Abstract
The objectives of this study are threefold: 1) to assess whether 7 days of oral glutamine (GLN) supplementation reduces exercise-induced intestinal permeability; 2) whether supplementation prevents the proinflammatory response; and 3) whether these changes are associated with upregulation of the heat shock response. On separate occasions, eight human subjects participated in baseline testing and in GLN and placebo (PLA) supplementation trials, followed by a 60-min treadmill run. Intestinal permeability was higher in the PLA trial compared with baseline and GLN trials (0.0604 ± 0.047 vs. 0.0218 ± 0.008 and 0.0272 ± 0.007, respectively; P < 0.05). IκBα expression in peripheral blood mononuclear cells was higher 240 min after exercise in the GLN trial compared with the PLA trial (1.411 ± 0.523 vs. 0.9839 ± 0.343, respectively; P < 0.05). In vitro using the intestinal epithelial cell line Caco-2, we measured effects of GLN supplementation (0, 4, and 6 mM) on heat-induced (37° or 41.8°C) heat shock protein 70 (HSP70), heat shock factor-1 (HSF-1), and occludin expression. HSF-1 and HSP70 levels increased in 6 mM supplementation at 41°C compared with 0 mM at 41°C (1.785 ± 0.495 vs. 0.6681 ± 0.290, and 1.973 ± 0.325 vs. 1.133 ± 0.129, respectively; P < 0.05). Occludin levels increased after 4 mM supplementation at 41°C and 6 mM at 41°C compared with 0 mM at 41°C (1.236 ± 0.219 and 1.849 ± 0.564 vs. 0.7434 ± 0.027, respectively; P < 0.001). GLN supplementation prevented exercise-induced permeability, possibly through HSF-1 activation.
Keywords: permeability, tight junction, exercise
intestinal permeability and systemic inflammation are associated with gastrointestinal (GI) distress, in which paracellular endotoxin leakage triggers an immune response, causing disruption to intestinal epithelial cell absorption mechanisms (39, 48). In animal models, endotoxemia causes leukocyte release of proinflammatory cytokines, which damage intestinal sodium-potassium pumps, leading to inhibition of electrolyte and water absorption (39, 48). The ultimate effect is fluid buildup in the gut, and possibly diarrhea (39). Exercise-induced hyperthermia in humans is associated with an increase in intestinal permeability, commonly called leaky gut (41, 44), which provokes an inflammatory cascade. This pathway may be responsible for exercise-induced GI distress in which tight junction (TJ) breakdown and increased permeability is the initial phase of the pathway.
Heat shock proteins are intracellular molecular chaperones that protect the cell through repair of unfolded proteins and contribute to cell maintenance (13, 37, 42). Heat shock protein 70 (HSP70) is induced when cells and animals are exposed to stress, and protects tissue from subsequent heat exposure (10, 13, 38). Human skeletal muscle levels of HSP70 increase in response to muscle-damaging exercise, preventing damage from further eccentric contractions (36). Additionally, HSP70 levels in peripheral blood mononuclear cells (PBMCs) are upregulated after endurance exercise and in response to heat acclimation (12, 26). Augmenting HSP70 levels by either conditioning stress or single gene transfer in intestinal epithelial cells increases TJ stability and reduces permeability to heat stress (10). In addition, we have shown that heat shock factor-1 (HSF-1), the transcription factor for HSP70, directly regulates the TJ protein occludin (11). HSP70 overexpression in rats by single gene transfer increases the expression of IκBα (nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha), which inhibits the nuclear translocation of nuclear factor-κB (NF-κB) (9). In exercising humans, HSP70 inhibition in PBMCs correlates with a rise in intestinal permeability (26). This demonstrates that augmenting the heat shock response may have dual mechanisms in protecting the gut under conditions of stress. One mechanism is through stabilization of the epithelial cell TJ proteins; the other is by reducing the inflammatory response in PBMCs. We hypothesize that enhancing HSP70 in intestinal cells may reduce exercise-induced intestinal permeability and GI distress.
Glutamine (GLN) is the most abundant amino acid in the human body. It is a major nutrient for enterocytes and immune cells, and reduces mortality when given intravenously to sepsis and burn patients (40, 56). GLN supplementation has been shown to reduce the symptoms of irritable bowel syndrome and Crohn's disease (23). The protective effects of GLN may occur through increasing HSF-1 and HSP70 synthesis. Oral GLN supplementation in rats increases HSP70 levels in the intestinal epithelial cells and reduces permeability. This mechanism is believed to be due to GLN activation of HSF-1 and stabilization of the TJ protein occludin (11). In addition, we have recently shown that 7 days of oral GLN supplementation upregulates HSP70 expression in human PBMCs in response to 60 min of high-intensity exercise (12).
Therefore, the purpose of this study was twofold: to test 1) whether 7 days of GLN supplementation reduces exercise-induced intestinal permeability through activation of the heat shock response (HSF-1 and HSP70) leading to occludin protein expression (in vitro studies); and 2) whether GLN suppresses the proinflammatory immune cascade by inhibiting the translocation of NF-κB in PBMCs as measured through the expression of IκBα. A third purpose was to determine whether these changes are associated with upregulation of the heat shock response. Through a combined model utilizing an in vivo exercise protocol in humans and in vitro heat stress experiments in an intestinal epithelial cell line (Caco-2) we demonstrate that GLN supplementation reduces exercise-induced intestinal permeability, possibly through heat shock response-induced inhibition in NF-κB pathway and increase in occludin protein expression.
MATERIALS AND METHODS
Human Protocol
Subjects.
The study was approved by the Human Research Review Committee of the University of New Mexico, Albuquerque, New Mexico. Eight endurance-trained adult men (n = 5) and women (n = 3) ages 18–45 were recruited from the university population. Fitness level was determined through maximal oxygen consumption testing and a physical activity questionnaire. Both men and women were included in the study because there is no reported difference between the groups in exercise-induced GI distress and HSP70 response to exercise (3, 16, 28). All subjects completed a health questionnaire, and procedures, discomforts, and risks were discussed before written informed consent was obtained. Subjects were excluded if they were taking medications (e.g., nonsteroidal anti-inflammatory drugs, antidepressants, or diuretics), or nutritional supplements. All testing was performed in the Exercise Laboratory at the University of New Mexico at 1,585 m of altitude.
Experimental design.
Using a double-blinded research design, each subject participated in baseline testing, and both a GLN and placebo (PLA) trial. The GLN and PLA trials were counterbalanced on the basis of subject number and were separated by a 4-wk washout period. During the first visit to the laboratory, baseline measurements (maximal oxygen consumption, body composition, and intestinal permeability) were performed on each subject. Afterward, each subject was provided a 7-day supplement bag containing GLN or PLA. Subjects were instructed to maintain current activity level during the supplementation period and to refrain from using caffeine the day of the trial. The morning of day 7 after an overnight fast, subjects reported to the laboratory and consumed their final supplement (GLN or PLA) 2 h prior to the exercise trial. Height and weight were measured; then a rectal probe was inserted for core temperature measurement. After 20 min of seated rest, a 20-ml blood sample was taken to measure baseline plasma levels of GLN, along with PBMC levels of IκBα. Each exercise trial consisted of a 60-min treadmill run at 65–70% of V̇o2max in an environmental chamber set at 30°C and a humidity range of 12–20%. Clothing was standardized (men wore shorts with no shirts, women wore shorts with tank tops), and trials were terminated early if subjects reached a core temperature of 40°C. Twenty minutes into the exercise trial, a 50-ml sugar probe solution (5 g lactulose, 2 g rhamnose) was consumed for measurement of intestinal permeability. Subjects consumed water ad libitum during and after each trial, but the consumption was not recorded. Seated posture-controlled venous blood samples were taken 20 min, 2 h, and 4 h after exercise for plasma and PBMC measurements. In addition, urine was collected at 5 h postexercise for measurement of intestinal permeability by quantifying the levels of lactulose (5 g) and rhamnose (2 g). One month later, subjects returned and performed the second exercise trial using the identical protocol (Fig. 1).
Fig. 1.
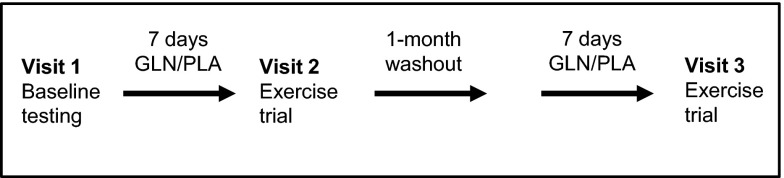
Experimental protocol.
GLN and PLA supplementation.
Subjects ingested 0.9 g/kg of fat-free mass per day for 7 days of either GLN mixed with sugar-free lemon drink powder or 2 g of sugar-free lemon drink PLA. The supplements were separated into three doses per day, taken in the morning, early afternoon, and evening.
Intestinal barrier permeability.
Assessment of intestinal permeability was quantified on the basis of absorption and urinary excretion of two sugar probes. Lactulose is a large disaccharide probe that is a marker of small intestine paracellular permeability. Rhamnose is a smaller monosaccharide that crosses the epithelia via the transcellular pathway (6, 34) and is a marker of intestinal absorption. Lactulose levels increase, whereas rhamnose stays constant or increases slightly, which provides evidence of stable absorption and increased paracellular movement. The sugars are calculated on the basis of amount recovered in urine (grams) multiplied by the urine volume recorded during the 5-h collection period. This value is then divided by the quantity ingested (5 g lactulose; 2 g rhamnose) to determine the percentage recovered for each sugar. The excretion percentages of the lactulose and rhamnose are used to then calculate a ratio (lactulose/rhamnose) whereby an increase in the ratio is a marker for an increase in intestinal permeability.
Subjects consumed a 50-ml solution containing 5 g lactulose (L7877; Sigma-Aldrich, St. Louis, MO), and 2 g rhamnose (R3875; Sigma-Aldrich). During the GLN and PLA trials the sugar drink was consumed 20 min into each exercise bout to ensure transit of the sugar probe solution when intestinal permeability would likely occur (41). Samples were separated into 30-ml aliquots and stored at −20° for subsequent analysis of the lactulose to rhamnose excretion ratio.
Urinary lactulose.
Lactulose was quantified using a standard enzymatic method developed by Behrens et al. (5) and Karaeren et al. (24). A 200-μl sample of urine was added to 100 μl of triethanolamine (TEA) buffer (5.6 g TEA and 740 mg MgSO4 in 50 ml of distilled H20; pH adjusted to 7.5 with 1 M NaOH; and total volume brought to 100 ml of stock solution). Beta-galactosidase (6.2 μl) was added to urine and TEA buffer, and incubated at room temperature for 2 h. After the incubation period, 2.73 ml of preprepared cocktail (1 ml TEA, 2 g ATP, 2 g NADP, 0.009 ml hexokinase glucose-6-phosphate dehydrogenase, and 1.721 ml of distilled H2O) was added per sample. Absorbance was measured at 340 nm (A1) against a blank water sample using a spectrophotometer (DU530; Beckman Coulter, Brea, CA). Phosphoglucoisomerase (7 μl) was then added to each sample, and absorbance was measured again at 340 nm (A2) against a blank water sample every 3 min until the reaction was stable. A blank sample containing water along with the cocktail and enzymes was subtracted from the summed absorbance. Concentration was calculated using the Beer-Lambert law to test NADH absorption.
Urinary rhamnose.
Rhamnose was quantified using a colorimetric enzyme immunoassay kit (K-RHAM; Megazyme, Wicklow, Ireland). Assay is sensitive to 1.3 mg/liter. Samples were not diluted, and all manufacturer directions were followed.
Blood sampling and analysis.
Seated posture-controlled venous blood was collected before exercise; and 20 min, 2 h, and 4 h after exercise from an antecubital vein. Blood samples were drawn into sterile syringes and immediately transferred into sterile vacutainers containing EDTA (BD Biosciences, Franklin Lakes, NJ). Vacutainer tops were removed and blood was injected along the sidewall to ensure sterile transfer. Blood was added to Histopaque (1077; Sigma Aldrich) in a 1:1 ratio (15 ml/15 ml) and centrifuged at 2,200 rpm for 30 min. The buffy coat containing the mononuclear cells was collected, transferred to a clean conical centrifuge tube, and resuspended with 10 ml of phosphate buffered saline (PBS) (4417; Sigma Aldrich). The mixture was centrifuged at 2,000 rpm for 10 min. The supernatant was removed and the mononuclear pellet was stored at −80°C for subsequent analysis of cellular levels of IκBα.
Glutamine.
Plasma GLN was assessed with a quantitative colorimetric enzyme assay kit (EGLN-100; BioAssay Systems, Hayward, CA) sensitive to 0.023 mM GLN. Samples were diluted 1:2 with distilled water. All materials and chemicals were provided by the manufacturer, and manufacturer directions were followed. Glutamate was measured in each sample and subtracted from the GLN absorbance of the respective sample.
IκBα gel electrophoresis.
Mononuclear cells were homogenized for 25 min with 200 μl of lysis buffer [150 mM NaCl, 20 mM HEPES, 2 mM EDTA, 0.2% sodium dodecyl sulfate (SDS), 0.5% sodium deoxycholate, 10% Triton X-100, 100 μM phenylmethylsulfonyl fluoride (PMSF), 100 μM vanadate, 1μg/ml leupeptin, 1μg/ml pepstatin A, 40 mM paranitrophenyl phosphate, and 1μg/ml aprotinin] and then centrifuged for 10 min. The supernatant was collected and protein measurement was performed using a protein assay kit (Bio-Rad Laboratories, Hercules, CA). Laemmli (161–0737; Bio-Rad) gel loading buffer was added to the lysate containing 15–20 μg of protein and boiled for 10 min. Proteins were then separated by SDS polyacrylamide gel electrophoresis, and transferred to a membrane (162–0094 nitrocellulose membrane; Bio-Rad). The membrane was incubated for 1 h in blocking solution (5% dry milk in Tris-buffered saline Tween 20 buffer) followed by incubation with IκBα (I0505; Sigma-Aldrich) antibody in a blocking solution. Each membrane was cut; the upper half was treated with antibody for β-actin (61–0120; Invitrogen), and the lower half was treated with IκBα. After incubation the membrane was washed with TBS-Tween then treated with horseradish peroxidase-conjugated secondary antibody (7074S, 7076S; Cell Signaling Technology, Danvers, MA). The membrane was developed using Western Blotting Luminol Reagants (SC-2048; Santa Cruz Biotechnology, Santa Cruz, CA) on Kodak BioMax MS film (F-BX57; Fisher Scientific, Pittsburgh, PA). Adobe Photoshop (San Jose, CA) was used to quantify protein expression and values were normalized to β-actin to control for protein loading. Protein levels were expressed relative to the preexercise time point.
In Vitro Protocol
Experimental procedure.
An in vitro experimental model was used to determine whether glutamine's protection of intestinal cells is mediated through HSF-1, HSP70, and occludin upregulation. A human carcinoma large intestinal epithelial cell line (Caco-2), widely used in in vitro epithelial model systems, was utilized in the present study (1, 10, 20). Caco-2 monolayers were supplemented with three concentrations of GLN (0, 4, and 6 mM) and exposed to control (37°C) or 75 min of heat stress (41.8°C). HSF-1, HSP70, and occludin were measured from cell lysate. Due to the carcinoma characteristics of the Caco-2 cell line, it is important to acknowledge that the responses may be different from those of normal, healthy intestinal epithelial cells.
Cell cultures.
Caco-2 cells (American Type Culture Collection, Rockville, MD) were maintained at 37°C in a culture medium composed of DMEM (4.5 mg/ml glucose, 50 U/ml penicillin, 50 U/ml streptomycin, 2 mM GLN, and 25 mM HEPES) supplemented with heat-inactivated 10% fetal bovine serum. Medium was changed every 2 days. After 80% confluency, Caco-2 cells were subcultured in 2-ml plates, and resupplemented for 7 days with three concentrations of GLN (0, 4, and 6 mM) (31).
Glutamine and heat shock treatment.
Supernatants of the confluent Caco-2 cell layers were replaced by DMEM supplemented for 7 days with GLN in three concentrations (0, 4, and 6 mM). Cells either remained in the control environment (37°C) or were incubated in a water bath for 75 min at 41.8°C followed by recovery incubation at 37°C for 5 h. The exposure to 41.8°C was to simulate an increase in intestinal temperature during heat stress.
Assessment of HSF-1, HSP70, and occludin protein expression by gel electrophoresis.
At the end of the 37°C and 41°C trials, Caco-2 monolayers were immediately rinsed in ice-cold PBS, and each plate was scraped for cell collection and frozen at −80°C. Cells were lysed, proteins were measured, and gel electrophoresis was performed as previously detailed. After transfer each membrane was cut; the lower half was treated with antibody for β-actin, and the upper half was treated with the appropriate primary antibodies (HSF-1, HSP70, and occludin) purchased from Stressgen (Victoria, BC, Canada), and then further developed as previously detailed. Adobe Photoshop was used to quantify protein expression and values were normalized to β-actin to control for protein loading. Protein levels were expressed relative to the control time point.
Statistical Analysis
All results are expressed as means ± SD and were checked for homogeneity of variance and normality. In the human experiment, a two-factor repeated measure ANOVA was used to analyze plasma GLN and PBMC levels of IκBα with condition (GLN, PLA) and various time points (preexercise and 20 min, 2 h, and 4 h postexercise) as the independent variables. A one-way ANOVA was used to measure intestinal permeability (ratio of lactulose to rhamnose) with condition (baseline, GLN, PLA) used as the independent variable. After main effect significance, a Tukey's test, which maintains alpha levels and is moderately conservative, was used for post hoc comparisons (26).
For the in vitro experiment, statistical significance was determined for HSF-1, HSP70, and occludin using a two-factor ANOVA with temperature (37°C and 41°C) and GLN supplementation (0, 4, and 6 mM) as the independent variables. Significance is reported at P < 0.05. SPSS (Armonk, NY) statistical software was used for data analysis.
RESULTS
Effect of 7-Day GLN Supplementation (Oral Route) in Exercising Humans
Subject physiological characteristics.
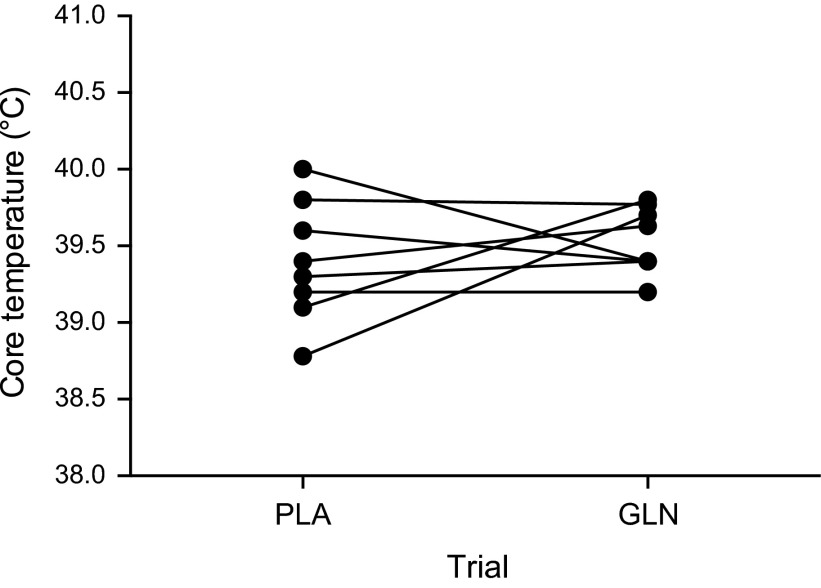
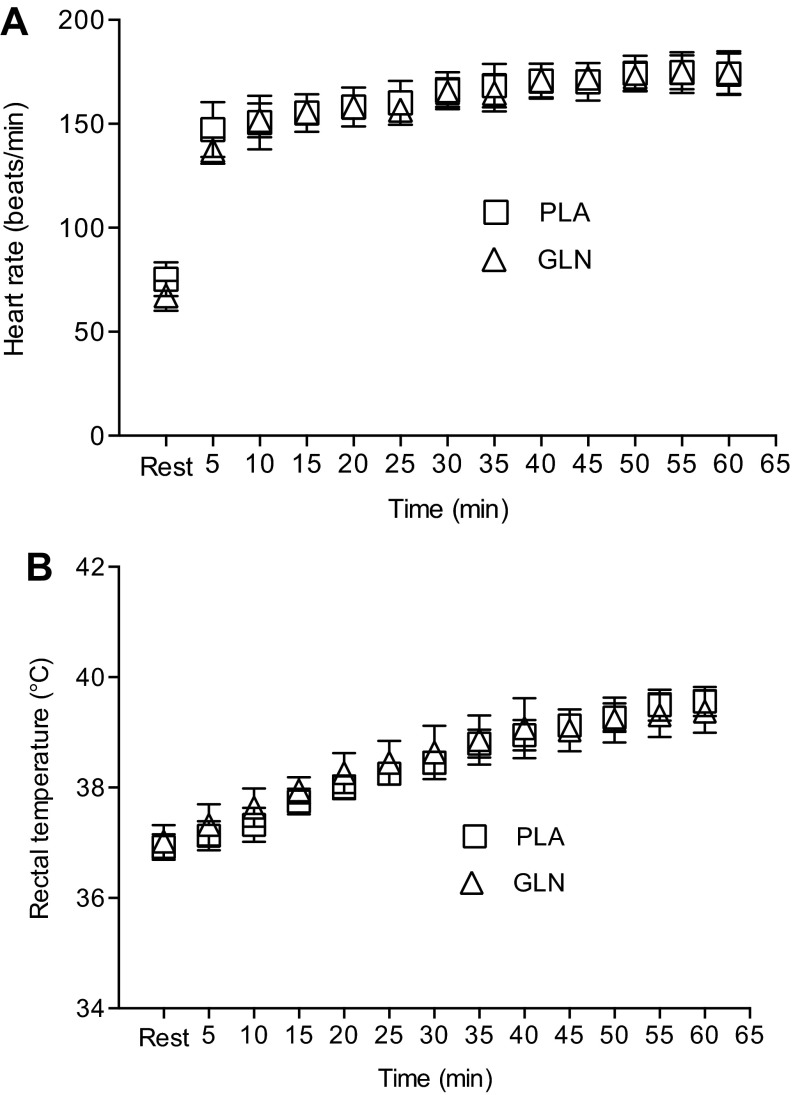
Eight subjects (5 men; 3 women) completed baseline, PLA, and GLN measurements (Table 1, Fig. 2, Fig. 3). Subject characteristics separated by gender are presented in Table 1. Treadmill speed and grade varied during the trials, and intensity was based on oxygen consumption levels. There was no difference between the PLA and GLN trials for exercise intensity reported as a relative percentage of V̇o2max (71.35 ± 0.01% vs. 74.34 ± 0.01%, respectively; P > 0.05). End exercise core temperature was not different between PLA and GLN trials (39.40 ± 0.13°C vs. 39.54 ± 0.07°C, respectively; P > 0.05) (Fig. 3). One subject completed 45 min of the 60-min protocol in both trials due to core temperature reaching termination criteria (40°C). In addition, another subject required probe replacement during the trial. End exercise heart rates were not different between PLA and GLN trials (173 ± 12 bpm vs. 174 ± 6 bpm, respectively) (Fig. 3).
Table 1.
Subject characteristics
| Men (n = 5) | Women (n = 3) | Combined (n = 8) | |
|---|---|---|---|
| Age, years | 27 ± 4 | 23 ± 4 | 25 ± 4 |
| Height, cm | 177 ± 5.4 | 171 ± 5.3 | 174 ± 5.6 |
| Weight, kg | 77.67 ± 17.82 | 64.36 ± 7.62 | 72.21 ± 16.39 |
| Body fat, % | 17.46 ± 10.74 | 26.93 ± 7.33 | 18.84 ± 9.32 |
| VO2max, ml·min−1·kg−1 | 52.83 ± 7.49 | 47.67 ± 2.51 | 51.11 ± 6.58 |
Data are means ± SD, n = 8.
Fig. 2.
End exercise core temperature for each subject during the placebo (PLA) and glutamine (GLN) trials. Data are expressed as individual subject's core temperature, n = 8.
Fig. 3.
Time course effect of 60 min of treadmill exercise on heart rate (A) and rectal temperature (B). Both heart rate and rectal temperature were not different between trials at any 5-min interval. Data are means ± SD, n = 8.
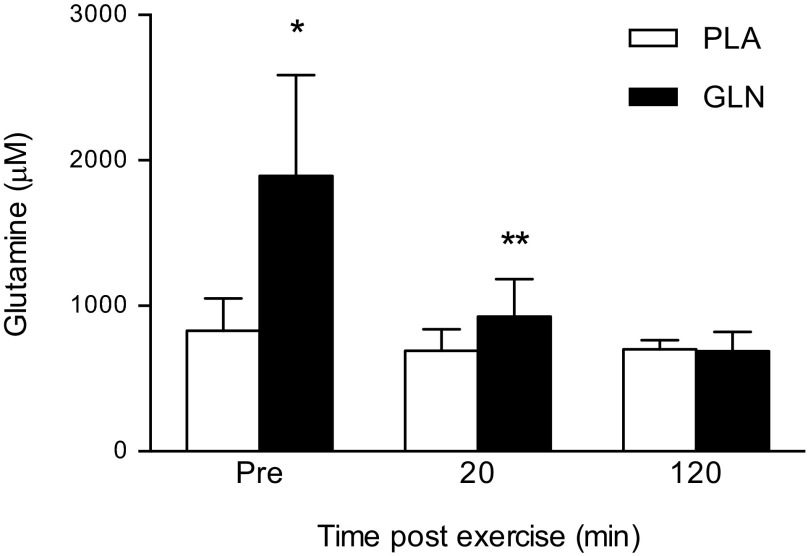
Oral GLN supplementation increased plasma glutamine levels.
Seven days of oral GLN supplementation increased plasma GLN levels by 128% (Fig. 4). The main effect was statistically significant [F(3, 7) = 20.13; P < 0.01]. Post hoc testing showed plasma GLN levels were significantly higher in the GLN trial at the preexercise time point compared with preexercise time point in the PLA trial (1,893 ± 694 μM vs. 828 ± 222 μM, respectively; P < 0.05). Plasma GLN levels declined significantly at the 20-min postexercise time point in the GLN trial (1,893 ± 694 μM vs. 926 ± 258 μM; P < 0.05).
Fig. 4.
Oral GLN supplementation increased plasma GLN levels. Plasma GLN was significantly higher at the preexercise time point in the GLN trial compared with preexercise in PLA trial. Plasma GLN was significantly lower at 20 min postexercise in the GLN trial compared with preexercise in the GLN trial. *P < 0.05 statistically significant from the same time point in the PLA trial; **P < 0.05 statistically significant from the GLN preexercise time point. Data are means ± SD, n = 8.
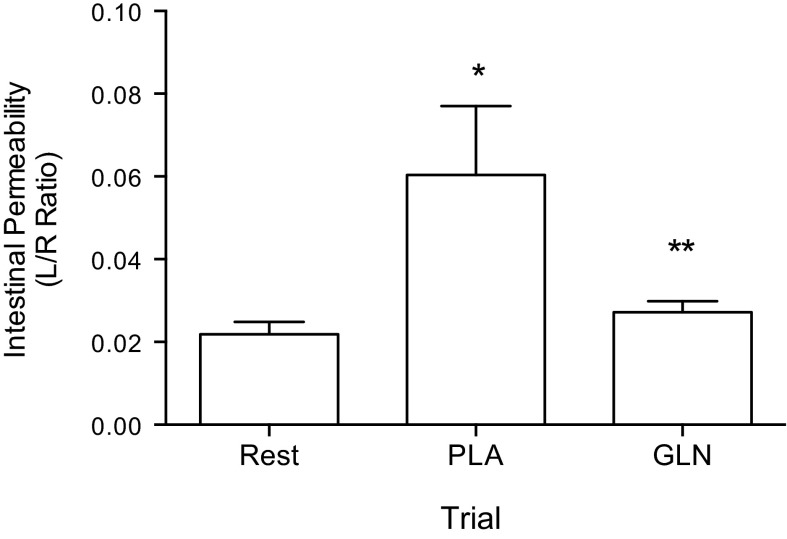
Oral GLN supplementation prevented exercise-induced intestinal permeability.
Exercise caused an increase in intestinal permeability (ratio of urinary lactulose to rhamnose) with the main effect of condition (baseline, GLN, or PLA) on postexercise intestinal permeability being statistically significant F(2, 7) = 6.060; P < 0.05 (Fig. 5). Post hoc testing showed intestinal permeability to be significantly higher in the PLA trial compared with rest (baseline) (0.0603 ± 0.047 vs. 0.0218 ± 0.008, respectively; P < 0.05). In addition, permeability was significantly higher in the PLA trial compared with the GLN trial (0.0603 ± 0.047 vs. 0.0272 ± 0.007; P < 0.05).
Fig. 5.
Glutamine prevented a rise in intestinal permeability as measured by the urinary excretion ratio of lactulose and rhamnose (L/R ratio). The L/R ratio was higher in the PLA trial compared with rest and GLN trials. *P < 0.05, statistically significant from the rest trial; **P < 0.05, statistically significant from the PLA. Data are means ± SD, n = 8.
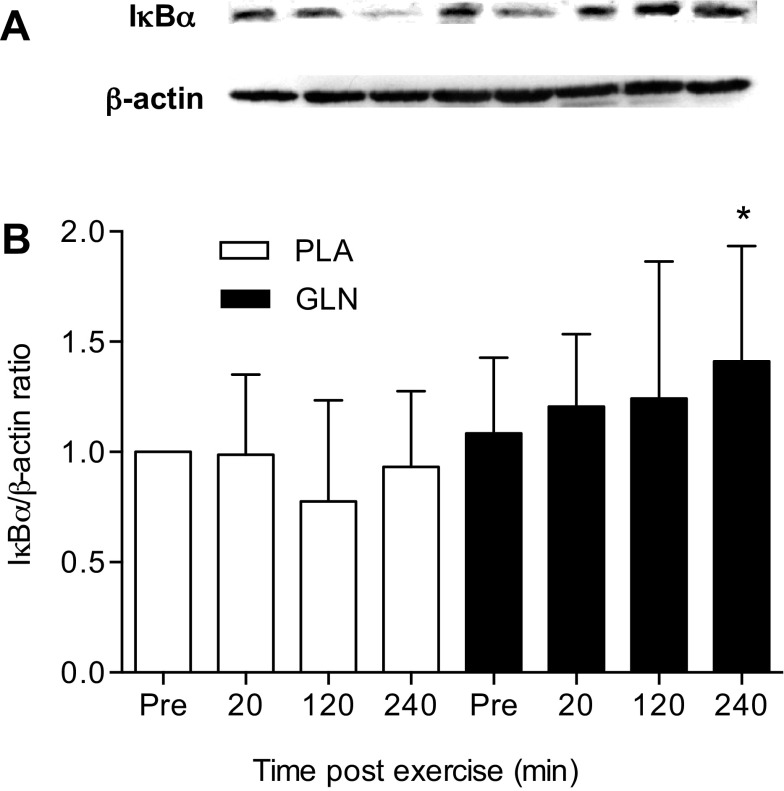
GLN increased the PBMC level of IκBα in response to exercise stress.
GLN supplementation enhanced IκBα levels in response to exercise [F(3,18) = 10.396; P < 0.05] (Fig. 6). IκBα levels were significantly higher in the GLN trial at the 4 h postexercise (240 min) time point compared with the 4-h postexercise (240 min) time point in the PLA trial (1.411 ± 0.523 vs. 0.933 ± 0.343; P < 0.05).
Fig. 6.
Effect of GLN supplementation on IκBα levels in PBMCs. The 240-min postexercise time point was higher in the GLN trial compared with the 240-min postexercise time point in the PLA trial. A: protein expression of IκBα and β-actin (loading control) were measured in PBMCs of subjects after 7 days of PLA and GLN supplementation. B: densitometric values of protein content were obtained using Photoshop software and normalized to β-actin and set to 1. *P < 0.05, statistically significant from the same time point in the PLA trial. Data are means ± SD, n = 8 for each time point.
In Vitro Experiment
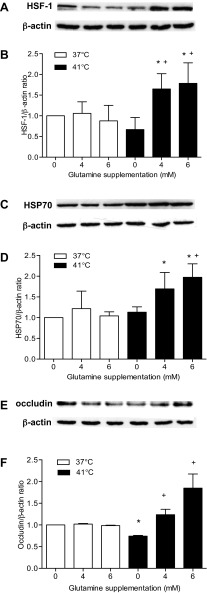
GLN supplementation increased HSF-1 and HSP70 levels in response to heat stress.
GLN combined with heat stress increased HSF-1 levels F(2,18) = 7.259; P < 0.05 (Fig. 7, A–D). Heat stress in the GLN-treated cells caused an increase in HSF-1 expression with statistical significance in the 4 mM 41°C trial compared with 0 mM at 37°C (control) (1.649 ± 0.369 vs. 1.000 ± 0.000, respectively; P < 0.05). HSF-1 was higher in the 4 mM 41°C and the 6 mM 41°C trials than in the 0 mM 41°C trial (1.649 ± 0.369, 1.785 ± 0.495 vs. 0.6681 ± 0.290, respectively; P = 0.001 and P < 0.01, respectively). However, heat stress applied to nonglutamine-supplemented cells did not increase HSF-1. HSP70 expression followed a similar trend as HSF-1. Again, the HSP70 response to heat stress was absent without GLN supplementation, but was significantly increased when heat stress was applied to GLN-treated cells [F(2,24) = 4.106, P < 0.05]. HSP70 response was significantly higher in both the 4 mM 41°C and 6 mM 41°C trials compared with the 0 mM 41°C trial (1.691 ± 0.395 and 1.973 ± 0.325 vs. 1.133 ± 0.129; P = 0.011 and P < 0.001, respectively).
Fig. 7.
Effect of GLN supplementation and heat stress on HSF-1, HSP70, and occludin protein expression in Caco-2 epithelial cells. HSF-1 (A and B) was significantly higher in the 4 and 6 mM 41°C trial compared with the 0 mM 37°C trial. A: HSF-1 and β-actin (loading control) protein expression. B: densitometry of protein content of the corresponding blots (A) was corrected for loading with β-actin and expressed as a ratio. *P < 0.05, statistically significant from the 0 mM 37°C trial; +P < 0.05, statistically significant from the 0 mM 41°C trial. Data are means ± SD, n = 4. HSP70 (C and D) protein expression was higher in the 4 and 6 mM 41°C trial compared with 0 mM 37°C trial. C: HSP70 and β-actin (loading control) protein expression. D: densitometry of protein content of the corresponding blots (C) was corrected for loading with β-actin and expressed as a ratio. *P < 0.05, statistically significant from the 0 mM 37°C trial; +P < 0.05, statistically significant from the 0 mM 41°C trial. Data are means ± SD, n = 4. Occludin expression (E and F) was significantly decreased in the 0 mM 41°C trial compared with the 0 mM 37°C trial. Occludin levels were higher in the 4 and 6 mM 41°C trials compared with the 0 mM 41°C trial. E: occludin and β-actin (loading control) protein expression. F: densitometry of protein content of the corresponding blots (E) was corrected for loading with β-actin and expressed as a ratio. *P < 0.05, statistically significant from the 0°C mM 37°C trial; +P < 0.05, statistically significant from the 0 mM 41°C trial. Data are means ± SD, n = 4.
GLN supplementation preserved the stability of occludin at the TJ.
When heat was applied in the absence of GLN, occludin levels declined, but the combined effect of GLN and heat increased occludin expression [F(2,18) = 7.711; P < 0.05] (Fig. 7, E and F). Occludin levels did not increase in control conditions, but were significantly reduced when cells were exposed to heat in the absence of GLN (0 mM 41°C) compared with control conditions (0 mM 37°C) (0.7434 ± 0.027 vs. 1.000 ± 0.000, respectively; P = 0.003). Occludin levels were preserved when cells were supplemented with GLN and exposed to heat stress. Occludin levels during both 4 mM 41°C and 6 mM 41°C trials were statistically higher compared with the 0 mM 41°C trial (1.236 ± 0.219 and 1.849 ± 0.564 vs. 0.7434 ± 0.027; P = 0.032 and P < 0.001, respectively), indicating stability of occludin at the TJ in response to heat stress.
DISCUSSION
The pathway leading to exercise-induced GI distress is complex. The stress of high-intensity exercise has been shown to increase intestinal permeability (41), stimulating a proinflammatory cascade of events (32), eventually causing GI distress (39). Here, we demonstrate that 7 days of oral GLN supplementation protected the gut during high-intensity endurance exercise by reducing intestinal permeability. Through a total body exercise model combined with a proof-of-concept in vitro design, we have shown that the mechanism may be through activation of HSF-1 and HSP70 leading to increased occludin protein expression at the TJ. In addition, using the same protocol and subjects, we have recently shown that oral GLN supplementation upregulates PBMC in vitro levels of HSP70 at the 4-h postexercise time point [see (10) and Fig. 6A] (12). In the present study, GLN supplementation increased the expression of dephosphorylated IκBα in human PBMCs at the 4-h postexercise time point, suggesting a blunted inflammatory response through HSP70 activation, and suppression of NF-κB during recovery from exercise stress. Thus we propose that the protective effects of GLN on the gut during vigorous exercise may be twofold: 1) through preserving the intestinal TJ barrier and reducing permeability; and 2) via modulation of the inflammatory response through activation of HSP70 and cytosolic housing of NF-κB.
Exercise-induced intestinal permeability has been previously demonstrated during high-intensity exercise (41, 51) and in heat stress trials (26). The current study shows that 60 min of high-intensity running (70% V̇o2max) caused an increase in intestinal permeability, which was completely ameliorated in the GLN trial, and demonstrates the protective effects of GLN supplementation on the gut. In clinical disease states the administration of GLN has been shown to be effective. For example, enteral GLN supplementation reduced intestinal permeability among patients undergoing systemic chemotherapy (30) and low-birth-weight infants with underdeveloped GI tracts (49). In addition, GLN administration in a rat jaundice model improved intestinal barrier function and reduced endotoxin levels (33). Our study demonstrates that oral GLN supplementation in humans reduces exercise-induced intestinal permeability, and although it was not reported in this study, may reduce plasma levels of endotoxin.
The protective effect of GLN in the gut may be through activation of HSF-1, leading to HSP70 expression. In an in vitro (Caco-2) model, we have shown that GLN supplementation with 4 mM and 6 mM upregulates HSF-1 and HSP70 in response to heat stress. Rats fed oral GLN supplementation for 5 days followed by induced heat stroke exhibited elevated HSF-1 and HSP70 expression in the gut, reduced permeability, and lower plasma endotoxin (46). Several follow-up studies have confirmed that glutamine's action against cellular stress occurs through the transcriptional activation of HSF-1 (18, 19, 35, 45). The GLN-induced increase in HSF-1 and HSP70 stimulation may occur through the hexosamine biosynthetic pathway (HBP) (18, 19). The HBP splits from the glycolytic pathway through fructose-6-phosphate, and GLN serves as a key substrate leading to the activation of O-linked N-acetylglucosamine (GlcNAc), which plays a critical role in transcription regulation of the stress response (18). Gong and Jing (18) demonstrated that inhibition of GlcNAc prevented a GLN-induced increase in HSF-1 and HSP70 in lipopolysaccharide (LPS)-treated cardiomyocytes. In addition, Wischmeyer's group showed that GLN-induced HSP70 expression is dependent upon activation of HBP in mouse embryonic fibroblasts (19). It is important to note that stimulation by GLN of the heat shock protein pathway has been shown only in response to physical (heat, exercise) or chemical (LPS) stress. In other words, GLN alone does not increase resting HSF-1 and HSP70 levels.
Occludin is a tetraspanning membrane protein, and along with claudins (claudin-1, claudin-2, and claudin-3), plays a key role in regulating paracellular absorption and secretion mechanisms in the GI tract (15, 43). Overexpression of occludin results in improved TJ resistance and reduced intestinal permeability (14). In an in vitro model, we have demonstrated reduced occludin levels in nonglutamine supplemented intestinal cells exposed to 75 min of heat stress (41°C) followed by 5 h at 37°C. This model was chosen in an attempt to simulate exercise stress and recovery. GLN supplementation at concentrations of 4 mM and 6 mM preserved occludin levels under heat stress conditions. The mechanism may be through HSF-1 regulation of occludin. Dokladny et al. (11) demonstrated that HSF-1 plays a central role in mediating heat-induced occludin expression. Caco-2 monolayers were supplemented with quercetin, a known HSF-1 inhibitor, and upon heat stress occludin levels were diminished, indicating HSF-1 regulation of occludin (11). Our results support this mechanism and further show that GLN activation of HSF-1 increased occludin levels. Our results are also consistent with previously published observations showing the importance of GLN in occludin protein expression. In Caco-2 cells GLN deprivation resulted in a decrease in occludin protein expression and a decrease in junctional localization (29).
Inflammatory cytokines released from leukocytes have also been linked to intestinal permeability and exercise-induced GI distress (22, 35). Tumor necrosis factor alpha (TNF-α), interluekin-1 beta (IL-1β), interferon-gamma (IFNγ), and additional cytokines have been shown to disrupt the epithelial cell barrier (2, 39, 57). The mechanism of cytokine-induced intestinal damage may occur through leukocyte (monocytes, macrophages, T cell) cytokine release, resulting in Na(+)/K(+)-ATPase inhibition, and leading to intestinal malabsorption and fluid accumulation in the gut. NF-κB is the transcription factor for many proinflammatory cytokines. Under control conditions it is inactively attached to IκBα in the cytosol (17, 32). Upon stimulation with LPS for example, IκBα is phosphorylated and degraded, releasing NF-κB, which translocates to the nucleus where it activates genes of inflammatory proteins (17, 32). The mechanism may be through stress-induced intestinal wall breakdown, which allows endotoxin leakage and activation of NF-κB in PBMCs, promoting proinflammatory cytokine release (9, 44). We have previously shown in animal studies that overexpression of HSP70 in the liver inhibited the LPS-induced increase in IκBα degradation and prevented NF-κB p65 cytoplasmic-to-nuclear translocation (9). Those results were further confirmed in human PBMCs in which adenovirus-directed overexpression of HSP70 inhibited the LPS-induced nuclear translocation of NF-κB p65. Together, those results indicate that HSP70 is involved in modulation of LPS-induced NF-κB in vivo. The cellular mechanisms of the inhibitory effect of HSP70 on NF-κB may be through HSP70 physical protein-to-protein interaction with the rel65 subunit of the NF-κB/IκBα complex preventing phosphorylation, nuclear translocation, and transcription of proinflammatory cytokines (7, 25, 55).
On the basis of current knowledge, the effect of exercise on modulation of NF-κB pathway is believed to be dependent on exercise type and intensity (8, 47, 52). In recent studies it has been shown that prolonged standardized physical training (6 wk) decreased mRNA levels of NF-κB and IκBα (47). On the other hand, an acute bout (1 h) of strenuous cycling exercise (80% of V̇o2max) resulted in activation of NF-κB pathways as determined by electrophoretic mobility shift assay (52). Similarly, short-term supramaximal anaerobic exercise resulted in a significant NF-κB activation and a decrease in IκB protein levels (8). In our present studies, oral GLN supplementation increased IκBα levels in PBMCs in response to exercise stress, and this effect was associated with a significant increase in HSP70 expression (12). In accordance with our results, in rats GLN improved survival during sepsis and this was associated with a suppressed DNA-binding of NF-kB, lower IL-6 plasma level, and increased HSP70 (27). The requirement of HSP70 in GLN-mediated protection against inflammatory injury has been documented in sepsis model in animals (45). GLN administration offered no protection against experimental sepsis in mice with specific deletion of the HSP70 gene. However, in mice with normal HSP70 expression, GLN treatment improved survival of septic mice, and this effect was associated with a reduced NF-κB activation and lower proinflammatory cytokine expression (45). Moreover, in lung epithelial cells, LPS administration resulted in activation of NF-κB as demonstrated by its nuclear translocation and increased phosphorylation, which was prevented by administration of GLN (21).
The effects of GLN supplementation on HSP70 regulation in PBMCs is not clear. Wischmeyer et al. (54) supplemented human PBMCs in a cell culture model and demonstrated activation of HSP70 and decreased TNF-α levels in response to LPS stimulation. Conversely, Andreasen et al. (4) intravenously supplemented men with GLN for 10 h followed by an endotoxin insult, but it did not lead to an increase in PBMC levels of HSP70. The conflicting results may be due to the dosage level because the cell model was supplemented at a much higher dose. Dokladny et al. (12) was the first to show that oral GLN supplementation elevated PBMC activation of HSP70 in response to exercise stress. These results may be due to the longer supplementation period (7 days), which resulted in a higher resting GLN level compared with the Andreasen study (765 μM vs. 1,893 μM) (4). Interestingly, postexercise plasma GLN levels in the GLN trials declined by 52%, whereas they declined by 21% in the Andreasen study (4), suggesting rapid GLN uptake by splanchnic and skeletal muscle tissue due to the stress of exercise (50).
The pathway leading to gut dysfunction is complicated and multifactorial, but a common cause is damage to the epithelial cell barrier. GLN is a primary metabolic fuel for intestinal cells and leukocytes, and may even exceed glucose and fatty acid metabolism (53). We have demonstrated that oral GLN supplementation protects the gut during recovery from high-intensity exercise. We propose two possible mechanisms by which GLN may exert its protective effects: 1) activation of HSF-1 and HSP70 leading to occludin stabilization and lowering intestinal permeability; and 2) stimulation of HSP70 and IκBα in PBMCs, thus inactivating the NF-κB proinflammatory pathway.
GRANTS
Partial support for this study was provided by National Institutes of Health National Center for Advancing Translational Sciences Grant UL1-TR000041.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.N.Z., K.R.L., L.K., C.M.M., S.S., K.D., and P.L.M. conception and design of research; M.N.Z., K.R.L., and K.D. performed experiments; M.N.Z., C.M.M., S.S., K.D., and P.L.M. analyzed data; M.N.Z., C.M.M., S.S., K.D., and P.L.M. interpreted results of experiments; M.N.Z. and K.D. prepared figures; M.N.Z. and L.K. drafted manuscript; M.N.Z., L.K., C.M.M., S.S., K.D., and P.L.M. edited and revised manuscript; M.N.Z., S.S., K.D., and P.L.M. approved final version of manuscript.
REFERENCES
- 1.Al-Sadi R, Guo S, Ye D, Dokladny K, Alhmoud T, Ereifej L, Said HM, Ma TY. Mechanism of IL-1beta modulation of intestinal epithelial barrier involves p38 kinase and activating transcription factor-2 activation. J Immunol 190: 6596–6606, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Sadi R, Ye D, Said HM, Ma TY. IL-1beta-induced increase in intestinal epithelial tight junction permeability is mediated by MEKK-1 activation of canonical NF-kappaB pathway. Am J Pathol 177: 2310–2322, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amorim F, Yamada P, Robergs R, Schneider S, Moseley P. Effects of whole-body heat acclimation on cell injury and cytokine responses in peripheral blood mononuclear cells. Eur J Appl Physiol 111: 1609–1618, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Andreasen AS, Pedersen-Skovsgaard T, Mortensen OH, van Hall G, Moseley PL, Pedersen BK. The effect of glutamine infusion on the inflammatory response and HSP70 during human experimental endotoxaemia. Crit Care 13: R7, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behrens RH, Docherty H, Elia M, Neale G. A simple enzymatic method for the assay of urinary lactulose. Clin Chim Acta 137: 361–367, 1984 [DOI] [PubMed] [Google Scholar]
- 6.Bjarnason I, MacPherson A, Hollander D. Intestinal permeability: an overview. Gastroenterology 108: 1566–1581, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Chen HW, Kuo HT, Wang SJ, Lu TS, Yang RC. In vivo heat shock protein assembles with septic liver NF-kappaB/I-kappaB complex regulating NF-kappaB activity. Shock 24: 232–238, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Cuevas MJ, Almar M, Garcia-Glez JC, Garcia-López D, De Paz JA, Alvear-Ordenes I, González-Gallego J. Changes in oxidative stress markers and NF-kappaB activation induced by sprint exercise. Free Radic Res 39: 431–439, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Dokladny K, Lobb R, Wharton W, Ma TY, Moseley PL. LPS-induced cytokine levels are repressed by elevated expression of HSP70 in rats: possible role of NF-kappaB. Cell Stress Chaperones 15: 153–163, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dokladny K, Moseley PL, Ma TY. Physiologically relevant increase in temperature causes an increase in intestinal epithelial tight junction permeability. Am J Physiol Gastrointest Liver Physiol 290: G204–G212, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Dokladny K, Ye D, Kennedy JC, Moseley PL, Ma TY. Cellular and molecular mechanisms of heat stress-induced up-regulation of occludin protein expression: regulatory role of heat shock factor-1. Am J Pathol 172: 659–670, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dokladny KZ, Mandell M, Bhattacharya D, Schneider S, Deretic V, Moseley PL. Regulatory coordination between two major intracellular homeostatic systems: heat shock response and autophagy. J Biol Chem 288: 14959–14972, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flanagan SW, Ryan AJ, Gisolfi CV, Moseley PL. Tissue-specific HSP70 response in animals undergoing heat stress. Am J Physiol Regul Integr Comp Physiol 268: R28–R32, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Furuse M, Fujimoto K, Sato N, Hirase T, Tsukita S. Overexpression of occludin, a tight junction-associated integral membrane protein, induces the formation of intracellular multilamellar bodies bearing tight junction-like structures. J Cell Sci 109: 429–435, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol 123: 1777–1788, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gillum TL, Kuennen MR, Schneider S, Moseley P. A review of sex differences in immune function after aerobic exercise. Exerc Immunol Rev 17: 104–121, 2011 [PubMed] [Google Scholar]
- 17.Gloire G, Legrand-Poels S, Piette J. NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol 72: 1493–1505, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Gong J, Jing L. Glutamine induces heat shock protein 70 expression via O-GlcNAc modification and subsequent increased expression and transcriptional activity of heat shock factor-1. Minerva Anestesiol 77: 488–495, 2011 [PubMed] [Google Scholar]
- 19.Hamiel CR, Pinto S, Hau A, Wischmeyer PE. Glutamine enhances heat shock protein 70 expression via increased hexosamine biosynthetic pathway activity. Am J Physiol Cell Physiol 297: C1509–C1519, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hidalgo IJ, Raub TJ, Borchardt RT. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 96: 736–749, 1989 [PubMed] [Google Scholar]
- 21.Hou YC, Chiu WC, Yeh CL, Yeh SL. Glutamine modulates lipopolysaccharide-induced activation of NF-kappaB via the Akt/mTOR pathway in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 302: L174–L183, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Jeukendrup AE, Vet-Joop K, Sturk A, Stegen JH, Senden J, Saris WH, Wagenmakers AJ. Relationship between gastro-intestinal complaints and endotoxaemia, cytokine release and the acute-phase reaction during and after a long-distance triathlon in highly trained men. Clin Sci 98: 47–55, 2000 [PubMed] [Google Scholar]
- 23.Jonas CR, Ziegler TR. Potential role of glutamine administration in inflammatory bowel disease. Nestle Nutr Workshop Ser Clin Perform Programme 2: 217–230, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Karaeren Z, Akbay A, Demirtas S, Ergüder I, Ozden A. A reference interval study of urinary lactulose excretion: a useful test of intestinal permeability in adults. Turk J Gastroenterol 13: 35–39, 2002 [PubMed] [Google Scholar]
- 25.Kizelsztein P, Komarnytsky S, Raskin I. Oral administration of triptolide ameliorates the clinical signs of experimental autoimmune encephalomyelitis (EAE) by induction of HSP70 and stabilization of NF-kappaB/IkappaBalpha transcriptional complex. J Neuroimmunol 217: 28–37, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Kuennen M, Gillum T, Dokladny K, Bedrick E, Schneider S, Moseley P. Thermotolerance and heat acclimation may share a common mechanism in humans. Am J Physiol Regul Integr Comp Physiol 301: R524–R533, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwon WY, Suh GJ, Kim KS, Jo YH, Lee JH, Kim K, Jung SK. Glutamine attenuates acute lung injury by inhibition of high mobility group box protein-1 expression during sepsis. Br J Nutr 103: 890–898, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Lambert GP, Broussard LJ, Mason BL, Mauermann WJ, Gisolfi CV. Gastrointestinal permeability during exercise: effects of aspirin and energy-containing beverages. J Appl Physiol 90: 2075–2080, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Li N, Lewis P, Samuelson D, Liboni K, Neu J. Glutamine regulates Caco-2 cell tight junction proteins. Am J Physiol Gastrointest Liver Physiol 287: G726–G733, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Yu Z, Liu F, Tan L, Wu B, Li J. Oral glutamine ameliorates chemotherapy-induced changes of intestinal permeability and does not interfere with the antitumor effect of chemotherapy in patients with breast cancer: a prospective randomized trial. Tumori 92: 396–401, 2006 [PubMed] [Google Scholar]
- 31.Lindemann G, Grohs M, Stange EF, Fellermann K. Limited heat-shock protein 72 induction in Caco-2 cells by L-glutamine. Digestion 64: 81–86, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Liu SF, Malik AB. NF-kappa B activation as a pathological mechanism of septic shock and inflammation. Am J Physiol Lung Cell Mol Physiol 290: L622–L645, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Margaritis VG, Filos KS, Michalaki MA, Scopa CD, Spiliopoulou I, Nikolopoulou VN, Vagianos CE. Effect of oral glutamine administration on bacterial tanslocation, endotoxemia, liver and ileal morphology, and apoptosis in rats with obstructive jaundice. World J Surg 29: 1329–1334, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Maxton DG, Bjarnason I, Reynolds AP, Catt SD, Peters TJ, Menzies IS. Lactulose, 51Cr-labelled ethylenediaminetetra-acetate, L-rhamnose and polyethyleneglycol 400 [corrected] as probe markers for assessment in vivo of human intestinal permeability. Clin Sci 71: 71–80, 1986 [DOI] [PubMed] [Google Scholar]
- 35.Morrison AL, Dinges M, Singleton KD, Odoms K, Wong HR, Wischmeyer PE. Glutamine's protection against cellular injury is dependent on heat shock factor-1. Am J Physiol Cell Physiol 290: C1625–C1632, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Morton JP, Kayani AC, McArdle A. The exercise-induced stress response of skeletal muscle, with specific emphasis on humans. Sports Med 39: 643–662, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Moseley PL. Heat shock proteins and the inflammatory response. Ann NY Acad Sci 856: 206–213, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Moseley PL, Gapen C, Wallen ES, Walter ME, Peterson MW. Thermal stress induces epithelial permeability. Am J Physiol Cell Physiol 267: C425–C434, 1994 [DOI] [PubMed] [Google Scholar]
- 39.Musch MW, Clarke LL, Mamah D, Gawenis LR, Zhang Z, Ellsworth W, Shalowitz D, Mittal N, Efthimiou P, Alnadjim Z, Hurst SD, Chang EB, Barrett TA. T cell activation causes diarrhea by increasing intestinal permeability and inhibiting epithelial Na+/K+-ATPase. J Clin Invest 110: 1739–1747, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oehler R, Pusch E, Dungel P, Zellner M, Eliasen MM, Brabec M, Roth E. Glutamine depletion impairs cellular stress response in human leucocytes. Br J Nutr 87: S17–S21, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Pals KL, Chang RT, Ryan AJ, Gisolfi CV. Effect of running intensity on intestinal permeability. J Appl Physiol 82: 571–576, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Ryan AJ, Flanagan SW, Moseley PL, Gisolfi CV. Acute heat stress protects rats against endotoxin shock. J Appl Physiol 73: 1517–1522, 1992 [DOI] [PubMed] [Google Scholar]
- 43.Schneeberger EE, Lynch RD. Structure, function, and regulation of cellular tight junctions. Am J Physiol Lung Cell Mol Physiol 262: L647–L661, 1992 [DOI] [PubMed] [Google Scholar]
- 44.Selkirk GA, McLellan TM, Wright HE, Rhind SG. Mild endotoxemia, NF-kappaB translocation, and cytokine increase during exertional heat stress in trained and untrained individuals. Am J Physiol Regul Integr Comp Physiol 295: R611–R623, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Singleton KD, Wischmeyer PE. Glutamine's protection against sepsis and lung injury is dependent on heat shock protein 70 expression. Am J Physiol Regul Integr Comp Physiol 292: R1839–R1845, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Singleton KD, Wischmeyer PE. Oral glutamine enhances heat shock protein expression and improves survival following hyperthermia. Shock 25: 295–299, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Sousa e Silva T, Longui CA, Rocha MN, Faria CD, Melo MR, Faria TG, de Souza JA, Rizzo LV. Prolonged physical training decreases mRNA levels of glucocorticoid receptor and inflammatory genes. Horm Res Paediatr 74: 6–14, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Suenaert P, Maerten P, Van Assche G, Van Driessche W, Geboes K, Bulteel V, Simaels J, Augustijns P, Ceuppens JL, Rutgeerts P, Perrier C. Effects of T cell-induced colonic inflammation on epithelial barrier function. Inflamm Bowel Dis 16: 1322–1331, 2010 [DOI] [PubMed] [Google Scholar]
- 49.van den Berg A, van Elburg RM, Twisk JW, Fetter WP. Glutamine-enriched enteral nutrition in very low birth weight infants. Design of a double-blind randomised controlled trial. BMC Pediatr 4: 17, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Hall G, Steensberg A, Fischer C, Keller C, Møller K, Moseley P, Pedersen BK. Interleukin-6 markedly decreases skeletal muscle protein turnover and increases nonmuscle amino acid utilization in healthy individuals. J Clin Endocrinol Metab 93: 2851–2858, 2008 [DOI] [PubMed] [Google Scholar]
- 51.van Nieuwenhoven MA, Brouns F, Brummer RJ. Gastrointestinal profile of symptomatic athletes at rest and during physical exercise. Eur J Appl Physiol 91: 429–434, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Vider J, Laaksonen DE, Kilk A, Atalay M, Lehtmaa J, Zilmer M, Sen CK. Physical exercise induces activation of NF-kappaB in human peripheral blood lymphocytes. Antioxid Redox Signal 3: 1131–1137, 2001 [DOI] [PubMed] [Google Scholar]
- 53.Windmueller HG, Spaeth AE. Identification of ketone bodies and glutamine as the major respiratory fuels in vivo for postabsorptive rat small intestine. J Biol Chem 253: 69–76, 1978 [PubMed] [Google Scholar]
- 54.Wischmeyer PE, Riehm J, Singleton KD, Ren H, Musch MW, Kahana M, Chang EB. Glutamine attenuates tumor necrosis factor-alpha release and enhances heat shock protein 72 in human peripheral blood mononuclear cells. Nutrition 19: 1–6, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Zheng Z, Kim JY, Ma H, Lee JE, Yenari MA. Anti-inflammatory effects of the 70 kDa heat shock protein in experimental stroke. J Cereb Blood Flow Metab 28: 53–63, 2008 [DOI] [PubMed] [Google Scholar]
- 56.Ziegler TR, Ogden LG, Singleton KD, Luo M, Fernandez-Estivariz C, Griffith DP, Galloway JR, Wischmeyer PE. Parenteral glutamine increases serum heat shock protein 70 in critically ill patients. Intensive Care Med 31: 1079–1086, 2005 [DOI] [PubMed] [Google Scholar]
- 57.Zolotarevsky Y, Hecht G, Koutsouris A, Gonzalez DE, Quan C, Tom J, Mrsny RJ, Turner JR. A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease. Gastroenterology 123: 163–172, 2002 [DOI] [PubMed] [Google Scholar]