Abstract
Obstructive sleep apnea is characterized by recurrent episodes of pharyngeal collapse, which result from a decrease in pharyngeal dilator muscle tone. The genioglossus is a major pharyngeal dilator that maintains airway patency during sleep. Early studies in animal and humans have demonstrated that electrical stimulation of this muscle reduces pharyngeal collapsibility, increases airflow, and mitigates obstructive sleep apnea. These findings impelled the development of fully implantable hypoglossal nerve stimulating systems (HGNS), for which feasibility trial results are now available. These pilot studies have confirmed that hypoglossal nerve stimulation can prevent pharyngeal collapse without arousing patients from sleep. Potentially, a substantial segment of the patient population with obstructive sleep apnea can be treated with this novel approach. Furthermore, the feasibility trial findings suggest that the therapeutic potential of HGNS can be optimized by selecting patients judiciously, titrating the stimulus intensity optimally, and characterizing the underlying function and anatomy of the pharynx. These strategies are currently being examined in ongoing pivotal trials of HGNS.
Keywords: hypoglossal nerve stimulating systems, obstructive sleep apnea, hypopnea, pharyngeal collapse
CONCEPTUAL BACKGROUND
obstructive sleep apnea is characterized by recurrent episodes of pharyngeal airway obstruction during sleep (35), leading to intermittent oxyhemoglobin desaturations and arousals from sleep. Recent evidence suggests that intermittent hypoxemia and sleep disruption contribute to substantial morbidity and mortality from metabolic dysregulation (glucose intolerance, hyperlipidemia, and nonalcoholic fatty liver disease), hypertension, cardiopulmonary disease, accelerated neurocognitive decline, and even tumor metastasis (26, 48).
Over the past 30 years, nasal continuous positive airway pressure (CPAP) has remained the mainstay of treatment for obstructive sleep apnea (1). Its efficacy is due to its ability to overcome increases in pharyngeal collapsibility, which is a fundamental defect in sleep apnea. Despite its efficacy in the laboratory, nasal CPAP has been plagued by significant problems with adherence (19, 47) that hinder its long-term effectiveness and ultimately its control of apnea-related morbidity and mortality. Moreover, CPAP does not address the root cause for obstructive sleep apnea, namely, increases in pharyngeal collapsibility during sleep.
In seminal studies, Remmers et al. (35) suggested that pharyngeal obstruction resulted from disturbances in upper airway neuromuscular control during sleep. At sleep onset, pharyngeal collapse is associated with a loss of genioglossus muscle tone (23). Under these circumstances, airway collapsibility increases, reflecting the influence of underlying alterations in pharyngeal structures or anatomy that predispose to airflow obstruction during sleep (33, 41). Airflow obstruction, however, can elicit reflex responses that increase pharyngeal muscle activity and restore airway patency during sleep (22, 33). More recently, it has been postulated that the upper airway requires two “hits” for the development of sleep apnea. First, underlying structural defects increase its collapsibility and predispose to airflow obstruction during sleep. Second, neuromuscular responses to airflow obstruction are blunted and fail to compensate for the obstruction and restore airway patency during sleep.
The genioglossus muscle protrudes the tongue, dilates the pharynx, and mitigates airflow obstruction during sleep (35). In early animal studies, exogenous electrical stimulation of the genioglossus muscle augmented the activity of this pharyngeal dilator and restored airway patency (30, 43). Hypoglossal nerve stimulation (HGNS) therapy was designed to recruit genioglossus activity and relieve airflow obstruction in sleeping patients with obstructive sleep apnea, (17, 27). In this review, we summarize results from early pilot studies on genioglossus and hypoglossus stimulation and update perspectives and therapeutic challenges for implantable hypoglossus nerve stimulation systems.
PILOT STUDIES
In pilot human studies, investigators have laid the groundwork for approaches to treat apneic patients with hypoglossal stimulation. These studies trialed three techniques for stimulating genioglossus muscle activity during sleep: submental transcutaneous stimulation, fine wire intramuscular lingual muscle stimulation, and direct hypoglossal nerve stimulation. The findings from each approach have been previously reviewed (17, 27) and are summarized below.
SUBMENTAL TRANSCUTANEOUS STIMULATION
Early studies with submental transcutaneous stimulation led invariably to arousals without consistent improvements in pharyngeal patency during sleep (2, 5, 13, 24). Initially, investigators documented improvements in airflow and reductions in sleep apnea severity with submental stimulation (15, 24), although later studies demonstrated frequent arousals from sleep without clear-cut improvements in airflow dynamics (2). Investigators have recently revisited the notion that prolonged, low-intensity transcutaneous stimulation during sleep can increase lingual muscle tone and improve airway patency during sleep (45). This concept is further supported by a recent study examining the effect of lingual “exercises” drawn from a speech therapy repertoire on sleep apnea severity (14). Daily training exercises improved sleep apnea over a period of weeks without noticeably increasing the bulk mass of the lingual musculature, suggesting a beneficial effect of low-level stimulation over time (see below).
DIRECT FINE WIRE STIMULATION
Intramuscular stimulation.
Difficulties in eliciting motor movement of the tongue during transcutaneous stimulation prompted investigators to stimulate the genioglossus directly with sublingual transmucosal and fine wire intramuscular electrodes. Indeed, direct stimulation of the hypoglossal nerve and genioglossus muscle produced pronounced muscle contraction and improvements in pharyngeal collapsibility and patency in isolated upper airway preparations in cats (8, 43), dogs (30), and rats (10). These animal findings motivated further studies examining effects of stimulation on airway patency in sleeping humans. In these experiments, sublingual transmucosal, and fine wire electrodes were used to stimulate the hypoglossus nerve (28, 31, 36, 39). Stimulation produced pure motor movement without significant discomfort during wakefulness.
Selective vs. combined lingual muscle stimulation.
In an early study examining effects of intramuscular fine wire stimulation, investigators demonstrated marked differences in tongue movement depending on the site of stimulation (39). Unilateral stimulation near the proximal hypoglossus nerve produced ipsilateral tongue deviation and “closed” the airway during sleep. In contrast, distal hypoglossal stimulation induced contralateral tongue deviation and variable degrees of airway opening during sleep. Striking differences in the responses to proximal and distal stimulation reflected distinct patterns of lingual muscle recruitment. The development of pharyngeal closure during proximal stimulation implicated lingual retrusor muscles, which pull the base of tongue posteriorly into the pharynx. In contrast, effects of distal stimulation suggested unopposed recruitment of the genioglossus, a powerful tongue protrusor, which pulls the tongue anteriorly and restores airway patency. Subsequently, investigators demonstrated in rats that lingual protrusor and retractor muscles appear to coactivate when ventilatory drive is elevated (9) and that combined stimulation of these muscle groups appear to stiffen the pharynx and stabilize its patency (10). Further studies in humans have confirmed that comparable improvements in pharyngeal patency occur in sleeping humans when the genioglossus is stimulated selectively or in combination with lingual retractor muscles (8, 29). Thus, unopposed stimulation of lingual protrusor or retractor muscles opens or closes the pharynx, respectively, whereas combined stimulation appears to stiffen the pharynx, helping to maintain airway patency during sleep.
Synchronizing stimulation with inspiration.
In early human studies, acute airflow responses to electrical stimulation during sleep were examined (8, 39). Brief stimulation pulses produced prompt changes in airflow with concomitant alterations in esophageal pressure, indicating a close temporal relationship between stimulation and the state of pharyngeal patency. In contrast, when stimulus intensity was increased to the point of arousing the patient from sleep, airway opening was sustained well beyond the burst duration. Time-linked responses in airway patency to stimulation suggested the lingual muscles could be stimulated selectively during sleep and that maximal therapeutic benefit was derived by applying the stimulation solely during the inspiratory phase of the respiratory cycle. These observations triggered the development of closed-loop methods for synchronizing electrical stimulation with the respiratory cycle, thereby minimizing the potential for neuromuscular fatigue and maximizing the life span of the power source.
DIRECT HYPOGLOSSUS STIMULATION
Electrode location.
Favorable results from animal and fine wire human studies impelled investigators to further quantify the effects of direct hypoglossal nerve stimulation on pharyngeal patency during sleep. In this study, five patients were implanted with hypoglossal cuff electrodes placed around the proximal or distal nerve trunk (just proximal to ramifying into the body of the genioglossus muscle) (8). Electrical stimulation was manually applied during sleep for single partially obstructed (flow-limited) inspirations, and generated increases in maximal inspiratory airflow of ∼150 to 200 ml/s. Airflow responded similarly during distal and proximal nerve stimulation, which stimulated tongue protrusors selectively or in combination with retractors. The magnitude of this response suggested that stimulation could achieve substantial improvements in pharyngeal patency during sleep and corresponding reductions in sleep apnea severity without arousing patients from sleep. The findings also provided sufficient impetus for efforts to develop a fully implantable therapeutic HGNS system.
FEASIBILITY TRIALS
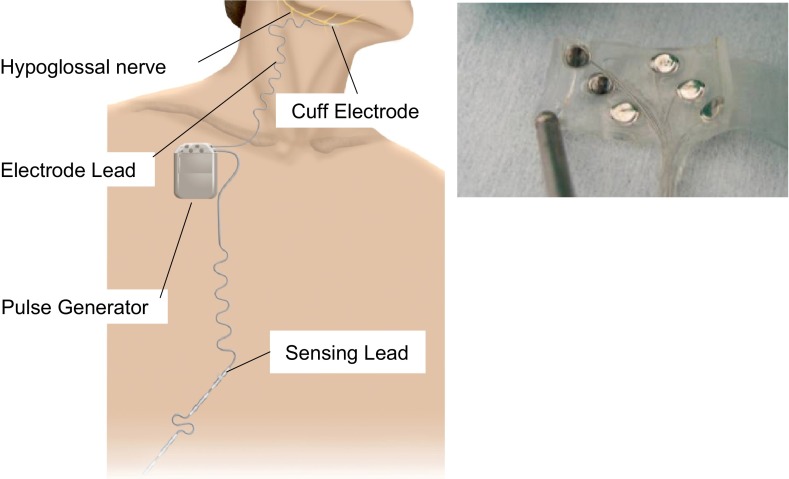
To date, four HGNS feasibility trials have been conducted to evaluate the performance and therapeutic potential of fully implantable hypoglossal nerve stimulation systems (4, 25, 38, 46). Each system includes 1) a circumferential nerve cuff electrode, 2) a stimulation lead, and 3) an implantable pulse generator (Fig. 1).
Fig. 1.
Left: hypoglossal nerve stimulating system components. Cuff electrode around the hypoglossal nerve, lead connected to implantable pulse generator, and respiratory sensing lead(s) to synchronize stimulation with inspiration. Right: flat electrode array used for asynchronous electrical stimulation. See text for details.
Synchronous (closed loop) stimulation.
In three systems, respiration has been monitored with sensing leads, which detect inspiratory effort (Fig. 1, left). Software algorithms embedded in the implantable pulse generator have been designed to predict the onset of inspiration and output a stimulus burst synchronized with the patient's inspiration (4, 38, 46). Stimulation induces increases in maximal inspiratory airflow, which coincided with the onset and offset of the stimulation bursts (38). Synchronized stimulation systems depolarize the entire hypoglossal nerve, which generates bulk tongue movement, as described for fine wire stimulation studies above.
Continuous (open loop) stimulation.
In another system, an array of six flat electrodes has been arranged circumferentially in the cuff electrode (Fig. 1, right). Rather than applying the stimulation during inspiration, this system stimulates specific electrodes continuously for a set duration. The pulse generator stimulates these electrodes in a predetermined sequence, targeting different nerve fibers in rotating fashion (25, 49). In each system, stimulation “on” periods have been programmed to avoid neuromuscular fatigue by maintaining an overall stimulation duty cycle of <50%. The flat electrode array also uses low-level stimulation, which increases background lingual muscle tone throughout the respiratory cycle (49).
In both open and closed loop stimulation systems, stimulus intensity can be adjusted by setting the pulse current amplitude (mA), frequency (Hz), and width (μs). Approaches to titrating stimulation, however, have not yet been standardized. Each system can improve airway patency by altering tongue position, stiffening pharyngeal structures, and/or strengthening upper airway muscles over time.
Each stimulation system has documented improvements in airflow dynamics and sleep apnea in the majority of patients. Favorable responses have been generally defined by a 50% or greater decrease in apnea-hypopnea index (AHI) to less than 20 episodes per hour. Despite variability in patient selection criteria among the trials, most have instituted an upper limit on the patient's body mass index. In all, responses have been reported in 74 patients with moderate to severe obstructive sleep apnea, who were generally middle-aged to older men with mild to moderate obesity (see Table 1). Trials have differed in patient selection criteria, based on the degree of obesity and sleep-disordered breathing event characteristics. Recent studies have excluded patients who were very obese. They have also utilized differing definitions of obstructive hypopneas (4, 46). In addition, one trial selected patients with a high proportion of obstructive hypopneas rather than complete apneas, for reasons described below (4). To date, relatively few women have been implanted, and optimal selection criteria have not been established for treating patients with hypoglossal stimulation therapy.
Table 1.
Baseline characteristics of patients enrolled in hypoglossal nerve stimulation feasibility trials
| Baseline Characteristics |
||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Multi-Center | n | Sex | Age | BMI | AHI | Synchronized Stimulation | Adverse Events* |
| Medtronic | Y | 8 | 8M:0F | 50 (8) | 28 (5) | 52 (20) | Y | 1, 1, 3, 4 |
| Apnex | Y | 21 | 14M:7F | 53 (10) | 32 (4) | 45 (18) | Y | 3, 5 |
| Inspire | Y | 31 | 30M:1F | 53 (12) | 29 (2) | Y | ||
| Phase I | 22 | 22M:0F | 56 (8) | 30 (3) | 44 (18) | 2, 5 | ||
| Phase II | Y | 9 | 8M:1F | 54 (12) | 29 (2) | 40 (10) | 3, 3 | |
| ImThera | N | 14 | 13M:1F | 50 (10) | 31 (4) | 45 (18) | N | 1 |
Values are expressed as means (SD)
1, lead failure; 2, infection; 3, device malfunction; 4, sensing problems; 5, miscellaneous.
Response to stimulation.
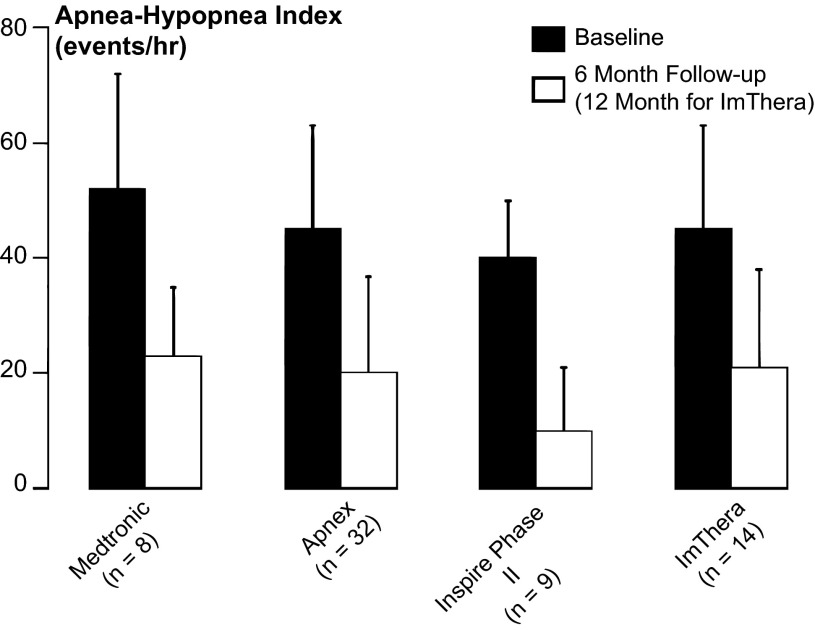
In the aggregate, feasibility trials for hypoglossal nerve-stimulating systems have demonstrated substantial responses in major polysomnographic metrics of sleep apnea severity, such as apnea-hypopnea index (Fig. 2). These improvements can be attributed to increases in maximal inspiratory airflow (VImax) and ventilation. Improvements in airflow have paralleled stimulation burst profiles and the degree of synchrony with each inspiration (see recording example on and off stimulation, Fig. 3) (39), suggesting that sleep apnea responses were a direct result of increases in airway patency rather than arousal from sleep. In further studies, improvements have been related to the stimulation intensity (37), the shape of the pharyngeal airway (3, 46), and the magnitude of decreases in pharyngeal collapsibility during sleep (29), which may be achieved by activating lingual protrusor muscles with or without concomitant activation of retractor muscles (29). These findings support the notion that the genioglossus, long considered to be a major pharyngeal dilator muscle (35), must act in concert with other pharyngeal muscles to fully stabilize airway patency during sleep. Thus, a complex interplay of device and functional factors will have to be considered to optimize therapeutic efficacy of HGNS.
Fig. 2.
Apnea-hypopnea indices (means ± SD) for each of four feasibility trials of hypoglossal nerve stimulation therapy. Baseline, before initiating hypoglossal nerve stimulation (solid bars) and at 6 mo, 12 mo, or last follow-up after the initiation of stimulation (open bars) (Plotted from data provided in Refs. 4, 25, 38, 46).
Fig. 3.
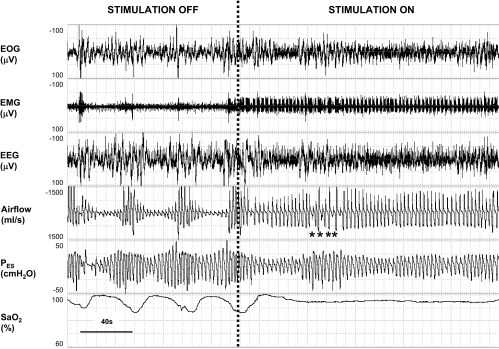
Representative recording example in one patient showing response in breathing pattern at the onset of hypoglossal stimulation during a continuous period of non-rapid eye movement sleep. Left: before stimulation was started, three obstructive hypopneas were evident, with periodic reductions in airflow terminated by microarousals from sleep [see rise in submental electromyogram (EMG) amplitude with resumption of tidal airflow] and oxyhemoglobin desaturations. Right: ∼20 s after the onset of the stimulus, tidal airflow stabilized, esophageal pressure swings were reduced, and arousals and oxyhemoglobin desaturations were abolished. EOG indicates electro-oculogram; EEG, C3-A2 electroencephalogram; PES, esophageal pressure; and SaO2, oxyhemoglobin saturation. *Inspirations in which stimulation was not applied, showing immediate reduction in airflow to unstimulated levels. Airflow increased promptly during subsequent stimulated breaths (38).
Beneficial responses to stimulation must be considered in light of potential immediate and long-term risks of adverse events. Acute surgical risks include those of anesthesia and analgesia (respiratory depression and airway obstruction), wound infections, hematomas, and nerve palsy. Long-term motor stimulation and repetitive tongue movements can produce soft tissue abrasions, muscle fatigue, changes in muscle fiber type, and lingual muscle hypertrophy. The chronic effects of stimulation and associated lingual muscle remodeling on upper airway function, however, have not been well characterized. It is also conceivable that stimulation intensity will need to be augmented over time to offset elevations in upper airway collapsibility that occur with increasing age (6, 21) and weight (18, 40). If excessive increases in collapsibility occur, stimulation efficacy may ultimately be limited if intensity surpasses the patient's arousal threshold.
APPROACHES FOR OPTIMIZING RESPONSES TO ELECTRICAL STIMULATION
Despite generally favorable responses in AHI, residual sleep apnea has been observed in many patients regardless of stimulating platform, thus, leading investigators to consider a variety of approaches to optimize or augment responses to electrical stimulation. Collectively, the feasibility studies offer interesting clues as to how responses can be enhanced by refining patient selection criteria and predicting physiological responses in airway patency. Post hoc analyses have led to several approaches for screening patients, characterizing airway anatomy and function, and titrating therapy to optimize treatment responses.
MEASUREMENTS OF UPPER AIRWAY COLLAPSIBILITY DURING SLEEP
Current evidence indicates that upper airway collapsibility varies along the continuum from health to disease, as reflected by differences in upper airway critical closing pressures (Pcrit) (12). Sleep apnea usually remits with treatment that decreases Pcrit below a threshold of approximately −5 cmH2O. Two factors determine the likelihood that HGNS will drive Pcrit below this threshold: 1) the baseline elevation of Pcrit and 2) the magnitude of the reduction in Pcrit with therapy (40, 42). In general, investigators have postulated that the likelihood of therapeutic success is greatest in patients whose Pcrit is just above the −5 cmH2O threshold (i.e., in the minimally negative range). This range coincides with those patients who exhibit a baseline predominance of obstructive hypopneas rather than complete apneas (11, 12). Accordingly, one feasibility study required that patients have predominantly obstructive hypopneas to be included in the trial, regardless of overall respiratory disturbance index (4). Another study found that HGNS responders had a lower body mass index (46), which may reflect lower levels of pharyngeal collapsibility during sleep. In these patients, observed reductions in Pcrit of 3 to 5 cmH2O with HGNS (28) would decrease Pcrit below the −5 cmH2O disease threshold, increasing the likelihood of favorable responses in sleep apnea.
PHARYNGEAL SHAPE AND SITE OF COLLAPSE
The shape of the pharyngeal lumen may also influence responses to HGNS. Leiter (20) postulated that tongue protrusion would yield greater improvements in pharyngeal patency when the pharynx is wider in the lateral than the antero-posterior dimension. Dotan et al. (3) confirmed that responses to electrical stimulation were greater in those with a low compared with high ratio of antero-posterior to lateral width of the pharyngeal lumen. Of note, the antero-posterior to lateral dimension was inversely related to cephalographic tongue size. Decreases in pharyngeal collapsibility (Pcrit) with electrical stimulation were also larger when stimulation increased the lateral dimension during drug-induced sleep endoscopy (DISE). In a separate HGNS study, a concentric rather than oblong pattern of luminal collapse during DISE was associated with poor responses to electrical stimulation, prompting investigators to exclude such patients from the later phase of their feasibility trial (46). Luminal shape may also determine the degree of coupling between tongue movements and luminal size, since stabilizing tongue position with HGNS can prevent collapse at the tongue base (16) or velopharynx through its zone of apposition with the soft palate (17). Thus, HGNS responses may be enhanced in patients demonstrating little collapse of the lateral pharyngeal walls during DISE (4, 46).
STIMULATION TITRATION
Therapeutic responses can also be optimized by titrating stimulation intensity during sleep (37). Compared with adjacent unstimulated breaths, stimulated breaths exhibit linear increases in maximal inspiratory airflow as stimulus intensity is increased progressively above the motor recruitment (capture) threshold (Fig. 4, left). At optimal HGNS stimulation levels, airflow peaked at normal tidal levels during sleep, and pharyngeal collapse (inspiratory flow limitation) was abolished in more than half of the patients, suggesting complete relief of airflow obstruction during sleep. Moreover, HGNS achieved normal levels of peak inspiratory airflow (Fig. 4, right; see shaded region), indicating that ventilation was restored to normal levels during sleep. These findings suggest that standardized protocols for HGNS titration are required to optimize therapeutic responses in sleep apnea to HGNS.
Fig. 4.
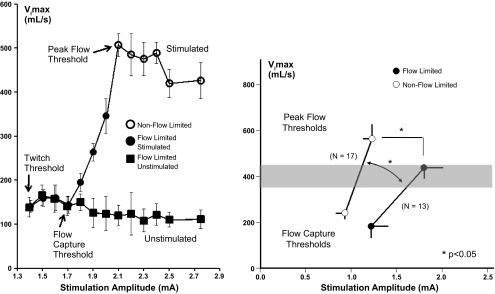
Left: maximal inspiratory airflow (VImax) responses to increasing levels of stimulation current (mA, milliamperes) on every other inspiration demonstrates progressive increases in inspiratory flow and the elimination of inspiratory flow limitation without arousing the patient from sleep. The findings imply that responses can be optimized by titrating stimulation intensity to maximize VImax (pharyngeal patency) during sleep. (37). Right: maximal inspiratory airflow (VImax) vs. stimulation current (milliamperes) in groups with (solid circles) and without (open circles) inspiratory flow limitation at the peak flow threshold. The flow response slope in the non-flow-limited group was greater than that in the flow-limited subgroup (1,241 ± 199 vs. 674 ± 167 ml·s−1·mA−1; n = 25; P < 0.05). Lower levels of stimulation current were required to achieve peak airflow in the non-flow-limited compared with flow-limited subgroup (1.23 ± 0.10 vs. 1.80 ± 0.20 mA; n = 25; P < 0.05), although peak inspiratory airflow did not differ between non-flow-limited and flow-limited subgroups (564 ± 58 vs. 438 ± 35 ml/s). Both groups attained normal or near-normal levels during sleep of ∼400 ml/s or greater (shaded region). (37).
SELECTIVE STIMULATION OF SPECIFIC LINGUAL MUSCLE GROUPS
The hypoglossal nerve innervates a variety of extrinsic and intrinsic lingual muscle groups (7). In principle, these muscles can be selectively stimulated to control the shape, position, and stiffness of the tongue and related pharyngeal structures. Extrinsic muscles, including lingual protrusor and retractor muscles, can either open or close the airway when stimulated in isolation, respectively (39). In contrast, pharyngeal collapsibility decreases similarly with combined stimulation of protrusors and retractor and selective protrusor muscle stimulation (29). Stimulating the genioglossus can also produce heterogeneous effects, depending on whether vertically oriented anterior or horizontally oriented posterior fibers are stimulated (3). Stimulating the vertical fibers has been shown to move the tongue forward, while pulling the lateral pharyngeal walls inward, leading to no significant change in pharyngeal collapsibility. In contrast, stimulating the horizontal fibers enlarged the pharynx and decreased collapsibility significantly. Thus, selectivity in stimulating specific hypoglossal nerve fibers may be required to achieve maximal decreases in pharyngeal collapsibility and maximize improvements in sleep apnea (25). A recently trialed multielectrode lead through which specific hypoglossal nerve fibers can be stimulated selectively should provide clinicians greater versatility in targeting those lingual muscles that best stabilize airway patency during sleep (49). It is also possible that specific fibers must be recruited to stabilize patency of specific pharyngeal segments (e.g., velopharynx vs. tongue base).
RESPONSES TO ACUTE AND CHRONIC HYPOGLOSSAL NERVE STIMULATION
Therapeutic responses may accrue from both the acute and chronic effects of hypoglossal stimulation. As shown in Figs. 3 and 4, prompt increases in airflow have been demonstrated when stimulation exceeds the motor recruitment threshold. As ventilation and oxygenation improve, however, alterations in afferent chemoreceptor and mechanoreceptor activity can decrease pharyngeal neuromuscular tone, leading to paradoxical increases in airway collapsibility (44). The influence of intrinsic neuromuscular tone on HGNS responses in pharyngeal patency, however, remains largely unexplored. It is also possible that stimulating the hypoglossus nerve chronically exerts long-term trophic effects on lingual muscles, including changes in muscle fiber strength and type (49), which may enhance their ability to maintain pharyngeal patency during sleep. Remodeling the lingual musculature, whether by applying subcapture hypoglossal stimulation, playing the didgeridoo (34), or training tongue muscles with lingual exercises (14), may also confer long-lasting beneficial effects that extend beyond the immediate treatment period (49).
CONCLUSIONS AND FUTURE DIRECTIONS
In summary, hypoglossal nerve stimulation decreases pharyngeal collapsibility, leading to increases in airflow and concomitant improvements in sleep apnea without arousing patients from sleep. Nevertheless, residual sleep apnea in treated patients suggests the need to develop appropriate selection criteria and methods for predicting and optimizing therapeutic responses. It may be possible to predict poor responses to HGNS based on 1) the severity of obesity and/or sleep apnea, 2) the presence of complete upper airway obstruction during sleep (obstructive apneas rather than hypopneas), and 3) collapse of the lateral pharyngeal walls during DISE. Moreover, responses may be optimized by titrating stimulation acutely on the basis of flow responses, assessing pharyngeal shape, and targeting specific lingual muscle groups. Additional gains may be achieved by modifying stimulation patterns to maintain intrinsic pharyngeal neuromuscular activity and augment lingual muscle tone over time. Further study will also be required to characterize effects of sleep stage, body position, and cranio-facial structures (32) on HGNS responses. Several sponsored clinical trials for HGNS systems are currently in progress, although responses in clinical outcomes to long-term stimulation still remain largely unexplored.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.R.S., P.L.S., and A.O. conception and design of research; A.R.S. drafted manuscript.
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health HL-50381, HL-32379, and Israel Binational Science Foundation no. 2011491.
REFERENCES
- 1.Basner RC. Continuous positive airway pressure for obstructive sleep apnea. N Engl J Med 356: 1751–1758, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Decker MJ, Haaga J, Arnold JL, Atzberger D, Strohl KP. Functional electrical stimulation and respiration during sleep. J Appl Physiol 75: 1053–1061, 1993 [DOI] [PubMed] [Google Scholar]
- 3.Dotan Y, Golibroda T, Oliven R, Netzer A, Gaitini L, Toubi A, Oliven A. Parameters affecting pharyngeal response to genioglossus stimulation in sleep apnoea. Eur Respir J 38: 338–347, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Eastwood PR, Barnes M, Walsh JH, Maddison KJ, Hee G, Schwartz AR, Smith PL, Malhotra A, McEvoy RD, Wheatley JR, O'Donoghue FJ, Rochford PD, Churchward T, Campbell MC, Palme CE, Robinson S, Goding GS, Eckert DJ, Jordan AS, Catcheside PG, Tyler L, Antic NA, Worsnop CJ, Kezirian EJ, Hillman DR. Treating obstructive sleep apnea with hypoglossal nerve stimulation. Sleep 34: 1479–1486, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edmonds LC, Daniels BK, Stanson AW, Sheedy PF, Shepard JWJ. The effects of transcutaneous electrical stimulation during wakefulness and sleep in patients with obstructive sleep apnea. Am Rev Respir Dis 146: 1030–1036, 1992 [DOI] [PubMed] [Google Scholar]
- 6.Eikermann M, Jordan AS, Chamberlin NL, Gautam S, Wellman A, Lo YL, White DP, Malhotra A. The influence of aging on pharyngeal collapsibility during sleep. Chest 131: 1702–1709, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eisele DW, Schwartz AR, Hari A, Thut DC, Smith PL. The effects of selective nerve stimulation on upper airway airflow mechanics. Arch Otolaryngol Head Neck Surg 121: 1361–1364, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Eisele DW, Smith PL, Alam DS, Schwartz AR. Direct hypoglossal nerve stimulation in obstructive sleep apnea. Arch Otolaryngol Head Neck Surg 123: 57–61, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Fuller D, Mateika JH, Fregosi RF. Co-activation of tongue protrudor and retractor muscles during chemoreceptor stimulation in the rat. J Physiol 507: 265–276, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuller D, Williams JS, Janssen PL, Fregosi RF. Effect of co-activation of tongue protrudor and retractor muscles on tongue movements and pharyngeal airflow mechanics in the rat. J Physiol 519: 601–613, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gleadhill IC, Schwartz AR, Schubert N, Wise RA, Permutt S, Smith PL. Upper airway collapsibility in snorers and in patients with obstructive hypopnea and apnea. Am Rev Respir Dis 143: 1300–1303, 1991 [DOI] [PubMed] [Google Scholar]
- 12.Gold AR, Schwartz AR. The pharyngeal critical pressure. The whys and hows of using nasal continuous positive airway pressure diagnostically. Chest 110: 1077–1088, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Guilleminault C, Powell N, Bowman B, Stoohs R. The effect of electrical stimulation on obstructive sleep apnea syndrome. Chest 107: 67–73, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Guimaraes KC, Drager LF, Genta PR, Marcondes BF, Lorenzi-Filho G. Effects of oropharyngeal exercises on patients with moderate obstructive sleep apnea syndrome. Am J Respir Crit Care Med 179: 962–966, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Hida W, Takishima T. The effect of submental electrical stimulation on sleep disordered breathing in patients with obstructive apnea. Sleep 16: S96–S97, 1993 [DOI] [PubMed] [Google Scholar]
- 16.Isono S, Tanaka A, Nishino T. Effects of tongue electrical stimulation on pharyngeal mechanics in anaesthetized patients with obstructive sleep apnoea. Eur Respir J 14: 1258–1265, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Kezirian EJ, Boudewyns A, Eisele DW, Schwartz AR, Smith PL, Van de Heyning PH, De Backer WA. Electrical stimulation of the hypoglossal nerve in the treatment of obstructive sleep apnea. Sleep Med Rev 14: 299–305, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Kirkness JP, Schwartz AR, Schneider H, Punjabi NM, Maly JJ, Laffan AM, McGinley BM, Magnuson T, Schweitzer M, Smith PL, Patil SP. Contribution of male sex, age, and obesity to mechanical instability of the upper airway during sleep. J Appl Physiol 104: 1618–1624, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kribbs NB, Pack AI, Kline LR, Smith PL, Schwartz AR, Schubert NM, Redline S, Henry JN, Getsy JE, Dinges DF. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis 147: 887–895, 1993 [DOI] [PubMed] [Google Scholar]
- 20.Leiter JC. Upper airway shape: Is it important in the pathogenesis of obstructive sleep apnea? Am J Respir Crit Care Med 153: 894–898, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Malhotra A, Huang Y, Fogel R, Lazic S, Pillar G, Jakab M, Kikinis R, White DP. Aging influences on pharyngeal anatomy and physiology: the predisposition to pharyngeal collapse. Am J Med 119: 72–14, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGinley BM, Schwartz AR, Schneider H, Kirkness JP, Smith PL, Patil SP. Upper airway neuromuscular compensation during sleep is defective in obstructive sleep apnea. J Appl Physiol 105: 197–205, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mezzanotte WS, Tangel DJ, White DP. Influence of sleep onset on upper-airway muscle activity in apnea patients versus normal controls. Am J Respir Crit Care Med 153: 1880–1887, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Miki H, Hida W, Chonan T, Kikuchi Y, Takishima T. Effects of submental electrical stimulation during sleep on upper airway patency in patients with obstructive sleep apnea. Am Rev Respir Dis 140: 1285–1289, 1989 [DOI] [PubMed] [Google Scholar]
- 25.Mwenge GB, Rombaux P, Dury M, Lengele B, Rodenstein D. Targeted hypoglossal neurostimulation for obstructive sleep apnoea. A 1-year pilot study. Eur Respir J 41: 360–367, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Nieto FJ, Peppard PE, Young T, Finn L, Hla KM, Farre R. Sleep disordered breathing and cancer mortality: results from the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med 186: 190–194, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oliven A. Treating obstructive sleep apnea with hypoglossal nerve stimulation. Curr Opin Pulm Med 17: 419–424, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Oliven A, O'hearn DJ, Boudewyns A, Odeh M, De BW, Van de HP, Smith PL, Eisele DW, Allan L, Schneider H, Testerman R, Schwartz AR. Upper airway response to electrical stimulation of the genioglossus in obstructive sleep apnea. J Appl Physiol 95: 2023–2029, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Oliven A, Odeh M, Geitini L, Oliven R, Steinfeld U, Schwartz AR, Tov N. Effect of coactivation of tongue protrusor and retractor muscles on pharyngeal lumen and airflow in sleep apnea patients. J Appl Physiol 103: 1662–1668, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Oliven A, Odeh M, Schnall RP. Improved upper airway patency elicited by electrical stimulation of the hypoglossus nerves. Respiration 63: 213–216, 1996 [DOI] [PubMed] [Google Scholar]
- 31.Oliven A, Schnall RP, Pillar G, Gavriely N, Odeh M. Sublingual electrical stimulation of the tongue during wakefulness and sleep. Respir Physiol 127: 217–226, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Oliven R, Tov N, Odeh M, Gaitini L, Steinfeld U, Schwartz AR, Oliven A. Interacting effects of genioglossus stimulation and mandibular advancement in sleep apnea. J Appl Physiol 106: 1668–1673, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Patil SP, Schneider H, Marx JJ, Gladmon E, Schwartz AR, Smith PL. Neuromechanical control of upper airway patency during sleep. J Appl Physiol 102: 547–556, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Puhan MA, Suarez A, Lo CC, Zahn A, Heitz M, Braendli O. Didgeridoo playing as alternative treatment for obstructive sleep apnoea syndrome: randomised controlled trial. Br Med J 332: 266–270, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol 44: 931–938, 1978 [DOI] [PubMed] [Google Scholar]
- 36.Schnall RP, Pillar G, Kelsen SG, Oliven A. Dilatory effects of upper airway muscle contraction induced by electrical stimulation in awake humans. J Appl Physiol 78: 1950–1956, 1995 [DOI] [PubMed] [Google Scholar]
- 37.Schwartz AR, Barnes M, Hillman D, Malhotra A, Kezirian E, Smith PL, Hoegh T, Parrish D, Eastwood PR. Acute upper airway responses to hypoglossal nerve stimulation during sleep in obstructive sleep apnea. Am J Respir Crit Care Med 185: 420–426, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwartz AR, Bennett ML, Smith PL, De Backer W, Hedner J, Boudewyns A, Van de HP, Ejnell H, Hochban W, Knaack L, Podszus T, Penzel T, Peter JH, Goding GS, Erickson DJ, Testerman R, Ottenhoff F, Eisele DW. Therapeutic electrical stimulation of the hypoglossal nerve in obstructive sleep apnea. Arch Otolaryngol Head Neck Surg 127: 1216–1223, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Schwartz AR, Eisele DW, Hari A, Testerman R, Erickson D, Smith PL. Electrical stimulation of the lingual musculature in obstructive sleep apnea. J Appl Physiol 81: 643–652, 1996 [DOI] [PubMed] [Google Scholar]
- 40.Schwartz AR, Gold AR, Schubert N, Stryzak A, Wise RA, Permutt S, Smith PL. Effect of weight loss on upper airway collapsibility in obstructive sleep apnea. Am Rev Respir Dis 144: 494–498, 1991 [DOI] [PubMed] [Google Scholar]
- 41.Schwartz AR, O'Donnell CP, Baron J, Schubert N, Alam D, Samadi SD, Smith PL. The hypotonic upper airway in obstructive sleep apnea: role of structures and neuromuscular activity. Am J Respir Crit Care Med 157: 1051–1057, 1998 [DOI] [PubMed] [Google Scholar]
- 42.Schwartz AR, Schubert N, Rothman W, Godley F, Marsh B, Eisele D, Nadeau J, Permutt L, Gleadhill I, Smith PL. Effect of uvulopalatopharyngoplasty on upper airway collapsibility in obstructive sleep apnea. Am Rev Respir Dis 145: 527–532, 1992 [DOI] [PubMed] [Google Scholar]
- 43.Schwartz AR, Thut DC, Russ B, Seelagy M, Yuan X, Brower RG, Permutt S, Wise RA, Smith PL. Effect of electrical stimulation of the hypoglossal nerve on airflow mechanics in the isolated upper airway. Am Rev Respir Dis 147: 1144–1150, 1993 [DOI] [PubMed] [Google Scholar]
- 44.Seelagy MM, Schwartz AR, Russ DB, King ED, Wise RA, Smith PL. Reflex modulation of airflow dynamics through the upper airway. J Appl Physiol 76: 2692–2700, 1994 [DOI] [PubMed] [Google Scholar]
- 45.Steier J, Seymour J, Rafferty GF, Jolley CJ, Solomon E, Luo Y, Man WD, Polkey MI, Moxham J. Continuous transcutaneous submental electrical stimulation in obstructive sleep apnea: a feasibility study. Chest 140: 998–1007, 2011 [DOI] [PubMed] [Google Scholar]
- 46.Van de Heyning PH, Badr MS, Baskin JZ, Cramer Bornemann MA, De Backer WA, Dotan Y, Hohenhorst W, Knaack L, Lin HS, Maurer JT, Netzer A, Odland RM, Oliven A, Strohl KP, Vanderveken OM, Verbraecken J, Woodson BT. Implanted upper airway stimulation device for obstructive sleep apnea. Laryngoscope 122: 1626–1633, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Weaver TE, Kribbs NB, Pack AI, Kline LR, Chugh DK, Maislin G, Smith PL, Schwartz AR, Schubert NM, Gillen KA, Dinges DF. Night-to-night variability in CPAP use over the first three months of treatment. Sleep 20: 278–283, 1997 [DOI] [PubMed] [Google Scholar]
- 48.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: A population health perspective. Am J Respir Crit Care Med 165: 1217–1239, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Zaidi FN, Meadows P, Jacobowitz O, Davidson TM. Tongue anatomy and physiology: The scientific basis for a novel targeted neurostimulation system designed for the treatment of obstructive sleep apnea. Neuromodulation 10.1111/j.1525-1403.2012.00514.x, 2012 [DOI] [PubMed] [Google Scholar]