Abstract
Animal studies indicate alpha-adrenergic coronary vasoconstriction helps maintain left ventricular function during physiological stress. Whether this process occurs in humans is unknown. In the current study, we used transthoracic Doppler echocardiography to test the effect of lower body negative pressure (LBNP) on coronary blood flow velocity (CBV, left anterior descending coronary artery) and myocardial function in eight young healthy subjects before and after systemic infusion of phentolamine, a nonselective alpha blocker. Heart rate (HR) and blood pressure (BP) were monitored on a beat-by-beat basis. Peak diastolic CBV and myocardial systolic and diastolic tissue velocities (Sm and Em), were quantified at baseline, and at −5 mmHg, −10 mmHg, and −15 mmHg LBNP. Coronary vascular resistance index (CVRI) was calculated as the quotient of diastolic BP and CBV. Phentolamine reduced baseline diastolic BP and increased HR but did not affect the reflex adjustments to LBNP. The reduction in CBV due to LBNP was blunted by phentolamine at −10 mmHg and −15 mmHg. Importantly, the increase in CVRI (i.e., coronary vasoconstriction) was abolished by phentolamine at −5 mmHg (0.21 ± 0.06 vs. 0.83 ± 0.13), −10 mmHg (0.24 ± 0.03 vs. 1.68 ± 0.31), and −15 mmHg (0.27 ± 0.10 vs. 2.34 ± 0.43). These data indicate that alpha-adrenergic coronary vasoconstriction is present during low levels of LBNP. With alpha blockade, more coronary flow is needed to maintain cardiac function. Our data suggest that alpha-adrenergic tone enhances coronary flow efficiency, presumably by redistributing flow from the epicardium to the endocardium.
Keywords: Doppler echocardiography, left ventricular function, sympathetic nervous system
over the last decade, advances in transthoracic Doppler echocardiography have enabled investigators to noninvasively assess coronary blood flow velocity (CBV) in the left anterior descending (LAD) artery as well as left ventricular (LV) myocardial function in conscious humans (17, 18, 35, 36). Animal experiments (10) and the clinical presentation of cardiac ischemia (2) indicate that diastolic CBV and LV systolic and diastolic function are intimately related; the excellent temporal resolution of Doppler echocardiography allows these relationships to be explored in healthy humans undergoing acute physiological stress (38). Using Doppler echocardiography in conjunction with pharmacological blockade, mechanistic insights about the coronary circulation can be obtained in vivo.
Although coronary blood flow is regulated primarily by local metabolic demand, changes in sympathetic tone can also affect it importantly (4, 24). For instance, lower body negative pressure (LBNP) (52) and isometric handgrip (33, 35) both evoke coronary vasoconstriction in healthy humans. Sympathetically mediated changes in coronary vasomotor tone are induced by activation of alpha- (vasoconstriction) and beta-adrenergic (vasodilation) receptors (4, 32). Compared with the beta-adrenergic effects of sympathetic activation, alpha-adrenergic effects on coronary blood flow are largely unknown in humans.
Activation of the sympathetic nervous system is associated with increases in heart rate (HR) and blood pressure (BP) that effectively increase myocardial oxygen demand and utilization. This increased oxygen utilization requires an increase in myocardial oxygen supply (11). Since the heart cannot significantly augment oxygen extraction, the ability to supply additional oxygen must be met by an increase in coronary blood flow. Coronary blood flow occurs predominantly during the diastolic period of each cardiac cycle; at higher heart rates (e.g., during exercise, hypoxemia, orthostasis) the diastolic period is reduced. Under these conditions, the subendocardial layers of the myocardium are thought to be more vulnerable to ischemia, and animal studies suggest that alpha-adrenergic coronary vasoconstriction helps to maintain uniform transmural blood flow from the epicardium to the endocardium (14, 25, 40).
We recently demonstrated that nonhypotensive LBNP, an established sympathoexcitatory stimulus that reduces central venous pressure (43, 51, 53, 55), evoked coronary vasoconstriction (i.e., a reduction in CBV) (33, 35). The reduction in CBV observed with LBNP was not associated with a fall in rate-pressure product (RPP), an index of myocardial oxygen demand, suggesting that activation of the sympathetic nervous system is capable of eliciting coronary vasoconstriction independent of changes in cardiac metabolic demand. To further examine the effects of sympathetic coronary vasoconstriction, in the current study we tested the effects of low-level LBNP on CBV and myocardial function before and after intravenous infusion of phentolamine, a nonselective alpha-adrenergic receptor antagonist. We hypothesized that in the presence of phentolamine a greater level of coronary blood flow would be required to maintain cardiac function.
METHODS
Experimental design and subjects.
This study used a repeated-measures, crossover design with the order of treatment (phentolamine, no phentolamine) being counterbalanced and tested on separate days. Eight healthy normotensive volunteers (4 men and 4 women: age 27 ± 1 yr, body mass index, 23.4 ± 0.9 kg/m2) were studied. This sample size was determined based on the CBV response to LBNP at −10 mmHg with and without phentolamine (posteriori power = 0.803). All subjects were nonsmokers and were not taking vasoactive medications or vitamin supplements. Subjects abstained from caffeine, alcohol, and exercise for 24 h before performing the studies. All subjects provided written informed consent; the experimental protocol was conducted according to the Declaration of Helsinki and was approved by the Institutional Review Board at the Pennsylvania State University College of Medicine.
Protocol.
All studies were conducted in the supine posture. Following enrollment and informed consent procedures, the subject was situated on an exam table with the lower body (to the level of the iliac crest) enclosed in an airtight tank. A tight fitting seal was placed around the waist. The tank was connected in series to a vacuum system that allowed for a consistent reduction in ambient pressure (i.e., suction of blood from the torso to the legs). Subjects were then outfitted with a three-lead ECG to monitor HR and a Finometer cuff for the beat-by-beat measurement of BP. HR, BP, and tank pressure were recorded using PowerLab (ADInstruments). Resting brachial artery BP was verified noninvasively using an oscillometric device (Dinamap XL, Critikon/GE).
Following a 5-min baseline, LBNP proceeded at −5 mmHg, −10 mmHg, and −15 mmHg (10 min each stage). These levels of LBNP were chosen based on prior research demonstrating reductions in central venous pressure and increases in muscle sympathetic nerve activity but no net change in HR or BP (9, 16, 31). In preliminary studies (n = 6) we found no change in LV size until LBNP exceeded −15 mmHg. Thus these levels of LBNP were expected to engage the sympathetic nervous system without drastically altering cardiac metabolic demand.
During one experimental trial, systemic alpha-adrenergic blockade was achieved by intravenous infusion of 6 mg phentolamine mesylate over 4.5 min (1 mg/min for 3 min followed by 2 mg/min for 1.5 min). This dose was chosen based on pilot studies in our laboratory (n = 4) demonstrating an ∼50% attenuation in the forearm vasoconstrictor response to the cold pressor test (hand in 1°C water) following phentolamine. Prior human investigations have also shown that 5–10 mg of intravenous phentolamine elicits physiological effects (8, 21, 50). Resting HR, BP, and echocardiographic parameters were obtained before and after phentolamine infusion (i.e., prior to LBNP). LBNP was then conducted identical to the procedures described above.
Echocardiographic measurements.
CBV and tissue Doppler imaging (TDI) indexes of systolic and diastolic LV function were examined at baseline and from minutes 6–10 at each level of LBNP (i.e., at physiological steady state). The same investigator (Z.G.) performed all echocardiography measurements and a prior publication from our laboratory reported high reproducibility (19).
CBV measurement was performed as described previously (18, 33–35). Briefly, the distal portion of the LAD was imaged in the apical four-chamber view using color flow mapping (velocity range set at ±19 cm/s). Care was taken to place the transducer in a position that allowed for a long-axis view of the LAD. With a sample volume (2.0 mm) positioned over the color Doppler signal in the LAD, we recorded CBV at the end of expiration. The Doppler tracing of the diastolic portion of each cardiac cycle was analyzed using Pro Solv 3.0 to obtain peak CBV. Consistent with previous studies, the coronary vascular resistance index (CVRI) was calculated offline as the quotient of diastolic BP and CBV (19).
Tissue Doppler imaging (TDI) was used to quantify LV systolic and diastolic function, as previously described by our laboratory (18, 38). The variables of interest included systolic (Sm), early diastolic (Em), and late diastolic (Am) myocardial velocity. Pulsed Doppler images were acquired in the apical four-chamber view with the sample volume placed within the intraventricular septum at the mitral annulus (41, 42, 48). To minimize the angle between the beam and the direction of annular motion, care was taken to keep the ultrasound beam perpendicular to the annulus plane (6). The TDI technique is relatively insensitive to changes in preload (1, 39, 48) and is able to detect both systolic and diastolic dysfunction represented as decreases of Sm and Em, respectively, within 5 s after an acute reduction in coronary blood flow (10). Thus the temporal resolution of TDI is excellent for detecting acute changes of coronary blood flow due to sympathetic activation (38).
Data analysis and statistics.
Because CBV and TDI images were examined from minutes 6–10 at each level of LBNP (i.e., at physiological steady state), the HR and BP values represent the average values of minutes 6–10 at each level of LBNP. All echocardiographic values are an average of three sequential cardiac cycles obtained at end-expiration. Rate-pressure product (RPP), an index of myocardial oxygen demand (20), was calculated as HR·systolic BP.
Normality was confirmed by the Kolmogorov-Smirnov test (i.e., P > 0.05 for all measurements). Next, a two drug (phentolamine, no phentolamine) by four time point (baseline, −5 mmHg, −10 mmHg, and −15 mmHg LBNP) repeated-measures analysis of variance was conducted for BP, HR, RPP, CBV, Sm, Em and Am. When a significant drug × time interaction was found, two-tailed paired t-tests were used for post hoc analysis. Paired t-tests were also used to compare baseline parameters (prior to LBNP) to determine if phentolamine altered resting hemodynamics. Data are presented as means ± SE and P values of <0.05 were considered to be statistically significant.
RESULTS
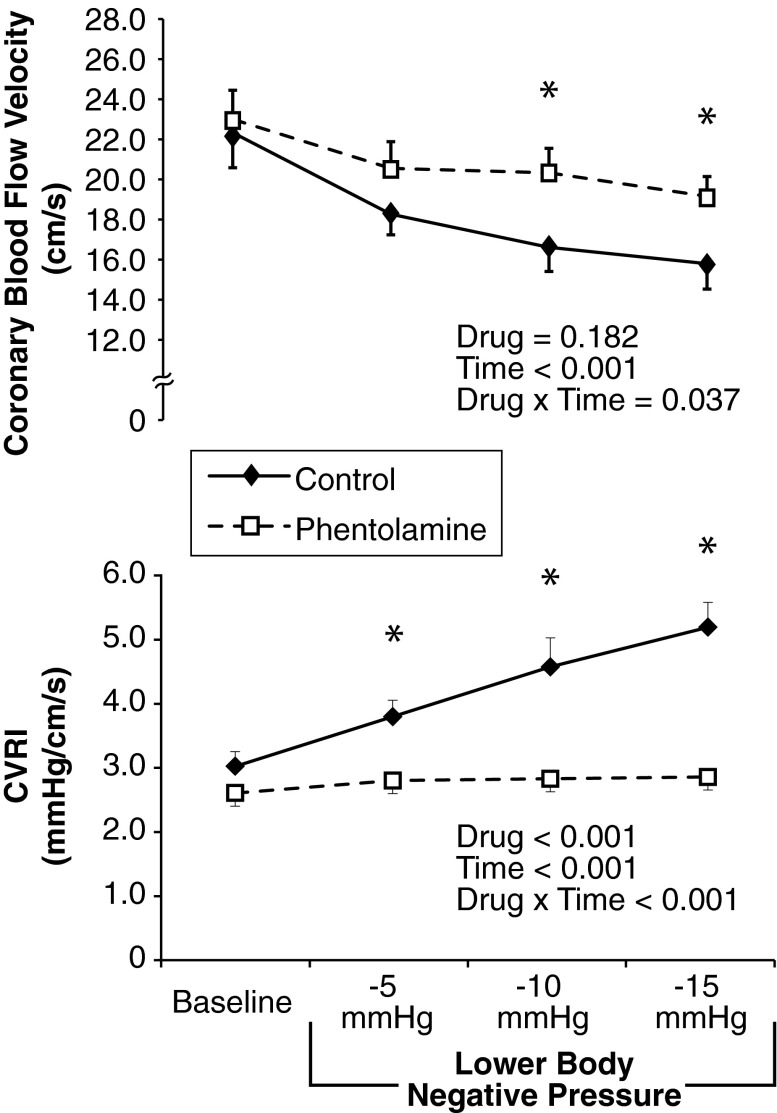
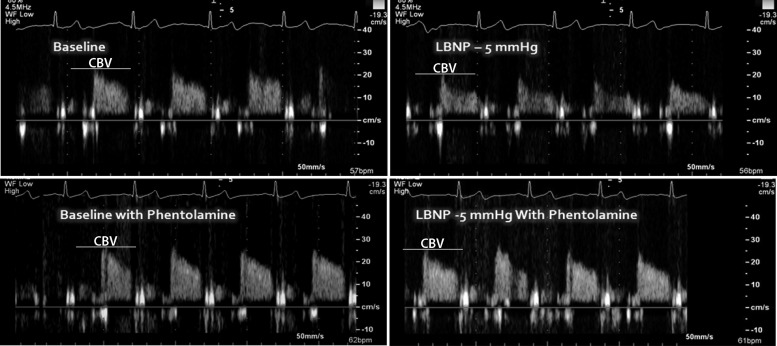
Intravenous phentolamine caused modest reductions in systolic (P = 0.043) and diastolic BP (P = 0.008, Table 1) but less clearly of mean BP (P = 0.074), and increased Sm without affecting Em or Am (Table 2). As shown in Table 1, progressive LBNP at −5, −10, and −15 mmHg for 10 min at each stage did not cause hypotension or tachycardia. Regardless of time, BP was lower with phentolamine and HR was higher (main effect of drug). In response to progressive LBNP, RPP increased to a similar extent under both control and phentolamine conditions. Despite this mild cardiac metabolic stimulus, CBV was consistently reduced with progressive LBNP (main effect for time, Fig. 1, top) and phentolamine blunted the reduction in CBV (drug × time interaction). Indeed, at −10 mmHg (P = 0.014) and −15 mmHg (P = 0.046), CBV was significantly greater with phentolamine compared with control. Phentolamine abolished the rise in CVRI (Fig. 1, bottom) at −5 mmHg (P = 0.006), −10 mmHg (P = 0.002), and −15 mmHg (P = 0.001) of LBNP. Representative recordings of CBV are displayed in Fig. 2.
Table 1.
Hemodynamic responses to progressive, low-intensity LBNP
| Preinfusion | Baseline | LBNP, −5 mmHg | LBNP, −10 mmHg | LBNP, −15 mmHg | Drug | Time | Drug × Time | |
|---|---|---|---|---|---|---|---|---|
| SBP, mmHg | ||||||||
| Control | 124 ± 7 | 130 ± 6 | 129 ± 6 | 130 ± 6 | 0.027 | 0.006 | 0.841 | |
| Phentolamine | 125 ± 4 | 118 ± 5* | 123 ± 6 | 126 ± 74 | 124 ± 7 | |||
| DBP, mmHg | ||||||||
| Control | 64 ± 4 | 68 ± 3 | 74 ± 6 | 81 ± 4 | 0.043 | 0.004 | 0.001 | |
| Phentolamine | 67 ± 4 | 53 ± 1* | 55 ± 1† | 55 ± 1† | 53 ± 1† | |||
| MAP, mmHg | ||||||||
| Control | 84 ± 4 | 86 ± 4 | 85 ± 3 | 86 ± 4 | 0.296 | 0.029 | 0.253 | |
| Phentolamine | 83 ± 2 | 79 ± 3 | 83 ± 3 | 84 ± 4 | 84 ± 4 | |||
| HR, beats/min | ||||||||
| Control | 61 ± 2 | 61 ± 2 | 60 ± 2 | 63 ± 2 | 0.005 | 0.114 | 0.911 | |
| Phentolamine | 66 ± 2 | 69 ± 2* | 70 ± 3 | 70 ± 3 | 73 ± 2 | |||
| RPP, beats/min·mmHg | ||||||||
| Control | 7,503 ± 325 | 7,808 ± 218 | 7,735 ± 212 | 8,194 ± 397 | 0.032 | 0.008 | 0.789 | |
| Phentolamine | 8,449 ± 218 | 8,258 ± 349 | 8,613 ± 362 | 8,780 ± 518 | 9,056 ± 552 |
Data are means ± SE. Eight young healthy subjects underwent testing in the supine posture; each stage was 10 min in duration.
LBNP, lower-body negative pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; HR, heart rate; RPP, rate-pressure product.
Significantly different from preinfusion;
significantly different from control condition.
Table 2.
Left ventricular responses (assessed by tissue Doppler imaging, TDI) to progressive, low-intensity LBNP
| PreInfusion | Base | LBNP, −5 mmHg | LBNP, −10 mmHg | LBNP, −15 mmHg | Drug | Time | Drug × Time | |
|---|---|---|---|---|---|---|---|---|
| Sm, cm/s | ||||||||
| Control | 7.9 ± 0.1 | 7.9 ± 0.1 | 7.9 ± 0.2 | 7.5 ± 0.3 | 0.037 | 0.139 | 0.792 | |
| Phentolamine | 8.0 ± 0.2 | *8.5 ± 0.2 | 8.4 ± 0.5 | 8.8 ± 0.3 | 8.1 ± 0.3 | |||
| Em, cm/s | ||||||||
| Control | 12.9 ± 0.5 | 12.8 ± 0.4 | 12.0 ± 0.5 | 10.5 ± 0.6 | 0.088 | 0.001 | 0.320 | |
| Phentolamine | 12.7 ± 0.3 | 12.6 ± 0.3 | 11.5 ± 0.6 | 10.9 ± 0.3 | 9.8 ± 0.4 | |||
| Am, cm/s | ||||||||
| Control | 6.1 ± 0.3 | 6.3 ± 0.6 | 5.7 ± 0.4 | 6.1 ± 0.5 | 0.055 | 0.513 | 0.195 | |
| Phentolamine | 7.0 ± 0.2 | 7.3 ± 0.5 | 7.2 ± 0.6 | 7.0 ± 0.4 | 6.2 ± 0.4 |
Data are means ± SE. Eight young healthy subjects underwent testing in the supine posture; each stage was 10 min in duration.
Sm, systolic myocardial velocity; Em, early diastolic myocardial velocity; Am, late diastolic myocardial velocity.
Significantly different from preinfusion.
Fig. 1.
Coronary responses to lower body negative pressure (LBNP). CVRI, coronary vascular resistance index. Data are means ± SE (n = 8). *Significant difference between conditions.
Fig. 2.
Representative recording of coronary blood flow velocity (Doppler echocardiography) from one subject during LBNP with and without phentolamine. The y-axis units are cm/s.
Table 2 displays the effects of progressive LBNP on TDI indexes of LV function. Baseline Sm was enhanced by phentolamine (P = 0.016) but alpha blockade did not affect the Sm response to LBNP over time. Em was reduced as LBNP progressed (main effect for time) but this reduction was not modified by phentolamine. Am tended to be higher at baseline under alpha blockade (P = 0.091), but phentolamine did not alter the Am response to progressive LBNP.
DISCUSSION
The purpose of this study was to determine the effect of alpha blockade on coronary blood velocity and TDI indexes of myocardial function in response to LBNP. We hypothesized that phentolamine would attenuate the LBNP-induced reduction in CBV because of its ability to block coronary alpha receptors. The results of this study confirm this hypothesis.
Autonomic regulation of coronary vasomotion has attracted the attention of physiologists and cardiologists for many decades (3, 7, 13, 44). It has long been known that sympathetically mediated coronary vasoconstriction is due to stimulation of alpha-adrenergic receptors (5, 22, 26). Furthermore, in the presence of coronary artery disease, unrestrained alpha-adrenergic vasoconstriction is thought to contribute to myocardial ischemia (4).
Prior human studies have reported that resting sympathetic coronary vascular tone is low (24). Specifically, intracoronary infusion of the alpha-adrenergic antagonist phentolamine, and/or the beta-adrenergic antagonists propranolol or metoprolol did not affect CVR or the coronary dilator response to papaverine in either heart transplant patients or healthy controls. Our current noninvasive data (prior to LBNP) support the concept that resting alpha-adrenergic tone is indeed low because neither CBV nor CVRI were altered by phentolamine. We should note that systemically infused phentolamine increased Sm in our study. We suspect this was due to the reduction in systolic BP and LV afterload.
LBNP is an established laboratory tool to elicit a reflex increase in sympathetic nerve activity (30, 53, 54) and peripheral vascular resistance (27, 55). At low levels (i.e., −5 to −15 mmHg), it is thought that the cardiopulmonary baroreceptors are the major afferent sensor initiating these reflex responses. Despite systemic increases in sympathetic activity and vascular resistance with low level LBNP (up to −20 mmHg), changes in HR and BP are small (9, 13, 43). Because HR and BP are important determinants of cardiac work, we felt that low-level LBNP would serve as a tool to examine sympathetic reflex responses on the coronary bed that were only minimally influenced by changes in cardiac metabolism. This is contrasted with other sympathetic stressors such as isometric handgrip, hypoxemia, and higher levels of LBNP that likely would have changed HR and/or BP along with increasing sympathetic nerve activity (28, 29, 37, 49).
Prior work from our laboratory has indicated that low-level LBNP elicits a reduction in CBV (i.e., myocardial oxygen supply) without significant changes in RPP (i.e., myocardial oxygen demand) (33, 35). The current data confirm and extend these prior findings in two ways. First, we demonstrate that activation of alpha-adrenergic receptors is the primary cause for the reduction in CBV in response to LBNP. Second, we show that in the presence of intact alpha-adrenergic constriction, LV performance is maintained at lower CBV values than with alpha blockade. Thus under physiological conditions (without alpha blockade), coronary vasoconstriction may facilitate redistribution of blood flow to the subendocardium. These data are consistent with prior animal studies (14, 25, 40) and to our knowledge are the first evidence of this process in humans.
Four general strengths of this study are worth noting: 1) this is a human in vivo study; 2) noninvasive imaging techniques were used and no anesthesia or sedation was employed; 3) subjects were under “true resting conditions” (i.e., less anxiety compared with catheterization studies); and 4) the LBNP protocol elicited similar HR and BP changes under both control and phentolamine conditions. We believe these are crucial advantages of the approach presented. Considering the complex nature of coronary blood flow regulation, potential species differences, and the general concerns noted with in vitro models, we believe studies in conscious humans are vital.
Several potential limitations should be noted. First, based on recent publications (46, 47), the dosage of phentolamine in our study may not have blocked alpha receptors completely. Since both phentolamine and LBNP have hypotensive effects, we were concerned that higher doses of phentolamine (12, 15, 45) would cause syncope. Based on our pilot data, we therefore purposely chose to administer 6 mg of phentolamine, a dose that elicited physiological effects (i.e., attenuated reduction in CBV), which is consistent with prior publications that infused 5–10 mg of phentolamine systemically (8, 21, 50). Second, both alpha-1 (epicardial vessels) and alpha-2 receptors (coronary microcirculation) subtypes are involved in producing coronary vasoconstriction (4). Because phentolamine blocks both subtypes, it is unclear which subtype is predominant in the LBNP-induced reduction in CBV. Third, beta-adrenergic receptors were not blocked in the current study. We were concerned about the use of simultaneous alpha- and beta-blockade during LBNP. Had we performed combined alpha- and beta-blockade in our subjects, both the change in HR due to phentolamine and the reflex tachycardia to LBNP would have been smaller, thereby enhancing the potential for serious hypotension. Last, we acknowledge that as we did not directly measure sympathetic activity (e.g., muscle sympathetic nerve activity and/or cardiac norepinephrine spillover), the contention that sympathetic outflow was increased during the LBNP intervention is based on prior research.
Conclusions.
Both human and animal studies indicate alpha-adrenergic vasoconstriction plays an important role in the regulation of coronary blood flow (4). When the coronary circulation is compromised by atherosclerosis and alpha-adrenergic vasoconstriction is unrestrained, myocardial perfusion can be reduced and ischemia may be noted (23). However, as suggested by this report, under physiological conditions alpha-adrenergic vasoconstriction may allow a more efficient distribution of blood flow throughout the myocardium during higher levels of physiological stress (14). In the current study, phentolamine attenuated the LBNP-induced reduction in CBV without altering LV systolic and diastolic function relative to control conditions. This suggests an important role of alpha-adrenergic tone in regulating coronary blood flow during stress in human subjects.
GRANTS
Grants R01-HL-070222 (L. I. Sinoway), UL1-TR000127 (L. I. Sinoway) as well as C06-RR016499 from the National Institutes of Health and Tobacco Settlement Funds (L. I. Sinoway) supported this study.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Z.G., L.I.S., and U.A.L. conception and design of research; Z.G. performed experiments; Z.G. and M.D.M. analyzed data; Z.G., M.D.M., L.I.S., and U.A.L. interpreted results of experiments; Z.G., M.D.M., L.I.S., and U.A.L. edited and revised manuscript; Z.G., M.D.M., L.I.S., and U.A.L. approved final version of manuscript; M.D.M. prepared figures; M.D.M. drafted manuscript.
ACKNOWLEDGMENTS
We are thankful to Cheryl Blaha and Jessica Mast for expert study coordination and nursing support. We also appreciate the technical assistance provided by Drs. Samson Spilk and Afsana Momen and the critical revision by Hardikkumar Patel. Last, the authors also express gratitude to Anne Muller for preparing the graphics and Jennie Stoner for outstanding secretarial skills.
REFERENCES
- 1.Abali G, Tokgozoglu L, Ozcebe OI, Aytemir K, Nazli N. Which Doppler parameters are load independent? A study in normal volunteers after blood donation. J Am Soc Echocardiogr 18: 1260–1265, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Alam M, Wardell J, Andersson E, Samad BA, Nordlander R. Effects of first myocardial infarction on left ventricular systolic and diastolic function with the use of mitral annular velocity determined by pulsed wave Doppler tissue imaging. J Am Soc Echocardiogr 13: 343–352, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Aung-Din R, Mitchell JH, Longhurst JC. Reflex α-adrenergic coronary vasoconstriction during hindlimb static exercise in dogs. Circ Res 48: 502–509, 1981 [DOI] [PubMed] [Google Scholar]
- 4.Barbato E. Role of adrenergic receptors in human coronary vasomotion. Heart 95: 603–608, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Baumgart D, Haude M, Gorge G, Liu F, Ge J, Grosse-Eggebrecht C, Erbel R, Heusch G. Augmented alpha-adrenergic constriction of atherosclerotic human coronary arteries. Circulation 99: 2090–2097, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Caiani EG, Weinert L, Takeuchi M, Veronesi F, Sugeng L, Corsi C, Capderou A, Cerutti S, Vaida P, Lang RM. Evaluation of alterations on mitral annulus velocities, strain, and strain rates due to abrupt changes in preload elicited by parabolic flight. J Appl Physiol 103: 80–87, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Chen DG, Dai XZ, Zimmerman BG, Bache RJ. Postsynaptic alpha 1- and alpha 2-adrenergic mechanisms in coronary vasoconstriction. J Cardiovasc Pharmacol 11: 61–67, 1988 [DOI] [PubMed] [Google Scholar]
- 8.Cook JS, Sauder CL, Ray CA. Melatonin differentially affects vascular blood flow in humans. Am J Physiol Heart Circ Physiol 300: H670–H674, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davy KP, Seals DR, Tanaka H. Augmented cardiopulmonary and integrative sympathetic baroreflexes but attenuated peripheral vasoconstriction with age. Hypertension 32: 298–304, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Derumeaux G, Ovize M, Loufoua J, Andre-Fouet X, Minaire Y, Cribier A, Letac B. Doppler tissue imaging quantitates regional wall motion during myocardial ischemia and reperfusion. Circulation 97: 1970–1977, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev 88: 1009–1086, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Esler MD, Julius S, Randall OS, Ellis CN, Kashima T. Relation of renin status to neurogenic vascular resistance in borderline hypertension. Am J Cardiol 36: 708–715, 1975 [DOI] [PubMed] [Google Scholar]
- 13.Feigl EO. Coronary physiology. Physiol Rev 63: 1–205, 1983 [DOI] [PubMed] [Google Scholar]
- 14.Feigl EO. The paradox of adrenergic coronary vasoconstriction. Circulation 76: 737–745, 1987 [DOI] [PubMed] [Google Scholar]
- 15.Frank SM, Raja SN. Reflex cutaneous vasoconstriction during cold pressor test is mediated through alpha-adrenoceptors. Clin Auton Res 4: 257–261, 1994 [DOI] [PubMed] [Google Scholar]
- 16.Furlan R, Jacob G, Palazzolo L, Rimoldi A, Diedrich A, Harris PA, Porta A, Malliani A, Mosqueda-Garcia R, Robertson D. Sequential modulation of cardiac autonomic control induced by cardiopulmonary and arterial baroreflex mechanisms. Circulation 104: 2932–2937, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Gao Z, Novick M, Muller MD, Williams RJ, Spilk S, Leuenberger UA, Sinoway LI. Exercise and diet-induced weight loss attenuates oxidative stress related-coronary vasoconstriction in obese adolescents. Eur J Appl Physiol 113: 519–528, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao Z, Spilk S, Momen A, Muller MD, Leuenberger UA, Sinoway LI. Vitamin C prevents hyperoxia-mediated coronary vasoconstriction and impairment of myocardial function in healthy subjects. Eur J Appl Physiol 112: 483–492, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao Z, Wilson TE, Drew RC, Ettinger J, Monahan KD. Altered coronary vascular control during cold stress in healthy older adults. Am J Physiol Heart Circ Physiol 302: H312–H318, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gobel FL, Norstrom LA, Nelson RR, Jorgensen CR, Wang Y. The rate-pressure product as an index of myocardial oxygen consumption during exercise in patients with angina pectoris. Circulation 57: 549–556, 1978 [DOI] [PubMed] [Google Scholar]
- 21.Halliwill JR, Minson CT, Joyner MJ. Effect of systemic nitric oxide synthase inhibition on postexercise hypotension in humans. J Appl Physiol 89: 1830–1836, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Heusch G. Alpha-adrenergic mechanisms in myocardial ischemia. Circulation 81: 1–13, 1990 [DOI] [PubMed] [Google Scholar]
- 23.Heusch G, Baumgart D, Camici P, Chilian W, Gregorini L, Hess O, Indolfi C, Rimoldi O. α-Adrenergic coronary vasoconstriction and myocardial ischemia in humans. Circulation 101: 689–694, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Hodgson JM, Cohen MD, Szentpetery S, Thames MD. Effects of regional alpha- and beta-blockade on resting and hyperemic coronary blood flow in conscious, unstressed humans. Circulation 79: 797–809, 1989 [DOI] [PubMed] [Google Scholar]
- 25.Huang AH, Feigl EO. Adrenergic coronary vasoconstriction helps maintain uniform transmural blood flow distribution during exercise. Circ Res 62: 286–298, 1988 [DOI] [PubMed] [Google Scholar]
- 26.Indolfi C, Piscione F, Villari B, Russolillo E, Rendina V, Golino P, Condorelli M, Chiariello M. Role of alpha 2-adrenoceptors in normal and atherosclerotic human coronary circulation. Circulation 86: 1116–1124, 1992 [DOI] [PubMed] [Google Scholar]
- 27.Johnson JM, Rowell LB, Niederberger M, Eisman MM. Human splanchnic and forearm vasoconstrictor responses to reductions of right atrial and aortic pressures. Circ Res 34: 515–524, 1974 [DOI] [PubMed] [Google Scholar]
- 28.Katkov VE, Chestukhin VV, Kakurin LI. Coronary circulation of the healthy man exposed to tilt tests, LBNP, and head-down tilt. Aviat Space Environ Med 56: 741–747, 1985 [PubMed] [Google Scholar]
- 29.Katkov VE, Chestukhin VV, Kakurin LI, Babin AM, Nikolaenko EM. Central and coronary circulation of the normal man during orthostatic and lower body negative pressure tests. Aviat Space Environ Med 58: A55-A60, 1987 [PubMed] [Google Scholar]
- 30.Kimmerly DS, O'Leary DD, Menon RS, Gati JS, Shoemaker JK. Cortical regions associated with autonomic cardiovascular regulation during lower body negative pressure in humans. J Physiol 569: 331–345, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kimmerly DS, Shoemaker JK. Hypovolemia and neurovascular control during orthostatic stress. Am J Physiol Heart Circ Physiol 282: H645–H655, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Lorenzoni R, Rosen SD, Camici PG. Effect of alpha 1-adrenoceptor blockade on resting and hyperemic myocardial blood flow in normal humans. Am J Physiol Heart Circ Physiol 271: H1302–H1306, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Momen A, Gao Z, Cohen A, Khan T, Leuenberger UA, Sinoway LI. Coronary vasoconstrictor responses are attenuated in young women as compared with age-matched men. J Physiol 588: 4007–4016, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Momen A, Kozak M, Leuenberger UA, Ettinger S, Blaha C, Mascarenhas V, Lendel V, Herr MD, Sinoway LI. Transthoracic Doppler echocardiography to noninvasively assess coronary vasoconstrictor and dilator responses in humans. Am J Physiol Heart Circ Physiol 298: H524–H529, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Momen A, Mascarenhas V, Gahremanpour A, Gao Z, Moradkhan R, Kunselman A, Boehmer J, Sinoway LI, Leuenberger UA. Coronary blood flow responses to physiological stress in humans. Am J Physiol Heart Circ Physiol 296: H854–H861, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muller MD, Gao Z, Drew RC, Herr MD, Leuenberger UA, Sinoway LI. Effect of cold air inhalation and isometric exercise on coronary blood flow and myocardial function in humans. J Appl Physiol 111: 1694–1702, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muller MD, Gao Z, Mast JL, Blaha CA, Drew RC, Leuenberger UA, Sinoway LI. Aging attenuates the coronary blood flow response to cold air breathing and isometric handgrip in healthy humans. Am J Physiol Heart Circ Physiol 302: H1737–H1746, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muller MD, Mast JL, Patel H, Sinoway LI. Cardiac mechanics are impaired during fatiguing exercise and cold pressor test in healthy older adults. J Appl Physiol 114: 186–194, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quinones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol 30: 1527–1533, 1997 [DOI] [PubMed] [Google Scholar]
- 40.Nathan HJ, Feigl EO. Adrenergic vasoconstriction lessens transmural steal during coronary hypoperfusion. Am J Physiol Heart Circ Physiol 250: H645–H653, 1986 [DOI] [PubMed] [Google Scholar]
- 41.Okada K, Mikami T, Kaga S, Onozuka H, Inoue M, Yokoyama S, Nishino H, Nishida M, Matsuno K, Iwano H, Yamada S, Tsutsui H. Early diastolic mitral annular velocity at the interventricular septal annulus correctly reflects left ventricular longitudinal myocardial relaxation. Eur J Echocardiogr 12: 917–923, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation 102: 1788–1794, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Padilla J, Young CN, Simmons GH, Deo SH, Newcomer SC, Sullivan JP, Laughlin MH, Fadel PJ. Increased muscle sympathetic nerve activity acutely alters conduit artery shear rate patterns. Am J Physiol Heart Circ Physiol 298: H1128–H1135, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Powell JR, Feigl EO. Carotid sinus reflex coronary vasoconstriction during controlled myocardial oxygen metabolism in the dog. Circ Res 44: 44–51, 1979 [DOI] [PubMed] [Google Scholar]
- 45.Raja SN, Turnquist JL, Meleka S, Campbell JN. Monitoring adequacy of alpha-adrenoceptor blockade following systemic phentolamine administration. Pain 64: 197–204, 1996 [DOI] [PubMed] [Google Scholar]
- 46.Sielatycki JA, Shamimi-Noori S, Pfeiffer MP, Monahan KD. Adrenergic mechanisms do not contribute to age-related decreases in calf venous compliance. J Appl Physiol 110: 29–34, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Snyder KA, Shamimi-Noori S, Wilson TE, Monahan KD. Age- and limb-related differences in the vasoconstrictor response to limb dependency are not mediated by a sympathetic mechanism in humans. Acta Physiol (Oxf) 205: 372–380, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sohn DW, Chai IH, Lee DJ, Kim HC, Kim HS, Oh BH, Lee MM, Park YB, Choi YS, Seo JD, Lee YW. Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol 30: 474–480, 1997 [DOI] [PubMed] [Google Scholar]
- 49.Somers VK, Mark AL, Zavala DC, Abboud FM. Influence of ventilation and hypocapnia on sympathetic nerve responses to hypoxia in normal humans. J Appl Physiol 67: 2095–2100, 1989 [DOI] [PubMed] [Google Scholar]
- 50.Sugawara J, Komine H, Hayashi K, Yoshizawa M, Otsuki T, Shimojo N, Miyauchi T, Yokoi T, Maeda S, Tanaka H. Systemic alpha-adrenergic and nitric oxide inhibition on basal limb blood flow: effects of endurance training in middle-aged and older adults. Am J Physiol Heart Circ Physiol 293: H1466–H1472, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Sundlöf G, Wallin BG. Effect of lower body negative pressure on human muscle nerve sympathetic activity. J Physiol 278: 525–532, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trimarco B, Vigorito C, Cuocolo A, Ricciardelli B, De Luca N, Volpe M, Lembo G, Condorelli M. Reflex control of coronary vascular tone by cardiopulmonary receptors in humans. J Am Coll Cardiol 11: 944–952, 1988 [DOI] [PubMed] [Google Scholar]
- 53.Victor RG, Leimbach WN., Jr. Effects of lower body negative pressure on sympathetic discharge to leg muscles in humans. J Appl Physiol 63: 2558–2562, 1987 [DOI] [PubMed] [Google Scholar]
- 54.Vissing SF, Scherrer U, Victor RG. Increase of sympathetic discharge to skeletal muscle but not to skin during mild lower body negative pressure in humans. J Physiol 481: 233–241, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zoller RP, Mark AL, Abboud FM, Schmid PG, Heistad DD. The role of low pressure baroreceptors in reflex vasoconstrictor responses in man. J Clin Invest 51: 2967–2972, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]