Abstract
Neuromuscular control relies on sensory feedback that influences responses to changing external demands, and the normal response is for movement and muscle activation patterns to adapt to repeated perturbations. People with knee osteoarthritis (OA) are known to have pain, quadriceps weakness, and neuromotor deficits that could affect adaption to external perturbations. The aim of this study was to analyze neuromotor adaptation during walking in people with knee OA (n = 38) and controls (n = 23). Disability, quadriceps strength, joint space width, malalignment, and proprioception were assessed. Kinematic and EMG data were collected during undisturbed walking and during perturbations that caused lateral translation of the foot at initial contact. Knee excursions and EMG magnitudes were analyzed. Subjects with OA walked with less knee motion and higher muscle activation and had greater pain, limitations in function, quadriceps weakness, and malalignment, but no difference was observed in proprioception. Both groups showed increased EMG and decreased knee motion in response to the first perturbation, followed by progressively decreased EMG activity and increased knee motion during midstance over the first five perturbations, but no group differences were observed. Over 30 trials, EMG levels returned to those of normal walking. The results illustrate that people with knee OA respond similarly to healthy individuals when exposed to challenging perturbations during functional weight-bearing activities despite structural, functional, and neuromotor impairments. Mechanisms underlying the adaptive response in people with knee OA need further study.
Keywords: proactive response, motor control, electromyographic, reactive response, afferent feedback
knee osteoarthritis (OA) affects 16% of adults >45 yr of age (38), with a majority of people with knee OA having significant disability (23).1 The medial compartment of the knee is most commonly affected by OA (12). Clinically, people with knee OA report significant pain, stiffness, and functional knee instability (FKI), all of which negatively impact activities of daily living (ADL) and quality of life (QOL) (13, 21, 68). The OA disease process involves morphological and compositional degeneration of all major knee tissues, including articular cartilage, meniscus, ligaments, subchondral/trabecular bone, and muscles (10, 46, 49, 53, 60). It has also been suggested that people with knee OA have deficits in afferent and efferent neural pathways demonstrated by decreases in proprioception, vibratory perception, muscle force control, and muscle strength (5, 6, 30, 34, 58, 71). Hence, interventions that focus on increasing muscle strength and those that aim at improving proprioception are included in the management of individuals with knee OA (7, 20, 48, 78, 82, 83).
The presence of pain, damage to joint structures, and afferent and efferent neural deficits could impair the ability of the neuromuscular system to sense and execute appropriate commands in response to external challenges to joint stability (31, 43). Interventions that rely on improving proprioception or improving neuromuscular control aim to do so by using error signals generated from external cues and clinician-controlled external perturbations to induce corrective reactions (3, 7, 19, 79). However, it is unknown whether people with knee OA, who have functional and structural impairments, are able to respond to external perturbations in a manner similar to people without knee symptoms or radiographic evidence of OA.
Ability to modify movement and muscle activation patterns is commonly assessed as a response to a series of external perturbations (24, 29, 52, 59, 62). Typical responses to perturbations include feedback (a.k.a. reactive) and feedforward (a.k.a. proactive) responses (37, 44, 52, 55, 57). Reactive responses occur during or shortly after a disturbance to restore balance, whereas proactive responses are those that occur prior to the onset of the disturbance and are thought to minimize the destabilization brought on by a disturbance (52, 55, 57). Proactive responses represent the ability of the nervous system to use sensory input to predict the effect of a disturbance and adjust the response accordingly (76). Nielsen and Sinkjaer (54) suggested that error signals (difference in anticipated and actual movement) generated from external perturbations during gait, when repeated, may constitute a substrate for motor learning. Hence, an analysis of short-term adaptation of muscle activations and movement patterns can provide insight into the ability of the nervous system in people with knee OA to integrate sensory input and produce appropriate reactive and proactive responses.
The aim of this study was to compare short-term adaptation in muscle activation and joint movement in response to repeated lateral perturbations during walking between people with and without radiographic and symptomatic knee OA. The operational definition of adaptation in the context of this study was an increase in knee motion and/or a decrease in the activation of muscles around the knee joint over repeated exposure of the perturbation. We hypothesized that people with radiographic and symptomatic knee OA would 1) show a diminished response in movement and muscle activation patterns compared with controls when exposed to the first novel perturbation, and 2) show less adaptation in movement and muscle activation patterns over repeated perturbations compared with controls.
EXPERIMENTAL PROCEDURES
Subjects
Thirty-eight individuals with diagnosed medial knee OA and 23 individuals without knee OA (Table 1) were referred from local physicians and recruited from communities in northern Delaware through newspaper advertisements. Standing semiflexed, posterior-anterior and sunrise view radiographs were taken of the more symptomatic knee in the OA subjects and in one knee of the control subject (side chosen at random). Participants in the OA group had Kellgren and Lawrence (K-L) grades of II or greater (39) in the medial tibiofemoral compartment, and K-L grade in the medial compartment was greater than that of the lateral compartment. If the participant had bilateral knee OA that fit the criteria, the more symptomatic knee was identified by the individual and used in the analysis. Participants were excluded if they had a history of other orthopedic injuries in the lower extremities (e.g., knee ligament injuries) or spine, used an assistive device, had a history of neurological injury, had a history of rheumatoid arthritis, were pregnant, or had undergone a joint replacement or skeletal realignment procedure in either lower extremity. The Institutional Review Board of the University of Delaware approved the research protocol, and all participants gave informed consent to participate.
Table 1.
Age, sex, BMI, and K-L grade in control and OA subjects
| Control | OA | P Value | |
|---|---|---|---|
| Age | 62.0 (10.5) | 66.6 (8.4) | 0.066 |
| BMI | 27.4 (5.3) | 29.7 (4.8) | 0.080 |
| Males/females | 12:11 | 16:21 | χ2 = 0.455, P = 0.500 |
| K-L 2 | NA | 15 (40%) | χ2 = 1.03, P = 0.60 |
| K-L 3 | NA | 12 (33%) | |
| K-L 4 | NA | 10 (27%) |
Values are means (SD).
BMI, body mass index; K-L, Kellgren and Lawrence grade; OA, osteoarthritis; NA, not applicable.
P values are from independent sample t-tests for age and BMI and from chi-square tests for sex and K-L distribution.
Assessment of Disability
Self-reported disability.
The Knee Injury and Osteoarthritis Outcome Score (KOOS), which is a self-reported measure of function that comprises five dimensions of knee function, i.e., pain, symptoms, ADL, sport and recreation function (sport), and knee-related QOL (47, 64), which were used to assess function. Each dimension is scored from 0 to 4, and then scores are transformed to a percentage score of 0 to 100, with 0 representing extreme knee problems and 100 representing no knee problems (64). The KOOS has been shown to be a valid, reliable, and responsive measurement of overall knee joint function in people with OA (47). For this study, KOOS subscales of symptoms, pain, and ADL were used.
Physical performance.
A timed stair-climbing test was used, where participants were timed with a stopwatch as they ascended and descended a set of 12 stairs (18 cm high). The participants were instructed to perform the task as quickly as they felt safe and comfortable. They were encouraged not to use the handrail but were not prohibited from doing so for safety. A longer time to complete the stair-climbing test represents worse functional limitations. Excellent test/retest reliability (Pearson r = 0.93) was reported for a similar stair-climbing task in people with knee OA (63).
Assessment of Functional Impairments
FKI.
FKI was assessed using the Knee Outcome Survey-Activities of Daily Living Scale (36) (KOS-ADLS). One question from the KOS-ADLS relating to functional stability of the knee has been shown to be a reliable measurement of self-reported knee instability in patients with knee OA (21). In this question, participants rated the severity of knee instability on a six-point scale in response to the question, “To what degree does giving way, buckling, or shifting of your knee affect your level of daily activity?”. A score of <4 indicated the presence of FKI, a score of 4 indicated FKI that does not impact daily activities, and a score of >4 indicated the absence of FKI.
Quadriceps strength.
Quadriceps femoris muscle strength was measured as the magnitude of the force output (in Newtons) during a maximal voluntary isometric contraction (MVIC) at 90° knee flexion on an isokinetic dynamometer (Kin Com Isokinetic International, Harrison, TN). Each participant practiced producing maximal quadriceps femoris muscle contractions against the dynamometer arm while verbal encouragement and visual feedback were provided to maximize volitional efforts. For the test, participants were asked to produce an MVIC of their quadriceps femoris muscle, and the highest trial with the greatest strength (highest force in Newtons) was used in the analysis. All strength data were normalized to the subject's BMI.
Proprioception.
Threshold to detect passive motion (TTDPM) was measured on a custom-built device (Fig. 1) with the subjects seated (75). The lower leg was secured in a pneumatic sleeve to minimize cutaneous cues, and headphones and blindfold were used to eliminate auditory and visual cues, respectively. TTDPM was tested at 15 and 45° from end of each subject's available knee extension range. The subjects were given three practice trials. In each recorded trial, the examiner tapped the subject on the shoulder to notify him/her that the device would start moving in the next 10 s. At a random interval within the 10 s, the device passively flexed or extended the lower leg of the subject at a velocity of 0.5°/s and an acceleration of 100°/s2. When the subject perceived the knee movement, they pressed a hand-held switch that disengaged the motor, and the degree of rotation was recorded. An average of three trials was taken. Previous studies using the proprioception-testing device have shown a test/retest reproducibility of 0.92 (75).
Fig. 1.
Device to measure proprioception.
Assessment of Structural Impairments
Medial joint space width.
Medial joint space width was measured on a posterior-anterior, weight-bearing, semiflexed radiograph as the narrowest distance between the femur and tibia (42).
Alignment.
Alignment was assessed using a standing, anterior-posterior radiograph in which the hip, knee, and ankle joints were visible. Alignment was determined by the angle (varus <180°, valgus >180°) of the mechanical axes of the femur and tibia (32).
The coefficient of variation for the radiographic measurements for the same rater was <3%.
Assessment of Response to Perturbations
Motion analysis.
Subjects walked at their self-selected speed over ground along a 13-m walkway. Kinematic data were collected at 120 Hz using a passive eight-camera system (VICON MX; Oxford Metrics, Oxford, UK). Joint centers of the lower limb were defined using 9.5 mm retroreflective markers placed bilaterally over the iliac crests, greater trochanters, lateral femoral condyles, lateral malleolus, and fifth metatarsal heads. Rigid thermoplastic shells affixed with four markers were attached to an elastic underwrap (SuperWrap; Fabrifoam, Exton, PA) surrounding the thigh and shank. Both shank and thigh shells were wrapped with Coban (St. Paul, MN) self-adherent wraps (3 M) to minimize movement (51). A marker triad placed on the sacrum and two additional markers on the heel counter of each subject's shoe along with the marker on the fifth metatarsal head were used to track pelvis and foot movement respectively. Inter- and intrarater reliability was established for marker placement in six young healthy subjects. The inter-class coefficients for both sagittal and frontal plane variables were >0.90.
Surface electromyography.
Muscle activity was recorded simultaneously at 1,080 Hz using a 16-channel system (MA300; Motion Lab Systems, Baton Rouge, LA). Preamplified surface electrodes (20-mm interelectrode distance, 12-mm disk diameter) were placed over the mid-muscle belly of the semitendinosis (MH), biceps femoris (LH), vastus medialis (MQ), vastus lateralis (LQ), and medial (MG) and lateral (LG) heads of the gastrocnemeii (14). electromyographic (EMG) signals during a MVIC and at rest were recorded for each muscle for use during postprocessing.
Disturbed walking paradigm.
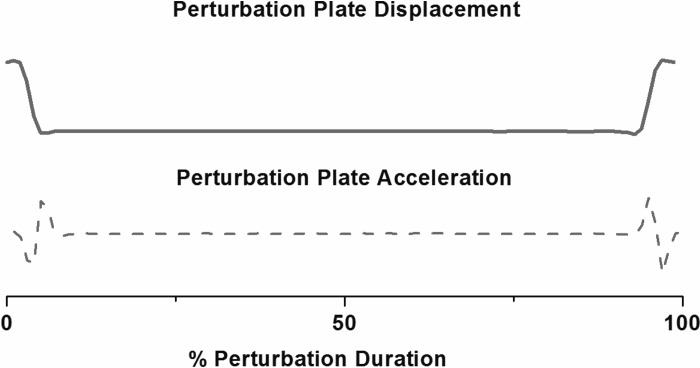
A custom-built, moveable platform (NSK, Tokyo, Japan) imbedded in the walkway was used to deliver the perturbations that consisted of a lateral translation of 5.8 cm at a speed of 40 cm/s in response to a signal from a switch mat mounted on the platform surface generated at initial contact (delay ≤10 ms; Fig. 2). The perturbation paradigm has been used in earlier studies from our group (44, 67). The paradigm is designed to challenge frontal plane stability in people with medial knee OA who are known to have abnormal frontal plane mechanics (2, 74). Data were collected during 10 trials in which the subject was aware that no movement would occur (“normal”). For safety, subjects were allowed to observe the perturbation, and if requested, they were allowed to perform one practice trial. No subject requested a practice trial. After 10 normal trials were collected the subjects were asked to continue walking at the same speed, and during one of these trials the platform would move, causing a perturbation (P). The trial number in which the platform first moved (P1) was randomized (between 1 and 5 trials). After the first perturbation trial (P1) was presented the subjects were informed that the platform would move during all subsequent trials, and five consecutive perturbation trials were collected. After initial inspection of the data, it appeared that adaptation continued beyond the fifth perturbed trial in some individuals. Therefore, to assess whether knee motion and muscle activity adapted to the extent that they were no different from level walking values, we collected data from a total of 30 consecutive perturbation trials in a subset of subjects (controls, n = 17; OA, n = 14).
Fig. 2.
Displacement and acceleration of the perturbation platform.
Data management.
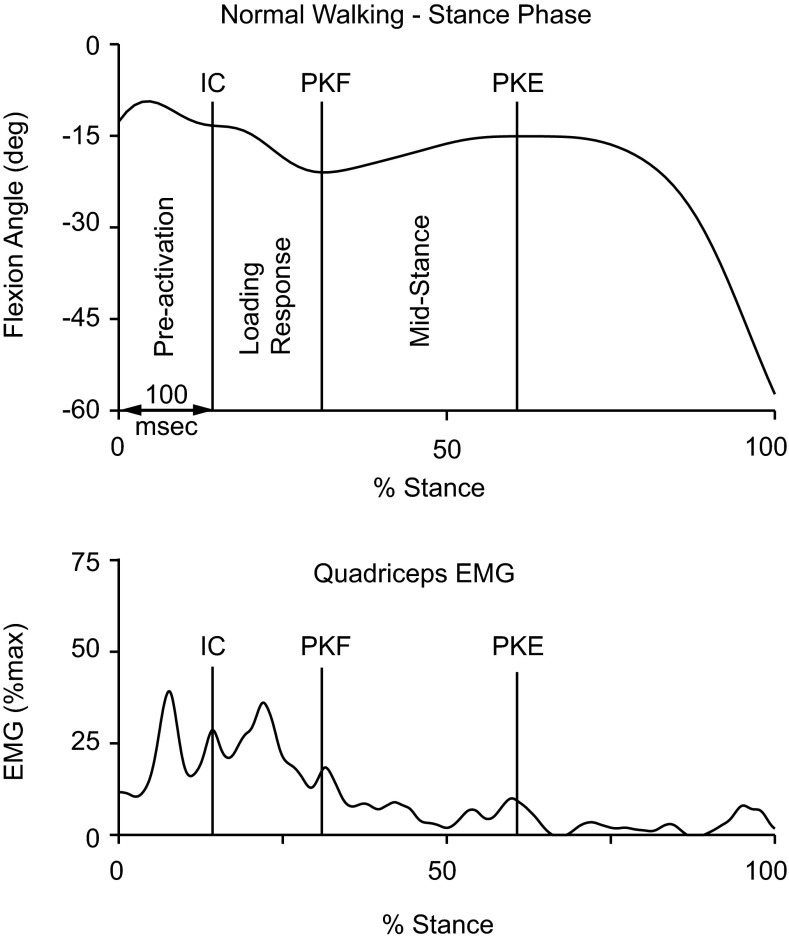
Marker trajectories were low-pass filtered (Butterworth 4th order, phase lag) with a cutoff frequency of 6 Hz using Visual 3D (C-Motion, Germantown, MD). Three-dimensional joint kinematics were calculated using rigid body analysis and Euler angles and referenced to the coordinate system from a standing posture. Sagittal plane knee angle variables included angle at initial contact and excursions over two intervals: loading response (from initial contact through peak knee flexion) and midstance (from peak knee flexion angle through peak knee extension) (Fig. 3).
Fig. 3.
Intervals of the stance phase used in the analysis. Knee flexion angle (top) and quadriceps electromyographic (EMG) activity (bottom). IC, initial contact; PKF, peak knee flexion; PKE, peak knee extension.
EMG data were high-pass filtered using a recursive fourth-order Butterworth filter with a cutoff of 20 Hz, and a full-wave rectified and linear envelope was created using a low-pass fourth-order recursive Butterworth filter with a cutoff of 20 Hz (Visual 3D; C-Motion). The level of resting EMG was subtracted from the linear envelope data from the active trials. The linear enveloped EMG data were then normalized to peak activity collected from a MVIC performed for each muscle group, so EMG data are reported as a percentage of the maximum. Linear envelope data were averaged over the following intervals: preactivation (100 ms prior to initial contact), loading response, and midstance (Fig. 3).
Statistical Analyses
Disability, functional impairments, and structural impairments.
Independent sample t-tests were used compare subject demographics, KOOS scores, stair-climbing test, quadriceps strength, TTDPM, medial joint space width, and alignment between the control and knee OA groups. Prevalence of FKI was compared across the groups using a chi-square test.
Proactive and reactive responses to first 5 perturbations.
A two-way mixed ANOVA was used to compare the kinematic (knee angle at initial contact, flexion excursion, extension excursion) and EMG (MQ, LQ, MH, LH, MG, and LG) responses during preactivation, loading response, and midstance phases. The anlyses were performed using a between-group factor (2 groups) and a within group repeated factor (6 trials). The two groups were controls and OA. The six trials were level walking (x̄ of 10 trials) and each of the first five perturbation trials (P1, P2… P5). Paired t-tests were used for post hoc comparisons of one trial to the next adjacent trial in each of the groups.
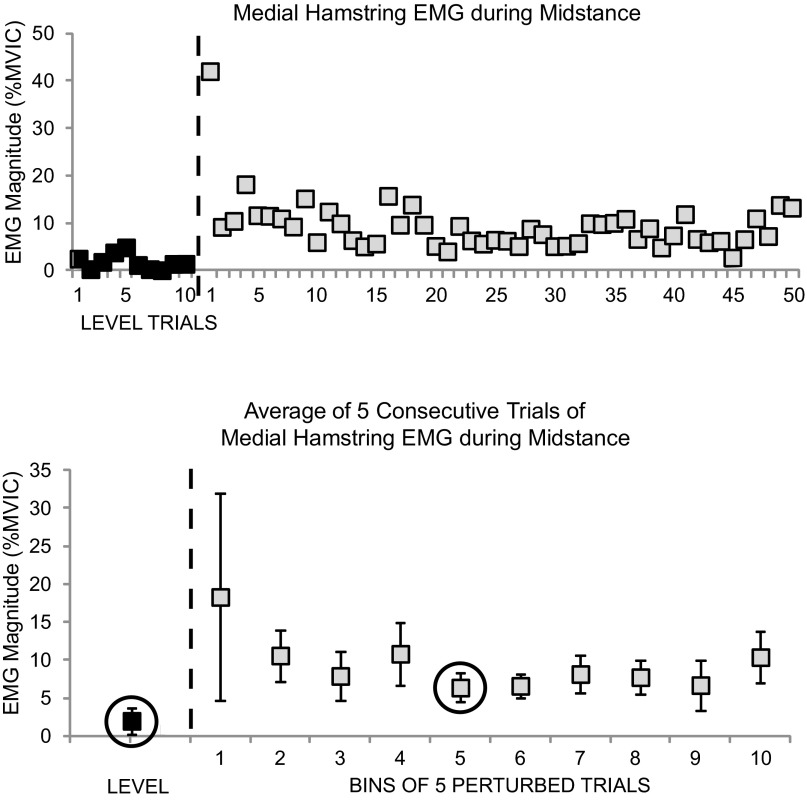
In the subset of individuals who completed 30 perturbation trials, exploratory analyses were done using a group by trial mixed ANOVA to compare the knee movement and muscle activity during level walking, with the amount of adaptation during the perturbations for the midstance phase only. The two groups were controls and OA. The two trial conditions were level walking (x̄ of 10 trials) and the “bin” of five trials with the lowest muscle activation or greater knee excursion (Fig. 4). The 30 trials were divided into bins of five trials, and mean extension excursion and activity of all muscles were calculated for each bin (Fig. 4). The greatest extension excursion and the lowest EMG activity in the bins were defined as the level of adaptation. The midstance phase was chosen because the time from initial contact to peak knee extension is within the duration of a long latency reflex, when adaptation is expected to occur.
Fig. 4.
Binning of average medial hamstring EMG data during the midstance interval from 1 subject to assess adaptation. Top: individual trials. Bottom: average of every 5 trials. ■, unperturbed trials 1–10; gray squares, perturbed trials 1–30. The black square inside a circle is that in which the maximum adaptation had occurred.
RESULTS
Disability, Functional Impairments, and Structural Impairments
The differences between control and OA subjects for age, BMI, and sex distribution (Table 1) were not significant (P > 0.05). The OA group had significantly higher reports of pain, knee-related symptoms, and difficulties with ADL (P < 0.001; Table 2). The OA group walked slower, took longer to complete the stair-climbing test, and had lower quadriceps strength and greater prevalence of symptomatic FKI (P <0.001–0.037). The differences in proprioception were not significant between OA and controls (P > 0.05). Subjects with knee OA had lesser medial joint space width and greater frontal plane varus (P < 0.001) compared with the control subjects.
Table 2.
Function, proprioception, structure, and strength variables in controls and OA subjects
| Control | OA | P Value | |
|---|---|---|---|
| KOOS | |||
| Symptoms | 98.9 (2.5) | 62.4 (14.6) | <0.001 |
| Pain | 99.5 (1.8) | 64.4 (14.9) | <0.001 |
| ADL | 99.7 (1.3) | 70.3 (17.0) | <0.001 |
| KOS-I* | |||
| <4 | 1 (4.3) | 15 (40%) | <0.001 |
| 4 | 0 (0) | 7 (18%) | |
| 5 | 22 (95.7) | 16 (42%) | |
| TTDPM (°) | |||
| At 15° flexion (into flexion) | 1.1 (1.1) | 1.1 (0.8) | 0.915 |
| At 15° flexion (into extension) | 0.8 (0.4) | 0.9 (0.5) | 0.355 |
| At 45° flexion (into flexion) | 0.9 (0.5) | 1.2 (0.7) | 0.107 |
| At 45° flexion (into extension) | 1.4 (0.9) | 1.8 (1.0) | 0.092 |
| Medial joint space width, mm | 4.3 (0.7) | 0.9 (1.5) | <0.001 |
| Alignment (°) | 178.5 (2.5) | 174.4 (3.8) | <0.001 |
| Stair climbing test, s | 10.0 (1.5) | 13.7 (5.1) | 0.001 |
| Walking speed, m/s | 1.6 (0.2) | 1.3 (0.2) | <0.001 |
| Quadriceps strength (N/BMI) | 25. 4 (10.1) | 20.9 (7.2) | 0.037 |
All values are means (SD), except for KOS-I (knee outcome survey/instability item score).
KOOS, Knee Injury and OA Outcome Score; ADL, activities of daily living; TTDPM, threshold to detect passive motion.
No. of participants (%).
Proactive and Reactive Responses to Perturbation
Preactivation.
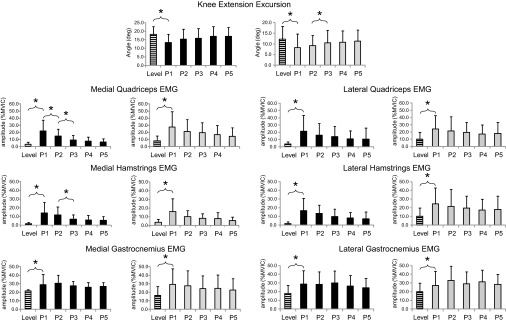
The data for knee angle at initial contact and muscle activity for all muscles are shown in Table 3. During the preactivation phase, prior to the foot contacting the platform, a main effect for trial type was observed. In P1, both groups demonstrated a similar pattern of greater knee flexion at initial contact and higher levels of muscle activity across all muscles (P ≤ 0.002). From P1 to P2, both groups increased their knee flexion at initial contact and showed an increase in muscle activity for LG (P ≤ 0.008). The activity for all other muscles did not show any further change after P1. Across all trials, the OA subjects maintained their knee in greater flexion at initial contact compared with the control group (P = 0.043).
Table 3.
Knee angle at initial contact and muscle activation for all muscles during the preactivation phase
| Variable | Level | P1 | P2 | P3 | P4 | P5 |
|---|---|---|---|---|---|---|
| Knee flexion at initial contact (°) | ||||||
| Control | 6.1 (4.3)* | 7.7 (6.2)* | 8.7 (5.9)* | 8.4 (6.3) | 8.0 (6.0) | 7.7 (6.0) |
| OA | 9.9 (6.8)* | 11.6 (7.1)* | 12.6 (6.9)* | 11.6 (7.3) | 11.9 (7.1) | 11.6 (7.4) |
| Medial quadriceps, %max | ||||||
| Control | 12.8 (8.2)* | 14.6 (11.6)* | 16.4 (10.7) | 14.6 (9.5) | 11.4 (8.1) | 12.6 (8.0) |
| OA | 14.6 (8.7)* | 18.3 (10.1)* | 18.5 (11.1) | 17.8 (9.3) | 18.5 (11.3) | 17.8 (10.5) |
| Lateral quadriceps, %max | ||||||
| Control | 15.0 (6.4)* | 17.6 (3.5)* | 18.6 (8.8) | 17.8 (10.6) | 16.3 (10.4) | 14.9 (9.7) |
| OA | 16.1 (8.3)* | 20.2 (10.8)* | 20.3 (11.7) | 21.9 (13.0) | 21.8 (12.0) | 20.0 (10.6) |
| Medial hamstrings, %max | ||||||
| Control | 16.3 (9.5)* | 21.4 (14.4)* | 23.2 (14.1) | 25.1 (14.6) | 23.9 (14.9) | 21.8 (12.2) |
| OA | 18.1 (12.2)* | 25.7 (22.5)* | 25.1 (15.1) | 27.1 (19.7) | 14.1 (14.7) | 25.0 (14.7) |
| Lateral hamstrings, %max | ||||||
| Control | 16.6 (6.7)* | 19.4 (10.8)* | 23.7 (14.6) | 23.9 (13.1) | 23.0 (12.0) | 20.7 (10.5) |
| OA | 21.5 (8.7)* | 28.2 (14.9)* | 27.5 (11.1) | 28.1 (104) | 26.8 (11.) | 26.6 (10.4) |
| Medial gastrocnemius, %max | ||||||
| Control | 2.4 (2.2)* | 4.2 (6.1)* | 6.0 (7.4) | 7.3 (6.7) | 6.6 (6.3) | 6.4 (6.8) |
| OA | 4.8 (5.9)* | 9.2 (11.5)* | 10.1 (10.4) | 9.9 (9.8) | 10.0 (9.9) | 9.2 (9.8) |
| Lateral gastrocnemius, %max | ||||||
| Control | 6.8 (12.3)* | 9.6 (18.5)* | 13.0 (17.6)* | 14.8 (19.0) | 12.2 (15.0) | 12.5 (14.5) |
| OA | 6.4 (8.0)* | 11.3 (13.3)* | 13.7 (12.8)* | 13.3 (15.3) | 13.0 (12.8) | 11.3 (11.5) |
Average of 10 unperturbed trials (level) and the first 5 perturbation trials (P1–P5). %Max, percentage of the maximum.
Statistically significant difference between adjacent trials (P < 0.05).
Loading response.
The data for knee flexion excursion and muscle activity for all muscles are shown in Table 4. During the loading response phase, as the limb accepts weight, a main effect for trial type was observed. In P1, both groups demonstrated a similar pattern of lesser knee flexion excursion during loading response and higher levels of muscle activity across all muscles (P ≤ 0.006). From P1 to P2, both groups showed a further decrease in their knee flexion excursion and increase in the activity in MH and LH muscles (P ≤ 0.029). There were no changes in the flexion excursion or muscle activity after P2. Across all trials, the OA group had less flexion excursion and higher LH activation during loading response (P ≤ 0.005).
Table 4.
Flexion excursion and muscle activation for all muscles during the loading response
| Variable | Level | P1 | P2 | P3 | P4 | P5 |
|---|---|---|---|---|---|---|
| Flexion excursion (°) | ||||||
| Control | 15.0 (2.7)* | 12.3 (4.1)* | 11.2 (3.9)* | 11.4 (4.4) | 12.2 (3.9) | 11.8 (3.6) |
| OA | 10.0 (3.8)* | 7.8 (3.8)* | 6.9 (3.5)* | 7.9 (3.9) | 8.0 (4.0) | 8.3 (3.4) |
| Medial quadriceps, %max | ||||||
| Control | 26.7 (15.8)* | 27.9 (19.6)* | 28.8 (17.1) | 30.4 (21.3) | 28.4 (17.6) | 28.0 (19.6) |
| OA | 30.4 (16.2)* | 37.7 (21.4)* | 39.3 (21.3) | 39.9 (26.6) | 37.5 (22.2) | 35.7 (21.9) |
| Lateral quadriceps, %max | ||||||
| Control | 26.7 (11.3)* | 32.7 (26.2)* | 28.3 (14.5) | 30.9 (18.2) | 30.8 (18.6) | 29.0 (17.5) |
| OA | 32.7 (14.0)* | 38.9 (19.4)* | 40.0 (23.3) | 40.4 (25.4) | 41.2 (22.3) | 40.7 (22.6) |
| Medial hamstrings, %max | ||||||
| Control | 6.8 (5.7)* | 12.2 (10.0)* | 17.9 (15.2)* | 20.9 (17.5) | 19.4 (15.0) | 18.2 (14.7) |
| OA | 11.0 (11.4*) | 18.8 (24.7)* | 22.0 (19.5)* | 23.7 (30.1) | 23.6 (28.1) | 22.0 (20.5) |
| Lateral hamstrings, %max | ||||||
| Control | 10.2 (7.9)* | 13.6 (12.0)* | 16.7 (12.6)* | 18.2 (12.4) | 18.7 (12.2) | 18.9 (14.2) |
| OA | 20.0 (12.1)* | 23.9 (15.2)* | 28.6 (14.9)* | 28.0 (13.9) | 28.7 (12.6) | 27.5 (15.4) |
| Medial gastrocnemius, %max | ||||||
| Control | 4.4 (3.7)* | 6.1 (5.7)* | 6.5 (5.9) | 7.5 (6.4) | 6.3 (5.4) | 4.7 (4.1) |
| OA | 9.4 (13.0)* | 10.7 (17.4)* | 11.9 (15.7) | 10.9 (13.7) | 11.0 (12.7) | 9.5 (13.5) |
| Lateral gastrocnemius, %max | ||||||
| Control | 8.2 (7.2)* | 10.4 (9.8)* | 11.1 (11.0) | 11.8 (12.2) | 11.8 (12.4) | 10.6 (11.2) |
| OA | 11.4 (10.2)* | 12.5 (12.1)* | 14.8 (12.4) | 14.8 (15.2) | 14.4 (14.2) | 12.4 (9.7) |
Average of 10 unperturbed trials (level) and the first 5 perturbation trials (P1–P5).
Statistically significant difference between adjacent trials (P < 0.05).
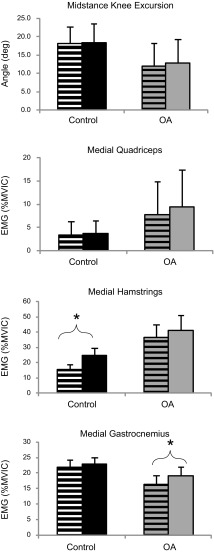
Midstance.
The data for knee extension excursion and muscle activity for all muscles are shown in Fig. 5. During the midstance phase as the stance knee extends, a main effect for trial type was observed for all variables (P < 0.001). In P1, subjects in both groups showed a reduction in the extension excursion and an increase in the activity of all muscles. Thereafter, the extension excursion increased from P1 to P2 (P < 0.001), and activity of MQ, LQ, MH, and LH showed a decrease from P1 to P2 and from P2 to P3 (P ≤ 0.023). Activity of MQ further decreased from P3 to P5 (P ≤ 0.034), and activity of MH also decreased from P4 to P5 (P = 0.011). Activity of MG decreased from P2 to P3 (P = 0.023) and did not change thereafter. Activity of LG did not change from P1 to P2 but showed a decrease from P3 to P5 (P ≤ 0.048). Across all trials, subjects with knee OA had smaller extension excursion and greater activation in MQ, LQ, and LH muscles (P ≤ 0.003).
Fig. 5.
Knee flexion (top) and muscle activation (bottom 3 graphs) during the midstance. Average of 10 unperturbed trials (level) and first 5 perturbation trials (P1–P5). *Statistically significant difference (P < 0.05).
Neuromuscular adaptation.
The data for knee extension excursion and muscle activity for MQ, MH, and MG for level walking and the greatest knee excursion or lowest level of EMG are shown in Fig. 6. Both groups showed an increase in knee extension excursion and decreased magnitude of muscle activation over the 30 consecutive perturbation trials. No statistical differences were observed between the extension excursion during level walking and the greatest extension excursion during the 30 trials or the activation of MQ, LQ, MH, and LH during level walking and the lowest EMG magnitude during the 30 perturbation trials, indicating that muscle responses and knee excursions had adapted to baseline levels. The magnitude of MG and LG activity in the 30 trials stayed greater than that of level walking in both groups (P ≤ 0.023).
Fig. 6.
Knee flexion and muscle activation during the midstance during level walking and after maximum adaptation had occurred. *Statistically significant difference (P < 0.05). OA, osteoarthritis.
DISCUSSION
The aim of this study was to compare short-term adaptation in muscle activation and joint movement in response to repeated lateral perturbations during walking between individuals with medial knee OA who present with significant functional and structural impairments and healthy asymptomatic controls. The hypotheses was that, compared with the control subjects, people with medial knee OA would exhibit a reduced ability to adapt their movement and muscle activation patterns due to structural, functional, and neuromotor impairments related to the OA disease process. However, our hypotheses were not supported by the data, with both groups demonstrating similar proactive and reactive responses to perturbations that challenged knee stability. The OA group had significant structural and functional impairments compared with the control group. Furthermore, the OA group also demonstrated patterns of higher muscle activation and less knee motion that have been shown in multiple earlier studies (33, 44, 66, 67), indicating that the OA cohort in this study had neuromuscular impairments, although we did not observe a difference in joint proprioception. The findings from this study show that people with knee OA use strategies that lead to neuromotor responses similar to that seen in healthy control subjects, when exposed to challenging perturbations. However, mechanisms underlying the adaptive response in people with knee OA need further study. In our paradigm, input was available from multiple lower extremity afferents, including muscle spindles, skin, and other lower extremity joints in people with significant knee OA-related impairments. Furthermore, the techniques used in our study may have limited sensitivity to detecting proprioceptive deficits (61). This redundant input may allow the generation of appropriate response to external challenges to stability while walking. Hence, the often-reported deficits in knee proprioception in people with knee OA, measured under carefully controlled conditions, may not be critical toward maintaining joint stability during daily activities. However, magnitudes and directions of perturbations other than those used in our study may yield different results. Future studies should also take into consideration the limited sensitivity of commonly used tests of proprioception and the role of muscle spindles in movement and position sense.
Disability, Functional and Structural Impairments, and Walking Patterns
Subjects in our OA group had significantly greater pain and self-reported and physical limitations compared with the control group, as demonstrated by lower KOOS scores, greater time taken to complete the stair-climbing test, and slower walking speed. The OA group also had greater quadriceps weakness, varus malalignment, and FKI. Pain at the knee has been shown to be associated with quadriceps inhibition (4, 70), but none of our subjects reported pain during maximal quadriceps strength assessment. Hence, the quadriceps strength deficits in the OA group may be more related to other mechanisms like loss of cross-sectional area (35) and change in muscle fiber type (18). The smaller medial joint space and greater frontal plane varus suggest that there was significant damage to the knee tissues in the OA group. It has been shown that varus malalignment is a reliable marker of OA incidence and progression with greater malalignment associated with greater medial cartilage loss, meniscal damage and extrusion, and greater bone marrow edema-like lesions (25, 72, 73).
We also observed less knee motion and higher muscle activation in people with knee OA compared with controls during walking. These findings have also been reported earlier in multiple studies (33, 44, 66, 67) and indicate that the cohort of knee OA subjects did have neuromotor impairments. Similar responses to perturbation in both groups, despite the profound differences in structure, function, and walking patterns, could be due to 1) the redundancy in afferent input from lower extremities, 2) the perturbation being of insufficient magnitude to challenge knee stability, or 3) other compensatory strategies. Since the perturbation was applied at the foot, it is quite likely that afferent information from multiple structures, including the sole of the foot, ankle and foot muscles, and ankle and foot joint receptors, would be available to the nervous system. None of our subjects had pain in other joints of the lower extremity, and hence, we could assume a normal afferent input from these structures. It is possible that the information from these structures was sufficient for the nervous system to generate adequate responses to these perturbations even in people with significant knee OA. Hence, the deficits in knee proprioception that have been reported in earlier studies may not impair the ability of people with knee OA to respond to external challenges to stability under functional weight-bearing conditions. It is less likely that the perturbation was of insufficient magnitude to challenge knee stability. Using the same perturbation paradigm, earlier we observed greater medial muscle cocontraction in people with knee OA compared with controls and in people with knee OA who have FKI compared with those who do not during standing and walking (44, 67). Hence, further work is needed to understand the strategies underlying the adaptive response in people with knee OA, perhaps with different directions and magnitudes of perturbations.
Although we observed differences in movement and muscle activation patterns, we did not see a difference in proprioception as assessed using the TTDPM technique between the OA and control groups. It has been suggested recently that the proprioception tests that are commonly used, including TTDPM, may not be sensitive to detect threshold of movement onset since they only assess that a movement occurred (61). Muscle spindles have been shown to be the primary afferent organ for movement and position sense detection, and muscle spindle discharge can vary depending on the length and history of muscle activity prior to propriception testing (61). It has been recommended that in tests of proprioception under relaxed conditions, like those used in our study, the participants should be asked to isometrically contract the agonist muscles at the joint angle at which the test is being performed to counter any thixotropic effects that may be present in the muscle or the spindles (61). The subjects in our study did not perform the isometric contraction, and hence, the findings may be affected by muscle thixotropy. Furthermore, these tests of proprioception also rely on memory, mood, motivation, and reaction time of the participants (71, 77). Hence, other tests, including vibratory perception, have been recommended, which partly overcome some of these limitations (71, 77). In fact, a recent review recommended that a new protocol for measurement of knee proprioception in people with knee OA is needed (40). Earlier studies that found differences in TTDPM between subjects with knee OA report large effect sizes between 0.47 and 2.7 (50, 58). Using these effect sizes, an α-level of 0.05, and a power of 80%, we had a sufficient sample size to detect differences. However, for one of the comparisons the P value was 0.092, suggesting that issues related to sample size may be present. Finally, the importance of proprioceptive deficits in knee OA has received some scrutiny, with large-scale longitudinal studies reporting weak or no associations between proprioceptive deficits and onset of symptomatic or radiographic knee OA or development of adverse OA outcomes (17, 69). The findings from our work also suggest that during functional weight-bearing activities, the redundancy in afferent feedback may be sufficient to allow adequate neuromotor responses.
Response to First Perturbation
The timing of the first perturbation trial was unknown to all of the subjects, and subjects in both the OA and control groups exhibited similar responses. Subjects in both groups responded to the first novel perturbation with a decrease in knee motion during loading response and midstance phases accompanied by an increase in muscle activity of all muscles studied. This first response (a.k.a. a “startle-like” response) is comparable with the responses elicited by a sudden high amplitude auditory stimulus (55, 56) and is characterized by cocontraction of muscles. The elevated EMG activity in conjunction with truncated knee motion illustrates a knee stiffening or “freezing” strategy (55) that may be an attempt to maintain knee stability.
Increased knee flexion and higher muscle activity observed during the preactivation phase were unexpected since subjects were unaware of when the first perturbation would take place (8, 26). This unexpected finding could have occurred because for safety reasons we allowed the subjects to observe the platform translate, and although they did not know when the first perturbation would occur, they may have been sufficiently unsure of the experience that their muscles were more active in anticipation of P1. Such a response has been reported previously (22, 45) and could lead to better stability under uncertain and challenging conditions.
Response to Repeated Perturbations
All subjects in the study showed an increase in knee motion and a reduction in muscle activity of quadriceps and hamstring muscles during the midstance phase on repeated exposure to the perturbations. Prior experience of a perturbation leads to the generation of proactive responses that works in conjunction with reactive response to maintain postural stability (52, 57). Adaptation is characterized by decrease in the reactive response to perturbations (57). Reactive response consists of short- and long-latency responses, and it has also been shown that the long-latency reflexes show the greatest habituation by a decrease in magnitude (24, 56, 65). For our subjects, the midstance phase likely corresponded with the interval of time when the long-latency reflex (>90 ms) occurs, and hence, adaptation was observed primarily in this phase. Although changes in reactive responses that occur in the time frame of a long-latency reflex may be too slow to affect dynamic stability directly, adaptation in the long-latency reflexes represents the influence of proactive responses that occur when the motor system predicts future motion based on past experiences (80). Classen et al. (9) proposed that short-term adaptation is the first step in skill acquisition, which would bode well for people with OA who may need to learn to stabilize their knees after joint structures become damaged.
It was interesting to see that the short-term adaptation was seen only during midstance phase, a point in the gait cycle when the second peak of external knee adduction moment (KAM) occurs. However, higher articular loads at the knee during loading response at first peak of KAM (11, 27, 41) that occurs during the loading response phase of gait are the hallmark of walking patterns in people with knee OA. No adaptation was observed during the loading response, and we did not observe an adaptive response with all of the subjects, which is most likely due to the loading response being a phase in which only short- and medium-latency reflexes, which usually do not show adaptation, are generated (24, 56).
The attenuation of the EMG during midstance was most pronounced in the quadriceps and hamstring muscles, whereas the gastrocnemius muscles showed less attenuation, and the magnitude was higher even after 30 trials. This finding is consistent with those of Nieuwenhuijzen Duysens, who found that sensorimotor adaptation is less in muscles, with special significance to the perturbation (55). In the current study the perturbation was applied at the foot-floor interface, so a sudden displacement of the foot is likely to elicit activation of muscles around the ankle joint to provide a more stable ankle, and adaptation could have decreased postural stability (28). The application of the perturbation at the foot-floor interface may be considered a limitation to this study since the perturbation may not have resulted in destabilization of the knee; however, the adaptation that was seen in the muscles that cross the knee and not the ankle suggest that the paradigm was appropriate for determining whether neuromuscular adaptation was different in OA subjects compared with controls.
Studies investigating the response to postural perturbations in individuals with knee OA are scarce. Earlier work from our group has found that, using the same perturbation paradigm as this study, people with medial knee OA generate higher medial muscle cocontraction during standing compared with controls, and people with medial knee OA who have FKI generate higher medial muscle cocontraction during walking compared with those without FKI (44, 67). However, the adaptive response to repeated perturbations was not investigated in these studies. Results from the current work build upon this earlier work and demonstrate that despite these differences in movement patterns, people with knee OA show similar decreases in response as controls if the perturbations are repeated. Using a knee-buckling paradigm in a unilateral stance, Irwin et al. (37) found no difference in the onset latencies of vastus lateralis or biceps femoris between people with knee OA and old or young adults. They recommended future studies to focus on muscle amplitudes instead of latencies, as has been done in the current study. Finally, Fallah-Yakhdani et al. (16) and Yakhdani et al. (81) assessed dynamic stability and variability during treadmill walking in subjects before and after total knee arthroplasty. They reported less variability in the affected extremity of OA subjects, which was associated with reduced fall risk. Furthermore, the OA subjects had greater cocontraction, and the affected extremity was more stable than the unaffected extremity. Our findings of less motion and greater muscle activation across all trials likely support the phenomenon of less variability. However, the changes in variability across repeated perturbations need further study.
Conclusions and Clinical Implications
The results from this study show that individuals with knee OA demonstrate similar responses to perturbations during walking as those without knee OA. Exercise programs focusing on joint stability and proprioception are becoming more popular in the rehabilitation of people with knee injuries (1, 15, 19). So-called “proprioceptive training” or “neuromuscular training” is purported to address knee control or alter walking patterns to lower knee contact loads, and they often involve activities that challenge knee stability in a safe and controlled manner (3, 7, 79). The role of diminished knee proprioception, if present in people with knee OA, toward maintaining knee stability during weight-bearing activities is questionable due to redundancy of afferent input. However, if these perturbation training-based programs are successful at altering movement patterns that can reduce articular loading during walking, they may have utility toward slowing structural progression of knee OA (7). Results from this study show that people with medial knee OA demonstrate changes in muscle activation and movement patterns when exposed to perturbations, but future studies would need to be done to investigate whether specific changes can be targeted and retained over a long period of time.
The results from this study need to be interpreted in light of certain limitations. The techniques used to assess proprioception may have had limited sensitivity, as discussed above. However, these techniques have been used in earlier studies in subjects with knee OA, allowing us to compare our findings with published literature. The perturbation paradigm used allowed subjects to continue walking after experiencing the perturbation to the end of the walkway and then back to the starting position. This period could have induced some “washout” of the adaptive response resulting from the perturbation. However, had subjects been able to experience the perturbation in consecutive strides, we speculate that the magnitude and rate of habituation may actually have been higher than observed here. Also, it is likely that the perturbation used, although appropriate to analyze reactive and proactive responses, was not of sufficient magnitude to elicit differential responses between groups. Finally, we did not adjust for multiple comparisons in the between-group analyses, and hence, P values close to 0.05 should be interpreted with caution.
In conclusion, the results from this study provide indirect evidence that the manner in which the nervous system processes sensory information in people with knee OA is similar to that in healthy control subjects. To our knowledge, this is the first study to demonstrate similar responses to repeated perturbations in people with symptomatic and radiographic knee OA and controls. The subjects with knee OA had significantly worse structure and function and differences in walking patterns compared with the control subjects but still showed similar adaptive response. Hence, compensatory strategies may be sufficient to allow people with knee OA to maintain stability when challenged during walking. However, the mechanisms underlying these responses will need further study.
GRANTS
Funding was provided by an International Society of Biomechanics Doctoral Dissertation Grant, an American College of Rheumatology-Research and Education Foundation Health Professional Graduate Student Preceptorship, a University of Delaware Graduate Fellowship, and National Institutes of Health Grants 1-P20-RR-016458-01 and 1-P20-RR-016458-06.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.K., C.B.S., D.S.R., and K.S.R. contributed to the conception and design of the research; D.K. performed the experiments; D.K., C.B.S., D.S.R., and K.S.R. analyzed the data; D.K., C.B.S., D.S.R., and K.S.R. interpreted the results of the experiments; D.K. and K.S.R. prepared the figures; D.K., C.B.S., D.S.R., and K.S.R. drafted the manuscript; D.K., C.B.S., D.S.R., and K.S.R. edited and revised the manuscript; D.K., C.B.S., D.S.R., and K.S.R. approved the final version of the manuscript.
ACKNOWLEDGMENTS
Currently. D. Kumar is affiliated with the Muscoloskeletal Quantitative Imaging Research Group, Department of Radiology, University of California, San Francisco, CA. Currently, K. S. Rudolph is affiliated with the Department of Physical Therapy, University of New England, Portland, ME.
REFERENCES
- 1.Ageberg E, Link A, Roos EM. Feasibility of neuromuscular training in patients with severe hip or knee OA: the individualized goal-based NEMEX-TJR training program. BMC Musculoskelet Disord 11: 126, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andriacchi TP, Mundermann A. The role of ambulatory mechanics in the initiation and progression of knee osteoarthritis. Curr Opin Rheumatol 18: 514–518, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Arampatzis A, Peper A, Bierbaum S. Exercise of mechanisms for dynamic stability control increases stability performance in the elderly. J Biomech 44: 52–58, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Arvidsson I, Eriksson E, Knutsson E, Arner S. Reduction of pain inhibition on voluntary muscle activation by epidural analgesia. Orthopedics 9: 1415–1419, 1986 [DOI] [PubMed] [Google Scholar]
- 5.Barrack RL, Skinner HB, Cook SD, Haddad RJ., Jr Effect of articular disease and total knee arthroplasty on knee joint-position sense. J Neurophysiol 50: 684–687, 1983 [DOI] [PubMed] [Google Scholar]
- 6.Barrett DS, Cobb AG, Bentley G. Joint proprioception in normal, osteoarthritic and replaced knees. J Bone Joint Surg Br 73: 53–56, 1991 [DOI] [PubMed] [Google Scholar]
- 6a.Barry BK, Sturnieks DL. How important are perturbation responses and joint proprioception to knee osteoarthritis? J Appl Physiol; 10.1152/japplphysiol.01207.2013 [DOI] [PubMed] [Google Scholar]
- 7.Bennell KL, Egerton T, Wrigley TV, Hodges PW, Hunt M, Roos EM, Kyriakides M, Metcalf B, Forbes A, Ageberg E, Hinman RS. Comparison of neuromuscular and quadriceps strengthening exercise in the treatment of varus malaligned knees with medial knee osteoarthritis: a randomised controlled trial protocol. BMC Musculoskelet Disord 12: 276, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatt T, Pai YC. Immediate and latent interlimb transfer of gait stability adaptation following repeated exposure to slips. J Mot Behav 40: 380–390, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Classen J, Liepert J, Hallett M, Cohen L. Plasticity of movement representation in the human motor cortex. Electroencephalogr Clin Neurophysiol Suppl 51: 162–173, 1999 [PubMed] [Google Scholar]
- 10.Conaghan PG, Felson D, Gold G, Lohmander S, Totterman S, Altman R. MRI and non-cartilaginous structures in knee osteoarthritis. Osteoarthritis Cartilage 14, Suppl A: A87–A94, 2006 [DOI] [PubMed] [Google Scholar]
- 11.D'Lima DD, Patil S, Steklov N, Slamin JE, Colwell CW., Jr The Chitranjan Ranawat Award: in vivo knee forces after total knee arthroplasty. Clin Orthop Relat Res 440: 45–49, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Dearborn JT, Eakin CL, Skinner HB. Medial compartment arthrosis of the knee. Am J Orthop 25: 18–26, 1996 [PubMed] [Google Scholar]
- 13.Dekker J, van Dijk GM, Veenhof C. Risk factors for functional decline in osteoarthritis of the hip or knee. Curr Opin Rheumatol 21: 520–524, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Delagi EI, Perotto A. Anatomic Guide for the Electromyographer, The Limbs. Springfield, IL: Charles C. Thomas, 1981 [Google Scholar]
- 15.Diracoglu D, Aydin R, Baskent A, Celik A. Effects of kinesthesia and balance exercises in knee osteoarthritis. J Clin Rheumatol 11: 303–310, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Fallah-Yakhdani HR, Abbasi-Bafghi H, Meijer OG, Bruijn SM, van den Dikkenberg N, Benedetti MG, van Dieën JH. Determinants of co-contraction during walking before and after arthroplasty for knee osteoarthritis. Clin Biomech (Bristol, Avon) 27: 485–494, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Felson DT, Gross KD, Nevitt MC, Yang M, Lane NE, Torner JC, Lewis CE, Hurley MV. The effects of impaired joint position sense on the development and progression of pain and structural damage in knee osteoarthritis. Arthritis Rheum 61: 1070–1076, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fink B, Egl M, Singer J, Fuerst M, Bubenheim M, Neuen-Jacob E. Morphologic changes in the vastus medialis muscle in patients with osteoarthritis of the knee. Arthritis Rheum 56: 3626–3633, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Fitzgerald GK, Childs JD, Ridge TM, Irrgang JJ. Agility and perturbation training for a physically active individual with knee osteoarthritis. Phys Ther 82: 372–382, 2002 [PubMed] [Google Scholar]
- 20.Fitzgerald GK, Piva SR, Gil AB, Wisniewski SR, Oddis CV, Irrgang JJ. Agility and perturbation training techniques in exercise therapy for reducing pain and improving function in people with knee osteoarthritis: a randomized clinical trial. Phys Ther 91: 452–469, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fitzgerald GK, Piva SR, Irrgang JJ. Reports of joint instability in knee osteoarthritis: its prevalence and relationship to physical function. Arthritis Rheum 51: 941–946, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Gruneberg C, Nieuwenhuijzen PH, Duysens J. Reflex responses in the lower leg following landing impact on an inverting and non-inverting platform. J Physiol 550: 985–993, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guccione AA, Felson DT, Anderson JJ, Anthony JM, Zhang Y, Wilson PW, Kelly-Hayes M, Wolf PA, Kreger BE, Kannel WB. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health 84: 351–358, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen PD, Woollacott MH, Debu B. Postural responses to changing task conditions. Exp Brain Res 73: 627–636, 1988 [DOI] [PubMed] [Google Scholar]
- 25.Hayashi D, Englund M, Roemer FW, Niu J, Sharma L, Felson DT, Crema MD, Marra MD, Segal NA, Lewis CE, Nevitt MC, Guermazi A. Knee malalignment is associated with an increased risk for incident and enlarging bone marrow lesions in the more loaded compartments: the MOST study. Osteoarthritis Cartilage 20: 1227–1233, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heiden TL, Sanderson DJ, Inglis JT, Siegmund GP. Adaptations to normal human gait on potentially slippery surfaces: the effects of awareness and prior slip experience. Gait Posture 24: 237–246, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Heinlein B, Kutzner I, Graichen F, Bender A, Rohlmann A, Halder AM, Beier A, Bergmann G. ESB Clinical Biomechanics Award 2008: Complete data of total knee replacement loading for level walking and stair climbing measured in vivo with a follow-up of 6–10 months. Clin Biomech (Bristol, Avon) 24: 315–326, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Hof AL, Elzinga H, Grimmius W, Halbertsma JP. Detection of non-standard EMG profiles in walking. Gait Posture 21: 171–177, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Horstmann GA, Gollhofer A, Dietz V. Reproducibility and adaptation of the EMG responses of the lower leg following perturbations of upright stance. Electroencephalogr Clin Neurophysiol 70: 447–452, 1988 [DOI] [PubMed] [Google Scholar]
- 30.Hortobagyi T, Garry J, Holbert D, Devita P. Aberrations in the control of quadriceps muscle force in patients with knee osteoarthritis. Arthritis Rheum 51: 562–569, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Hsieh RL, Lee WC, Lo MT, Liao WC. Postural stability in patients with knee osteoarthritis: comparison with controls and evaluation of relationships between postural stability scores and international classification of functioning, disability and health components. Arch Phys Med Rehabil 94: 340–346, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Hsu RW, Himeno S, Coventry MB, Chao EY. Normal axial alignment of the lower extremity and load-bearing distribution at the knee. Clin Orthop Relat Res 215–227, 1990 [PubMed] [Google Scholar]
- 33.Hubley-Kozey C, Deluzio K, Dunbar M. Muscle co-activation patterns during walking in those with severe knee osteoarthritis. Clin Biomech (Bristol, Avon) 23: 71–80, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Hurley MV, Scott DL, Rees J, Newham DJ. Sensorimotor changes and functional performance in patients with knee osteoarthritis. Ann Rheum Dis 56: 641–648, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ikeda S, Tsumura H, Torisu T. Age-related quadriceps-dominant muscle atrophy and incident radiographic knee osteoarthritis. J Orthop Sci 10: 121–126, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Irrgang JJ, Snyder-Mackler L, Wainner RS, Fu FH, Harner CD. Development of a patient-reported measure of function of the knee. J Bone Joint Surg Am 80: 1132–1145, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Irwin KE, Wening JD, Bhatt T, Pai YC. Does knee osteoarthritis alter the neuromuscular responses to a perturbation during single lower limb stance? J Geriatr Phys Ther 28: 93–101, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Jordan JM, Helmick CG, Renner JB, Luta G, Dragomir AD, Woodard J, Fang F, Schwartz TA, Abbate LM, Callahan LF, Kalsbeek WD, Hochberg MC. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol 34: 172–180, 2007 [PubMed] [Google Scholar]
- 39.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 16: 494–502, 1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knoop J, Steultjens MP, van der Leeden M, van der Esch M, Thorstensson CA, Roorda LD, Lems WF, Dekker J. Proprioception in knee osteoarthritis: a narrative review. Osteoarthritis Cartilage 19: 381–388, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Kumar D, Manal KT, Rudolph KS. Knee joint loading during gait in healthy controls and individuals with knee osteoarthritis. Osteoarthritis Cartilage 21: 298–305, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lequesne M. Quantitative Measurements of Joint Space During Progression of Osteoarthritis: “Chondrometry”. Rosemont, IL: American Academy of Orthopedic Surgeons, 1995, p. 427–444 [Google Scholar]
- 43.Lewek MD, Chmielewski TL, Risberg MA, Snyder-Mackler L. Dynamic knee stability after anterior cruciate ligament rupture. Exerc Sport Sci Rev 31: 195–200, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Lewek MD, Ramsey DK, Snyder-Mackler L, Rudolph KS. Knee stabilization in patients with medial compartment knee osteoarthritis. Arthritis Rheum 52: 2845–2853, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lewek MD, Rudolph KS, Snyder-Mackler L. Control of frontal plane knee laxity during gait in patients with medial compartment knee osteoarthritis. Osteoarthritis Cartilage 12: 745–751, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X, Benjamin Ma C, Link TM, Castillo DD, Blumenkrantz G, Lozano J, Carballido-Gamio J, Ries M, Majumdar S. In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthritis Cartilage 15: 789–797, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liang MH, Larson MG, Cullen KE, Schwartz JA. Comparative measurement efficiency and sensitivity of five health status instruments for arthritis research. Arthritis Rheum 28: 542–547, 1985 [DOI] [PubMed] [Google Scholar]
- 48.Lin DH, Lin CH, Lin YF, Jan MH. Efficacy of 2 non-weight-bearing interventions, proprioception training versus strength training, for patients with knee osteoarthritis: a randomized clinical trial. J Orthop Sports Phys Ther 39: 450–457, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Lohmander LS. Articular cartilage and osteoarthrosis. The role of molecular markers to monitor breakdown, repair and disease. J Anat 184: 477–492, 1994 [PMC free article] [PubMed] [Google Scholar]
- 50.Lund H, Juul-Kristensen B, Hansen K, Christensen R, Christensen H, Danneskiold-Samsoe B, Bliddal H. Movement detection impaired in patients with knee osteoarthritis compared to healthy controls: a cross-sectional case-control study. J Musculoskelet Neuronal Interact 8: 391–400, 2008 [PubMed] [Google Scholar]
- 51.Manal K, McClay I, Stanhope S, Richards J, Galinat B. Comparison of surface mounted markers and attachment methods in estimating tibial rotations during walking: an in vivo study. Gait Posture 11: 38–45, 2000 [DOI] [PubMed] [Google Scholar]
- 52.Marigold DS, Patla AE. Strategies for dynamic stability during locomotion on a slippery surface: effects of prior experience and knowledge. J Neurophysiol 88: 339–353, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Mosher TJ, Dardzinski BJ. Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Musculoskelet Radiol 8: 355–368, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Nielsen JB, Sinkjaer T. Afferent feedback in the control of human gait. J Electromyogr Kinesiol 12: 213–217, 2002 [DOI] [PubMed] [Google Scholar]
- 55.Nieuwenhuijzen PH, Duysens J. Proactive and reactive mechanisms play a role in stepping on inverting surfaces during gait. J Neurophysiol 98: 2266–2273, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Nieuwenhuijzen PH, Schillings AM, Van Galen GP, Duysens J. Modulation of the startle response during human gait. J Neurophysiol 84: 65–74, 2000 [DOI] [PubMed] [Google Scholar]
- 57.Pai YC, Bhatt TS. Repeated-slip training: an emerging paradigm for prevention of slip-related falls among older adults. Phys Ther 87: 1478–1491, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pai YC, Rymer WZ, Chang RW, Sharma L. Effect of age and osteoarthritis on knee proprioception. Arthritis Rheum 40: 2260–2265, 1997 [DOI] [PubMed] [Google Scholar]
- 59.Pai YC, Wening JD, Runtz EF, Iqbal K, Pavol MJ. Role of feedforward control of movement stability in reducing slip-related balance loss and falls among older adults. J Neurophysiol 90: 755–762, 2003 [DOI] [PubMed] [Google Scholar]
- 60.Peterfy CG, Gold G, Eckstein F, Cicuttini F, Dardzinski B, Stevens R. MRI protocols for whole-organ assessment of the knee in osteoarthritis. Osteoarthritis Cartilage 14, Suppl A: A95–A111, 2006 [DOI] [PubMed] [Google Scholar]
- 61.Proske U, Gandevia SC. The proprioceptive senses: their roles in signaling body shape, body position and movement, and muscle force. Physiol Rev 92: 1651–1697, 2011 [DOI] [PubMed] [Google Scholar]
- 62.Quintern J, Berger W, Dietz V. Compensatory reactions to gait perturbations in man: short- and long-term effects of neuronal adaptation. Neurosci Lett 62: 371–376, 1985 [DOI] [PubMed] [Google Scholar]
- 63.Rejeski WJ, Ettinger WH, Jr, Schumaker S, James P, Burns R, Elam JT. Assessing performance-related disability in patients with knee osteoarthritis. Osteoarthritis Cartilage 3: 157–167, 1995 [DOI] [PubMed] [Google Scholar]
- 64.Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther 28: 88–96, 1998 [DOI] [PubMed] [Google Scholar]
- 65.Rothwell JC, Day BL, Berardelli A, Marsden CD. Habituation and conditioning of the human long latency stretch reflex. Exp Brain Res 63: 197–204, 1986 [DOI] [PubMed] [Google Scholar]
- 66.Rutherford DJ, Hubley-Kozey CL, Stanish WD. Changes in knee joint muscle activation patterns during walking associated with increased structural severity in knee osteoarthritis. J Electromyogr Kinesiol 23: 704–711, 2013 [DOI] [PubMed] [Google Scholar]
- 67.Schmitt LC, Rudolph KS. Muscle stabilization strategies in people with medial knee osteoarthritis: the effect of instability. J Orthop Res 26: 1180–1185, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schmitt LC, Fitzgerald GK, Reisman AS, Rudolph KS. Instability, laxity, and physical function in patients with medial knee osteoarthritis. Phys Ther 88: 1506–1516, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Segal NA, Glass NA, Felson DT, Hurley M, Yang M, Nevitt M, Lewis CE, Torner JC. Effect of quadriceps strength and proprioception on risk for knee osteoarthritis. Med Sci Sports Exerc 42: 2081–2088, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shakespeare DT, Stokes M, Sherman KP, Young A. Reflex inhibition of the quadriceps after meniscectomy: lack of association with pain. Clin Physiol 5: 137–144, 1985 [DOI] [PubMed] [Google Scholar]
- 71.Shakoor N, Agrawal A, Block JA. Reduced lower extremity vibratory perception in osteoarthritis of the knee. Arthritis Rheum 59: 117–121, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sharma L, Chmiel JS, Almagor O, Felson D, Guermazi A, Roemer F, Lewis CE, Segal N, Torner J, Cooke TD, Hietpas J, Lynch J, Nevitt M. The role of varus and valgus alignment in the initial development of knee cartilage damage by MRI: the MOST study. Ann Rheum Dis 72: 235–240, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sharma L, Eckstein F, Song J, Guermazi A, Prasad P, Kapoor D, Cahue S, Marshall M, Hudelmaier M, Dunlop D. Relationship of meniscal damage, meniscal extrusion, malalignment, and joint laxity to subsequent cartilage loss in osteoarthritic knees. Arthritis Rheum 58: 1716–1726, 2008 [DOI] [PubMed] [Google Scholar]
- 74.Sharma L, Hurwitz DE, Thonar EJ, Sum JA, Lenz ME, Dunlop DD, Schnitzer TJ, Kirwan-Mellis G, Andriacchi TP. Knee adduction moment, serum hyaluronan level, and disease severity in medial tibiofemoral osteoarthritis. Arthritis Rheum 41: 1233–1240, 1998 [DOI] [PubMed] [Google Scholar]
- 75.Swanik CB, Lephart SM, Rubash HE. Proprioception, kinesthesia, and balance after total knee arthroplasty with cruciate-retaining and posterior stabilized prostheses. J Bone Joint Surg Am 86-A: 328–334, 2004 [DOI] [PubMed] [Google Scholar]
- 76.Tang PF, Woollacott MH, Chong RK. Control of reactive balance adjustments in perturbed human walking: roles of proximal and distal postural muscle activity. Exp Brain Res 119: 141–152, 1998 [DOI] [PubMed] [Google Scholar]
- 77.Thorlund JB, Shakoor N, Ageberg E, Sandal LF, Block JA, Roos EM. Vibratory perception threshold in young and middle-aged patients at high risk of knee osteoarthritis compared to controls. Arthritis Care Res (Hoboken) 64: 144–148, 2012 [DOI] [PubMed] [Google Scholar]
- 78.Tsauo JY, Cheng PF, Yang RS. The effects of sensorimotor training on knee proprioception and function for patients with knee osteoarthritis: a preliminary report. Clin Rehabil 22: 448–457, 2008 [DOI] [PubMed] [Google Scholar]
- 79.Tunay VB, Baltaci G, Atay AO. Hospital-based versus home-based proprioceptive and strengthening exercise programs in knee osteoarthritis. Acta Orthop Traumatol Turc 44: 270–277, 2010 [DOI] [PubMed] [Google Scholar]
- 80.Wagner MJ, Smith MA. Shared internal models for feedforward and feedback control. J Neurosci 28: 10663–10673, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yakhdani HR, Bafghi HA, Meijer OG, Bruijn SM, van den Dikkenberg N, Stibbe AB, van Royen BJ, van Dieën JH. Stability and variability of knee kinematics during gait in knee osteoarthritis before and after replacement surgery. Clin Biomech (Bristol, Avon) 25: 230–236, 2010 [DOI] [PubMed] [Google Scholar]
- 82.Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, Bierma-Zeinstra S, Brandt KD, Croft P, Doherty M, Dougados M, Hochberg M, Hunter DJ, Kwoh K, Lohmander LS, Tugwell P. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage 16: 137–162, 2008 [DOI] [PubMed] [Google Scholar]
- 83.Zhang W, Nuki G, Moskowitz RW, Abramson S, Altman RD, Arden NK, Bierma-Zeinstra S, Brandt KD, Croft P, Doherty M, Dougados M, Hochberg M, Hunter DJ, Kwoh K, Lohmander LS, Tugwell P. OARSI recommendations for the management of hip and knee osteoarthritis: part III: Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage 18: 476–499, 2010 [DOI] [PubMed] [Google Scholar]