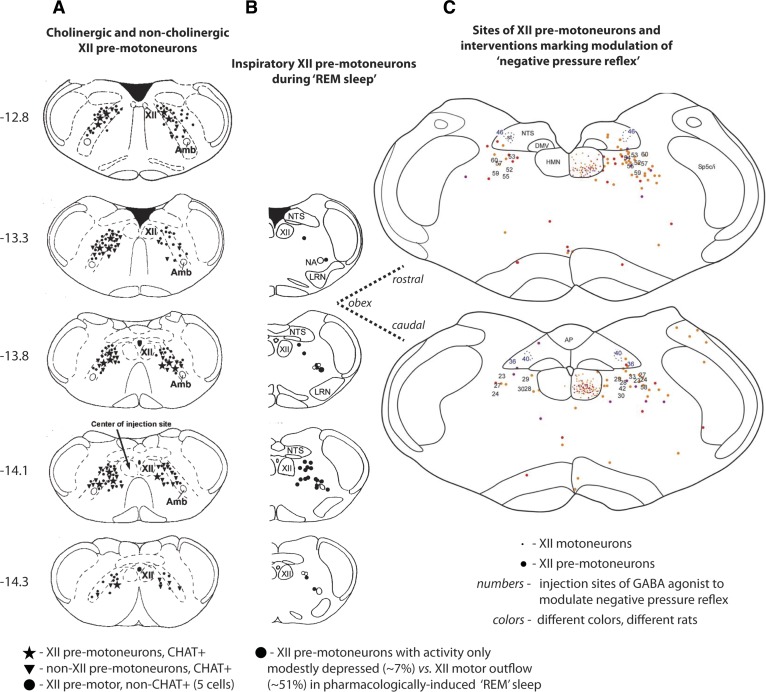
The source of inspiratory drive to hypoglossal motoneurons is different from phrenic motoneurons, being predominantly from the reticular formation lateral to the hypoglossal motor pool for the former, and from bulbo-spinal dorsal and ventral respiratory group neurons for the latter (3, 84, 96). This reticular region is also the major source of cholinergic hypoglossal premotoneurons (104) (Fig. 3A), as well as inspiratory hypoglossal premotoneurons that remain significantly more active than hypoglossal motor activity during REM sleep (108) (Fig. 3B). Regions of the medullary reticular formation rostral to obex are also critical to the circuitry of the “negative pressure reflex” (14) (Fig. 3C) and opioid-induced suppression of hypoglossal motor activity (67). The reticular formation provides a major source of tonic drive to respiratory neurons and motoneurons, and this tonic drive is particularly affected in sleep (77). In summary, the reticular region lateral to the hypoglossal motor pool acts as a “state-dependent interface” for respiratory and reflex inputs to the hypoglossal (and likely other) cranial motor pools.
Fig. 3.
A: sections of rat brain stem showing the location of cholinergic [choline acetyltransferase positive (CHAT+)] hypoglossal (XII) premotoneurons (★) in the reticular formation lateral to the hypoglossal motor nucleus (HMN) (104). Also shown are non-XII premotor cholinergic cells (▼) and noncholinergic XII premotoneurons (●). B: the location of inspiratory XII premotoneurons that exhibit sustained or only modest depression of activity during carbachol-induced “REM sleep” [mean decrease of 7 ± 14 (SD)% from 69 ± 34 to 65 ± 37 Hz] (108). Some cells showed increased activity in the “REM” periods; in contrast, the suppression of hypoglossal motor activity (51 ± 22%) was significantly larger. C: the location of XII premotoneurons in the reticular formation adjacent to the HMN are indicated by the larger colored dots (the smaller dots indicate motoneurons in the HMN) (14). The sites of interventions with the GABAA receptor agonist muscimol to modulate the “negative pressure reflex” are also shown (sites are indicated by the numbers on the corresponding sections). Muscimol injections at and up to 500 μm rostral to the obex abolished the negative pressure reflex; injections caudal to obex did not. The sections in A and B are taken from 12.8 to 14.3 caudal to the skull reference point bregma and are plotted according to a standard brain atlas (83). The sections in C are plotted relative to obex and correspond most closely to the sections indicated by the dashed lines. AP, area postrema; DMV, dorsal motor nucleus of the vagus; NTS, nucleus of the solitary tract; LRN, lateral reticular nucleus; Amb, nucleus ambiguus. [From Volgin et al. (104), Woch et al. (108), and Chamberlin et al. (14).]
The “Negative Pressure Reflex”
Subatmospheric (“negative”) pressures in the upper air space elicit reflex activation of the pharyngeal musculature. This reflex has been extensively characterized in both animals and human subjects (42). There are certain characteristics of this reflex that make it particularly pertinent to pharyngeal motor control and OSA pathogenesis. For example, different individuals have characteristically different “strengths” of response to a given stimulus of negative airway pressure when measured in wakefulness, and whether someone is a “big” or “small” responder is repeatable on different days (42, 46). Different individuals also experience characteristically different degrees of suppression of their reflex responses to airway negative pressure from wakefulness to sleep: some lose their reflex at sleep onset, whereas others maintain it, at least to some degree (94). Different individuals also exhibit different pharyngeal dilator muscle responses to the hypoxia and hypercapnia that accumulates during hypopneas and apneas, as well as also exhibiting different arousal responses to the same stimuli (82, 107, 111, 113). These examples of person-specific physiological traits coexist with person-specific anatomical traits; for example, some individuals have larger upper airways than others, some have longer collapsing segments than others, while some have thicker palates or more parapharyngeal or submental fat deposits (17, 20, 22).
Key to the pathogenesis of OSA within an individual is the interaction of the mechanical (anatomical) factors with the effectiveness of upper airway neuromuscular (reflex) compensation (42, 82, 111, 113). In this view, an individual with a robust neuromuscular response would be better able to maintain (or restore) a patent upper airway, even with a high “mechanical load” (i.e., an air space that is anatomically predisposed to collapse), compared with an equally anatomically predisposed individual with relatively weak neuromuscular compensatory responses (82, 111, 113). Thus any suppression in reflex responses (e.g., caused by the common mediators underpinning the effects of sleep, sedation or anesthesia; see upper airway motor control: a common mechanism underpinning the effects of sleep, sedation, and anesthesia above and Fig. 2) would lead to an increased tendency to develop OSA. In this scheme, individuals with already “weak” responses to subatmospheric airway pressure (42, 46), or those with large decrements at sleep onset (94), would be most susceptible.
The Brain Stem Circuitry of the Negative Pressure Reflex
Afferents in the superior laryngeal, glossopharyngeal, and trigeminal nerves mediate the reflex effects of subatmospheric airway pressure on the pharyngeal musculature (42, 45). The principal site of central termination of upper airway afferents is the nucleus of the solitary tract, with additional projections to the trigeminal sensory nucleus (52). Superior laryngeal nerve and glossopharyngeal inputs are thought to reach the hypoglossal motor nucleus via the nucleus of the solitary tract, whereas trigeminal inputs reach the hypoglossal motor nucleus via the sensory trigeminal nucleus (56).
Injection of pseudorabies virus into the genioglossus muscle is used to identify hypoglossal premotoneurons, and this approach has been applied to identify the anatomy and circuitry of the negative pressure reflex (14). As introduced in the mechanism of genioglossus muscle inhibition in rem sleep above, some hypoglossal premotor neurons are clustered in the reticular formation lateral to the hypoglossal motor pool (18, 30, 103). Microinjection of GABAA receptor agonists into this same region elicits an increase in respiratory-related genioglossus activity (14). This result indicates the presence of a tonic inhibitory drive to the hypoglossal motor pool that arises from this region containing hypoglossal premotor neurons (14). Notably, microinjection of GABAA receptor agonists into this region also abolishes the negative pressure reflex that beforehand activated the genioglossus (14) (Fig. 3). This result positions this region as a component of the brain stem circuitry mediating the negative pressure reflex.
The components of the circuitry mediating the “negative pressure reflex” have been proposed by Chamberlin and colleagues (14), based on the above-cited neuroanatomy, identification of hypoglossal premotoneurons, and responses to GABAA receptor agonists in different medullary regions. The circuit includes mechano-sensitive afferents in the superior laryngeal nerve that terminate in the interstitial nucleus of the nucleus of the solitary tract (34). The solitary tract has strong projections to the lateral reticular formation (88) with the circuit completed by projections to the hypoglossal motor nucleus (9). It is also important to note that, in rats, tonic activity in superior laryngeal nerve afferents inhibits upper airway motor activity, with subatmospheric upper airway pressure suppressing this tonic inhibitory superior laryngeal nerve activity, so leading to motor activation (89). What this circuitry has been taken to suggest is that abolition of the negative pressure reflex with local inhibition of relay neurons in the periobex region, i.e., where the GABAA receptor agonists were applied (14), requires the presence of inhibitory relays in the reflex pathway, in both the nucleus of the solitary tract and the periobex region (14). These have yet to be identified electrophysiologically, likewise the sites where the state-dependent modulators identified in Fig. 2 exert their actions. These remain important avenues for further study, given the major impact of state-dependent upper airway reflexes in maintaining a patent upper air space, and the ability of neuromuscular compensation to restore patency once closed (22, 42, 82, 111, 113).
Applied Physiology and Clinical Implications
Despite considerable insights from basic and translational work on upper airway reflexes, the importance of the negative pressure reflex has been questioned. For example, the observation that the negative pressure reflex is normal, if not augmented, in OSA patients compared with controls during wakefulness has led some to suggest that other factors must be underlying pharyngeal collapse (5). Similarly, the observation that the pharyngeal airway may close during expiration rather than inspiration might suggest that the tonic activity of the upper airway muscles may be more important than the activation of the muscles in response to collapsing perturbations during inspiration (69). However, we have speculated the reflex to be important for several reasons. First, pharyngeal collapse is highly variable, with many patients experiencing progressive upper airway narrowing during inspiration rather than exclusively expiratory narrowing (49). Thus mechanisms that protect the pharyngeal airway area are likely to be important (61, 100). Second, as stated, OSA pathogenesis is highly variable across patients (20). Thus, although group averages suggest preserved negative pressure reflexes in OSA, a subset of OSA patients likely exists in whom augmentation of reflex activity may be protective of pharyngeal patency. Third, recent emphasis has been placed on the concept of negative effort dependence (NED) (61, 100). NED refers to the progressive reductions in airflow which can occur in the face of increasing driving pressure (29). Such progressive diminution in airflow-type behavior defining NED is in contrast to the traditional “Starling Resistor” model in which airflow remains constant over a range of driving pressures (13). Although NED has been recognized for years, there has been a more recent emphasis on the magnitude of the decline in inspiratory flow in selected patients (61). The mechanisms underlying NED are debated, but are likely to be a function of within-breath changes in pharyngeal mechanics; thus deficiencies in the negative pressure reflex may theoretically be important in patients with marked NED.
In summary, we have selected three illustrative topics to provide insights into the pathogenesis of sleep apnea and the potential for therapeutic manipulation. Insights from basic neurobiology and human physiology are clearly complementary in determining the translational potential for various interventions. Only through further research into underlying mechanisms are new therapeutic strategies likely to emerge.
GRANTS
This work was supported by funds from the Canadian Institutes of Health Research (CIHR, Grant MT-15563), and a collaborative research grant from Eli Lilly and Company. R. L. Horner is currently supported by funds from the Canadian Institutes of Health Research (CIHR, Grant MT-15563) and a Tier I Canada Research Chair in Sleep and Respiratory Neurobiology. S. W. Hughes is Principal Research Scientist, Lilly Research Laboratories, UK. A. Malhotra has received consulting and/or research income from Philips, SGS, SHC, Pfizer, Apnex and Apnicure, but has relinquished all outside personal income since May 2012. A. Malhotra is funded by National Heart, Lung, and Blood Institute Grants R01-HL-110350, P01-HL-095491, K24-HL-093218, R01-HL-090897, and NIH-R01-HL085188 and American Heart Association Grant 0840159N.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.L.H., S.W.H., and A.M. conception and design of research; R.L.H., S.W.H., and A.M. interpreted results of experiments; R.L.H. prepared figures; R.L.H. and A.M. drafted manuscript; R.L.H., S.W.H., and A.M. edited and revised manuscript; R.L.H., S.W.H., and A.M. approved final version of manuscript.
REFERENCES
- 1.Bayliss DA, Barrett PQ. Emerging roles for two-pore-domain potassium channels and their potential therapeutic impact. Trends Pharmacol Sci 29: 566–575, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci 6: 565–575, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Bellingham MC, Berger AJ. Presynaptic depression of excitatory synaptic inputs to rat hypoglossal motoneurons by muscarinic M2 receptors. J Neurophysiol 76: 3758–3770, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Berg AP, Talley EM, Manger JP, Bayliss DA. Motoneurons express heteromeric TWIK-related acid-sensitive K+ (TASK) channels containing TASK-1 (KCNK3) and TASK-3 (KCNK9) subunits. J Neurosci 24: 6693–6702, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berry RB, White DP, Roper J, Pillar G, Fogel RB, Stanchina M, Malhotra A. Awake negative pressure reflex response of the genioglossus in OSA patients and normal subjects. J Appl Physiol 94: 1875–1882, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Berry RB, Yamaura EM, Gill K, Reist C. Acute effects of paroxetine on genioglossus activity in obstructive sleep apnea. Sleep 22: 1087–1092, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Bilston LE, Gandevia SC. Biomechanical properties of the human upper airway and their effects on its behavior during breathing and in obstructive sleep apnea. J Appl Physiol. In press [DOI] [PubMed] [Google Scholar]
- 8.Boissard R, Gervasoni D, Schmidt MH, Barbagli B, Fort P, Luppi PH. The rat ponto-medullary network responsible for paradoxical sleep onset and maintenance: a combined microinjection and functional neuroanatomical study. Eur J Neurosci 16: 1959–1973, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Borke RC, Nau ME, Ringler RL., Jr Brain stem afferents of hypoglossal neurons in the rat. Brain Res 269: 47–55, 1983 [DOI] [PubMed] [Google Scholar]
- 10.Brooks PL, Peever JH. Glycinergic and GABAA-mediated inhibition of somatic motoneurons does not mediate REM sleep motor atonia. J Neurosci 28: 3535–3545, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brooks PL, Peever JH. Identification of the transmitter and receptor mechanisms responsible for REM sleep paralysis. J Neurosci 32: 9785–9795, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown EN, Lydic R, Schiff ND. General anesthesia, sleep, coma. N Engl J Med 363: 2638–2650, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butler JP, Owens RL, Malhotra A, Wellman A. CrossTalk opposing view: the human upper airway during sleep does not behave like a Starling resistor. J Physiol 591: 2233–2234, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chamberlin NL, Eikermann M, Fassbender P, White DP, Malhotra A. Genioglossus premotoneurons and the negative pressure reflex in rats. J Physiol 579: 515–526, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chami HA, Baldwin CM, Silverman A, Zhang Y, Rapoport D, Punjabi NM, Gottlieb DJ. Sleepiness, quality of life, and sleep maintenance in REM versus non-REM sleep-disordered breathing. Am J Respir Crit Care Med 181: 997–1002, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conwell W, Patel B, Doeing D, Pamidi S, Knutson KL, Ghods F, Mokhlesi B. Prevalence, clinical features, and CPAP adherence in REM-related sleep-disordered breathing: a cross-sectional analysis of a large clinical population. Sleep Breath 16: 519–526, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev 90: 47–112, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dobbins EG, Feldman JL. Differential innervation of protruder and retractor muscles of the tongue in rat. J Comp Neurol 357: 376–394, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Douglas NJ. Respiratory physiology: understanding the control of ventilation. In: Principles and Practice of Sleep Medicine (5th Ed.), edited by Kryger MH, Roth T, Dement WC. St. Louis, MO: Elsevier, Saunders, 2011, p. 250–258 [Google Scholar]
- 20.Eastwood PR, Malhotra A, Palmer LJ, Kezirian EJ, Horner RL, Ip MS, Thurnheer R, Antic NA, Hillman DR. Obstructive sleep apnoea: from pathogenesis to treatment. Current controversies and future directions. Respirology 15: 587–595, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eastwood PR, Platt PR, Shepherd K, Maddison K, Hillman DR. Collapsibility of the upper airway at different concentrations of propofol anesthesia. Anesthesiology 103: 470–477, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Eckert DJ, Malhotra A. Pathophysiology of adult obstructive sleep apnea. Proc Am Thorac Soc 5: 144–153, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eckert DJ, Malhotra A, Lo YL, White DP, Jordan AS. The influence of obstructive sleep apnea and gender on genioglossus activity during rapid eye movement sleep. Chest 135: 957–964, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eckert DJ, Owens RL, Kehlmann GB, Wellman A, Rahangdale S, Yim-Yeh S, White DP, Malhotra A. Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin Sci (Lond) 120: 505–514, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea: identification of novel therapeutic targets. Am J Respir Crit Care Med. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25a.Eckert DJ, Younes MK. Arousal from sleep: implications for obstructive sleep apnea pathogenesis and treatment. J Appl Physiol; 10.1152/japplphysiol.00649.2013 [DOI] [PubMed] [Google Scholar]
- 26.Egan TM, North RA. Acetylcholine hyperpolarizes central neurones by acting on an M2 muscarinic receptor. Nature 319: 405–407, 1986 [DOI] [PubMed] [Google Scholar]
- 27.Eikermann M, Malhotra A, Fassbender P, Zaremba S, Jordan AS, Gautam S, White DP, Chamberlin NL. Differential effects of isoflurane and propofol on upper airway dilator muscle activity and breathing. Anesthesiology 108: 897–906, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eiseman NA, Westover MB, Ellenbogen JM, Bianchi MT. The impact of body posture and sleep stages on sleep apnea severity in adults. J Clin Sleep Med 8: 655–666, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elliott EA, Dawson SV. Test of wave-speed theory of flow limitation in elastic tubes. J Appl Physiol 43: 516–522, 1977 [DOI] [PubMed] [Google Scholar]
- 30.Fay RA, Norgren R. Identification of rat brainstem multisynaptic connections to the oral motor nuclei using pseudorabies virus. III. Lingual muscle motor systems. Brain Res Brain Res Rev 25: 291–311, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci 9: 370–386, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Fregosi RF, Ludlow CL. Activation of upper airway muscles during breathing and swallowing. J Appl Physiol; 10.1152/japplphysiol.00670.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fuller PM, Saper CB, Lu J. The pontine REM switch: past and present. J Physiol 584: 735–741, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Furusawa K, Yasuda K, Okuda D, Tanaka M, Yamaoka M. Central distribution and peripheral functional properties of afferent and efferent components of the superior laryngeal nerve: morphological and electrophysiological studies in the rat. J Comp Neurol 375: 147–156, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Goh AS, Issa FG, Sullivan CE. Upper airway dilating forces during wakefulness and sleep in dogs. J Appl Physiol 61: 2148–2155, 1986 [DOI] [PubMed] [Google Scholar]
- 36.Grace KP, Hughes SW, Horner RL. Identification of a pharmacological target for genioglossus reactivation throughout sleep. Sleep. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grace KP, Hughes SW, Horner RL. Identification of the mechanism mediating genioglossus muscle suppression in REM sleep. Am J Respir Crit Care Med 187: 311–319, 2013 [DOI] [PubMed] [Google Scholar]
- 38.Grace KP, Hughes SW, Shahabi S, Horner RL. K+ channel modulation causes genioglossus inhibition in REM sleep and is a strategy for reactivation. Respir Physiol Neurobiol 188: 277–288, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Heinzer RC, White DP, Jordan AS, Lo YL, Dover L, Stevenson K, Malhotra A. Trazodone increases arousal threshold in obstructive sleep apnoea. Eur Respir J 31: 1308–1312, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hillman DR, Walsh JH, Maddison KJ, Platt PR, Kirkness JP, Noffsinger WJ, Eastwood PR. Evolution of changes in upper airway collapsibility during slow induction of anesthesia with propofol. Anesthesiology 111: 63–71, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Horner RL. Emerging principles and neural substrates underlying tonic sleep-state-dependent influences on respiratory motor activity. Philos Trans R Soc Lond B Biol Sci 364: 2553–2564, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horner RL. Neural control of the upper airway: integrative physiological mechanisms and relevance for sleep disordered breathing. Compr Physiol 2: 479–535, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Horner RL. Neuromodulation of hypoglossal motoneurons during sleep. Respir Physiol Neurobiol 164: 179–196, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Horner RL. The tongue and its control by sleep state-dependent modulators. Arch Ital Biol 149: 406–425, 2011 [DOI] [PubMed] [Google Scholar]
- 45.Horner RL, Innes JA, Holden HB, Guz A. Afferent pathway(s) for pharyngeal dilator reflex to negative pressure in man: a study using upper airway anaesthesia. J Physiol 436: 31–44, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horner RL, Innes JA, Murphy K, Guz A. Evidence for reflex upper airway dilator muscle activation by sudden negative airway pressure in man. J Physiol 436: 15–29, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jordan AS, White DP, Lo YL, Wellman A, Eckert DJ, Yim-Yeh S, Eikermann M, Smith SA, Stevenson KE, Malhotra A. Airway dilator muscle activity and lung volume during stable breathing in obstructive sleep apnea. Sleep 32: 361–368, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karschin C, Dissmann E, Stuhmer W, Karschin A. IRK(1–3) and GIRK(1–4) inwardly rectifying K+ channel mRNAs are differentially expressed in the adult rat brain. J Neurosci 16: 3559–3570, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kezirian EJ, White DP, Malhotra A, Ma W, McCulloch CE, Goldberg AN. Interrater reliability of drug-induced sleep endoscopy. Arch Otolaryngol Head Neck Surg 136: 393–397, 2010 [DOI] [PubMed] [Google Scholar]
- 50.Khan A, Harrison SL, Kezirian EJ, Ancoli-Israel S, O'Hearn D, Orwoll E, Redline S, Ensrud K, Stone KL. Obstructive sleep apnea during rapid eye movement sleep, daytime sleepiness, and quality of life in older men in osteoporotic fractures in men (MrOS) sleep study. J Clin Sleep Med 9: 191–198, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khoo MC. Using loop gain to assess ventilatory control in obstructive sleep apnea. Am J Respir Crit Care Med 163: 1044–1045, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol 101: 618–627, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kubin L, Davies RO, Pack L. Control of upper airway motoneurons during REM sleep. News Physiol Sci 13: 637–656, 1998 [DOI] [PubMed] [Google Scholar]
- 54.Kuna ST, Remmers JE. Premotor input to hypoglossal motoneurons from Kolliker-Fuse neurons in decerebrate cats. Respir Physiol 117: 85–95, 1999 [DOI] [PubMed] [Google Scholar]
- 55.Liu X, Sood S, Liu H, Nolan P, Morrison JL, Horner RL. Suppression of genioglossus muscle tone and activity during reflex hypercapnic stimulation by GABA(A) mechanisms at the hypoglossal motor nucleus in vivo. Neuroscience 116: 249–259, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Lowe AA. Tongue movements–brainstem mechanisms and clinical postulates. Brain Behav Evol 25: 128–137, 1984 [DOI] [PubMed] [Google Scholar]
- 57.Lu J, Nelson LE, Franks N, Maze M, Chamberlin NL, Saper CB. Role of endogenous sleep-wake and analgesic systems in anesthesia. J Comp Neurol 508: 648–662, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature 441: 589–594, 2006 [DOI] [PubMed] [Google Scholar]
- 59.Luppi PH, Gervasoni D, Verret L, Goutagny R, Peyron C, Salvert D, Leger L, Fort P. Paradoxical (REM) sleep genesis: the switch from an aminergic-cholinergic to a GABAergic-glutamatergic hypothesis. J Physiol (Paris) 100: 271–283, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Lydic R, Baghdoyan HA. Sleep, anesthesiology, and the neurobiology of arousal state control. Anesthesiology 103: 1268–1295, 2005 [DOI] [PubMed] [Google Scholar]
- 61.Malhotra A, Butler JP, Wellman A. The pharyngeal airway: is bigger really better? Chest 141: 1372–1375, 2012 [DOI] [PubMed] [Google Scholar]
- 62.Malhotra A, Pillar G, Fogel RB, Beauregard J, Edwards JK, Slamowitz DI, Shea SA, White DP. Genioglossal but not palatal muscle activity relates closely to pharyngeal pressure. Am J Respir Crit Care Med 162: 1058–1062, 2000 [DOI] [PubMed] [Google Scholar]
- 63.McCarley RW, Greene RW, Rainnie D, Portas CM. Brainstem neuromodulation and REM sleep. Semin Neurosci 7: 341–354, 1995 [Google Scholar]
- 64.McGinty D, Szymusiak R. The sleep-wake switch: a neuronal alarm clock. Nat Med 6: 510–511, 2000 [DOI] [PubMed] [Google Scholar]
- 65.Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, Harris RA, Harrison NL. Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature 389: 385–389, 1997 [DOI] [PubMed] [Google Scholar]
- 66.Mokhlesi B, Punjabi NM. “REM-related” obstructive sleep apnea: an epiphenomenon or a clinically important entity? Sleep 35: 5–7, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Montandon G, Qin W, Liu H, Ren J, Greer JJ, Horner RL. Pre-Bötzinger complex neurokinin-1 receptor-expressing neurons mediate opioid-induced respiratory depression. J Neurosci 31: 1292–1301, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moore JT, Chen J, Han B, Meng QC, Veasey SC, Beck SG, Kelz MB. Direct activation of sleep-promoting VLPO neurons by volatile anesthetics contributes to anesthetic hypnosis. Curr Biol 22: 2008–2016, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morrell MJ, Arabi Y, Zahn B, Badr MS. Progressive retropalatal narrowing preceding obstructive apnea. Am J Respir Crit Care Med 158: 1974–1981, 1998 [DOI] [PubMed] [Google Scholar]
- 70.Morrison JL, Sood S, Liu H, Park E, Nolan P, Horner RL. GABA-A receptor antagonism at the hypoglossal motor nucleus increases genioglossus muscle activity in NREM but not REM sleep. J Physiol 548: 569–583, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morrison JL, Sood S, Liu H, Park E, Nolan P, Horner RL. Role of inhibitory amino acids in control of hypoglossal motor outflow to genioglossus muscle in naturally sleeping rats. J Physiol 552: 975–991, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morrison JL, Sood S, Liu X, Liu H, Park E, Nolan P, Horner RL. Glycine at the hypoglossal motor nucleus: genioglossus activity, CO2 responses and the additive effects of GABA. J Appl Physiol 93: 1786–1796, 2002 [DOI] [PubMed] [Google Scholar]
- 73.Nelson LE, Guo TZ, Lu J, Saper CB, Franks NP, Maze M. The sedative component of anesthesia is mediated by GABA(A) receptors in an endogenous sleep pathway. Nat Neurosci 5: 979–984, 2002 [DOI] [PubMed] [Google Scholar]
- 74.Nelson LE, Lu J, Guo T, Saper CB, Franks NP, Maze M. The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology 98: 428–436, 2003 [DOI] [PubMed] [Google Scholar]
- 75.Nichols CG, Lopatin AN. Inward rectifier potassium channels. Annu Rev Physiol 59: 171–191, 1997 [DOI] [PubMed] [Google Scholar]
- 76.Nikolov EN, Ivanova-Nikolova TT. Dynamic integration of alpha-adrenergic and cholinergic signals in the atria: role of G protein-regulated inwardly rectifying K+ channels. J Biol Chem 282: 28669–28682, 2007 [DOI] [PubMed] [Google Scholar]
- 77.Orem J, Kubin L. Respiratory physiology: central neural control. In: Principles and Practice of Sleep Medicine (3rd Ed.), edited by Kryger MH, Roth T, Dement WC. Philadelphia, PA: Saunders, 2000, p. 205–220 [Google Scholar]
- 78.Orem JM, Lovering AT, Vidruk EH. Excitation of medullary respiratory neurons in REM sleep. Sleep 28: 801–807, 2005 [DOI] [PubMed] [Google Scholar]
- 79.Pace-Schott EF, Hobson JA. The neurobiology of sleep: genetics, cellular physiology and subcortical networks. Nat Rev Neurosci 3: 591–605, 2002 [DOI] [PubMed] [Google Scholar]
- 80.Pagnotta SE, Lape R, Quitadamo C, Nistri A. Pre- and postsynaptic modulation of glycinergic and gabaergic transmission by muscarinic receptors on rat hypoglossal motoneurons in vitro. Neuroscience 130: 783–795, 2005 [DOI] [PubMed] [Google Scholar]
- 81.Park E, Younes M, Liu H, Liu X, Horner RL. Systemic vs. central administration of common hypnotics reveals opposing effects on genioglossus muscle activity in rats. Sleep 31: 355–365, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Patil SP, Schneider H, Marx JJ, Gladmon E, Schwartz AR, Smith PL. Neuromechanical control of upper airway patency during sleep. J Appl Physiol 102: 547–556, 2007 [DOI] [PubMed] [Google Scholar]
- 83.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic, 1998 [Google Scholar]
- 84.Peever JH, Shen L, Duffin J. Respiratory pre-motor control of hypoglossal motoneurons in the rat. Neuroscience 110: 711–722, 2002 [DOI] [PubMed] [Google Scholar]
- 85.Pillar G, Malhotra A, Fogel RB, Beauregard J, Slamowitz DI, Shea SA, White DP. Upper airway muscle responsiveness to rising Pco2 during NREM sleep. J Appl Physiol 89: 1275–1282, 2000 [DOI] [PubMed] [Google Scholar]
- 86.Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic control of motoneuronal excitability. Physiol Rev 80: 767–852, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roda F, Pio J, Bianchi AL, Gestreau C. Effects of anesthetics on hypoglossal nerve discharge and c-Fos expression in brainstem hypoglossal premotor neurons. J Comp Neurol 468: 571–586, 2004 [DOI] [PubMed] [Google Scholar]
- 88.Ross CA, Ruggiero DA, Reis DJ. Projections from the nucleus tractus solitarii to the rostral ventrolateral medulla. J Comp Neurol 242: 511–534, 1985 [DOI] [PubMed] [Google Scholar]
- 89.Ryan S, McNicholas WT, O'Regan RG, Nolan P. Reflex respiratory response to changes in upper airway pressure in the anaesthetized rat. J Physiol 537: 251–265, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saboisky JP, Chamberlin NL, Malhotra A. Potential therapeutic targets in obstructive sleep apnoea. Expert Opin Ther Targets 13: 795–809, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Saper CB, Lu J, Chou TC, Gooley J. The hypothalamic integrator for circadian rhythms. Trends Neurosci 28: 152–157, 2005 [DOI] [PubMed] [Google Scholar]
- 92.Schwartz AR, Barnes M, Hillman D, Malhotra A, Kezirian E, Smith PL, Hoegh T, Parrish D, Eastwood PR. Acute upper airway responses to hypoglossal nerve stimulation during sleep in obstructive sleep apnea. Am J Respir Crit Care Med 185: 420–426, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schwartz AR, Smith PL, Oliven A. Electrical stimulation of the hypoglossal nerve: a potential therapy. J Appl Physiol. In Press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shea SA, Edwards JK, White DP. Effect of wake-sleep transitions and rapid eye movement sleep on pharyngeal muscle response to negative pressure in humans. J Physiol 520: 897–908, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Siegel JM. Brainstem mechanisms generating REM sleep. In: Principles and Practice of Sleep Medicine (3rd Ed.), edited by Kryger MH, Roth T, Dement WC. Philadelphia, PA: Saunders, 2000, p. 112–133 [Google Scholar]
- 96.Smith JC, Abdala AP, Koizumi H, Rybak IA, Paton JF. Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J Neurophysiol 98: 3370–3387, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Soja PJ, Lopez-Rodriguez F, Morales FR, Chase MH. Effects of excitatory amino acid antagonists on the phasic depolarizing events that occur in lumbar motoneurons during REM periods of active sleep. J Neurosci 15: 4068–4076, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stanchina ML, Malhotra A, Fogel RB, Ayas N, Edwards JK, Schory K, White DP. Genioglossus muscle responsiveness to chemical and mechanical stimuli during non-rapid eye movement sleep. Am J Respir Crit Care Med 165: 945–949, 2002 [DOI] [PubMed] [Google Scholar]
- 99.Steenland HW, Liu H, Horner RL. Endogenous glutamatergic control of rhythmically active mammalian respiratory motoneurons in vivo. J Neurosci 28: 6826–6835, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Strohl K, Butler J, Malhotra A. Mechanical properties of the upper airway. Compr Physiol 2: 1853–1872, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Talley EM, Lei Q, Sirois JE, Bayliss DA. TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron 25: 399–410, 2000 [DOI] [PubMed] [Google Scholar]
- 102.Töpert C, Döring F, Wischmeyer E, Karschin C, Brockhaus J, Ballanyi K, Derst C, Karschin A. Kir2.4: a novel K+ inward rectifier channel associated with motoneurons of cranial nerve nuclei. J Neurosci 18: 4096–4105, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Travers JB, Norgren R. Afferent projections to the oral motor nuclei in the rat. J Comp Neurol 220: 280–298, 1983 [DOI] [PubMed] [Google Scholar]
- 104.Volgin DV, Rukhadze I, Kubin L. Hypoglossal premotor neurons of the intermediate medullary reticular region express cholinergic markers. J Appl Physiol 105: 1576–1584, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wellman A, Jordan AS, Malhotra A, Fogel RB, Katz ES, Schory K, Edwards JK, White DP. Ventilatory control and airway anatomy in obstructive sleep apnea. Am J Respir Crit Care Med 170: 1225–1232, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wiegand L, Zwillich CW, White DP. Collapsibility of the human upper airway during normal sleep. J Appl Physiol 66: 1800–1808, 1989 [DOI] [PubMed] [Google Scholar]
- 108.Woch G, Ogawa H, Davies RO, Kubin L. Behavior of hypoglossal inspiratory premotor neurons during the carbachol-induced, REM sleep-like suppression of upper airway motoneurons. Exp Brain Res 130: 508–520, 2000 [DOI] [PubMed] [Google Scholar]
- 109.Wu J, Xu H, Shen W, Jiang C. Expression and coexpression of CO2-sensitive Kir channels in brainstem neurons of rats. J Membr Biol 197: 179–191, 2004 [DOI] [PubMed] [Google Scholar]
- 110.Xie A, Teodorescu M, Pegelow DF, Teodorescu MC, Gong Y, Fedie JE, Dempsey JA. Effects of stabilizing or increasing respiratory motor outputs on obstructive sleep apnea. J Appl Physiol 115: 22–33, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Younes M. Contributions of upper airway mechanics and control mechanisms to severity of obstructive apnea. Am J Respir Crit Care Med 168: 645–658, 2003 [DOI] [PubMed] [Google Scholar]
- 112.Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med 169: 623–633, 2004 [DOI] [PubMed] [Google Scholar]
- 113.Younes M. Role of respiratory control mechanisms in the pathogenesis of obstructive sleep disorders. J Appl Physiol 105: 1389–1405, 2008 [DOI] [PubMed] [Google Scholar]
- 115.Younes M, Ostrowski M, Thompson W, Leslie C, Shewchuk W. Chemical control stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med 163: 1181–1190, 2001 [DOI] [PubMed] [Google Scholar]
- 116.Younes Y, Park E, Horner RL. Pentobarbital sedation increases genioglossus respiratory activity in sleeping rats. Sleep 30: 478–488, 2007 [DOI] [PubMed] [Google Scholar]