Abstract
Massage is an ancient manual therapy widely utilized by individuals seeking relief from various musculoskeletal maladies. Despite its popularity, the majority of evidence associated with massage benefits is anecdotal. Recent investigations have uncovered physiological evidence supporting its beneficial use following muscle injury; however, the effects of massage on healthy, unperturbed skeletal muscle are unknown. Utilizing a custom-fabricated massage mimetic device, the purpose of this investigation was to elucidate the effects of various loading magnitudes on healthy skeletal muscle with particular interest in the gene expression profile and modulation of key immune cells involved in the inflammatory response. Twenty-four male Wistar rats (200 g) were subjected to cyclic compressive loading (CCL) over the right tibialis anterior muscle for 30 min, once a day, for 4 consecutive days using four loading conditions: control (0N), low load (1.4N), moderate load (4.5N), and high load (11N). Microarray analysis showed that genes involved with the immune response were the most significantly affected by application of CCL. Load-dependent changes in cellular abundance were seen in the CCL limb for CD68+ cells, CD163+ cells, and CD43+cells. Surprisingly, load-independent changes were also discovered in the non-CCL contralateral limb, suggesting a systemic response. These results show that massage in the form of CCL exerts an immunomodulatory response to uninjured skeletal muscle, which is dependent upon the applied load.
Keywords: cyclic compressive loading, inflammation, macrophage, neutrophil, mechanosensitivity
massage is defined as a “mechanical manipulation of body tissues with rhythmical pressure and stroking for the purpose of promoting health and well-being” (9). According to the American Massage Therapy Association, the benefits of massage therapy include alleviation of pain, tension headaches, and depression, while promoting sleep and improved quality of life (1). Although it has recently gained a considerable presence in the health care system (22, 32), the evidence in support of massage as a beneficial clinical modality remains mostly anecdotal. Most reports on the effects of massage have focused on the recovery of muscle function after exercise. Our animal models of massage indicate that massage limits damage and enables the repair and regenerative process (48). However, a comprehensive literature review indicated that scientific evidence relating massage application to postexercise muscle recovery and function is conflicting (4). Moreover, mechanisms underlying the damage-reducing effects of massage are just starting to be investigated and some studies are focusing on the immune system. Increases in the number of natural killer cells (23, 26, 37) and lymphocytes (23, 26, 35, 37), as well as their cytotoxic capacity (2, 26), have been observed with massage in immune-compromised individuals [AIDS/HIV (26, 37)], subjects with cancer (23), preterm infants (2), and healthy individuals (34, 35). Increases in cell number occur after 1 day (34) to several weeks of intervention. These studies provide important evidence for the influence of massage on the immune response; however, they also greatly underscore the methodological limitations for massage studies involving human subjects, such as application technique, the amount of load applied, and frequency and duration of sessions.
The fabrication and utilization of a novel massage device which applies predetermined loads to muscle has allowed for a more controlled exploration of the mechanical effects of massage in skeletal muscle (8). Cyclic compressive loading (CCL), a massage mimetic, enhanced functional recovery of force production when immediately applied after a bout of eccentric exercise to tibialis anterior muscle in a rabbit model (8) and also showed recovery of the muscle's mechanical properties such as stress relaxation and creep (19). Cellular mechanisms that potentially contribute to the recovery of function are starting to be investigated. It was noted that leukocyte infiltration was decreased in exercised muscle after CCL in rabbits (8), and biopsies taken from exercised and subsequently massaged quadriceps muscles of human volunteers showed an attenuation of proinflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) (10). A recent investigation demonstrated a reduction in inflammatory cells in muscle that was immediately subjected to CCL after exercise, compared with muscles that were not (20). This immunomodulatory effect was also found to be time dependent such that the functional recovery was attenuated if the application of CCL was delayed after exercise (20). Collectively, these studies indicate that massage (either applied manually or through CCL) modulates the inflammatory response in skeletal muscle following exercise and consequently influences secondary muscle damage, which is usually associated with inflammation (27).
Although it is becoming clear that the immune system is involved in response to massage after a damaging event, it remains to be determined whether noninjured muscle responds in similar fashion and if the immune system is involved when previous muscle damage is not induced. Additionally, differential responses provoked by variable loads applied to unperturbed muscle have yet to be evaluated. Therefore the purpose of this study was to investigate the immunomodulatory responses to CCL of nondamaged, unperturbed muscle in relation to magnitude of applied load. We hypothesize that CCL modulates the inflammatory environment in a load-dependent manner. Utilizing our custom-fabricated cyclic compressive loading device (CCLD) modified from the previously described device (8, 19–21, 27), we will determine the effect of massage mimetic on rat tibialis anterior muscle in response to different load magnitudes.
METHODS
Twenty-four male Wistar rats (200 g, Harlan Laboratories, Indianapolis, IN) were used in this study. Rats were housed in cages within the animal housing facility at the University of Kentucky, with access to food and water ad libitum. All procedures were approved by the University of Kentucky's Institutional Animal Care and Use Committee. Rats were randomly assigned to one of four CCL groups (n = 6): control at 0N (C); low load at 1.4N (LL); moderate load at 4.5N (ML); and high load at 11N (HL). The HL was determined from previous work with rabbits (8), the LL is the minimal load applied by the CCLD, and the ML load was determined by scaling down the optimal load determined for rabbit tibialis anterior for use in rats. All groups (excluding control group) received a bout of CCL for 30 min (on their right limb) over 4 consecutive days.
Cyclic compressive loading of muscle.
Rats were anesthetized using isoflurane and placed lateral recumbent on a heated sling with one limb secured to a small platform by coban or athletic tape encircling the talocrural joint/midfoot. The tibialis anterior (TA) muscle was placed facing superiorly for the application of cyclic compressive loads by a custom-fabricated CCLD adapted for rats (Fig. 1) (8). A spring-loaded strut mechanism was designed to allow a cylinder to roll longitudinally over a contoured mass of tissue and displace vertically in response to the normal force exerted upward from the tissue to the roller during an oscillating movement. Utilizing a Hookean model, the vertical displacement of the roller was resisted by adjusting the deformation length of two identical compressive springs attached to the struts suspending the roller's axle (Fig. 1). For the LL group the springs were disengaged and the weight of the chassis assembly represented the minimal applied load (1.4N). With the springs engaged, the desired amount of preloaded force was adjusted using a rigidly fixed micrometer head with a stroke length of 25 mm and a resolution of 0.01 mm (Fig. 1). A force transducer was mounted in series between the micrometer head and compression spring-loading mechanism, which, after calibration, enabled continuous, real-time readings of the normal force applied to the roller. The output signal from the force transducer was routed through strain-gauge signal conditioning amplifier and low-pass filtered at 100 Hz through an integrated second-order recursive Butterworth filter (Vishay 2310B, Vishay Micro-Measurements, Raleigh, NC). The spring-loaded roller mechanism was calibrated prior to application of CCL by displacing the roller using a DFS digital v-block mounted to a force gauge (Shimpo, Itasca, IL). Positioned directly below the mechanism, the micrometer was adjusted every 0.5 mm throughout its range while recording the output voltage of the transducer and force gauge at each position. The horizontal position of the roller system, and subsequently the contact point of the roller, was controlled using a linear actuator with a stroke length of 120 mm driven by a servomotor (Parker Hannifin, Irwin, PA), controlled via Motion Planner software (Compumotor, Rohnert Park, CA) (Fig. 1). Data from the motor and force transducer were acquired with WinDaq data acquisition (Dataq Instruments, Akron, OH) at 250 samples per second.
Fig. 1.
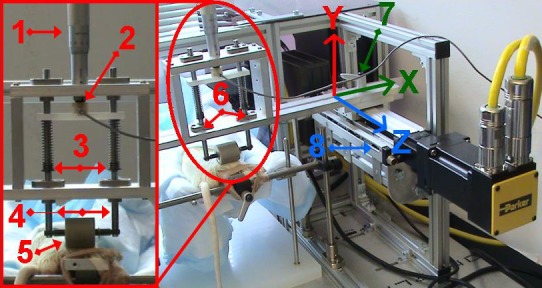
Custom-fabricated cyclic compressive loading device (CCLD) has the ability to quantify compressive loads to soft tissues during cyclic motions, from a range of 1.4N to 100N, utilizing a spring-loaded roller mechanism. A vertically mounted micrometer (1) allows precise adjustment of the compressive load applied to two springs (3) arranged in parallel with a known Hooke's spring constant, to allow accurate calibration of forces applied through the 25 mm roller (5) in series with a force transducer (2). Struts (4) rigidly attached to the roller's axle and distal end of compressive springs to allow vertical movement (y). The horizontal position (x) of the roller assembly was secured with clamps (7) and linear translation (z), stroke length, and frequency were controlled by a servo-motor and linear actuator (8) providing positional feedback and data in real time.
For CCL application the roller was placed over the skin overlying the TA muscle, immediately proximal to the lateral malleolus of the hindlimb, and cycled 15 mm proximal and distal along the length of the TA muscle at a frequency of 0.5 Hz. The contralateral left limb in all groups was not subjected to CCL. The control group (0N load) was anesthetized and placed lateral recumbent on the CCLD platform for 30 min. Upon completion of the CCL session or sham treatment, the rats were returned to their cages and allowed to recover every day.
Rats were euthanized 24 h after the fourth CCL session by IP injection of Euthasol (sodium pentobarbital) (NLS Animal Health, Pittsburgh, PA). The TA muscles from both legs were dissected, sectioned into medial and lateral portions, flash frozen in liquid nitrogen, and maintained at −80°C for future analysis.
Microarray analysis.
Total RNA was isolated from the TA muscle using the ToTALLY RNA kit (Ambion-Applied Biosystems, Foster City, CA), according to manufacturer's instructions. RNA integrity and concentration were determined using the Agilent Bioanalyzer (Agilent Technologies, Santa Clara, CA). Microarray was used as a screening tool to determine changes in gene transcription with compressive load. Real-time RT-PCR was then utilized to verify findings. Hypothesizing that the high load of 11N would cause a substantial inflammatory response, we focused on elucidating the differences that may exist between the LL and ML for therapeutic benefit. Therefore samples from three animals were chosen at random from each of the Control, LL, and ML groups for microarray and RT-PCR analysis. Samples with a RNA Integrity Number (RIN) of 8 or greater were analyzed at the Microarray Core Facility at the University of Kentucky. One sample per chip (Affymetrix GeneChip) was assayed from each group: Control, LL, and ML. The microarray was performed using Command and Expression Console Software (Affymetrix). Output generated was analyzed using Partek software version 6.5 (Partek). Statistical analysis of the microarray was performed using Robust Multichip Average (RMA) for chip normalization. Following normalization, a one-way ANOVA (P < 0.01 significance) was used to compare treatment groups at the gene level. Genes which were found to be differentially expressed were analyzed for gene ontology using Database for Annotation, Visualization, and Integrated Discovery. Functional Annotated Clusters meeting an acceptable enrichment score of ≥1.3 (minus log scale, equivalent or less than significance of 0.05) (25) under medium stringency (15 total) were included in the overall analysis. Patterns of gene expression were determined for differentially expressed genes. Individual genes were selected for verification via RT-PCR based on a 1.8-fold change cutoff and mean overall abundance.
RT-PCR.
RNA was isolated from all six animals per group using the RNeasy Fibrous Tissue Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. RNA integrity and concentration were determined using the Agilent Bioanalyzer (Agilent Technologies) in addition to Nano Drop (Thermo Fisher Scientific, West Palm Beach, FL). cDNA was obtained from 400 ng RNA with RNA integrity number (RIN) of 8 or higher using ReadyScript cDNA Synthesis Mix according to manufacturer's instructions (Sigma-Aldrich, St. Louis, MO). cDNA concentration was measured using a Nano Drop (Thermo Fisher Scientific) and samples were diluted to 50 ng/μl per well for RT-PCR analysis. Primers were designed using TaqMan Probe and Primers from Applied Biosystems (Foster City, CA), (Rn primer numbers: CXCR5: Rn02132880_s1, CD74: Rn00565062_m1, CCR2: Rn01637698_s1, LYZ2: Rn00562794_m1, LILRB4: Rn01399943_m1). PCR reactions were assembled using protocols from Applied Biosciences utilizing TaqMan Gene Expression Master Mix and protocol (Applied Biosystems). Reactions were performed using the ABI Prism 7700 Sequence Detection System (Applied Biosystems) using standard cycling conditions.
Hematoxylin and eosin staining.
TA muscle cross sections were cut at 8 μm; every fifth section was fixed using methanol (100%) and reacted for standard hematoxylin and eosin staining. A total of four sections per muscle were captured using Axioimager upright microscope (Zeiss Gottingen, Germany). Cross-sectional area of individual muscle fibers as well as total area of tissue sections were measured using Zeiss AxioVision Software (Zeiss). The total muscle fiber cross-sectional area was subtracted from the total overall muscle area of the specific field and expressed as percentage of total area. This measure is an estimation of edema in the muscle tissue.
Immunohistochemistry: Neutrophil and Macrophage Quantification.
TA muscle cross sections were cut as above and fixed in ice-cold acetone (100%). Sections were blocked in 3% H2O2 in PBS, followed by normal horse serum (ImmPRESS-Vector Laboratories Burlingame, CA). Primary antibodies were applied and incubated overnight at 4°C at 1:100 dilution for 1) Mouse anti-Rat CD43 (neutrophil marker); 2) ED1+ Mouse anti-Rat CD68; and 3) ED2+ Mouse anti-Rat CD163 (macrophage markers) (all three antibodies from Serotec, Raleigh, NC). ImmPRESS anti-Mouse IgG (Vector Laboratories) secondary antibody with fluorescein (for ED1 and ED2) or cyanine-3 (Cy3 for CD43) was applied in amplification buffer [Tyramide Signal Amplification (TSA), Perkin Elmer, Waltham, MA]. Then 4′6-diamidino-2-phenoylindole (DAPI) was applied at 0.01 μM to visualize nuclei. All histology images were obtained using a Zeiss Axio Imager M1 microscope (Carl Zeiss Microimaging GmbH, Göttingen, Germany).
Stereological point counting.
Total cellular abundance was measured using a random stereological point counting technique. Using a Zeiss Axio Imager M1 microscope at 200X magnification, one randomly selected field from each of the four sections per muscle was photographed and used for cell counting. Cells in the interstitial space outside muscle fibers were counted as total cellular abundance on the H&E slides. For determination of ED1 and ED2 cellular abundance, we counted cells that were positive for primary antibody and also reacted with DAPI. Cell counts from each of the four sections per muscle were averaged and expressed as cells per field of 0.15 mm2.
Statistical analyses.
Microarray statistical analyses were performed as described above. All remaining statistical analyses were performed using IBM SPSS 18.0 (SPSS, Chicago, IL) and SigmaPlot (Systat Software, San Jose, CA). For all variables, mean and standard error are reported. Levene's statistic for homogeneity of variance was violated across groups when comparing gene abundance following RT-PCR. Therefore five one-way Welch's ANOVAs were performed with Games-Howell post hoc tests to determine significant differences among groups. Student's paired t-tests were used to compare differences between designated CCL hindlimbs and the contralateral control hindlimbs for each condition (C, LL, ML, HL). One-way ANOVAs with Holm-Sidak post hoc analysis were used to compare significant differences between groups. Biological data violating normality were log10 transformed prior to one-way ANOVA analysis. Simple linear regressions were performed to assess the relationships between magnitude of load and cellular abundance for both the CCL and non-CCL limbs (H&E, ED1+, ED2+, and CD43+ staining). Statistical significance was assumed at p ≤ 0.05.
RESULTS
Loads applied to TA muscle.
The cyclic compressive loads applied to the right TA during the 4 days of massage mimetic were 4.58 ± 0.14N, 4.52N ± 0.07N, 4.53N ± 0.06N, and 4.53 ± 0.05N for the ML group and 11.01 ± 0.02N, 10.84 ± 0.16N, 11.17 ± 0.04N, 10.80 ± 0.41N for the HL group for days 1–4, respectively. Because the springs were disengaged for the LL group, the applied load was the chassis weight, or 1.4N.
Microarray and gene ontology.
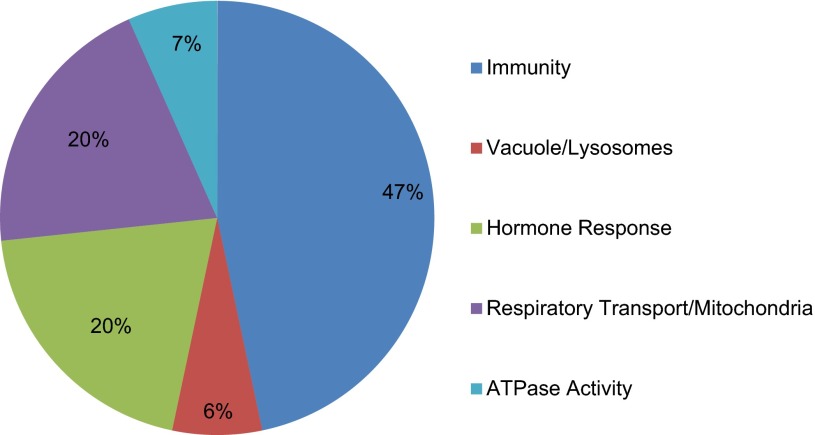
Microarray analysis was performed to identify differences in gene expression profiles between groups with distinct magnitudes of CCL. Results indicated that 534 genes were differentially expressed between the three groups. Functional annotated clustering resulted in 107 clusters, 15 of which met the cutoff criteria of an acceptable enrichment score of ≥1.3 (p ≤ 0.05) (25). These clusters were combined into five categories based on associated functions to better represent the systems most affected by massage treatment (Fig. 2). Interestingly, 47% of the functional clusters were associated with “immunity” or the “immune response,” indicating that CCL has the ability to modulate the expression of genes associated with immune function. Other clusters that were highly represented consisted of genes that have functions involving vacuoles/lysosomes, hormone responses, respiratory transport/mitochondria, and ATPase activity (Fig. 2).
Fig. 2.
Immune related genes are abundantly changed in response to cyclic compressive loading (CCL) percentage of associated clusters. Representation of the systems most affected by CCL intervention after combining 15 significant gene clusters with associated systems meeting an enrichment score of ≥1.3 from Database for Annotation, Visualization, and Integrated Discovery.
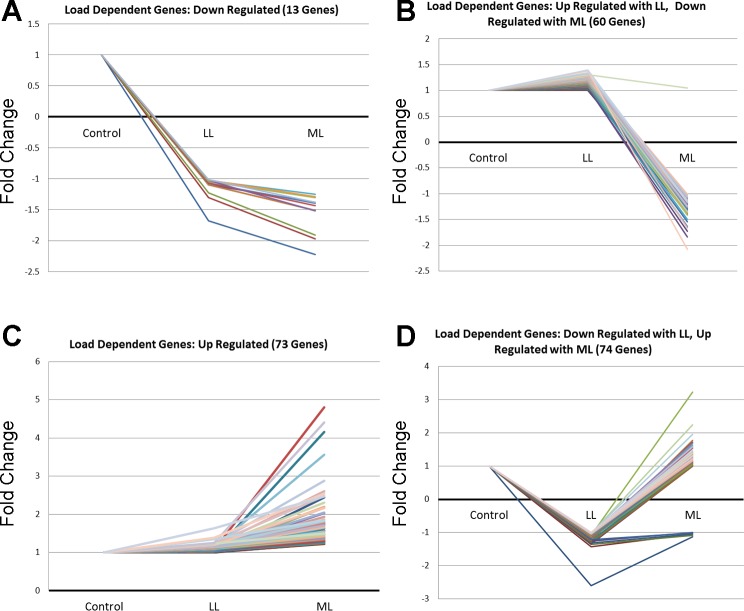
Expression pattern analysis was performed using significant genes meeting a minimum 1.2-fold change in either the LL or ML condition and were mapped for mechanical load-dependent changes. A total of 13 genes were downregulated from Control to LL and remained relatively constant from LL to ML (Fig. 3A); 60 genes were slightly upregulated or remained constant from Control to LL and downregulated from LL to ML (Fig. 3B); 72 genes were slightly upregulated or remained constant from Control to LL and upregulated from LL to ML (Fig. 3C); and the majority of genes within the 1.2 cutoff (74) were downregulated from Control to LL and either remained constant or were upregulated from LL to ML (Fig. 3D). A majority of significant gene expression changes take place under the ML conditions (Fig. 3, B, C, and D). A listing of the specific genes with mechanomodulatory responses is shown in Table 1.
Fig. 3.
Load-dependent gene expression changes. Significant genes meeting a minimum 1.2-fold change in either the low load (LL) or moderate load (ML) condition were mapped for load-dependent changes. A: set of genes downregulated with both LL and ML; B: genes upregulated with LL and downregulated with ML; C: genes upregulated with both LL and ML; and D: genes downregulated with LL and upregulated with ML.
Table 1.
Genes that display mechanodependency with cyclic compressive loading application
| Gene | Low Load | Moderate Load |
|---|---|---|
| Genes downregulated (fold change) | ||
| Mx1 | −1.67888 | −2.22632 |
| Prnd | −1.30217 | −1.97309 |
| Crhr2 | −1.23096 | −1.91271 |
| Zim1 | −1.09821 | −1.38117 |
| Adamtsl2 | −1.09717 | −1.51432 |
| Plin5 | −1.09453 | −1.5082 |
| Akr1c14 | −1.07731 | −1.39781 |
| Paqr4 | −1.06431 | −1.43816 |
| Hist2 h4 | −1.04552 | −1.30481 |
| Fbp2 | −1.03018 | −1.51608 |
| Cd320 | −1.02864 | −1.25421 |
| Ndufs1 | −1.02437 | −1.29286 |
| Apln | −1.01045 | −1.379 |
| Genes upregulated with LL, downregulated with ML (fold change) | ||
| Sdhd | 1.00453 | −1.26477 |
| Gp1ba | 1.0074 | −1.30918 |
| Sfrs3 | 1.01574 | −1.39536 |
| Alpl | 1.01612 | −1.26399 |
| Ndufc1 | 1.02241 | −1.30661 |
| Cyp3a73 | 1.02253 | −1.26853 |
| Sdc3 | 1.02365 | −1.26772 |
| Iscu | 1.03122 | −1.16401 |
| Alas2 | 1.0363 | −1.72545 |
| Cyc1 | 1.03824 | −1.17463 |
| Gpr119 | 1.03894 | −1.20054 |
| Abcb9 | 1.04282 | −1.27781 |
| Atp5j | 1.05327 | −1.21322 |
| Coq3 | 1.05538 | −1.27781 |
| Hbb | 1.05692 | −1.84194 |
| Ppp1r16b | 1.06384 | −1.2883 |
| Atp5o | 1.06733 | −1.25577 |
| Gpihbp1 | 1.08041 | −1.53934 |
| Trim65 | 1.08103 | −1.11891 |
| Nr4a1 | 1.09142 | −1.34926 |
| Hbb | 1.09253 | −1.63692 |
| Cd38 | 1.09713 | −1.64601 |
| Fam101b | 1.10063 | −1.2491 |
| Adamts15 | 1.11163 | −1.13041 |
| Olr610 | 1.12396 | −1.12905 |
| Kras | 1.12451 | −1.11356 |
| Kctd1 | 1.12787 | −1.08052 |
| Ramp2 | 1.1294 | −1.25453 |
| Ptprb | 1.13947 | −1.37989 |
| Cmtm8 | 1.13964 | −1.31341 |
| Flt4 | 1.14106 | −1.20383 |
| Kank3 | 1.1419 | −1.25887 |
| Fmo2 | 1.14564 | −1.14626 |
| Tinagl1 | 1.15087 | −1.49893 |
| Pcdh19 | 1.15164 | −1.2619 |
| Ppm1f | 1.15716 | −1.1208 |
| Tmcc2 | 1.16269 | −1.07494 |
| Fabp4 | 1.16452 | −1.41451 |
| Olr1071 | 1.16607 | −1.19932 |
| Clec1a | 1.1693 | −1.33944 |
| Myg1 | 1.17301 | −1.02413 |
| Sult1a1 | 1.17627 | −1.26284 |
| Cadps2 | 1.17838 | −1.07281 |
| Plekhf1 | 1.18948 | −1.14489 |
| tGap1 | 1.19962 | −1.06295 |
| Pald | 1.20786 | −1.15809 |
| Lsm6 | 1.21436 | −1.14579 |
| Olr883 | 1.23224 | −1.05987 |
| Sh2d3c | 1.24691 | −1.11927 |
| Hecw2 | 1.25509 | −1.35093 |
| Gpr56 | 1.26422 | −1.24234 |
| Lrrc8c | 1.26899 | −1.02307 |
| Fam117b | 1.27727 | −1.01271 |
| Pparg | 1.30015 | −1.06719 |
| Ccrn4l | 1.30033 | −1.65959 |
| Dusp14 | 1.30929 | 1.05216 |
| RGD1309437 | 1.32925 | −1.24698 |
| Mcf2l | 1.34381 | −1.13001 |
| Cxcr5 | 1.37685 | −2.0812 |
| Sema6b | 1.39766 | −1.1656 |
| Dopey2 | 1.0006 | 1.24357 |
| Genes upregulated | ||
| Zdhhc9 | 1.00062 | 1.40561 |
| RT1-DMa | 1.00119 | 1.63748 |
| Cd6 | 1.00381 | 1.45596 |
| Lilra5 | 1.00807 | 1.49524 |
| Cfp | 1.01148 | 1.41999 |
| Ostalpha | 1.01301 | 2.44534 |
| Dnajb12 | 1.01628 | 1.23203 |
| Slc35e3 | 1.01862 | 1.32156 |
| Mt1a | 1.01949 | 1.56986 |
| Sln | 1.01985 | 4.16476 |
| Stk40 | 1.02245 | 1.36316 |
| Sfpi1 | 1.02355 | 1.41548 |
| Use1 | 1.03004 | 1.26069 |
| Lilrb3l | 1.03401 | 1.5607 |
| Cited2 | 1.03441 | 1.29743 |
| Itgb2 | 1.03531 | 1.64192 |
| Ptpn6 | 1.03674 | 1.33613 |
| Stambp | 1.03962 | 1.28958 |
| Gatsl2 | 1.04169 | 1.30993 |
| Grina | 1.04347 | 1.25651 |
| Lrrc2 | 1.0445 | 1.54859 |
| Arhgap20 | 1.0449 | 1.27054 |
| Tex14 | 1.04669 | 1.54502 |
| Amica1 | 1.04726 | 1.30095 |
| Manba | 1.04779 | 1.35059 |
| Coro1a | 1.05034 | 1.48764 |
| Mt1a | 1.05219 | 1.73638 |
| Irf5 | 1.05294 | 1.44123 |
| Nkain1 | 1.05896 | 1.85526 |
| Flnc | 1.05928 | 1.35474 |
| RGD1565761 | 1.06243 | 1.30353 |
| RT1-Ba | 1.06244 | 2.18034 |
| Mapkapk3 | 1.06265 | 1.3596 |
| Atp8b4 | 1.06338 | 1.38296 |
| Clec7a | 1.06435 | 1.66454 |
| Nol3 | 1.06931 | 1.42261 |
| Ccr2 | 1.07254 | 4.80733 |
| Abca1 | 1.08232 | 1.79046 |
| Ccl6 | 1.08235 | 2.03713 |
| Dusp10 | 1.08294 | 1.65548 |
| Gm2a | 1.09346 | 1.77622 |
| Apoe | 1.09527 | 1.45713 |
| Ctse | 1.1028 | 1.33291 |
| Klra5 | 1.10803 | 1.36754 |
| Cytip | 1.11044 | 1.50308 |
| Serping1 | 1.11465 | 1.47324 |
| Dclk1 | 1.115 | 2.19401 |
| Ninj1 | 1.13061 | 1.42488 |
| Unc93b1 | 1.13281 | 1.76976 |
| Klra2 | 1.13891 | 1.69752 |
| Pycard | 1.14134 | 1.37783 |
| Gpnmb | 1.14236 | 3.55754 |
| Kdelr1 | 1.1429 | 1.42426 |
| Lgals3 | 1.14296 | 2.04704 |
| Pcbd1 | 1.14534 | 1.9332 |
| Cd180 | 1.14658 | 1.45831 |
| Ahnak2 | 1.15179 | 2.17046 |
| Ctxn3 | 1.15287 | 1.55471 |
| Mpeg1 | 1.16012 | 1.66003 |
| Ctsl1 | 1.16037 | 1.51657 |
| Myh8 | 1.17525 | 2.61133 |
| Cd74 | 1.17749 | 2.30762 |
| RT1-Db1 | 1.1826 | 1.70608 |
| Prg4 | 1.19939 | 1.82368 |
| Glipr1 | 1.20672 | 2.16827 |
| Zmynd17 | 1.23146 | 2.88109 |
| RT1-Da | 1.234 | 2.4826 |
| Fbln2 | 1.24561 | 1.50472 |
| Aph1b | 1.25502 | 4.40816 |
| Laptm5 | 1.35195 | 1.85288 |
| Lyz2 | 1.39196 | 2.54273 |
| Ly49 si2 | 1.63562 | 2.46929 |
| Akr1b10 | −2.59901 | −1.13041 |
| Hoxc4 | −1.42813 | −1.04026 |
| Genes downregulated with LL, upregulated with ML (fold change) | ||
| Olr729 | −1.34991 | −1.09039 |
| Ptgir | −1.33699 | 1.00575 |
| Rnf213 | −1.31342 | 1.01908 |
| Mrp63 | −1.31199 | 1.0089 |
| Gnb3 | −1.30672 | −1.04567 |
| H19 | −1.29318 | 1.22315 |
| Slamf9 | −1.28122 | 1.07424 |
| Cyb561d1 | −1.26229 | −1.00186 |
| Klk1c10 | −1.24063 | 1.05775 |
| Ncam1 | −1.2352 | 1.77688 |
| Meox1 | −1.21478 | −1.0094 |
| Prosc | −1.16397 | 1.1136 |
| Ccr9 | −1.16342 | 1.07631 |
| Pole | −1.16318 | 1.163 |
| Galnt4 | −1.16222 | 1.0394 |
| Zdhhc24 | −1.16181 | 1.10229 |
| Prkag3 | −1.1614 | 1.2858 |
| P2rx3 | −1.15877 | 1.04953 |
| Zfp352 | −1.15459 | 1.05273 |
| Chrnd | −1.15136 | 1.71459 |
| Fermt3 | −1.14936 | 1.15321 |
| Lrrn4 | −1.1361 | 1.20956 |
| Xpc | −1.13104 | 1.17023 |
| Tmc2 | −1.12634 | 1.16931 |
| Slc9a5 | −1.11922 | 1.17323 |
| Gcap14 | −1.09836 | 1.18077 |
| Slc7a7 | −1.09698 | 1.15508 |
| Ddi2 | −1.09646 | 1.34899 |
| Mfsd5 | −1.08427 | 1.13588 |
| Car6 | −1.08327 | 1.41657 |
| Th | −1.07913 | 3.23794 |
| Eif2c4 | −1.07635 | 1.12661 |
| Litaf | −1.07426 | 1.18742 |
| Wdfy1 | −1.07423 | 1.23392 |
| Tlr5 | −1.07029 | 1.29908 |
| Irf8 | −1.06447 | 1.55221 |
| Mapt | −1.0558 | 1.19734 |
| Rbpj | −1.05443 | 1.15795 |
| Nckap1l | −1.05107 | 1.63277 |
| Rasgef1a | −1.05033 | 1.19355 |
| Stat5b | −1.04826 | 1.14749 |
| Col19a1 | −1.04676 | 1.24115 |
| Trim37 | −1.04657 | 1.33013 |
| Chrng | −1.04424 | 1.6884 |
| Gpr114 | −1.04422 | 1.1999 |
| Fibcd1 | −1.04361 | 1.16704 |
| Tmem9 | −1.04267 | 1.27268 |
| Olr1588 | −1.04032 | 1.37464 |
| Csf3r | −1.03939 | 1.25327 |
| Myo1f | −1.03779 | 1.57667 |
| Cables2 | −1.03537 | 1.21487 |
| Adrbk2 | −1.03379 | 1.19979 |
| Abcc3 | −1.03241 | 1.28234 |
| Nlrp3 | −1.02915 | 1.41129 |
| Tubb6 | −1.02808 | 2.24517 |
| Zswim1 | −1.02678 | 1.23981 |
| Shisa4 | −1.0262 | 1.17146 |
| Izumo1 | −1.0259 | 1.1788 |
| Gna15 | −1.02457 | 1.30425 |
| Cd37 | −1.0235 | 1.41633 |
| N4bp2l1 | −1.02272 | 1.21849 |
| Ptpn21 | −1.01806 | 1.20019 |
| Lilrb4 | −1.01642 | 1.96546 |
| Ccdc6 | −1.01429 | 1.22081 |
| Cd97 | −1.01173 | 1.30407 |
| Selplg | −1.01008 | 1.44315 |
| Aoah | −1.00955 | 1.38569 |
| Cd84 | −1.00852 | 1.59057 |
| Sash3 | −1.00601 | 1.47232 |
| Arhgap30 | −1.00459 | 1.31292 |
| Dock2 | −1.00111 | 1.48195 |
| Itgb7 | −1.00031 | 1.31802 |
RT-PCR quantification.
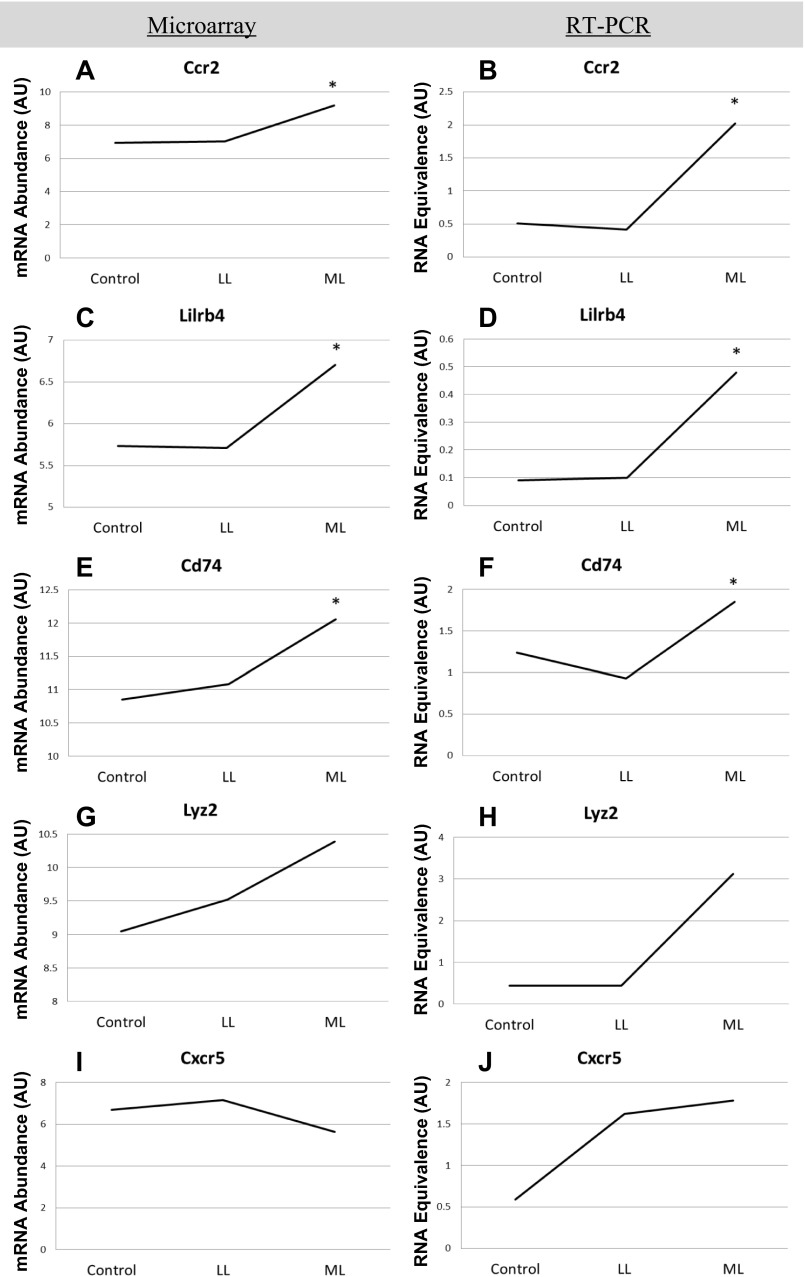
Five genes were selected to corroborate the array results: Chemokine (C-C motif) receptor-2 (CCR2), Leukocyte immunoglobulin-like receptor (subfamily B, member 4) (LILRB4), Cd74 molecule major histocompatibility complex (Class II) (CD74), Lysozyme 2 (LYZ2), and Chemokine (C-X-C motif) receptor-5 (CXCR5). These genes showed at least a 1.8-fold increase or decrease in expression between any of the three conditions and were of interest because of their association with the immune response (Fig. 4). No change in CCR2 gene expression was detected from Control to LL in either microarray (Fig. 4A) or RT-PCR (Fig. 4B). However, CCR2 gene expression was elevated in ML compared with control and LL using both techniques (Fig. 4, A and B). Gene expression pattern for LILRB4 was very similar to CCR2 such that no difference was observed between Control and LL, but an elevated level was detected in ML compared with control and LL using both microarray (Fig. 4C) and RT-PCR (Fig. 4D). CD74 gene expression was also not different between control and LL, and again a higher level of CD74 gene expression was observed in ML compared with control and LL for both techniques (Fig. 4, E and F). Therefore a load-dependent increase was observed for all three of these genes, indicating their involvement with CCL at moderate loads. Lyz2 gene expression as measured by microarray was higher in the ML compared with Control and LL (Fig. 4G), but the RT-PCR analysis did not confirm these data even though a similar pattern was observed (Fig. 4H). CXCR5 gene expression was elevated in ML compared with control and LL when measured by microarray (Fig. 4I), but these data were not corroborated by RT-PCR analysis (Fig. 4J). In general the results from the microarray were mimicked by RT-PCR analysis, and therefore immune-related processes involved in the response to CCL were further investigated.
Fig. 4.
Verification of mRNA abundance for selected genes. Side-by-side comparisons of gene abundance per loading condition, as measured by microarray (n = 3 per group) and RT-PCR (n = 6 per group). Five genes meeting a minimum fold change of 1.8 were selected to corroborate microarray results. Chemokine (CC-motif) receptor-2 (CCR2), Leukocyte immunoglobulin-like receptor (subfamilty B, member 4) (LILRB4), Cd74 molecule major histocompatibility complex (Class II) (CD74), Lysozyme 2 (LYZ2), and Chemokine (C-X-C motif) receptor-5 (CXCR5). *indicates significant difference from both Control and LL conditions.
Histological analysis.
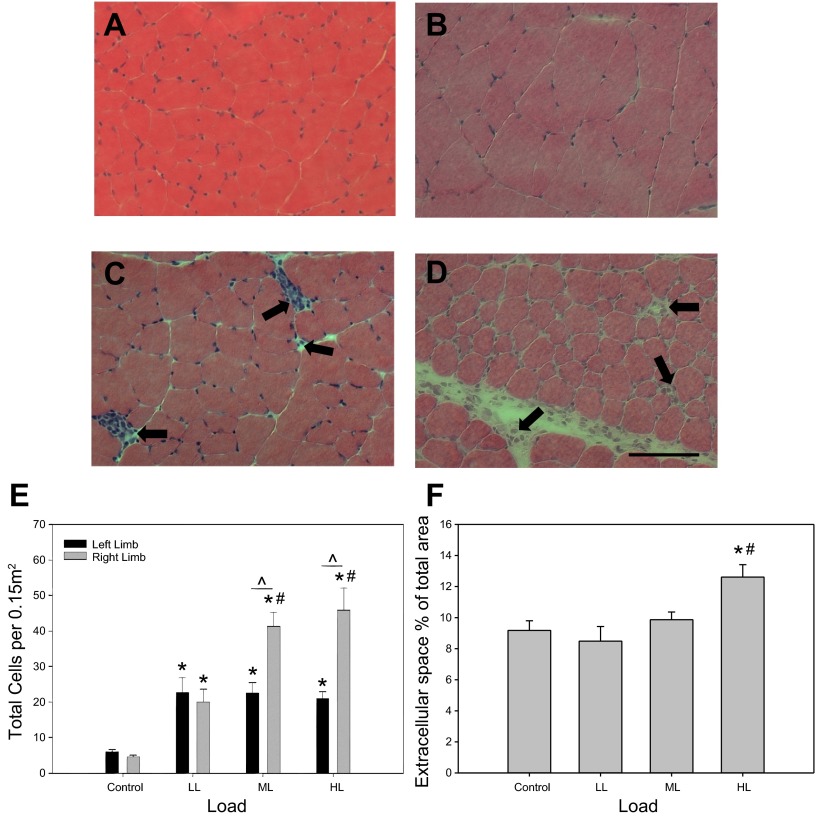
No gross morphological differences in cellular integrity, such as fiber necrosis and centronucleation, were observed between the different load levels (Fig. 5, A–D). However, a significant difference in extracellular space was detected in the HL group vs. all the other groups, while no significant differences were detected between the LL, ML, and control groups (Fig. 5F). The increase in extracellular space in the HL group is likely a result of increased edema in the muscle as a consequence of some tissue damage. The cellular immune response to CCL was investigated in the TA muscle by counting the number of interstitial cells. A load-dependent increase in cellular abundance was observed in the muscles of ML and HL that underwent CCL (Fig. 5E). Using a simple linear regression model, the fitted data resulted in R2 = 0.57, indicating the 57% of the variability in cellular infiltration could be accounted for by magnitude of the applied load (Table 2). Surprisingly, cellular abundance observed in the contralateral non-CCL limbs was significantly higher for LL, ML, and HL vs. the reference non-CCL control animals, suggesting a systemic physiological effect of CCL on cellular infiltration (Fig. 5E). This systemic “crossover” effect is, however, not load-dependent such that higher CCL loads do not increase cellular abundance in the contralateral limb (P value = 0.080).
Fig. 5.
Load-dependent changes in cellular abundance and in extracellular space in CCL-treated muscle. Representative cross sections of the right, CCL-treated tibialis anterior (TA) muscle stained for H&E of control (A), low load (B), moderate load (C), and high load (D). Bar in D indicates 100 μm. Cell quantification across loading conditions (E) with values shown as means ± SE. No differences were detected between limbs in the control condition; therefore one bar is shown for simplification. Extracellular space (F) represented as a percentage of the total area. *Significant difference from Control. #Significant difference from LL. ^Significant difference between limbs within loading group.
Table 2.
Cellular abundance is load dependent in the CCL-treated muscle and load independent in the contralateral muscle
| CLL-Treated Limb |
Contralateral Non-CCL-Treated Limb |
|||
|---|---|---|---|---|
| R2 | P value | R2 | P value | |
| Total cells | 0.57 | <0.001 | 0.13 | 0.08 |
| CD43+ cells | 0.20 | 0.030 | 0.00 | 0.760 |
| CD68+ cells | 0.54 | <0.001 | 0.01 | 0.632 |
| CD163+ cells | 0.51 | <0.001 | 0.02 | 0.509 |
CCL, cyclic compressive loading.
Immune cell abundance.
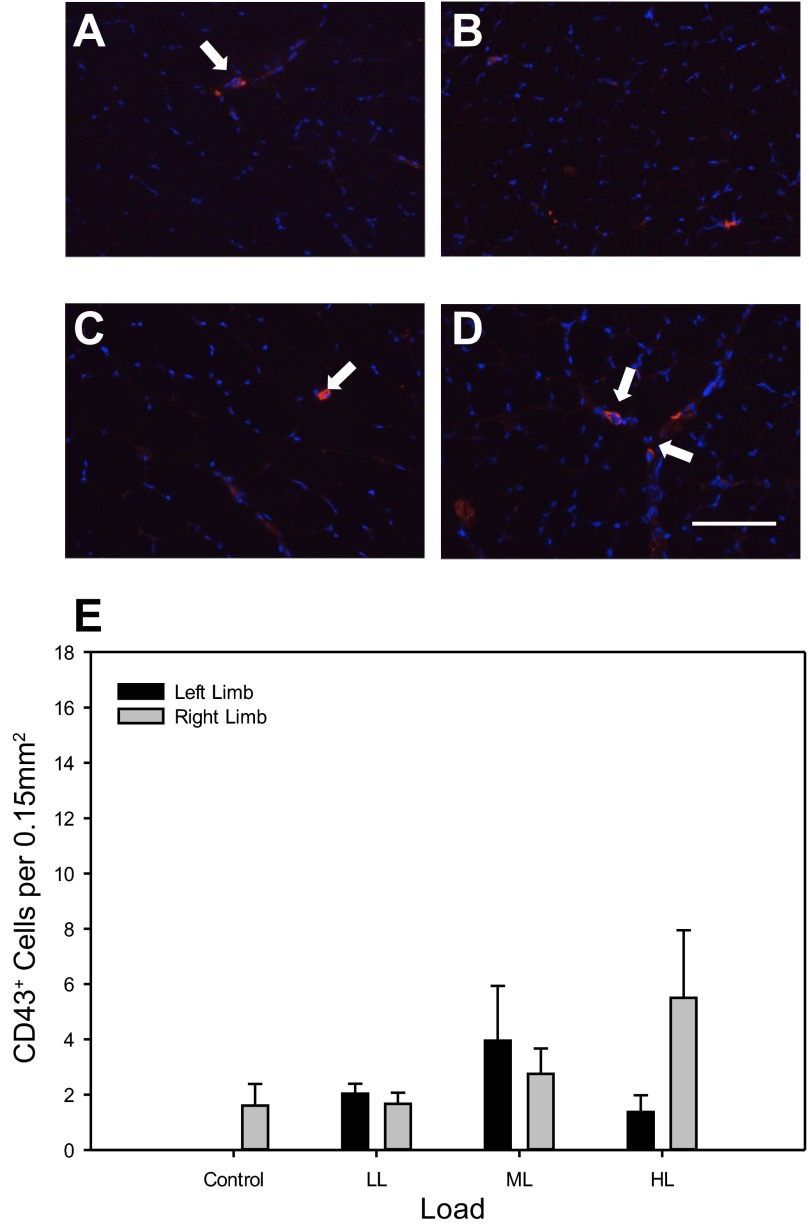
Key monocytes involved in the inflammatory response were analyzed. CD43+ cell staining was rare in TA muscle sections of all conditions (Fig. 6, A–D). No significant differences in CD43+ cell abundance were found in the CCL-treated muscle, the contralateral non-CCL limb, or between limbs across all conditions (Fig. 6E).
Fig. 6.
Abundance of CD43+ cells is not different after four bouts of CCL representative cross sections of the right, CCL-treated TA muscle of Control (A), low load (B), moderate load (C), and high load (D) immunoreacted for CD43 as a marker for the identification of neutrophils (red) and stained for 4′6-diamidino-2-phenoylindole (DAPI) (blue). Bar in D indicates 100 μm. Quantification of CD43+ cell number (E) with values as means ± SE. No differences were detected between limbs in the control condition; therefore one bar is shown for simplification.
CD68+ cell abundance was rare or absent in control conditions but was elevated with increasing loads (Fig. 7A). Following 4 consecutive days of CCL, muscles receiving HL had a significantly larger quantity of CD68+ cells compared with Control, LL, and ML, while ML was higher than control (Fig. 7B). For the LL and ML no differences were detected between the CCL and the contralateral non-CCL limb (Fig. 7B). However, CD68+ cell abundance was significantly higher in the CCL-treated muscle of the HL group only (Fig. 7B), indicating that CCL at a higher load induces a local proinflammatory response. CD163+ cell presence was detected in TA muscles across all loading groups with a higher level of staining in the HL group (Fig. 7C). Quantification indicated a significantly higher abundance of CD163+ cells in the HL group compared with control, LL, and ML (Fig. 8D). In addition, CD163+ cell abundance was significantly higher in the CCL muscle compared with the non-CCL contralateral muscle of the HL group only (Fig. 8D).
Fig. 7.
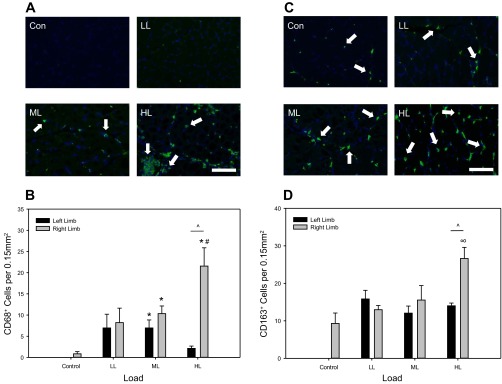
Abundance of CD68+ cells increases in a load-dependent manner, while CD163+ cells respond only to high load. A: representative cross sections of the right, CCL-treated TA muscle of Control (Con), low load (LL), moderate load (ML), and high load (HL) immunoreacted for CD68 (ED1) (green) and stained for DAPI (blue). Bar in (HL) indicates 100 μm. B: quantification of CD68+ cell number with values as means ± SE. No differences were detected between limbs in the control condition; therefore one bar is shown for simplification. *Significant difference from Control. #Significant difference from LL. ^Significant difference between limbs within loading group. C: representative cross sections of the right, CCL-treated TA muscle of Control (Con), low load (LL), moderate load (ML), and high load (HL) immunoreacted for CD163 (ED2) (green) and stained for DAPI (blue). Bar in (HL) indicates 100 μm. D: quantification of CD163+ cell number with values as means ± SE. No differences were detected between limbs in the control condition; therefore one bar is shown for simplification. ∞ Significant difference from Control, LL, and ML. ^Significant difference between limbs within loading groups.
Similar to total cellular infiltrate, abundance of CD68+, CD163+, and CD43+ cells was found to be load dependent in the CCL limb but not within the contralateral non-CCL limb (Table 2). Although the resultant R2 value is low at 0.20, and significance was not detected when comparing within group, the regression for CD43+ cell abundance is significant as they do become more abundant at high loads (Table 2). These correlations suggest induction of a proinflammatory response with the application of high-load CCL.
Discussion.
Beneficial physiological responses to massage have been observed in muscle which has been previously injured (4, 8, 10, 19–21). However, the mechanism(s) by which massage exerts its effects, and how different loads change the response in nondamaged muscle, are currently unknown. Therefore the aim of this investigation was to determine potential immunomodulatory targets of massage mimetic CCL application in noninjured, unperturbed tissue relative to magnitude of applied load. Our results demonstrate that 1) expression of genes associated with the immune response were the most affected with the application of CCL; 2) various genes involved with the inflammatory response display mechanodependency and have disparate responses to the magnitude of load applied to the muscle; 3) magnitude of applied load significantly influences the abundance of immune cells in the muscle; 4) a significant increase in abundance of both CD68+ and CD163+ cells is observed in muscle subjected to higher loads of CCL, signifying an inflammatory response; and 5) CCL has a systemic effect, increasing abundance of immune cells in the contralateral non-CCL-treated muscle independent of level of loading.
Effect of CCL on immune system associated genes.
We show in this study that 47% of the significant functional gene clusters obtained from gene ontology are associated with the immune response following four daily bouts of CCL. The importance of this finding lies partly in the fact that we investigated uninjured muscle tissue, which indicates the muscle itself, and likely resident cells, are capable of generating a large change in expression of immune-related genes. It was previously shown that massage induced a beneficial immune response in patients with breast cancer (5, 23, 24), in children and adults with HIV (12, 26, 38), and in preterm infants (2). Moreover, genes involved in the immune system were the largest functional group changed in white blood cells as response to massage (15) and immune function was improved after either a single bout or repeated sessions in healthy individuals (34, 35). These human studies all indicate that the innate immune system is capable of responding to changes in mechanical activity exerted on muscles. Mechanotransduction is a powerful tool with regard to cell signaling: transferring mechanical energy into a chemical response results in quicker signal transduction from the cell surface to the nucleus than that of a ligand-receptor interaction (47). Manual therapies like massage utilize this principal and, according to our data, have a measureable effect on the expression of immune system-related genes. Recently, it was shown that massage therapy attenuated proinflammatory signaling after exercise-induced damage (10), but to our knowledge the current study is the first to show changes in expression of immune-related genes in healthy uninjured muscle tissue.
Mechanodependency of inflammatory responses.
The gene expression pattern analysis suggests a load-dependent response to CCL. Most genes remained relatively constant with low load (1.4N) but exhibited a change with moderate load (4.5N). We validated the gene expression pattern of four genes involved in the regulation of the immune system and the inflammatory response: CCR2 interacts with various proinflammatory cytokines to regulate cell chemotaxis as a surface cell receptor expressed in immune cells such as neutrophils and macrophages (46). CCL2, also known as monocyte chemoattractant protein-1, is the primary ligand for the CCR2 receptor (29, 30, 33), and the CCR2/CCL2 interaction has shown to be a critical regulator of the autonomous inflammatory response in skeletal muscle (30, 33). Mice deficient in CCL2, as well as CCR2 receptor null mice, display a delayed inflammatory response that results in deficient regeneration (29, 30, 33). CCR2 null mice also display a retarded inflammatory process characterized by diminished macrophage recruitment and adipocyte infiltration (30, 33). Thus these proteins are intricately involved in the inflammatory response of skeletal muscle. CD74 is a transmembrane protein expressed on antigen-presenting cells and is a receptor for macrophage inhibitory factor (MIF) (36, 49); LILRB4 is a leukocyte Ig-like receptor that is expressed on antigen presenting cells, displays an immune inhibitory nature, and has been suggested to play a role in regulating both the innate and adaptive immune systems (6). LYZ2 is a lysosomal enzyme involved in lysosomal activities such as reducing local expression of proinflammatory cytokines [tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), interferon-gamma (INF-γ), interleukin-8 (IL-8), and interleukin-17 (IL-17)], while increasing the expression of anti-inflammatory cytokines [interleukin-4 (IL-4) and transforming growth factor-beta (TGF-β) (28)]. The load-dependent alterations in the abundance of these genes may be a contributing factor to the reduction of proinflammatory cytokines such as TNF-α and IL-6 observed in previous investigations (10) and ultimately influence immune cell accumulation.
Our histological data indeed suggest that cellular abundance is affected by the magnitude of compressive load applied and is elevated at higher loads. Notably, in the HL condition there is a significant increase in CD68+ and CD163+ cells. CD68+ and CD163+ are antibodies used to identify antigens ED1+ and ED2+ displayed by macrophage cells of the proinflammatory M1 and anti-inflammatory (or resident) M2 populations in rats, respectively (14, 44, 45). M1 macrophages are considered to be phagocytic in nature (7, 18, 31, 48) and are responsible for the removal of cellular debris resulting from the initial injury. M2 macrophages are resident in the tissue, classified as nonphagocytic, and serve to aid in repair and regeneration (7, 41, 43, 48). Macrophage phenotype is thought to be plastic and subject to influences from the microenvironment, such that they can undergo phenotype transition depending on the presence and/or absence of certain cytokines: proinflammatory cytokines such as IL-6, IL-8, and TNF-α promote the action of M1 macrophages, whereas anti-inflammatory cytokines (e.g., IL-4, TGF-β, and IL-10) induce the activation of M2 macrophages (39, 40, 43, 48). However, large quantities of M1 macrophages have been correlated with the severity of the immune response. Therefore high CD68+ cell abundance paired with an increase in extracellular space (edema) in the muscles subjected to high loads (11N) most likely signifies an actual injury to the muscle, but at the lower loads this injury is likely very subtle or nonexistent. The increase in CD163+ abundance is likely due to an increased need for repair and regeneration of tissue damage induced by the HL. Various clinical treatments utilize the application of high force (e.g., deep tissue or cross-friction massage) to induce local inflammation, with the goal of promoting repair and regeneration. It is unknown at this time whether or not the proinflammatory response elicited by the application of high-load CCL in this study would be of benefit in those situations. Acute vs. chronic injury environments differ considerably, and in some chronic situations inducing a local inflammatory response, elicited by high load application, may be of benefit. The results presented in this investigation are within the acute stages of the inflammatory response; however, they do not evaluate the acute response of a single bout of CCL, as four bouts were applied. Of note is that we did not observe an increase in CD43+ cell abundance (neutrophil marker) with CCL at any level of load. Neutrophil infiltration usually peaks between 6 and 12 h following a perturbation and subsides within 24 h (41–43). Therefore, when considering the temporal nature of the inflammatory response, it was not surprising that we did not detect an abundance of CD43+ cells in the muscle tissue 4 days from the initial bout of CCL. However, it has been suggested previously that neutrophil infiltration may not be a required component of the inflammatory process in certain types of skeletal muscle injury (e.g., exercise-induced damage) (27). Hence neutrophils may not be modulated with massage application. The data from our study imply that the moderate load induces an immunomodulatory response largely mediated by macrophages, which is likely beneficial; however, high loads induce an inflammatory response indicative of damage.
Systemic effect of CCL.
An unexpected result from our study was the fact that CCL induced a load-independent cellular immune response in the contralateral leg. This mimics the cross-education (crossover) effect of exercise in which the contralateral leg improves in performance even though only the ipsilateral leg is exercised (50). With exercise this effect has been attributed to neurally mediated mechanisms residing at the level of the spinal cord (50). It is possible that the crossover effect of CCL as observed here is also mediated through neuronal mechanisms; however, we suggest that it is mainly induced by an endocrine-like mechanism because previous studies have shown that massage can induce changes in the number of circulating immune cells and their function (34, 35). We suggest that CCL2 produced by resident skeletal muscle macrophages is potentially responsible for the mobilization of monocytes from the bone marrow (30, 33) and ultimately the recruitment of proinflammatory macrophages to the muscle tissue; CCL application may enhance this effect.
Mechanical considerations regarding CCL application.
Previous studies have shown a load-dependent effect of CCL on recovery of function after muscle-damaging events (21), but to our knowledge this report is the first to show load-dependent changes in immune markers in uninjured muscle tissue. The advantage of our device is that loads can be carefully controlled, unlike in the human condition where loads are quite often not described, vary during the massage session, or are described in subjective terms such as “within the range of comfort,” “light or moderate pressure,” or “as tolerated” (11, 13, 15–17). With over 75 different methods associated with massage therapy (3), application of the treatment should be carefully considered based on the desired outcome. The present study most closely mimics a bidirectional, effleurage technique, and it is extremely difficult to infer whether or not a different mode of application, or combination of strokes, would have the same results.
Conclusion
When a massage mimetic is applied to healthy skeletal muscle, the inflammatory response is load dependent and associated with mechanosensitive regulation of various immune related genes, which may be due to muscle damage when high loads are applied. Here, we have also demonstrated a systemic response to compressive loading, resulting in an increase of CD68+ (ED1+) cells in the contralateral limbs. Although this study provides some preliminary insight into the physiological mechanisms behind massage in skeletal muscle, further studies are needed to establish the efficacy behind its use. Immunomodulatory properties of massage make this manual therapy an attractive alternative intervention to pharmaceuticals for various inflammatory conditions. As massage is increasingly integrated into conventional medicine, determining the appropriate parameters for application will become increasingly important to maximize beneficial outcomes.
GRANTS
This work was supported by the National Athletic Trainer's Association Osternig Research Award, the National Athletic Trainers' Association Research and Education Foundation, the University of Kentucky College of Health Sciences pilot grant program, National Institute on Aging Grant AG-042699, and the National Heart, Lung, and Blood Institute Grant T32-HL-086341, Research Training in Muscle Biology of the Cardiopulmonary System.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.W.-B., T.A.B., and E.E.D.-V. conception and design of research; C.W.-B. and T.A.B. performed experiments; C.W.-B., T.A.B., and E.E.D.-V. analyzed data; C.W.-B., T.A.B., and E.E.D.-V. interpreted results of experiments; C.W.-B. prepared figures; C.W.-B. and E.E.D.-V. drafted manuscript; C.W.-B., T.A.B., and E.E.D.-V. edited and revised manuscript; C.W.-B., T.A.B., and E.E.D.-V. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors would like to acknowledge Sarah Abshire, Amy Confides, Jyothi Mula, R. Grace Walton, and Tommy Cunningham for technical assistance, in addition to Arnold J. Stromberg and Yanling Hu for statistical instruction.
REFERENCES
- 1.American Massage Therapy Association. amtamassage. http://www.AMTA./org [4/232013, 2013]
- 2.Ang JY, Lua JL, Mathur A, Thomas R, Asmar BI, Savasan S, Buck S, Long M, Shankaran S. A randomized placebo-controlled trial of massage therapy on the immune system of preterm infants. Pediatrics 130: e1549–e1558, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes PM, Powell-Griner E, McFann K, Nahin RL. Complementary and alternative medicine use among adults: United States, 2002. Adv Data 1–19, 2004 [PubMed] [Google Scholar]
- 4.Best TM, Hunter R, Wilcox A, Haq F. Effectiveness of sports massage for recovery of skeletal muscle from strenuous exercise. Clin J Sport Med 18: 446–460, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Billhult A, Lindholm C, Gunnarsson R, Stener-Victorin E. The effect of massage on immune function and stress in women with breast cancer–A randomized controlled trial. Auton Neurosci 150: 111–115, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Brown DP, Jones DC, Anderson KJ, Lapaque N, Buerki RA, Trowsdale J, Allen RL. The inhibitory receptor LILRB4 (ILT3) modulates antigen presenting cell phenotype and, along with LILRB2 (ILT4), is upregulated in response to Salmonella infection. BMC Immunol 10: 56, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butterfield TA, Best TM, Merrick MA. The dual roles of neutrophils and macrophages in inflammation: A critical balance between tissue damage and repair. J Athl Train 41: 457–465, 2006 [PMC free article] [PubMed] [Google Scholar]
- 8.Butterfield TA, Zhao Y, Agarwal S, Haq F, Best TM. Cyclic compressive loading facilitates recovery after eccentric exercise. Med Sci Sports Exerc 40: 1289–1296, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cafarelli E, Flint F. The role of massage in preparation for and recovery from exercise: An overview. Sports Med 14: 1–9, 1992 [DOI] [PubMed] [Google Scholar]
- 10.Crane JD, Ogborn DI, Cupido C, Melov S, Hubbard A, Bourgeois JM, Tarnopolsky MA. Massage therapy attenuates inflammatory signaling after exercise-induced muscle damage. Sci Transl Med 4: 119ra113, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Diego MA, Field T. Moderate pressure massage elicits a parasympathetic nervous system response. Int J Neurosci 119: 630–638, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Diego MA, Field T, Hernandez-Reif M, Shaw K, Friedman L, Ironson G. HIV adolescents show improved immune function following massage therapy. Int J Neurosci 106: 35–45, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Diego MA, Field T, Sanders C, Hernandez-Reif M. Massage therapy of moderate and light pressure and vibrator effects on EEG and heart rate. Int J Neurosci 114: 31–44, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Dijkstra CD, Dopp EA, Joling P, Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: Distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology 54: 589–599, 1985 [PMC free article] [PubMed] [Google Scholar]
- 15.Donoyama N, Ohkoshi N. Effects of traditional Japanese massage therapy on gene expression: Preliminary study. J Altern Complement Med 17: 553–555, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Field T, Diego M, Hernandez-Reif M. Moderate pressure is essential for massage therapy effects. Int J Neurosci 120: 381–385, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Field T, Diego MA, Hernandez-Reif M, Deeds O, Figuereido B. Moderate versus light pressure massage therapy leads to greater weight gain in preterm infants. Infant Behav Dev 29: 574–578, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frenette J, St-Pierre M, Cote CH, Mylona E, Pizza FX. Muscle impairment occurs rapidly and precedes inflammatory cell accumulation after mechanical loading. Am J Physiol Regul Integr Comp Physiol 282: R351–R357, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Haas C, Best TM, Wang Q, Butterfield TA, Zhao Y. In vivo passive mechanical properties of skeletal muscle improve with massage-like loading following eccentric exercise. J Biomech 45: 2630–2636, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haas C, Butterfield TA, Abshire S, Zhao Y, Zhang X, Jarjoura D, Best TM. Massage timing affects postexercise muscle recovery and inflammation in a rabbit model. Med Sci Sports Exerc 45: 1105–1112, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haas C, Butterfield TA, Zhao Y, Zhang X, Jarjoura D, Best TM. Dose-dependency of massage-like compressive loading on recovery of active muscle properties following eccentric exercise: Rabbit study with clinical relevance. Br J Sports Med 47: 83–88, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Herman PM, Poindexter BL, Witt CM, Eisenberg DM. Are complementary therapies and integrative care cost-effective?: A systematic review of economic evaluations. BMJ Open 2: e001046, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernandez-Reif M, Field T, Ironson G, Beutler J, Vera Y, Hurley J, Fletcher MA, Schanberg S, Kuhn C, Fraser M. Natural killer cells and lymphocytes increase in women with breast cancer following massage therapy. Int J Neurosci 115: 495–510, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Hernandez-Reif M, Ironson G, Field T, Hurley J, Katz G, Diego M, Weiss S, Fletcher MA, Schanberg S, Kuhn C, Burman I. Breast cancer patients have improved immune and neuroendocrine functions following massage therapy. J Psychosom Res 57: 45–52, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Huang da W, Sherman BT, Tan Q, Collins JR, Alvord WG, Roayaei J, Stephens R, Baseler MW, Lane HC, Lempicki RA. The DAVID Gene Functional Classification Tool: A novel biological module-centric algorithm to functionally analyze large gene lists. Genome Biol 8: R183, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ironson G, Field T, Scafidi F, Hashimoto M, Kumar M, Kumar A, Price A, Goncalves A, Burman I, Tetenman C, Patarca R, Fletcher MA. Massage therapy is associated with enhancement of the immune system's cytotoxic capacity. Int J Neurosci 84: 205–217, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Lapointe BM, Frenette J, Cote CH. Lengthening contraction-induced inflammation is linked to secondary damage but devoid of neutrophil invasion. J Appl Physiol 92: 1995–2004, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Lee M, Kovacs-Nolan J, Yang C, Archbold T, Fan MZ, Mine Y. Hen egg lysozyme attenuates inflammation and modulates local gene expression in a porcine model of dextran sodium sulfate (DSS)-induced colitis. J Agric Food Chem 57: 2233–2240, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Lu H, Huang D, Ransohoff RM, Zhou L. Acute skeletal muscle injury: CCL2 expression by both monocytes and injured muscle is required for repair. FASEB J 25: 3344–3355, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinez CO, McHale MJ, Wells JT, Ochoa O, Michalek JE, McManus LM, Shireman PK. Regulation of skeletal muscle regeneration by CCR2-activating chemokines is directly related to macrophage recruitment. Am J Physiol Regul Integr Comp Physiol 299: R832–R842, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McLoughlin TJ, Mylona E, Hornberger TA, Esser KA, Pizza FX. Inflammatory cells in rat skeletal muscle are elevated after electrically stimulated contractions. J Appl Physiol 94: 876–882, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Myklebust M, Iler J. Policy for therapeutic massage in an academic health center: A model for standard policy development. J Altern Complement Med 13: 471–475, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Pillon NJ, Bilan PJ, Fink LN, Klip A. Cross-talk between skeletal muscle and immune cells: Muscle-derived mediators and metabolic implications. Am J Physiol Endocrinol Metab 304: E453–E465, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Rapaport MH, Schettler P, Bresee C. A preliminary study of the effects of a single session of Swedish massage on hypothalamic-pituitary-adrenal and immune function in normal individuals. J Altern Complement Med 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rapaport MH, Schettler P, Bresee C. A preliminary study of the effects of repeated massage on hypothalamic-pituitary-adrenal and immune function in healthy individuals: A study of mechanisms of action and dosage. J Altern Complement Med 18: 789–797, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shachar I, Haran M. The secret second life of an innocent chaperone: The story of CD74 and B cell/chronic lymphocytic leukemia cell survival. Leuk Lymphoma 52: 1446–1454, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Shor-Posner G, Hernandez-Reif M, Miguez MJ, Fletcher M, Quintero N, Baez J, Perez-Then E, Soto S, Mendoza R, Castillo R, Zhang G. Impact of a massage therapy clinical trial on immune status in young Dominican children infected with HIV-1. J Altern Complement Med 12: 511–516, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Shor-Posner G, Miguez MJ, Hernandez-Reif M, Perez-Then E, Fletcher M. Massage treatment in HIV-1 infected Dominican children: A preliminary report on the efficacy of massage therapy to preserve the immune system in children without antiretroviral medication. J Altern Complement Med 10: 1093–1095, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Stout RD, Jiang C, Matta B, Tietzel I, Watkins SK, Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol 175: 342–349, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Stout RD, Suttles J. Functional plasticity of macrophages: Reversible adaptation to changing microenvironments. J Leukoc Biol 76: 509–513, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tidball JG. Inflammatory cell response to acute muscle injury. Med Sci Sports Exerc 27: 1022–1032, 1995 [DOI] [PubMed] [Google Scholar]
- 42.Tidball JG. Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol 288: R345–R353, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Tidball JG, Villalta SA. Regulatory interactions between muscle and the immune system during muscle regeneration. Am J Physiol Regul Integr Comp Physiol 298: R1173–R1187, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van den Berg TK, Dopp EA, Dijkstra CD. Rat macrophages: Membrane glycoproteins in differentiation and function. Immunol Rev 184: 45–57, 2001 [DOI] [PubMed] [Google Scholar]
- 45.van den Berg TK, Puklavec MJ, Barclay AN, Dijkstra CD. Monoclonal antibodies against rat leukocyte surface antigens. Immunol Rev 184: 109–116, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Volpe S, Cameroni E, Moepps B, Thelen S, Apuzzo T, Thelen M. CCR2 acts as scavenger for CCL2 during monocyte chemotaxis. PLos One 7: e37208, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: Mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol 10: 75–82, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Waters-Banker C, Dupont-Versteedgen EE, Kitzman PH, Butterfield TA. Investigating the mechanisms of massage efficacy: The role of mechanical immunomodulation. J Athl Train In press: 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng YX, Yang M, Rong TT, Yuan XL, Ma YH, Wang ZH, Shen LS, Cui L. CD74 and macrophage migration inhibitory factor as therapeutic targets in gastric cancer. World J Gastroenterol 18: 2253–2261, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou S. Chronic neural adaptations to unilateral exercise: Mechanisms of cross education. Exerc Sport Sci Rev 28: 177–184, 2000 [PubMed] [Google Scholar]