Abstract
Human listeners are sensitive to interaural time differences (ITDs) in the envelopes of sounds, which can serve as a cue for sound localization. Many high-frequency neurons in the mammalian inferior colliculus (IC) are sensitive to envelope-ITDs of sinusoidally amplitude-modulated (SAM) sounds. Typically, envelope-ITD-sensitive IC neurons exhibit either peak-type sensitivity, discharging maximally at the same delay across frequencies, or trough-type sensitivity, discharging minimally at the same delay across frequencies, consistent with responses observed at the primary site of binaural interaction in the medial and lateral superior olives (MSO and LSO), respectively. However, some high-frequency IC neurons exhibit dual types of envelope-ITD sensitivity in their responses to SAM tones, that is, they exhibit peak-type sensitivity at some modulation frequencies and trough-type sensitivity at other frequencies. Here we show that high-frequency IC neurons in the unanesthetized rabbit can also exhibit dual types of envelope-ITD sensitivity in their responses to SAM noise. Such complex responses to SAM stimuli could be achieved by convergent inputs from MSO and LSO onto single IC neurons. We test this hypothesis by implementing a physiologically explicit, computational model of the binaural pathway. Specifically, we examined envelope-ITD sensitivity of a simple model IC neuron that receives convergent inputs from MSO and LSO model neurons. We show that dual envelope-ITD sensitivity emerges in the IC when convergent MSO and LSO inputs are differentially tuned for modulation frequency.
Keywords: inferior colliculus, amplitude modulation, interaural time differences, computational modeling, sound localization
psychophysical studies have shown that human listeners can detect interaural time differences (ITDs) conveyed via the envelopes of high-frequency sounds (Bernstein and Trahiotis 1994, 2002; Henning 1974; Klumpp and Eady 1956; McFadden and Pasanen 1976; Nuetzel and Hafter 1976). Physiologically, the superior olivary complex (SOC) is considered to be the primary site for encoding ITDs in the ascending auditory pathway. Both principal nuclei of the SOC, the medial superior olive (MSO) and the lateral superior olive (LSO), contain high-frequency neurons that are sensitive to the envelope-ITD of sinusoidally amplitude-modulated (SAM) tones, although they appear to encode ITD in different ways.
MSO neurons receive predominantly excitatory inputs from both ears (Batra et al. 1997; Caird and Klinke 1983; Goldberg and Brown 1968; Langford 1984; Yin and Chan 1990), resulting in peak discharge rate when neural activities from both sides arrive coincidentally. These neurons are called “peak type” because their ITD tuning functions align at their peaks for sounds of different frequencies. In contrast, most LSO neurons receive excitatory input from the ipsilateral ear and inhibitory input from the contralateral ear (Batra et al. 1997; Boudreau and Tsuchitani 1968; Caird and Klinke 1983; Finlayson and Caspary 1989; Joris 1996; Joris and Yin 1995; Sanes 1990; Tollin and Yin 2005). Because of this excitation-inhibition (EI) interaction, coincidental arrival of binaural inputs produces maximal cancellation and therefore minimum discharge rate. These cells are said to be “trough type” because their ITD tuning functions align at the trough for different modulation frequencies.
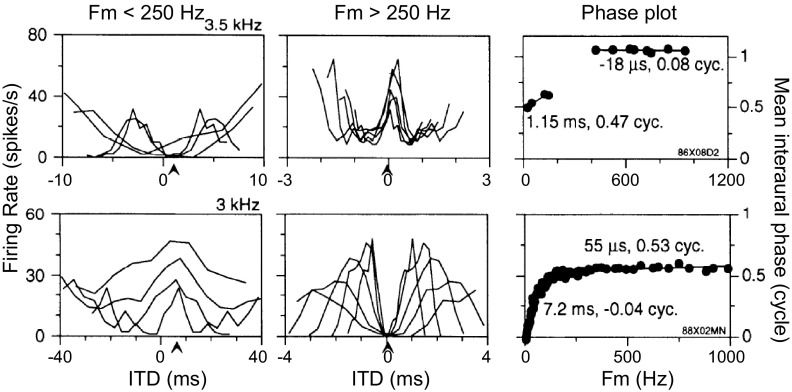
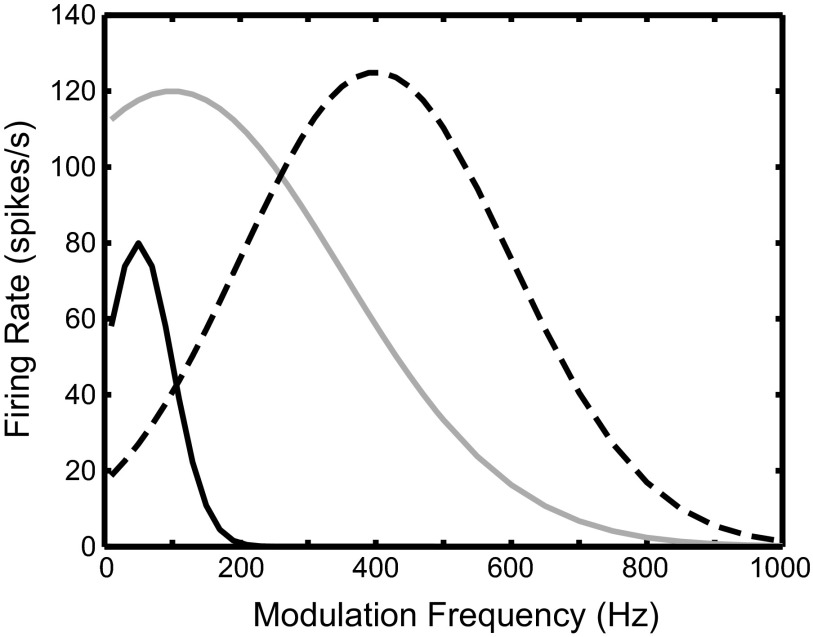
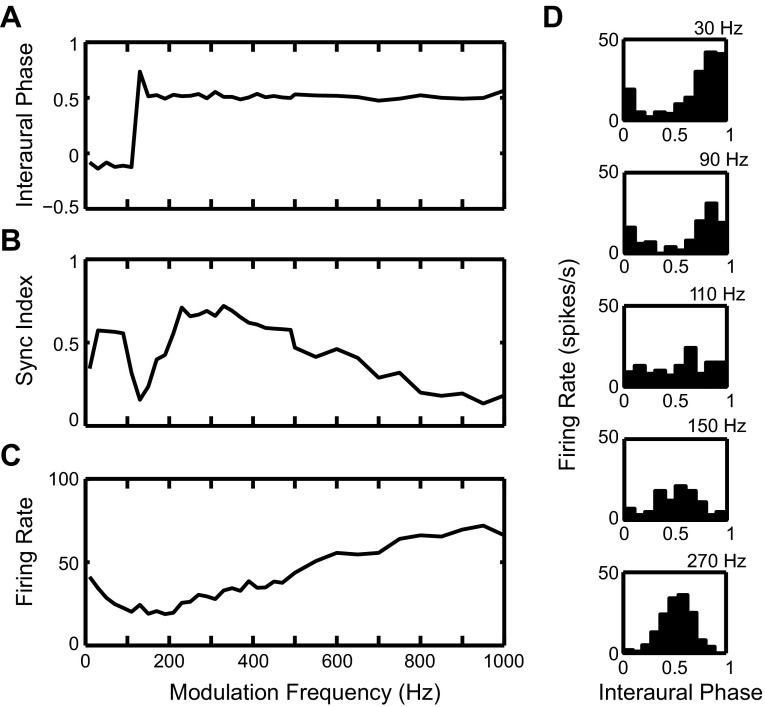
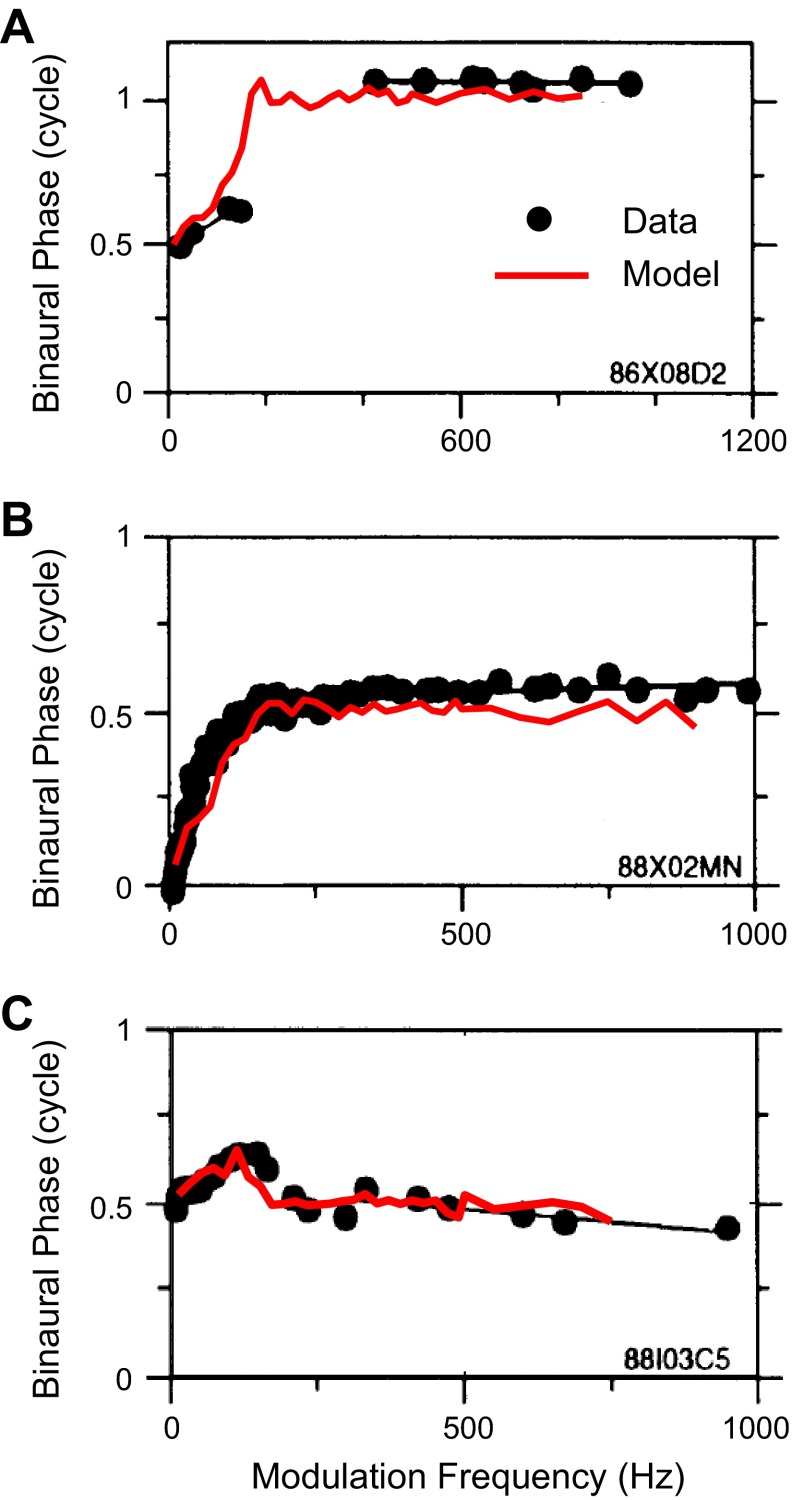
The SOC transmits the encoded envelope-ITD information to the next major nucleus in the auditory pathway, the inferior colliculus (IC), either via direct excitatory projections or via intervening inhibitory neurons such as those of the dorsal nucleus of the lateral lemniscus (DNLL). Many high-frequency neurons in the IC are sensitive to envelope-ITDs of SAM tones (Batra et al. 1989, 1993; Griffin et al. 2005; Yin et al. 1984) and also to ITDs in the cochlear-generated (CG) envelopes of broadband noise (Devore and Delgutte 2010; Joris 2003). In many cases, the ITD-tuning characteristics of IC neurons are similar to those of SOC neurons; thus the responses of these neurons can be explained by a single input from an SOC neuron with similar ITD sensitivity. However, some IC neurons exhibit emergent properties in their responses to envelope-ITD that have not been observed in the SOC. To describe neural sensitivity to envelope-ITD, the mean interaural phase of the neural responses is often plotted as a function of the modulation frequency (fm) (Batra et al. 1993, 1997; Yin and Kuwada 1983; Yin et al. 1984). For simple coincidence detectors, there is a linear relationship between phase and fm. The slope of the linear fit, termed the characteristic delay (CD), corresponds to the envelope-ITD at which the rate-ITD curves align. The phase intercept for zero fm, termed the characteristic phase (CP), is 0 cycle for peak-type neurons and 0.5 cycle for trough-type neurons. One type of IC neuron with emergent ITD sensitivity shows two linear segments with different slopes (CDs) in their phase plot, with a half-cycle change in CP between the two segments (Batra et al. 1993). We refer to these IC cells as dual-CP neurons. Some of the dual-CP neurons show trough-type responses at low fm and peak-type responses at high fm (Fig. 1, top), while others show the opposite pattern: peak-type responses at low fm and trough-type responses at high fm (Fig. 1, bottom). The transition between peak-type and trough-type responses tends to occur for fm between 150 and 250 Hz, and a drop in firing rate typically occurs in the transition region in the examples shown by Batra et al. (1993). The phase plots of these dual-CP neurons are suggestive of convergent inputs from the MSO and the LSO onto single high-frequency IC neurons.
Fig. 1.
Experimental data for 2 high-frequency inferior colliculus (IC) neurons showing dual characteristic delays (CDs) in response to binaural sinusoidally amplitude-modified (SAM) tones. Each row represents 1 IC neuron. Left and center: rate-interaural time difference (ITD) functions for modulation frequency (fm) < 250 Hz (left) and > 250 Hz (center). Each curve represents a rate-ITD function for a single fm. Note that the peaks are lined up for peak-type responses (bottom left and top center); the troughs are lined up for trough-type responses (top left and bottom center). Right: plot of the interaural phase as a function of fm (phase plot). Note the 2 linear segments in the phase plot. One linear segment is for fm < 250 Hz; another linear segment (with a different CP) is for fm > 250 Hz. [Figure from Batra et al. (1993) with permission.]
In a combined experimental and modeling study of dual ITD sensitivity in IC, we first extend the experimental results of Batra et al. (1993) by showing that some neurons in the IC of unanesthetized rabbit exhibit the dual type of ITD sensitivity to amplitude-modulated broadband noise. By independently manipulating the interaural correlation of the waveform fine structure and the modulation of the envelope, we reveal distinct types of sensitivity to the ITD in the slower, externally imposed amplitude envelope (ITDAM) versus the ITD in the faster envelope induced by cochlear filtering of the noise waveform (ITDCG). The responses of these IC neurons are also suggestive of convergent inputs from the MSO and the LSO. Anatomical tracer studies have demonstrated convergence of projections from MSO and LSO onto the IC at the level of fibro-dendritic laminae (Loftus et al. 2004) and also onto highly local neighborhoods of physiologically characterized IC neurons (Loftus et al. 2010), making it likely that convergence actually occurs onto the same IC cell.
We developed a computational model of the binaural circuits up through the IC to test whether a convergence of inputs from LSO and MSO is adequate to explain the dual types of envelope-ITD sensitivity (peak type and trough type) of high-frequency IC neurons for both SAM tones and broadband noises. Using either existing models of SOC neurons or quantitative descriptions of available experimental data for the MSO and the LSO, we show that the IC model is able to reproduce the complex responses observed in the IC, including both the fm dependence of the responses to SAM tones and the dual types of envelope-ITD sensitivity seen in the response to broadband noise.
METHODS
We first present the methods for the in vivo electrophysiology experiments. The details of the computational modeling are then presented.
In Vivo Electrophysiology
Surgical preparation.
Female Dutch Belted rabbits were prepared for chronic in vivo electrophysiology as described by Devore and Delgutte (2010). Briefly, under aseptic conditions, a stainless steel recording chamber and brass head post were affixed to the skull with dental acrylic and stainless steel screws. Custom ear mold impressions were made with vinyl polysiloxane (Reprosil). After a 1-wk recovery period, animals were gradually accustomed to head restraint and subsequently underwent a second aseptic surgical procedure in which an ∼3-mm craniotomy was made inside the recording chamber (coordinates from bregma: −10.5 mm AP and 3 mm ML). All procedures were approved by the animal care and use committees of both the Massachusetts Eye and Ear Infirmary and the Massachusetts Institute of Technology.
Experimental procedures.
Sound stimuli were generated by a 24-bit digital-to-analog converter (National Instruments NIDAC 4461) at a sampling rate of 50 kHz. Stimuli were digitally filtered to compensate for the transfer function of the acoustic assemblies, which consisted of a pair of Beyer-Dynamic (DT-48) speakers attached to sound tubes running through the custom-fitted ear molds. A probe-tube microphone (Etymotic ER-7C) measured acoustic pressure in the ear canal at the end of the sound delivery tube. At the start of each recording session, we determined the system transfer function in each ear with a broadband chirp stimulus and generated digital equalization filters over the range of 0.1–22.5 kHz.
Single neurons were isolated with epoxy-insulated tungsten electrodes (A-M Systems) inserted through a 25-gauge guide tube and advanced with a remote-controlled hydraulic micropositioner (David Kopf Instruments 650). The neural signal was amplified, band-pass filtered (0.3–3 kHz), and fed to a software spike detector triggering on level crossings. Animals were monitored on a closed-circuit video system throughout recording sessions. During the final recording session, recording sites were marked by electrolytic lesions (10-μA direct current for 30–45 s); subsequent histological examination verified the lesion sites to be within the IC.
Characterization of ITD sensitivity.
When a single neuron was well isolated, its characteristic frequency (CF) was determined with an automatic tracking procedure (Kiang and Moxon 1974) and the threshold for contralateral broadband noise was determined by measuring responses as a function of level. We then characterized ITD sensitivity, using three types of broadband noise stimuli. The first type comprised unmodulated broadband noises with the same waveforms at the two ears, so that their waveforms were interaurally correlated (unmod_corr). The second type comprised uncorrelated broadband noises, created with a Gramm-Schmidt orthogonalization procedure (Culling et al. 2001), that were amplitude modulated at the same frequency and depth at both ears (mod_uncorr). The third type comprised broadband noises with both correlated fine structure and the same amplitude modulation at both ears (mod_corr). The modulation depth was always 100%, and fm was typically 100 Hz, although 40 Hz was used for some neurons.
For each of the three stimulus conditions, ITD sensitivity was assessed by measuring neural responses as a function of ITD applied to the whole waveform of the noises. We assessed ITD sensitivity over the range of ±2 ms in 0.2-ms steps for unmod_corr stimuli, ±20 ms in 2-ms steps for 40-Hz modulated stimuli, and ±10 ms in 1-ms steps for 100-Hz modulated stimuli. Noise stimuli were 200 ms in duration and were presented at a rate of 2/s at 15–20 dB above the unit's threshold. In all experiments, ITD sensitivity was first characterized for the unmod_corr stimuli. Only high-CF (>1,500 Hz) neurons exhibiting ITD sensitivity to the unmod_corr stimulus were further studied using the mod_uncorr and/or mod_corr stimuli in random order. For characterization of ITD sensitivity, ITD was randomly varied from trial to trial until 10 repeats were obtained at each ITD.
Data analysis.
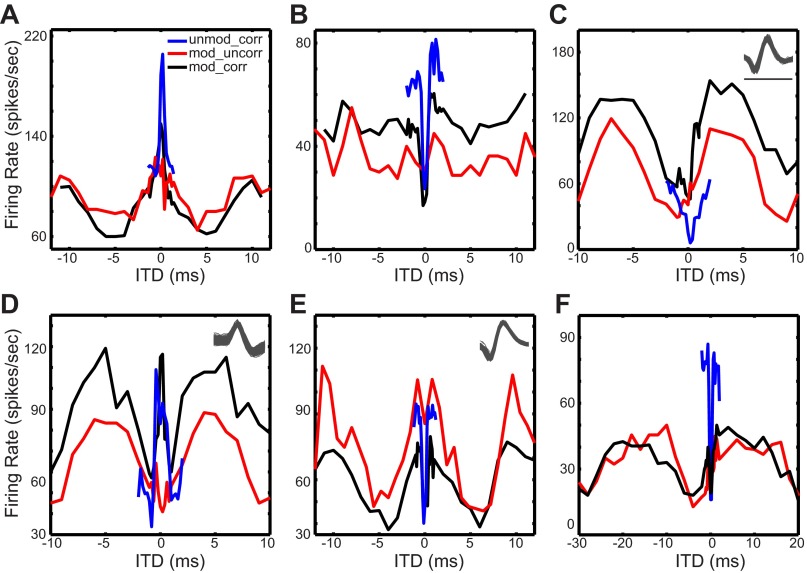
To ensure that we included responses from only single units, as opposed to multiunit responses, analysis was restricted to recording sites in which the spike amplitude and waveshape were stable and exceeded the noise floor by at least three standard deviations. Figure 5, C–E, insets, show representative spike waveforms from three IC neurons.
Fig. 5.
Neural sensitivity to ITD. Average firing rate as a function of ITD for the 3 broadband noise stimulus conditions: unmodulated noise with correlated fine structure (unmod_corr, blue lines), modulated noise with correlated fine structure (mod_corr, black lines), and modulated noise with uncorrelated fine structure (mod_uncorr, red lines). The unit characteristic frequencies (CFs, in kHz) are 3.79 (A), 3.0 (B), 2.39 (C), 3.66 (D), 1.7 (E), and 6.88 (F). The fm of the SAM noise was 100 Hz except for the data in F, which were obtained with a SAM frequency of 40 Hz. Unit quality is illustrated by superimposed waveforms of >500 spikes recorded in response to the mod_corr stimulus for the units shown in C–E. Scale bar in C is 1 ms; all spike waveforms are plotted on the same timescale.
For each noise type, we computed a rate-ITD function by first counting the spikes over the 200-ms stimulus duration and averaging across all trials for each ITD. Rate-ITD curves were smoothed by a three-point digital filter with weights (1/6, 2/3, 1/6). ITD sensitivity to the unmod_corr stimuli was classified as peak type, trough type, or biphasic/intermediate with the classification scheme of Devore and Delgutte (2010).
Modeling Procedures
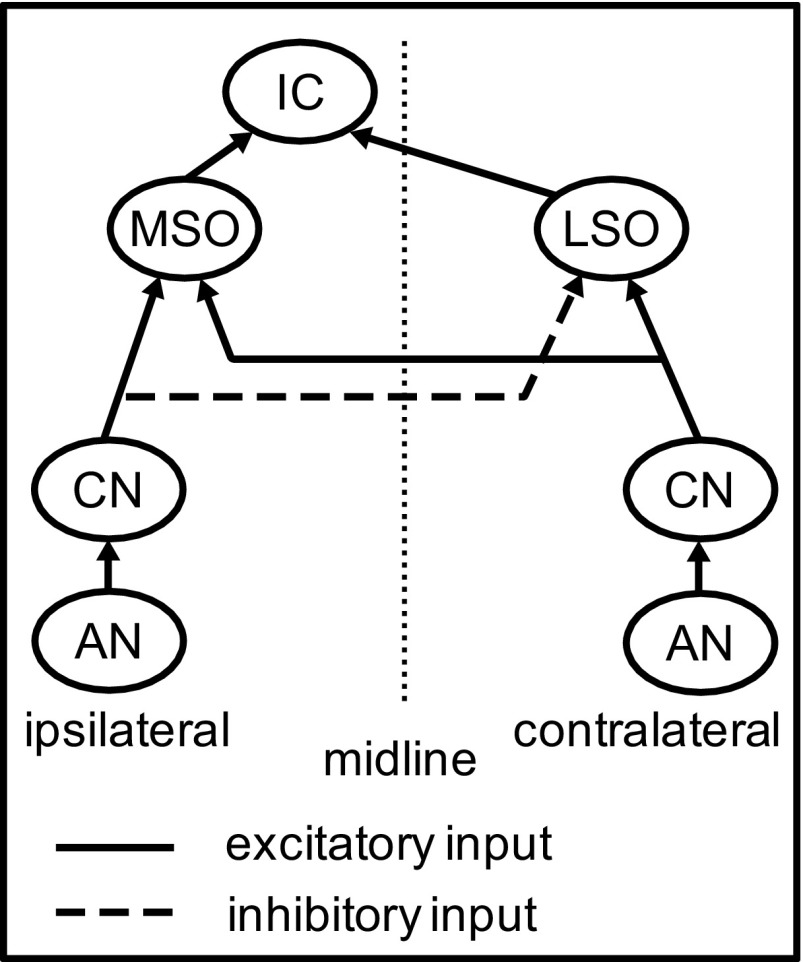
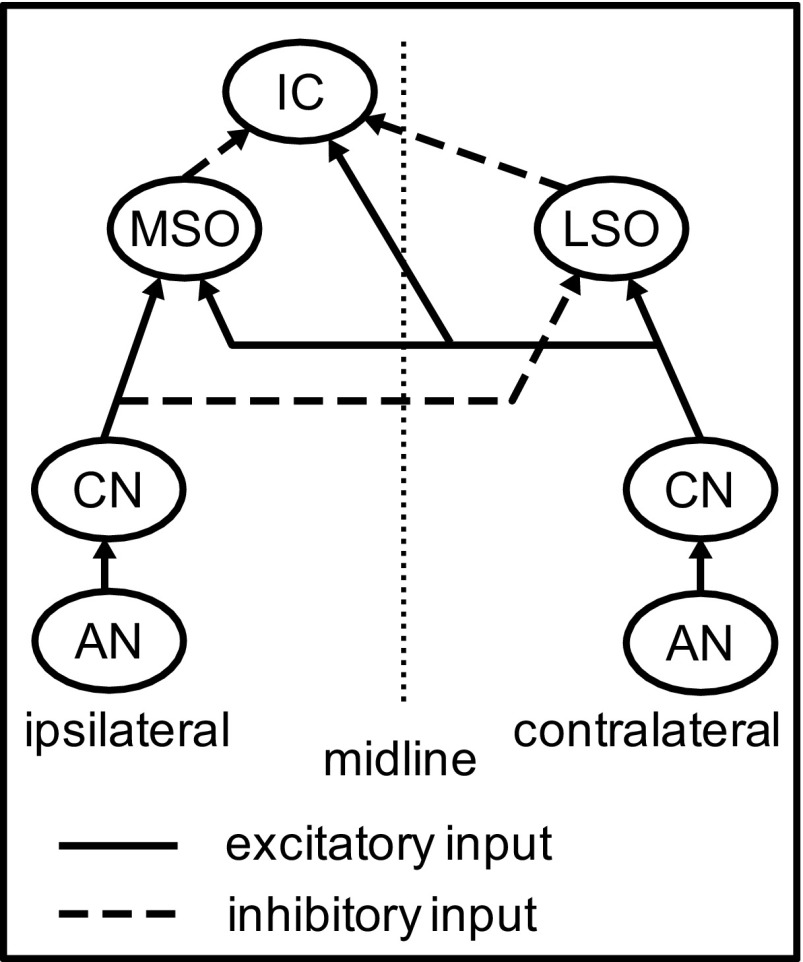
The IC model developed in the present study consists of four stages, as shown in Fig. 2. The first stage represents auditory nerve (AN) responses to SAM tones and broadband noises. The second stage represents the cochlear nucleus (CN), which is modeled here as a simple relay, as is the medial nucleus of the trapezoid body (MNTB). The third stage models the responses of high-frequency MSO and LSO neurons to binaural SAM tones and noises. The MSO and LSO models were either Hodgkin-Huxley (HH)-type models developed in previous studies (Colburn et al. 2009; Wang and Colburn 2012) or phenomenological models that directly simulate the experimentally measured discharge rates of MSO and LSO neurons in response to binaural SAM tones. The fourth stage models the responses of an IC cell with convergent input from MSO and LSO. In all simulations, the stimulus duration was the same as in the experimental data, i.e., 5 s for SAM tone stimuli and 200 ms for broadband noise stimuli.
Fig. 2.
Diagram of the IC model with excitatory inputs from the medial (MSO) and lateral (LSO) superior olive. The cochlear nucleus (CN) is modeled as simple relay. [For the phenomenological MSO and LSO models, the response patterns of MSO and LSO inputs are specified computationally without explicitly describing the inputs to these MSO and LSO neurons from the auditory nerve (AN) or the CN.]
AN models.
Two types of AN models were used: a simplified AN model based on Poisson processes and a detailed AN model with realistic cochlear processing (Zilany et al. 2009). The simplified AN model was used to simulate IC responses to binaural SAM tones, and the detailed AN model was used to simulate responses to binaural broadband noises. The simplified AN model lacks an explicit description of cochlear processing, and thus is not suitable for simulating responses to noise. Spikes generated by the AN models directly drive synaptic inputs to the MSO and LSO models since the CN and the MNTB are modeled as simple relays.
The simplified AN model is based on a point-process AN description similar to those used in previous modeling studies (Colburn et al. 2009; Zhou et al. 2005) except that there is no refractoriness in the simplified model (i.e., it is approximated as a Poisson process). The instantaneous firing rate of the Poisson process for a single AN fiber in response to SAM tones is characterized by four parameters: the modulation frequency fm, the average firing rate Ravg, the synchronization index SI to the modulation, and the average response phase φ. The rate function is periodic, with a period T, equal to the inverse of the modulation frequency fm. The parameters SI and φ determine the temporal aspects of the AN spike trains. The unit for φ is cycles, and its value was always 0.5 in the AN model.
To generate spikes, we first determined the expected number of spikes for each modulation cycle by multiplying the average firing rate Ravg by the modulation period T. If the expected number of spikes was not an integer, it was rounded either up or down (independently for each cycle) with a random number generator. Then the spike times tk within each cycle were drawn repeatedly from a Gaussian distribution with mean Tφ and standard deviation T/(2F) until the expected number of spikes was generated. The synchrony index SI is determined by the value of the parameter F, which corresponds to the inverse of the coefficient of variation of the distribution of spike jitters. Specifically, SI can be calculated by inverting the formula F = π/(). For cases when the Gaussian sample tk fell outside of the period (tk < 0 or tk > T), the model constrained the event time to be within the current period by replacing tk with its circular residue i.e., = tk mod T whenever tk > T and = tk mod T + T for the case that tk < 0. The spike times were generated by applying the above procedure for each period until enough spikes were generated for the whole duration of the stimulus, i.e., the overall number of spikes matched the theoretical total spike count 5 × Ravg for a 5-s stimulus duration.
Multiple model AN fibers were used to provide synaptic inputs to the MSO and LSO models on each side. AN fibers on the same side have the same values for the four parameters of the rate function. AN fibers from the two sides also share their input parameters except that fm is 1 Hz lower on one side than the other side. The difference in fm produces binaural modulation beats and gives rise to an interaural phase difference that traverses the full cycle at a rate equal to the 1-Hz beat frequency.
For simulating responses to binaural broadband noises, a more detailed AN model, developed by Zilany et al. (2009), was used to provide synaptic inputs to the MSO and LSO models. This AN model incorporates detailed descriptions of cochlear processing including frequency tuning, the cochlear amplifier, inner hair cell (IHC) transduction, the transmission at IHC-AN synapses, and refractoriness in AN fibers. These explicit descriptions of cochlear processing, which are lacking in the simplified AN model, are used to simulate AN responses to broadband noises. In the present study, the CF of the AN model was always 6,000 Hz and the stimulus level was fixed at 60 dB SPL for each ear.
MSO and LSO models.
Two types of models were used for the high-CF MSO and LSO neurons: HH models and phenomenological models with Poisson time-varying rate functions chosen to simulate experimental data. The HH-type models were implemented with NEURON (Hines and Carnevale 1997), a broad-purpose neuron simulation environment. For both types of MSO and LSO models, MATLAB (MathWorks, Natick, MA) was used to initialize the model parameters before each simulation and to analyze the results after each simulation.
HH-TYPE MSO AND LSO MODELS.
The HH-type LSO cell model was the same as in a previous study of LSO responses to monaural SAM tones (Wang and Colburn 2012). It is a single-compartment model with three ion channels in the membrane: a sodium channel, a high-threshold potassium channel (KHT), and a “leak” channel. The biophysical parameters of this model, including the passive membrane properties and the mathematical characterization of the ion channels, were as described by Wang and Colburn (2012). The LSO model receives 20 excitatory AN inputs from the ipsilateral side and 20 inhibitory inputs from the contralateral side.
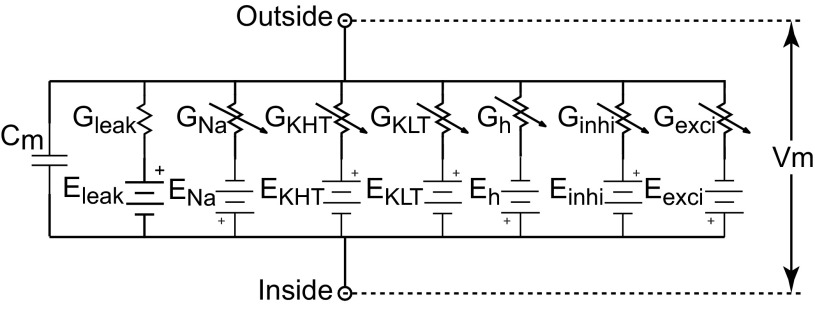
The HH-type MSO model was the same single-compartment model used previously to predict responses of MSO neurons to bilateral electrical stimulation through cochlear implants (Colburn et al. 2009). The model contained all the ion channels used in the LSO model and two additional ion channels: the h-current (Ih) channel and the low-threshold potassium channel (KLT) (Fig. 3). Parameter values for these channels were the same as used by Rothman and Manis (2003a, 2003b) to describe their experimental measurements of bushy cell membrane potentials in the ventral CN. The Ih channel was included in the MSO model to depolarize the resting potential so that the KLT channel would be partially activated at rest. This resting activation of the KLT channel gives rise to faster membrane time constants near resting potential and therefore better phase locking at high fm. The MSO model receives 10 excitatory AN inputs from each side. Table 1 summarizes the parameters for the HH-type MSO and LSO models.
Fig. 3.
Circuit diagram for the Hodgkin-Huxley (HH)-type model for the MSO with 5 explicit channels in addition to the synaptic channels: a leak channel, a sodium channel (Na), a low-threshold potassium channel (KLT), a high-threshold potassium channel (KHT), and an h current (Ih) channel. The two synaptic channels, Gexci and Ginhi, represent excitatory and inhibitory synaptic inputs. The diagram for the HH-type LSO model is similar to the MSO model except that the LSO model contains no KLT channel and no Ih channel. The membrane parameters for the MSO and LSO models are summarized in Table 1. Cm, membrane capacitance; Vm, membrane potential.
Table 1.
Default parameters for HH-type LSO model and single-compartment MSO model
| Cm | gleak | gNa | gKHT | gKLT | gh | τE | τE_rise | τI | τI_rise | No. of ANs on each side | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LSO model | 31.4 pF | 31.4 nS | 8,000 nS | 1,200 nS | 0 nS | 0 nS | 2 ms | 0.2 ms | 5 ms | 0.5 ms | 20 |
| MSO model | 12 pF | 2 nS | 1,000 nS | 150 nS | 200 nS | 20 nS | 0.1 ms | 0.01 ms | N/A | N/A | 10 |
HH, Hodgkin-Huxley; LSO, lateral superior olive; MSO, medial superior olive; AN, auditory nerve; N/A, not applicable. See text for parameter definitions.
The HH-type MSO and LSO models used here were developed previously to describe MSO and LSO responses to other types of stimuli. In the present study, the membrane descriptions of these models, including the passive properties and characterization of the ion channels, are the same as in the original studies; however, the model for AN inputs, which was designed to describe AN responses to binaural high-frequency waveforms, differed from that in the original studies.
The HH-type MSO and LSO models receive inputs from either the simplified or the detailed AN model. Unless otherwise noted, the interaural delay between the AN inputs on the two sides was zero for both MSO and LSO models. Each spike generated by the AN model elicits an excitatory or inhibitory postsynaptic conductance (PSC), and the total excitatory (resp. inhibitory) synaptic conductance waveform is the superposition of all the excitatory (resp. inhibitory) PSCs occurring during the stimulus. Each PSC was simulated as the difference of two exponentials (rising and falling components), and the excitatory and inhibitory peak conductances (GE and GI, respectively) were specified separately. Values of GE and GI were varied to explore effects of the relative strengths of the AN inputs to MSO and LSO. Specifically, each excitatory PSC was expressed as
where τE_rise and τE refer to the rise and decay time constants for excitation, respectively. The normalization factor to ensure the peak conductance is GE is given by Gnorm = exp(−tp/τE) − exp(−tp/τE_rise), where the peak latency
with τE > τE_rise. Inhibition is given in the same form with corresponding parameters τI_rise, τI, and GI. The reversal potential was 0 mV for excitatory synapses and −70 mV for inhibitory synapses. The resting potential was −63.6 mV for the MSO model and −64.9 mV for the LSO model. Note that Table 1 shows faster time constants for the MSO and slower time constants for the LSO.
PHENOMENOLOGICAL MSO AND LSO MODELS.
The second type of models for MSO and LSO neurons is phenomenological point-process models with rate functions based on experimental data from the MSO and the LSO in response to SAM tones with fm chosen to create binaural beats (Joris 1996; Yin and Chan 1990). These models were only used to simulate the IC responses to SAM tones, and no AN inputs were generated explicitly in these models. For each fm, the spike patterns for the MSO and LSO models were generated by the same method as for the simplified AN model, with the key difference that the instantaneous firing rate was phase locked to the 1-Hz binaural beat frequency rather than to fm. For the MSO model, the synchronization SI to the beat frequency was fixed at 0.25 and the interaural phase φ was fixed at 0 for all fm, as appropriate for a peak-type neuron. For the LSO model, SI was fixed at 0.33 and φ was fixed at 0.5 for all fm, as appropriate for a trough-type neuron. These values are based on the average SI and φ measured experimentally in MSO and LSO neurons (Joris 1996). The distinguishing feature of these models is the binaural rate-fm function representing the average firing rate over the entire stimulus duration in response to binaural SAM tones. Specifically, three synthetic functions were used as the binaural rate-fm function for the MSO model, and the same three functions were also used for the LSO model (Fig. 4). These synthetic functions were chosen to approximate the range of binaural rate-fm functions observed experimentally in MSO and LSO neurons (Joris 1996; Yin and Chan 1990).
Fig. 4.
Synthetic binaural rate-fm functions of the phenomenological models. Each curve was used for both the MSO model and the LSO model. Different curves represent different shapes of the binaural rate-fm function observed empirically in the MSO and the LSO: sharp low pass (black solid curve), broad low pass (gray curve), and band pass (dashed curve). The firing rate plotted here is computed as the average firing rate over the whole duration of the SAM tone stimulus (5 s). The synchronization index (SI) is fixed at 0.25 for the MSO and at 0.33 for the LSO for all fm.
Unless otherwise noted, the internal interaural delays for the MSO and the LSO models were zero, which gave rise to a constant interaural phase as a function of fm. In a few cases, nonzero internal interaural delays were introduced to reproduce the fm-dependent phase curves observed in the experimental data. In these cases, the interaural phase of the MSO and the LSO model responses was varied linearly with fm, with a slope equal to desired interaural delay.
IC model.
The IC model is a leaky integrate-and-fire (LIF) model that only contains a leak channel with constant resistance and the excitatory synaptic conductances derived from the spike inputs from the MSO and the LSO models. The membrane potential Vm of the IC model fluctuates as the input spikes arrive. Whenever Vm reaches a threshold Vth, an output spike is generated by transiently setting Vm to 0 mV. After each output spike, Vm is reset to the resting potential Vrest and held constant at Vrest for a duration Tref to simulate the absolute refractory period. The spikes generated from the MSO and LSO models drive the IC model via the same synaptic model as described for the AN to SOC synapses, but with different parameters. Two variants of the IC model were developed; in one version the inputs from the SOC are excitatory, while they are inhibitory in the other version. For both variants, only one MSO input and one LSO input were used to drive the IC model. The model parameters for both model versions are summarized in Table 2.
Table 2.
Default parameters for IC model
| MSO-IC Synapse |
LSO-IC Synapse |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cm | gleak | τE | τE_rise | τI | τI_rise | No. of inputs | τE | τE_rise | τI | τI_rise | No. of inputs |
| 31.4 pF | 31.4 nS | 1 ms | 0.1 ms | 5 ms | 0.5 ms | 1 | 1 ms | 0.1 ms | 5 ms | 0.5 ms | 1 |
IC, inferior colliculus. See text for parameter definitions.
RESULTS
Physiological Evidence for Dual ITD Sensitivity in IC with Broadband Noise
We characterized the envelope-ITD sensitivity of 30 high-frequency neurons (mean CF: 4,098 ± 1,728 Hz) in the IC of unanesthetized rabbits (n = 3). These data were collected as part of a larger study of spatial tuning for a large sample (n = 791) of IC neurons (Devore and Delgutte 2010). In that study, we showed that high-frequency IC neurons (CF > 1,850 Hz) typically exhibit sensitivity to ITD in the CG envelope of broadband noise stimuli (ITDCG), while low-frequency IC neurons are sensitive to ITD in the noise waveform fine structure (Devore and Delgutte 2010). Each of the 30 neurons in the present study was classified as ITDCG sensitive according to prior criteria (Devore and Delgutte 2010).
Our goal was to further characterize the ITD sensitivity of these high-frequency ITDCG-sensitive neurons in the presence of an externally imposed SAM envelope. Figure 5 shows the response of six ITDCG-sensitive neurons to three different types of broadband noise stimuli (see methods). The rate-ITD curves in response to binaurally identical noise waveforms with no amplitude modulation (unmod_corr, Fig. 5) typically exhibit either a single peak (Fig. 5, A and D) or a single trough (Fig. 5, B, C, E, and F), as expected for neurons that are ITDCG sensitive (Devore and Delgutte 2010; Joris 2003). We next assessed ITD sensitivity to the externally imposed AM envelope (ITDAM) by measuring the response to interaurally uncorrelated broadband noise with 100-Hz SAM (mod_uncorr, Fig. 5). Five of the six neurons in Fig. 5 (excluding Fig. 5B) exhibit cyclic variations in firing rate at the period of the externally imposed SAM. In contrast, the neuron in Fig. 5B shows no evidence for ITDAM sensitivity, as evidenced by the flat mod_uncorr ITD curve. The sharp peak or trough resulting from ITDCG sensitivity is not observed in the mod_uncorr condition because, for uncorrelated inputs, the envelopes generated by cochlear filtering will also be uncorrelated and thus there can be no ITD sensitivity to CG envelopes (Joris 2003). Overall, we observed ITDAM sensitivity with mod_uncorr stimuli in 90% (27/30) of the ITDCG-sensitive neurons.
There was a great diversity in the type of ITD sensitivity to each stimulus among our sample of units exhibiting both ITDCG and ITDAM sensitivity. In some neurons, the type of ITDCG sensitivity was similar to that for ITDAM. For example, the neuron in Fig. 5A responds to ITDCG with a single peak centered around 0 ms (unmod_corr). The response to ITDAM in the 100-Hz AM envelope (mod_uncorr) shows similar sensitivity, with a peak occurring around 0 ms ITD. Similarly, the neuron in Fig. 5C shows trough-type sensitivity to both ITDCG and ITDAM. However, in the majority of neurons, the sensitivity to ITDCG differed from that observed in response to ITDAM (Fig. 5, D–F). For example, the unit in Fig. 5D exhibits peak-type ITDCG sensitivity but trough-type ITDAM sensitivity, while the unit in Fig. 5E exhibits a prominent trough in response to ITDCG but appears peaklike in response to ITDAM. Finally, the unit in Fig. 5F exhibits trough-type sensitivity to ITDCG, but this trough is located on the rising slope of the ITD tuning curve to the mod_uncorr stimulus, indicating a different type of sensitivity to the two types of envelope. In all cases (Fig. 5, A–F), the ITD tuning curve for the mod_corr stimulus was approximately the superposition of the response to ITDCG and ITDAM. The responses of the neurons in Fig. 5, D–F, are reminiscent of dual-CP neurons observed in the IC by Batra et al. (1993) in response to SAM tones and are also suggestive of convergent inputs from MSO and LSO.
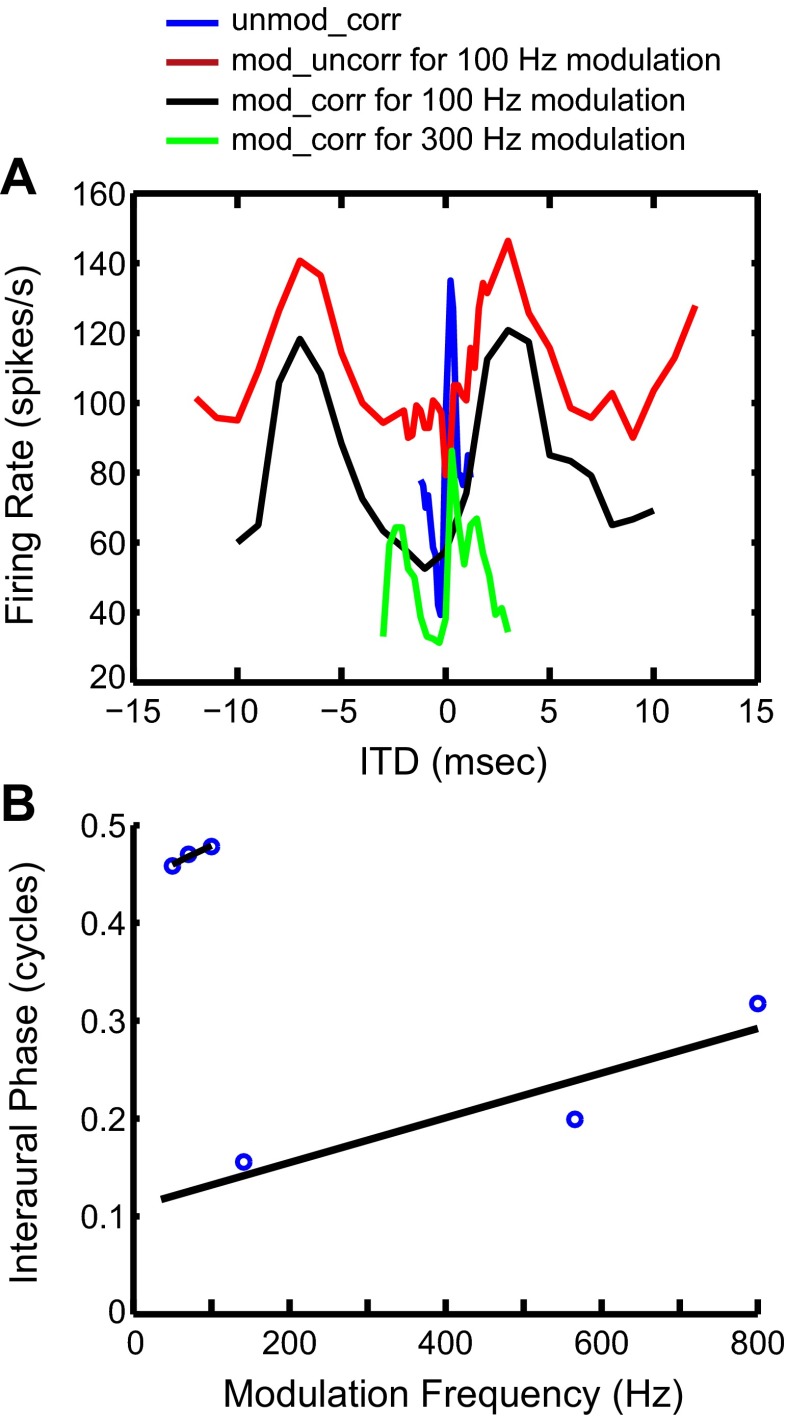
In one neuron (Fig. 6), we tested ITD sensitivity using binaural SAM tones in addition to the three broadband noise stimuli. With noise stimuli, this neuron shows trough-type sensitivity to ITDAM and intermediate-type (biphasic) sensitivity to ITDCG (Fig. 6A). With SAM tones, the phase plot of this neuron shows two linear segments (Fig. 6B). The low-fm segment has a CP close to 0.5, characteristic of a trough-type response. The high-fm segment has a CP around 0.1, which is significantly different from zero and therefore corresponds to an intermediate-type response. Thus the dual ITD sensitivity observed with noise stimuli is qualitatively consistent with the dual sensitivity observed with SAM tones in this neuron.
Fig. 6.
Responses of a single IC neuron to both SAM tones and broadband noises. A: rate-ITD functions for binaural broadband noises. Different colors represent different stimulus conditions, as indicated in key. The peak in the blue curve is not visible in the 100 Hz modulation case because in that case the ITD step is too large to allow the peak to appear. B: phase plot of this IC neuron in response to binaural SAM tones with 1-Hz difference in fm between the 2 ears (binaural beat). Two black lines are linear fits for low fm region and high fm region.
Because most neurons in our sample were only tested at one fm, their sensitivity to ITDAM could not be quantitatively determined from a phase plot. Rather, we characterized the types of ITD sensitivity by defining at what phase of the ITDAM curve—peak, trough, or slope—the main feature of the ITDCG response appeared. For example, if a peak-type ITDCG response appears in the trough of the ITDAM curve, we classify the delay interaction as peak-trough. This method effectively assumes that the neuron's CD is small relative to the period of the low-frequency modulations, so that the response phase at fm differs little from the CP. Table 3 lists the number of units falling into the different response classes. Overall, dual ITD sensitivity was observed in 14 of the 27 neurons (52%) sensitive to both ITDCG and ITDAM. Dual ITD sensitivity is thus common with broadband noise stimuli. Table 3 further shows that neurons with trough-type ITDAM sensitivity and peak-type ITDCG sensitivity were more common than the opposite pattern.
Table 3.
Breakdown of phase interactions for envelope-sensitive IC neurons
| Phase of AM Envelope Rate-ITD Curve at Which CG Envelope Feature Appears |
||||
|---|---|---|---|---|
| Type of CG ITD Tuning | Peak | Slope | Trough | Total |
| Peak | 5 | 1 | 7 | 13 |
| Intermediate | 0 | 2 | 2 | 4 |
| Trough | 2 | 2 | 6 | 10 |
| Total | 7 | 5 | 15 | 27 |
ITD, interaural time difference; CG, cochlear generated; AM, amplitude modulated.
We next use computational models to explicitly test whether convergent inputs from peak-type (MSO) and trough-type (LSO) inputs onto single IC neurons can generate the diversity in response profiles observed with noise stimuli in our sample of neurons as well as with SAM tones in the study of Batra et al. (1993).
Modeling IC Responses to Binaural SAM Tones
Results with HH-type MSO and LSO models.
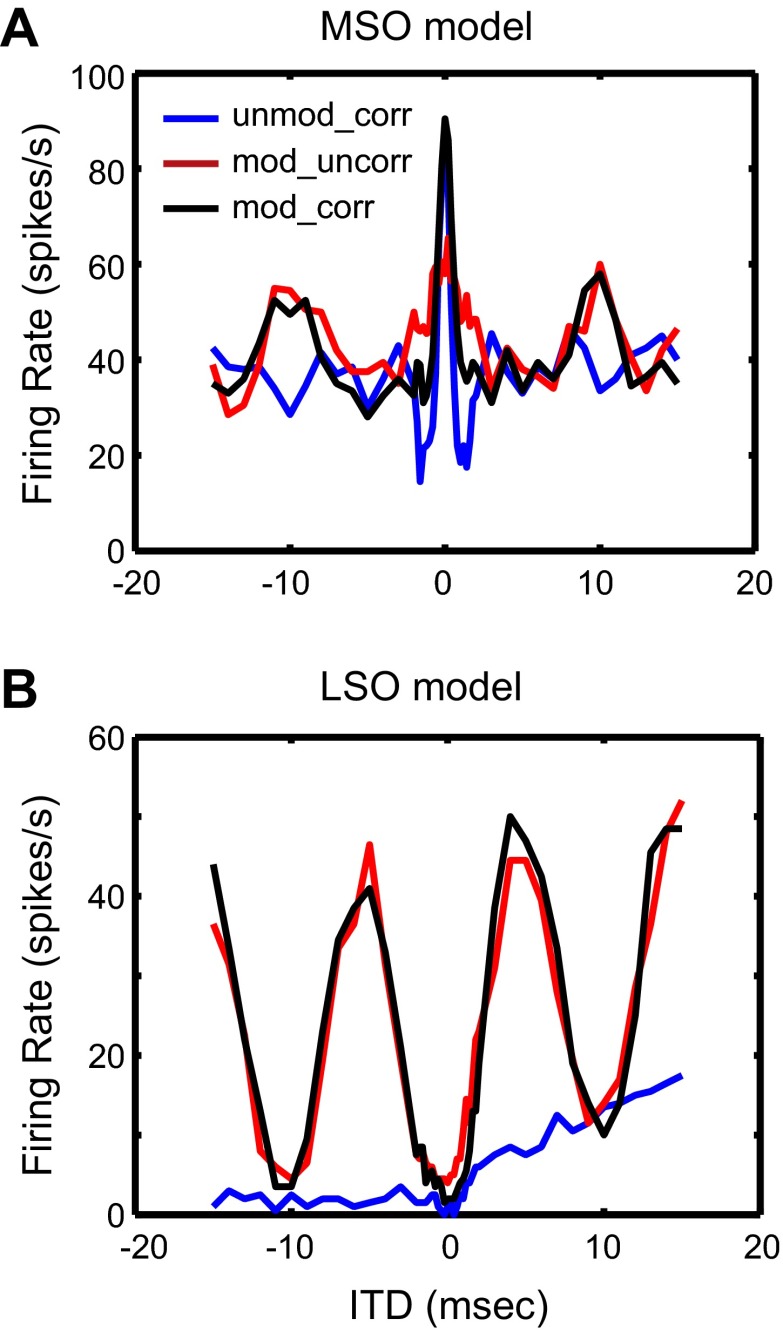
The IC model with convergent inputs from the MSO and the LSO is first used to simulate IC data for SAM tones. This section presents simulation results for inputs from existing HH-type MSO and LSO models. The parameters for the passive membrane properties and the ion channels in the MSO and LSO models were the same as in the original studies, i.e., Colburn et al. (2009) for the MSO and Wang and Colburn (2012) for the LSO. The average firing rate of the AN model Ravg was 150 spikes/s and the synchronization index SI was 0.7, as is typical for physiological measurements from the AN (Joris and Yin 1992, 1998). Typical responses of the MSO and LSO models to binaural SAM tones are shown as a function of fm in Fig. 7. Figure 7A shows the average firing rate for both models, while Fig. 7B shows the corresponding SI with respect to the beat frequency. By construction, the mean interaural response phase was 0 at all fm for the MSO model and 0.5 for the LSO model (not shown).
Fig. 7.
Responses of the HH-type MSO and LSO models to binaural SAM tones. A: binaural rate-fm functions. B: binaural sync-fm functions. Dashed lines show the output of the MSO model, and solid lines show the output of the LSO model. The membrane parameters for these models are given in Table 1, and the synaptic parameters are given here: for the AN to LSO synapse: strE = 1 nS, strI = 1.6 nS, τE = 2 ms, τE_rise = 0.2 ms, τI = 5 ms, τI_rise = 0.5 ms, NE = NI = 20; for the AN to MSO synapse: strE = 15 nS, τE = 0.1 ms, τE_rise = 0.01 ms, NE = 10.
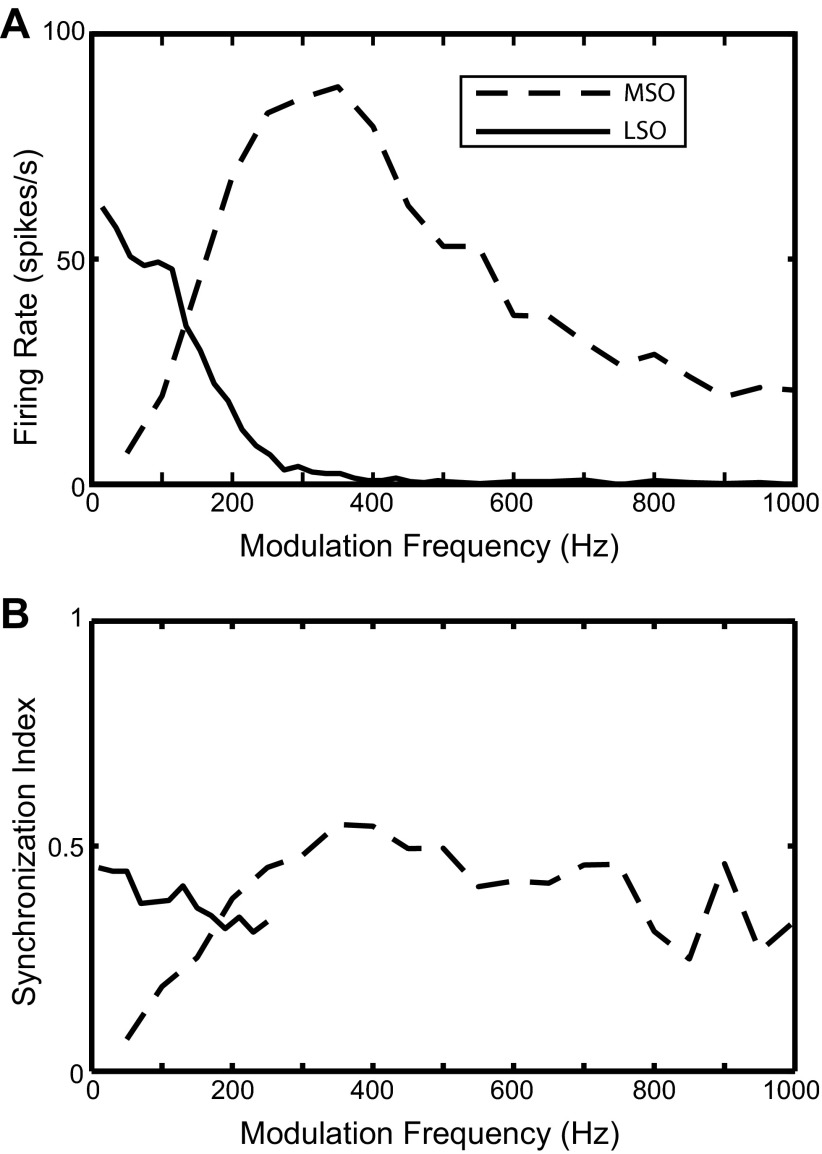
For the MSO model, the simulated binaural rate-fm function has a band-pass shape with a maximum at 350 Hz and steep cutoffs on both sides. The corresponding binaural sync-fm function gradually increases with fm up to 350 Hz and then plateaus around 0.5. To investigate the underlying mechanism for the band-pass characteristic of the rate-fm function, the MSO model was tested with constant current injections. The result shows that the MSO model only produces one spike at the stimulus onset in response to current steps up to 5 nA (not shown). This onset-type response is not caused by either the KLT channel or the Ih channel, since removing these channels did not significantly affect the response of the MSO model to current injections. Instead, the onset response might be created by the interaction between the sodium and potassium channels (both KHT and KLT). When the KLT channel was eliminated and both the Na and KHT conductances were increased by a factor of 8, the MSO model produced sustained response to current injections and the rate-fm function became flatter than in the standard MSO model. These results suggest that the intrinsic properties in the MSO model are likely the main mechanism underlying the onset response to current injections and the band-pass tuning in the firing rate.
For the LSO model, the simulated binaural rate-fm function is low pass, with essentially no response above 400 Hz (Fig. 7A). The SI is close to 0.5 at low fm and decays with increasing fm until it becomes statistically insignificant above 250 Hz (Fig. 7B). The low-pass characteristic of the rate-fm function in the LSO model was produced without a KLT channel. Adding KLT channels did not significantly affect the low-pass profile of the binaural rate-fm function in most cases, although when the KLT channel conductance was strong the rate-fm function sometimes exhibited a high-pass segment at low fm, producing a band-pass fm tuning in the firing rate (results not shown). These results suggest that the KLT channel is not necessary to produce the low-pass feature in the LSO responses to binaural SAM tones. On the other hand, for SAM tones presented monaurally to the ipsilateral ear, the rate-fm functions are also typically low pass but there is more variability across LSO neurons than in the binaural condition. In the monaural condition, KLT channels were shown likely to be the main mechanism underlying the low-pass fm tuning in the firing rate of LSO cells (Wang and Colburn 2012). These contrasting results suggest that the low-pass character of the rate-fm function in response to binaural and monaural SAM tones may be produced by different mechanisms. A likely explanation for the low-pass character of the binaural response is the interaction between ipsilateral excitation and contralateral inhibition received by the LSO. At low fm, both the excitatory and the inhibitory synaptic conductances are phase locked to the modulation. Therefore, when the excitation and inhibition are out of phase, the excitation is only slightly suppressed by the inhibition. At higher fm, the inhibition is no longer phase locked to the modulation because of its long time constant. This unmodulated inhibition suppresses the excitation more effectively than phase-locked inhibition, thus giving rise to a decreasing firing rate as fm increases.
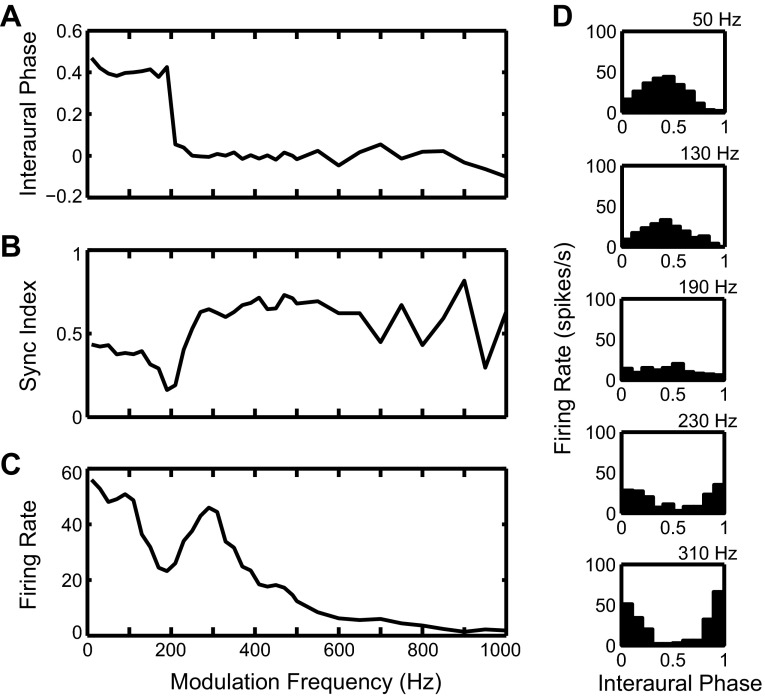
The responses of the MSO and LSO models shown in Fig. 7 were used as excitatory inputs to the IC cell model. Figure 8 addresses the temporal structure of the IC responses. Figure 8, A–C, show measures of temporal structure as a function of frequency, and Fig. 8D shows period histograms with respect to the binaural beat frequency. At low fm (50 and 130 Hz), the period histograms show a single peak around 0.5 cycle, suggesting a dominance of the trough-type input from LSO. At high fm (230 and 310 Hz), the peak of the period histograms shifts to 0 cycle, suggesting a dominance of the peak-type input from MSO. At an intermediate frequency (190 Hz), the period histogram is nearly flat and shows a decreased firing rate compared with responses at lower and higher fm.
Fig. 8.
Responses of the leaky integrate-and-fire (LIF) IC cell model with inputs from HH-type MSO and LSO models. A: interaural phase as a function of fm (phase plot). B: binaural sync-fm function. C: binaural rate-fm function. D: period histogram for 5 fm. The synaptic parameters for the IC model shown in Fig. 3 are given here: for the MSO to IC synapse: strE = 17.5 nS, τE = 1 ms, τE_rise = 0.1 ms; for the LSO to IC synapse: strE = 18.25 nS, τE = 1 ms, τE_rise = 0.1 ms.
The mean interaural response phase (Fig. 8A) shows a sharp transition from 0.5 to 0 for fm around 200 Hz. Both the average firing rate (Fig. 8C) and the synchronization index to the beat frequency (Fig. 8B) show a dip at the frequency of this phase transition, with elevated response on both sides of the dip. Together, these results indicate a transition from trough-type to peak-type response, similar to those seen in the experimental IC data (Fig. 1, top).
The results in Fig. 8 suggest that the IC model with convergent inputs from MSO and LSO can reproduce the trough-type to peak-type transition seen in some IC neurons. The key features of the model are the band-pass firing rate profile for the MSO and the low-pass profile for the LSO. At low fm, the IC model shows the trough-type response inherited from its LSO input. As fm increases, the LSO input rate decreases and the MSO input starts to dominate. The IC model then switches to the peak-type response inherited from its MSO input at high fm. Near the transition frequency, the IC model receives weak inputs that are out of phase, resulting in a low firing rate and a flat period histogram. Note, however, that, although this IC model captures the half-cycle phase transition, it does not reproduce the large CD at low fm (>1 ms) observed in some experimental data (Fig. 1, top). This issue is discussed in the following sections.
The IC model structure of Fig. 2 reproduces the IC responses with trough-type to peak-type transition. Some IC neurons show peak-type responses at low fm and trough-type responses at high fm (Fig. 1, bottom). In principle, a band-pass LSO input combined with a low-pass MSO input could reproduce the peak-type to trough-type transition. The HH-type LSO model used here was able to produce a band pass-shaped binaural rate-fm function when it included a KLT channel (results not shown). The HH-type MSO model, however, never gave rise to a binaural low-pass fm tuning in the firing rate for any of the parameters explored. To reproduce the peak-type to trough-type transition based on low-pass MSO input and band-pass LSO input, the structure of the IC model shown in Fig. 2 was modified. In the modified IC model structure (Fig. 9), the MSO and LSO inputs become inhibitory, and an additional excitatory monaural input (assumed to be from the contralateral CN) was added in order to induce discharges in the IC cell model. While Fig. 9 shows direct inhibitory projections from MSO and LSO to the IC, it is anatomically more likely that one or both of these projections would be implemented via an inhibitory interneuron that is assumed to act as a simple relay (see discussion). The same MSO and LSO inputs based on HH-type models as in Fig. 7 were used in the modified IC model, but now these inputs produced inhibitory PSCs in the IC cell model, and the synaptic strengths and time constants were also different from those used in the standard IC model in Fig. 2 (see Table 2). The additional monaural excitatory input in the modified IC model was the same AN input that was used to drive MSO and LSO models (Ravg = 150 spikes/s, SI = 0.7, φ = 0.5).
Fig. 9.
Diagram of the modified IC model with convergent inhibitory input from the MSO and the LSO and an additional excitatory CN input. Descriptions for the AN, CN, MSO, and LSO modules in the modified model are the same as the model in Fig. 2.
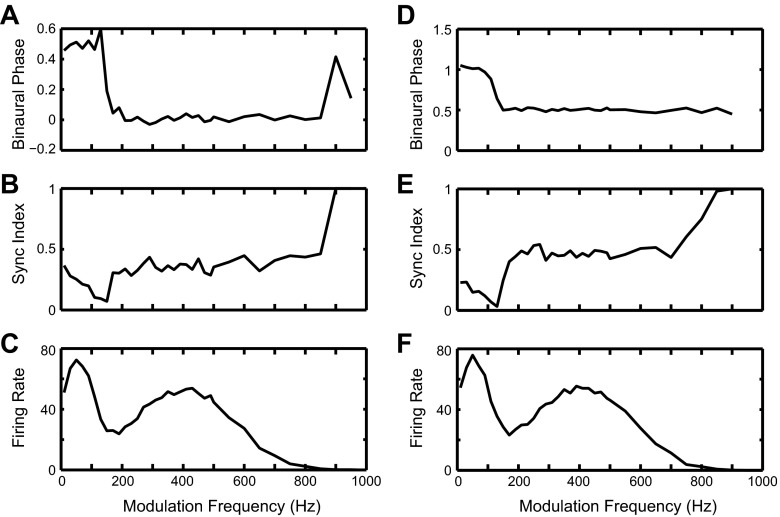
Figure 10 shows the responses of the modified IC model to SAM tones in the same format used for the original model in Fig. 8. The period histograms (Fig. 10D) show a peak near 0 cycle at low fm (30 and 90 Hz), a peak near 0.5 cycle at high fm (150 and 270 Hz), and a flat shape with decreased firing at 110 Hz. The phase curve (Fig. 10A) shows a sharp transition from a little less than 0 to 0.5 for fm around 120 Hz. The synchronization to the binaural beat (Fig. 10B) is substantial for fm above and below the phase transition, with a dip near the transition. The model also shows a broad trough in firing rate around the transition similar to the experimental data (Fig. 10C). These results suggest that the combination of a low-pass LSO input and a band-pass MSO input can also account for the peak-type to trough-type transition observed in some IC neurons, if these inputs are inhibitory and combined with another excitatory input.
Fig. 10.
Responses of the modified IC cell model in Fig. 9 with HH-type MSO and LSO models. Same format as Fig. 8. A: interaural phase as a function of fm (phase plot). B: binaural sync-fm function. C: binaural rate-fm function. D: period histogram for 5 fm. The synaptic parameters for the IC model shown in Fig. 9 are given here: for the MSO to IC synapse: strI = 0.5 μS, τI = 5 ms, τI_rise = 0.5 ms; for the LSO to IC synapse: strI = 0.5 μS, τI = 5 ms, τI_rise = 0.5 ms; for the CN to IC synapse: strE = 20 nS, τE = 1 ms, τE_rise = 0.1 ms.
Results with phenomenological MSO and LSO models.
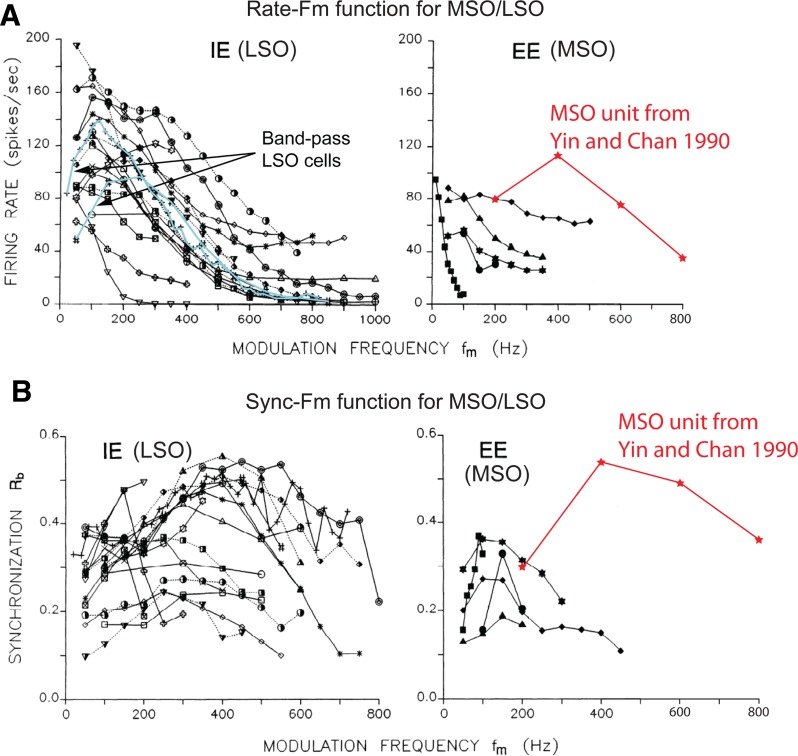
The HH-type LSO model gives rise to both low pass- and band pass-shaped binaural rate-fm functions. In contrast, only band pass-shaped binaural rate-fm functions were obtained with the MSO model. To test the validity of these constraints, the available data for the MSO and LSO are compared to model simulations. Joris (1996) reported binaural SAM tone responses for 21 high-frequency LSO units (CF > 2 kHz) and five MSO units (CF > 5 kHz). The majority of his LSO neurons exhibited prominent low-pass character in their binaural rate-fm functions. A few LSO neurons, however, showed band pass-shaped binaural rate-fm functions (Fig. 11A, left). The binaural sync-fm functions for the LSO units (Fig. 11B, left) were more heterogeneous than the rate-fm functions. Most of the SI values were between 0.2 and 0.5, and the dependence on fm was rather shallow. Experimental data from the MSO in response to binaural SAM tones are much more limited. The firing rates in the five MSO cells from Joris (1996), shown in Fig. 11A, right, all decay as fm increases, i.e., the functions are low pass. The amplitude and steepness of the rate decay vary among these five MSO cells. Yin and Chan (1990) reported the SAM tone response for another high-frequency MSO unit (red curves in Fig. 11, CF = 15 kHz). This unit showed band-pass characteristics in both firing rate and SI, with a peak at 400 Hz.
Fig. 11.
Experimental data for the MSO and the LSO in response to binaural SAM tones. A: binaural rate-fm functions for the LSO (left) and the MSO (right). Each curve represents 1 neuron in the MSO or LSO. Examples of band-pass LSO neurons are highlighted with light blue curves (left). B: binaural sync-fm functions for the LSO (left) and the MSO (right). Each curve represents 1 neuron in the MSO or the LSO. [Figure adapted from Joris (1996) with permission; data for additional MSO unit (red curves) from Yin and Chan (1990).]
The responses of the HH-type MSO and LSO models (Fig. 7) are consistent with the experimental data in some respects. The binaural rate-fm function of the LSO model (Fig. 7A) captures the predominant low-pass trend seen in Fig. 11A, left. The band-pass feature in the binaural rate-fm function of the MSO model is similar to the MSO unit in Yin and Chan (1990) but not to the five units in Joris (1996). The SI values of the model responses and the experimental data are also in the same range. Despite this agreement, the fact remains that some features of the experimental data are not captured by these HH-type models, most notably the fact that all MSO cells in Joris (1996) show a low-pass instead of a band-pass characteristic in their binaural rate-fm function.
To test our IC model using MSO and LSO inputs that represent the full range of the experimental data, phenomenological MSO and LSO models (Fig. 4) were used as inputs to the basic IC model of Fig. 2. Three synthetic binaural rate-fm functions were generated to cover the range of observations from both the MSO and the LSO: sharp low-pass (black solid curve in Fig. 4), broad low-pass (gray curve in Fig. 4) and band-pass (dashed curve, Fig. 4). For all three possible binaural rate-fm functions, the binaural sync-fm function was a constant at 0.25 for the MSO model and at 0.33 for the LSO model. Each MSO binaural rate-fm function was paired with each LSO binaural rate-fm function. Each pair of binaural rate-fm functions was used to generate convergent MSO and LSO inputs to the IC model with the original model structure in Fig. 2.
Among the nine possible pairs of inputs, only two pairs of MSO and LSO inputs reproduced the key features of the data from dual-CP neurons, that is, a half-cycle phase transition and a trough in the firing rate at the transition frequency. The first pair comprises the band-pass MSO input (Fig. 4) and the sharp low-pass LSO input (Fig. 4), which gave rise to IC responses with a trough-type to peak-type transition (Fig. 12, A–C). The second pair comprises the sharp low-pass MSO input (Fig. 4) and the band-pass LSO input (Fig. 4), which produced IC responses with a peak-type to trough-type transition (Fig. 12, D–F). The results in Fig. 12, D–F, suggest that the peak-type to trough-type transition could also be explained by convergent excitatory inputs from the MSO and the LSO, as an alternative to the convergent inhibitory input mechanism used in the modified IC model (Fig. 9). When the IC model responses using other pairs of inputs were examined, the phase plots showed a half-cycle phase transition in some cases, but the corresponding IC firing rate did not show a dip in the transition region (results not shown). These results suggest that only when MSO and LSO inputs are tuned to well-separated fm regions with minimum overlap can they explain the responses of those dual-CP IC neurons that show a dip in their firing rate at the transition frequency. Although all the examples of dual-CP neurons shown by Batra et al. (1993) featured a dip in firing rate at the phase transition, there may exist neurons with little or no dip. Reponses of such neurons could be explained by other pairs of MSO and LSO inputs, e.g., combination of broad low-pass and band-pass inputs.
Fig. 12.
Responses of the IC cell model with inputs from phenomenological MSO and LSO models. A–C: same format as Fig. 8, A–C. The binaural rate-fm function is band pass for the MSO (Fig. 4, dashed curve) and sharp low pass for the LSO (Fig. 4, black solid curve). For the MSO to IC synapse: strE = 18 nS, τE = 1 ms, τE_rise = 0.1 ms; for the LSO to IC synapse: strE = 20 nS, τEe = 1 ms, τE_rise = 0.1 ms. D–F: same format as Fig. 8, A–C. The binaural rate-fm function is sharp low pass for the MSO (Fig. 4, black solid curve) and band pass for the LSO (Fig. 4, dashed curve), opposite to the combination used in A–C. For the MSO to IC synapse: strE = 19 nS, τE = 1 ms, τE_rise = 0.1 ms; for the LSO to IC synapse: strE = 18 nS, τE = 1 ms, τE_rise = 0.1 ms.
Both neurons in Fig. 1 show a rising slope in their phase plots at low fm, a feature that was not reproduced by the IC model. This slope corresponds to a nonzero CD of the IC neuron. Because the interaural delays for the MSO and LSO models used so far were always fixed at zero, it is not surprising that the IC model responses do not exhibit the rising slope at low fm. Figure 13 shows that the nonzero CD in the experimental IC data can be reproduced by introducing nonzero internal interaural delays in the SOC inputs. The experimental phase plots from Fig. 1 are replotted in Fig. 13, A and B, to facilitate comparison. Superimposed upon the data are the phase plots generated by the IC model with convergent inputs from the same pairs of phenomenological MSO and LSO models used in Fig. 12, except that nonzero interaural delays were introduced for the sharp low-pass MSO and LSO inputs. (The interaural delays for the band-pass MSO and LSO inputs were still zero.) For both neurons in Fig. 13, A and B, the IC model not only reproduces the half-cycle phase transition described above but also captures the rising slope at low fm in the phase plot.
Fig. 13.
Measured and simulated phase plots for 3 high-frequency intermediate-type IC neurons. Black dots represent experimental data. Black lines are the linear fit for the experimental data at low fm and high fm. Red curve represents the simulated phase plot of the IC model. A: empirical phase plot is the same as that shown in Fig. 1 (top right). The inputs to the IC model are the same as in Fig. 12, A–C, except that an interaural delay of 1 ms is included in the LSO input. B: empirical phase plot is the same as that shown in Fig. 1 (bottom right). The inputs to the IC model are the same as in Fig. 12, A–C, except that an interaural delay of 3 ms is included in the MSO input. C: empirical phase plot is for a high-frequency IC neuron with nonlinear phase plot [adapted from Batra et al. 1993 with permission]. To simulate this phase plot, the IC model receives 2 LSO inputs. The binaural rate-fm function is sharp low pass for one LSO input (black solid curve in Fig. 4) and band pass for the other LSO input (dashed curve in Fig. 4). The interaural delay was 2 ms for the low-pass LSO input and 0 for the band-pass LSO input. Note the curvature in both empirical and simulated phase plots at low fm (<250 Hz). For the low-pass LSO input to the IC: strE = 18 nS, τE = 1 ms, τE_rise = 0.1 ms; for the band-pass LSO input to the IC: strE = 15 nS, τE = 1 ms, τE_rise = 0.1 ms.
Besides IC neurons with dual CPs, high-frequency IC neurons can exhibit other types of nonlinear phase plots in response to envelope-ITDs (Batra et al. 1993). Some neurons show peak-type or trough-type responses at high modulation frequencies and more complex nonlinear patterns at low modulation frequencies (e.g., Fig. 13C). A version of the IC model with convergent inputs from two phenomenological LSO models can reproduce the phase plot in Fig. 13C if the two LSO inputs have different binaural rate-fm functions and also different CDs (Fig. 13C). In this case, the binaural rate-fm function for one LSO input was sharp low pass and the function for the other LSO input was band pass. The low-pass LSO input had a 2-ms CD, which caused the interaural phase of the IC to deviate from 0.5 at low fm. The band-pass input had a zero CD, which gave rise to a constant interaural phase of 0.5. This band-pass LSO input caused the interaural phase of the IC to recover back to 0.5 when this input became dominant at high fm. This result shows that the IC model structure with convergent inputs can account for nonlinear phase plots in the IC other than those with dual CPs.
Modeling IC Responses to Binaural Broadband Noises
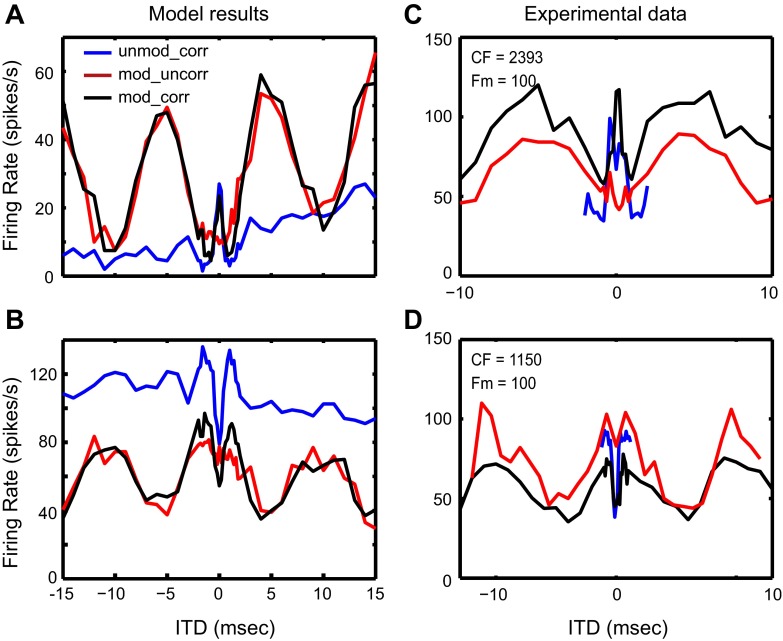
The IC model was also used to simulate the dual types of ITD sensitivity observed in response to binaural noises (Fig. 5, D and E; the same data are replotted in Fig. 15, C and D). For these simulations, the simplified AN model was replaced by a detailed AN model (Zilany et al. 2009) to provide realistic simulations of AN responses to noise. Only HH-type MSO and LSO models were used for these simulations. The membrane descriptions of the MSO, LSO, and IC as well as the synaptic parameters for the AN-MSO and AN-LSO synapses were the same as used in modeling response to binaural SAM tones. The only model parameters that had to be changed to reproduce the IC data for broadband noises were the synaptic strengths for the MSO-IC and LSO-IC synapses (see discussion).
Fig. 15.
A and B: rate-ITD functions of the IC model with inputs from HH-type MSO and LSO models in response to broadband noises. A: IC model responses with convergent excitatory inputs from the MSO and the LSO models (same structure as Fig. 2). The synaptic parameters for this IC model are given here: for the MSO to IC synapse: strE = 18 nS, τE = 1 ms, τE_rise = 0.1 ms; for the LSO to IC synapse: strE = 19 nS, τE = 1 ms, τE_rise = 0.1 ms. B: IC model responses with convergent inhibitory inputs from the MSO and the LSO models and an additional excitatory CN input (same structure as Fig. 9). The synaptic parameters for this IC model are given here: for the MSO to IC synapse: strI = 10 nS, τI = 5 ms, τI_rise = 0.5 ms; for the LSO to IC synapse: strI = 100 nS, τI = 5 ms, τI_rise = 0.5 ms; for the CN to IC synapse: strE = 20 nS, τE = 1 ms, τE_rise = 0.1 ms. C and D: experimental data for the IC in responses to broadband noises. C: same as Fig. 5D. D: same as Fig. 5E.
Responses of the MSO and LSO models to broadband noises are shown in Fig. 14. The MSO model ITD tuning function shows a large peak in firing rate at zero ITD in response to ITDCG and small modulations in firing rate in response to ITDAM (Fig. 14A). The low firing rates and weak sensitivity to ITDAM in the MSO model are consistent with the fm (100 Hz) being below the peak of the band-pass response of the MSO model to SAM tones (Fig. 7). In contrast, the LSO model response exhibits strong modulations in firing rate as a function of the ITDAM and a weak trough-type sensitivity to ITDCG. The strong sensitivity to AM envelope ITDs is consistent with the low-pass response of the LSO model to binaural SAM tones, which gives rise to a high firing rate at 100 Hz (Fig. 7). The low firing rate in response to ITDCG is probably due to the strong contralateral inhibition suppressing the ipsilateral excitation nearly completely for ITDs near zero. The slow increasing trend in the response to ITDCG for positive ITDs results from an onset response due to the delay of the inhibition relative to excitation for positive ITDs, i.e., it represents sensitivity to onset ITD rather than envelope ITD.
Fig. 14.
Rate-ITD functions of the HH-type MSO and LSO models to binaural broadband noises. A: MSO model. B: LSO model. Different colors represent different stimulus conditions (same as Fig. 5). The membrane parameters for these models are given in Table 1, and the synaptic parameters are given here: for the AN to MSO synapse: strE = 15 nS, τE = 0.1 ms, τE_rise = 0.01 ms, NE = 10; for the AN to LSO synapse: strE = 1 nS, strI = 1.6 nS, τE = 2 ms, τE_rise = 0.2 ms, τI = 5 ms, τI_rise = 0.5 ms, NE = NI = 20.
When MSO and LSO responses in Fig. 14 are used to drive the original IC model via excitatory synapses (Fig. 2), the IC model shows a peak-type response to ITDCG and a trough-type response to ITDAM (Fig. 15A), comparable to the experimental data in Fig. 15C. To reproduce the opposite response pattern with peak-type response to ITDAM and trough-type response to ITDCG that is also observed, although less frequently, in the experimental data (Fig. 15D), the modified IC model (Fig. 9) with convergent inhibitory inputs from the MSO and the LSO was used. The resulting IC model responses (Fig. 15B) qualitatively capture the dual types of ITD sensitivity in the IC neuron shown in Fig. 15D. Although the general shapes of the curves in Fig. 15 are similar between the experimental data and the model results, the relative heights of the three curves in the model do not match the neural data. Additional inputs to the IC, such as a monaural inhibitory input, may produce different DC shifts in the three curves and lead to improved matches with the experimental data.
The simulation results for broadband noises presented above were made at a fixed sound level (60 dB SPL), consistent with the experimental IC data, which were also obtained at a single sound level for each neuron. To investigate how sound level affects IC responses, the sound level of the broadband noise was varied, with all parameters of the IC model kept fixed. For both the MSO and the LSO models, the ITD sensitivity to CG envelopes was not significantly affected by sound level; however, the modulation of the firing rate caused by ITDAM was strongest at intermediate levels (60–70 dB) for both the MSO and LSO models. The weak ITDAM sensitivity at low levels is probably due to the low overall firing rate of the input and the concomitant small changes in the firing rate when ITD changes. High sound levels also give rise to weak envelope-ITD sensitivity because saturation at the hair cell synapse decreases the modulation depth of the inputs. The effects of sound level on the IC model response were similar to those on the MSO and LSO models, since the responses of the IC model closely resemble the superposition of the MSO and LSO model responses.
DISCUSSION
The experimental and computational modeling results presented here address interaural sensitivity to the temporal structure of the envelopes of high-frequency waveforms. Specifically, attention was focused on previously published complex responses of IC neurons to SAM tone stimuli (e.g., Batra et al. 1993) and new data on IC neuron responses to broadband noise waveforms. Results show that a relatively straightforward conceptual structure is consistent with almost all aspects of these data.
As shown by the empirical and computational results presented above, the responses to broadband noise in neurons with dual-type ITD sensitivity are consistent with the responses of these neurons to SAM tones as a function of fm. While the dual types of envelope-ITD sensitivity observed by Batra et al. (1993) were separately evoked by SAM tones of different frequencies, our results with SAM broadband noise show that the two types of sensitivity can be simultaneously observed with the same stimulus. Using convergent inputs from the MSO and the LSO, the IC model reproduced the complex forms of ITD sensitivity observed in the data for both SAM tones and broadband noises. For the IC model to reproduce responses to SAM tones, it was critical that the firing rates of the MSO and the LSO inputs be tuned to different fm regions with minimal overlap, i.e., one had to be sharply low pass and the other one had to be band pass. The same IC model could reproduce the IC data for broadband noises, although different synaptic strengths for the SOC to IC synapses were required. In the following sections, these basic conclusions are discussed in more detail, first for the responses to SAM tones and then for the response to broadband noises.
IC Responses to SAM Tones
Comparison with previous IC models with convergent inputs from SOC.
Using a physiologically based computational model of the IC, we showed that convergent inputs from the MSO and the LSO onto single IC neurons are sufficient to create the nonlinear phase plots observed in dual-CP IC neurons in response to SAM tones. The key assumptions in this model are that each IC cell receives independent inputs from SOC neurons with different types of ITD sensitivity (i.e., peak and trough) and different binaural fm tuning. An IC model based on similar assumptions was developed previously to explain the complex phase plots of low-frequency IC neurons sensitive to ITD in the temporal fine structure (Shackleton et al. 2000). In that model, the inputs to the IC cell are either from two MSO cells or from one MSO cell and one LSO cell and the two input cells typically have different CFs. Because of the different frequency tuning between the two inputs, the response of the IC cell transitions from being dominated by the lower-CF input at low frequencies to being dominated by the higher-CF input at high frequencies.
The present IC model is similar to the model of Shackleton et al. (2000) in that both models use two SOC cells with different frequency and ITD tuning to provide convergent input to the IC; however, the two models differ in the origin of the frequency tuning in the input SOC cells. The different frequency tunings for the MSO and LSO cells of Shackleton et al. (2000) directly result from the different CFs of their low-frequency AN fiber inputs, which in turn reflects different sites of innervation along the cochlea. The discharge rates of most high-frequency AN fibers, however, do not show a systematic dependence on fm over the range investigated (Joris and Yin 1992, 1998). Therefore, in our model, the different fm tunings in the binaural rate-fm function of high-frequency MSO and LSO cells are not inherited from the AN inputs. Instead, for the MSO model, intrinsic membrane properties, i.e., the interaction between sodium and potassium channels, appear to be the main mechanism for the band-pass characteristic in the binaural rate-fm function. For the LSO model, the interaction between the faster ipsilateral excitation and the slower contralateral inhibition seems to be the main factor underlying the low pass-shaped binaural rate-fm function.
The two mechanisms for producing different frequency tuning are not mutually exclusive, that is, the CFs of the MSO input and the LSO input could also be mismatched for some high-frequency IC neurons and this CF mismatch could contribute to the nonlinear phase plot of dual-CP neurons. When the CFs of the MSO and LSO inputs are mismatched, the CF of at least one of these inputs must be different from the carrier frequency of the SAM tone stimuli. To test the possible effect of CF on the responses of the MSO and LSO models, the detailed AN model (Zilany et al. 2009) was used to provide inputs to the MSO and LSO models, and the responses to binaural SAM tones were obtained when CF of the AN input to the MSO or LSO model was systematically varied around the fixed carrier frequency of the SAM tone. Results showed that changing the CF of the AN fibers could, in some cases, slightly sharpen the fm tuning in the MSO and the LSO model. As a result, MSO and LSO models with different CFs allowed the IC model to produce slightly improved matches with the experimental data compared with the case when MSO and LSO models with the same CF were used to drive the IC model. However, in general a CF mismatch between the MSO and LSO inputs did not appear to be sufficient by itself to create the different fm tuning in the firing rates of the MSO and LSO inputs.
Alternative mechanisms for creating nonlinear phase plots.
In addition to convergent inputs from SOC discussed above, other mechanisms have been proposed to account for nonlinear phase plots in low-CF SOC neurons. Batra et al. (1997) suggested that additional monaural excitatory and inhibitory inputs with different delays may converge onto coincidence detector cells in the SOC and influence ITD tuning. They developed a model to show that such connections can account for certain types of nonlinearities in the phase plots. It is not clear, however, whether their model can reproduce the dual CPs observed in high-CF IC neurons. Batra et al. (1997) further suggested that phase plots in the SOC and the IC show different kinds of nonlinearities, and, in particular, phase plots with multiple linear segments have only been observed in the IC. Therefore, mechanisms like the one proposed by Batra et al. (1997) that operate at the site of primary binaural interaction using monaural inputs would not be expected to explain the emergent nonlinearities in the IC, including phase plots with dual CPs.
Bonham and Lewis (1999), on the other hand, showed that a simple coincidence detector with CF-mismatched AN inputs can produce a curvature in the phase plot for pure-tone stimuli. Day and Semple (2011) also showed that a model MSO cell with CF-mismatched AN inputs can reproduce the nonlinear phase plots observed in MSO neurons for low-frequency pure tones. The key factor in these two models is the frequency-dependent cochlear delay mismatch induced by the mismatch in the CFs of the AN inputs from the two sides. This mismatch in cochlear delay, however, is likely to be very small for high-CF AN fibers, which have smaller group delays than low-CF AN fibers. In addition, the function relating the group delay to cochlear place is downward convex, meaning that the same CF mismatch (in octaves) will produce a smaller delay mismatch in the high-CF region than in the low-CF region (Joris et al. 2006; Recio-Spinoso et al. 2005). Therefore, the mechanism based on mismatched CFs is unlikely to produce sufficient differences in group delays to account for the nonlinear phase plots of high-CF IC neurons.
Convergent inhibitory inputs from SOC to IC.
We found that convergent inhibitory inputs from peak-type and trough-type neuron models can explain the peak-type to trough-type transition observed in some IC neurons. The connection structure in Fig. 9 was assumed because the binaural rate-fm functions of the HH-type MSO and LSO models were band pass and low pass, respectively, making it impossible to create a peak-type to trough-type transition with purely excitatory inputs. However, direct inhibitory projections from the MSO and the LSO to the IC as assumed in Fig. 9 are undoubtedly an oversimplification of actual neural connections. Most MSO cells contain excitatory neurotransmitters (Kumoi et al. 1993; Oliver et al. 1995), and the contralateral projection from the LSO to the IC is also excitatory (Oliver et al. 1995). Thus the direct inhibitory projections as shown in Fig. 9 are probably physiologically unrealistic. A more likely neural connectivity is that the MSO and the LSO influence the IC response via inhibitory interneurons that could be located either in the DNLL or in the IC itself. The DNLL contains primarily GABAergic neurons that receive inputs from MSO and LSO and project to the IC (Adams and Mugnaini 1984; Helfert and Aschoff 1997). The IC contains many inhibitory interneurons that could influence the responses of principal IC neurons via local circuits (Gonzalez-Hernandez et al. 1996). Although the actual neural connections may differ from cell to cell, the critical feature to obtain a peak-type to trough-type transition is the conversion of excitatory MSO and LSO inputs into inhibitory inputs.
One way to create the peak-type to trough-type transition without using inhibition is to combine a low-pass MSO input and a band-pass LSO input in a single IC cell as shown with the phenomenological MSO and LSO models (Fig. 12, D–F). This mechanism is appealing for its simplicity. Although most of the LSO cells studied by Joris (1996) showed a low-pass characteristic in their binaural rate-fm functions, a few did show band-pass binaural rate-fm function. In addition, all MSO cells in Joris (1996) had low-pass binaural rate-fm functions. So it is possible that some IC neurons generate a peak-type to trough-type transition via convergent input from a band-pass LSO cell and a low-pass MSO cell.
Limitation of the HH-type MSO model.
Our HH-type MSO model predicts band-pass shapes for binaural rate-fm function, consistent with one MSO cell in Yin and Chan (1990), but it does not reproduce the low pass-shaped binaural rate-fm functions observed by Joris (1996) in all of his MSO cells. To rule out the possibility that the limited range of model responses is due to the particular characteristics of our MSO model, we also tested a multicompartment MSO model developed by Mathews et al. (2010). The parameters of this model were adjusted to generate realistic rate-ITD tuning functions for low-frequency pure tones (Brughera et al. 2013). The simplified AN model (based on Poisson processes describing responses to SAM tones) was used to drive the compartmental MSO model. Although the structure and parameters of the two MSO models differed, the multicompartment model also predicted band-pass binaural rate-fm functions. This is not unexpected because both MSO models tested show phasic responses to current injections, i.e., they fire just one spike at the onset. Phasic cell models typically respond poorly to low-frequency inputs (Gai et al. 2010), therefore giving rise to band-pass binaural fm tuning in the firing rate. The low-pass binaural rate-fm functions seen in the majority of available MSO data (Fig. 11A, right) are somewhat unexpected and may be due to certain intrinsic properties in the MSO cell that are not captured by the HH-type MSO models or to additional inputs from lower nuclei. In any case, more data are needed to confirm the prevalence of low-pass fm tuning in high-frequency MSO cells and to elucidate the mechanism underlying the low pass-shaped binaural rate-fm function.
Large internal delay for SOC input to IC.
To account for the nonlinear phase plot of the IC neuron in Fig. 13C, a 2-ms CD was required for the low-pass LSO input that controlled the behavior of the IC model neuron at low fm. Other dual-CP neurons in the IC also show large CDs at low fm (Fig. 1). The CDs measured from MSO and LSO in response to SAM tones were always lower than 1 ms (Joris 1996), but only a small number of neurons have been studied, so it is possible that units with large CDs do exist in the MSO and LSO but have not been sampled yet. Alternatively, such long delays could be generated at sites farther up the auditory pathway than the SOC, such as the DNLL and the IC. For example, an IC neuron with a monaural input from one side and another monaural input from the other side could give rise to long delays (larger than 2 ms) if one monaural input has longer synaptic delays than the other input. If this IC neuron also receives an input from the MSO or the LSO that is tuned to higher fm, this neuron might exhibit intermediate-type responses or dual types of ITD sensitivity with long delays at low fm.
IC Responses to Broadband Noises
Relationship with IC responses to SAM tones.
The IC model presented here suggests that convergent inputs from the MSO and the LSO can account for the dual types of envelope-ITD sensitivity in the IC in response to either SAM tones or broadband noises. One of the IC neurons studied with broadband noise was also examined with binaural SAM tones (Fig. 6). With SAM tone stimulation, this neuron showed a trough-type response to low fm and intermediate-type response to high fm (Fig. 6B). This pattern is consistent with the trough-type ITD sensitivity to slower AM envelopes and the intermediate-type ITD sensitivity to faster CG envelopes (Fig. 6A) obtained with broadband noise stimuli. These very limited data provide direct evidence that the dual types of envelope-ITD sensitivity observed with SAM tones and broadband noises could be due to a common mechanism.
The IC models used to reproduce the SAM tone data and the broadband noise data, respectively, shared the same membrane properties for the MSO, LSO, and IC and the same parameters for the AN-MSO and AN-LSO synapses. The only model parameters that had to be changed to reproduce both sets of IC data were the synaptic strengths for the MSO-IC and LSO-IC synapses. Specifically, for the IC model with excitatory inputs from the MSO and the LSO used to reproduce trough-type to peak-type transitions (Fig. 2), the synaptic strengths of the SOC inputs to the IC were increased by a few percent to account for the noise data; for the IC model with inhibitory inputs from the MSO and the LSO used to reproduce peak-type to trough-type transitions (Fig. 9), the synaptic strengths of the SOC inputs to the IC had to be decreased by at least a factor of five. This parameter difference between the IC models used with SAM tones and broadband noises might be due to the different sound levels used in the two sets of experiments. To explore this hypothesis, the detailed AN model was used as input to the SOC models with SAM tone stimulation so that the sound level could be varied; however, varying the sound level did not lead to a unified IC model with common parameters (results not shown). It is possible that a broader parameter space of the membrane description of the MSO, LSO, and IC neurons has to be explored to reproduce the two sets of IC data using a unified IC model. Alternatively, stimulation with broadband noise might recruit additional inputs to the IC that are not activated by narrowband stimulation with SAM tones and not explicitly included in the IC model structure.
Other response patterns in IC responses to SAM noise.
Besides IC neurons with dual types of ITD sensitivity (Fig. 5, D and E), other IC neurons exhibit complex patterns in responses to ITDAM and ITDCG. For example, some neurons are sensitive to ITDCG but not ITDAM (Fig. 5B). The lack of ITDAM sensitivity in these neurons could be due to the low firing rate of the SOC input in response to the slow envelopes (fm = 40 or 100 Hz) used in the experiments. Some MSO and LSO neurons show band-pass characteristics in their binaural rate-fm functions (Fig. 11), providing a possible basis for low firing rates at low fm. Yet another type of IC neuron shows intermediate-type responses to ITDCG (Fig. 6). The biphasic rate-ITD functions of these neurons may come from a combination of peak-type and trough-type inputs with slightly different interaural delays. Finally, some IC neurons are sensitive to ITDAM, but neither the peak nor the trough in their rate-ITD tuning curve occurs at zero ITD (Fig. 5F). Their responses to AM envelopes may be explained by long interaural delays from SOC inputs or by binaural interactions occurring de novo in the DNLL or IC, as discussed above for IC responses to SAM tones. In summary, the responses of essentially every IC neuron in our sample could potentially be explained by simple combinations of SOC inputs to the same IC cell model.
Relative delay between MSO and LSO inputs.
As discussed above, the inhibitory inputs to the IC in the model structure of Fig. 9 are presumed to come from inhibitory interneurons in the DNLL or the IC even though the simulations ignored the effect of these intervening synapses. In particular, the additional delay in the inhibitory inputs relative to the direct inputs from the SOC was not included. To investigate the effect of delays in the inhibition on the IC responses, one of the inhibitory inputs in the modified IC model (Fig. 9) was delayed relative to the other input (up to 50 ms), creating a relative time shift between the MSO and the LSO inputs. The IC model responses to SAM tones and SAM noises were not significantly affected by the delay in the inhibition (results not shown). This is because, for SAM tones, the MSO and LSO models are tuned to different fm, with the MSO being band pass and the LSO being low pass. Therefore, in the fm range where one input is dominant over the other input, a relative delay between the two inputs does not significantly change the IC response. Furthermore, even in the fm region where both the MSO and LSO inputs are activated, because the delay created by the inhibitory interneurons is much smaller than the binaural beat period (1 s), this delay is not sufficient to greatly affect the IC responses. In the SAM noise cases, consistent with the different fm tuning in response to SAM tones, each SOC input (MSO or LSO) is primarily driven by one type of envelope-ITD. Specifically, the MSO response is strongly modulated by ITD in the faster CG envelope while the LSO response is strongly modulated by ITD in the slower AM envelope (cf. Fig. 14). As a result, the IC model response is dominated by one of the two SOC inputs and is not greatly sensitive to the time shift between the MSO and LSO inputs.
Functional significance of neurons with dual types of envelope-ITD sensitivity.
Neurons with dual types of sensitivity were quite common among IC neurons that were sensitive to ITDCG and ITDAM of broadband noise, forming roughly one-half of the sample. While Batra et al. (1993) did not specify the incidence of dual-CP neurons with SAM tone stimulation, it is clear from the examples shown that different types of ITD sensitivity to low and high fm are common in their data. If, as our modeling suggests, such dual types of sensitivity to envelope-ITD are created via convergent inputs from MSO and LSO, an obvious question is, what is the functional significance of such convergence?
For neurons with dual ITD sensitivity, the sensitivity occurring at higher fms is likely to be the most functionally relevant because the changes in interaural phase within the natural range of ITDs for low fms are too small to significantly modulate the neurons' firing rates. This is shown in the example neurons in Fig. 5, where the variations in firing rate within the ±300-μs natural range of ITDs for the rabbit are much greater for the unmod_corr stimulus (which contains CG envelope ITDs) than for the mod_uncorr stimulus (which has imposed AM). When both ITDCG and ITDAM are present in the mod_corr stimulus, the sensitivity within the natural ITD range resembles that for CG envelope only, with the effect of the imposed AM being mainly that of raising or lowering the overall firing rate. Thus ITD sensitivity to the low fms tested is very weak within the natural range and unlikely to be functionally significant in most situations.
What then is the function of the SOC input tuned to low fms that gives rise to ITDAM tuning in the model? In cases when ITD sensitivity to low frequencies is trough type and sensitivity to high frequencies is peak type, the low-frequency input is thought to come from LSO. While LSO neurons are sensitive to envelope ITDs, they are also sensitive to interaural level differences (ILDs) via an EI interaction (Batra et al. 1997; Boudreau and Tsuchitani 1968; Caird and Klinke 1983; Finlayson and Caspary 1989, Joris 1996; Joris and Yin 1995; Sanes 1990; Tollin and Yin 2005). As suggested by Batra et al. (1993), high-CF IC neurons with trough-type ITD sensitivity at low fms and peak-type sensitivity at high frequencies are therefore likely to be sensitive to both ILD and envelope ITDs within the natural range. Such neurons, which were common in our sample, might play a role in the integration of directional information from ILD and envelope ITD, which are the two main acoustic cues for azimuthal localization at high frequencies. A specific functional role for neurons with peak-type to trough-type transitions is harder to define, as the ITD sensitivity to low-frequency AM, presumably inherited from the peak-type MSO input, would be too weak in most circumstances. Interestingly, such neurons were uncommon in our sample.
In reverberant environments, the ITD sensitivity of high-frequency IC neurons to CG envelope of broadband noise is severely degraded (Devore and Delgutte 2010). In such cases, neurons with trough-type to peak-type transitions would still be sensitive to ILD via their trough-type LSO input. Perceptual and physiological data suggest that ILD is a relatively robust cue in reverberation (Devore and Delgutte 2010; Rakerd et al. 2006). Similar situations would occur in multisource environments, where the spatial separation of two noise sources causes a decorrelation of the ear input signals and their envelopes. This decorrelation in turn leads to major changes in firing rate of IC neurons sensitive to ITD in CG envelopes (Day et al. 2012). If these neurons have dual ITD sensitivity, they could provide a cue to the spatial separation of sound sources via the changes in firing rate resulting from decorrelation, while also preserving directional information through the ILD sensitivity inherited from their putative LSO input. In short, IC neurons with dual ITD sensitivity would be expected to convey information about the complex, dynamic changes in binaural cues occurring in multisource and reverberant environments. Whether the decoding stages further up the auditory pathway can make use of such information remains to be determined.
Conclusion
In summary, we have shown that the dual types of envelope-ITD sensitivity observed in some high-CF IC neurons can be accounted for by a relatively simple IC model. The convergent inputs from MSO and LSO with different binaural fm tuning in firing rate are sufficient to explain the transition between peak-type and trough-type responses in the IC in response to SAM tones. The same neural mechanism can also explain the combination of peak-type and trough-type responses to broadband noises with both ITDCG and ITDAM. Similar mechanisms based on convergent inputs from the SOC have been shown to underlie complex phase plots in low-CF IC neurons (Shackleton et al. 2000), consistent with the contention that the binaural mechanisms are similar between the low- and high-frequency regions (Colburn and Esquissaud 1976).
GRANTS
This work was supported by National Institute on Deafness and Other Communication Disorders Grants 5R01 DC-00100 (H. S. Colburn), R01 DC-002258 (B. Delgutte), P30 DC-004463 (Boston University), and P30 DC-005209 (Massachusetts Eye and Ear Infirmary). S. Devore was partly supported by training grant T32 DC-000038 and by a Helen Carr Peake fellowship from MIT.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.W., S.D., B.D., and H.S.C. conception and design of research; L.W. and S.D. performed experiments; L.W., S.D., and H.S.C. analyzed data; L.W., S.D., B.D., and H.S.C. interpreted results of experiments; L.W. and S.D. prepared figures; L.W. drafted manuscript; L.W., S.D., B.D., and H.S.C. edited and revised manuscript; L.W., S.D., B.D., and H.S.C. approved final version of manuscript.
REFERENCES
- Adams JC, Mugnaini E. Dorsal nucleus of the lateral lemniscus: a nucleus of GABAergic projection neurons. Brain Res Bull 13: 585–590, 1984 [DOI] [PubMed] [Google Scholar]
- Batra R, Kuwada S, Fitzpatrick DC. Sensitivity to interaural temporal disparities of low- and high-frequency neurons in the superior olivary complex. I. Heterogeneity of responses. J Neurophysiol 78: 1222–1236, 1997 [DOI] [PubMed] [Google Scholar]
- Batra R, Kuwada S, Stanford TR. Temporal coding of envelopes and their interaural delays in the inferior colliculus of the unanesthetized rabbit. J Neurophysiol 61: 257–268, 1989 [DOI] [PubMed] [Google Scholar]
- Batra R, Kuwada S, Stanford TR. High-frequency neurons in the inferior colliculus that are sensitive to interaural delays of amplitude-modulated tones: evidence for dual binaural influences. J Neurophysiol 70: 64–80, 1993 [DOI] [PubMed] [Google Scholar]
- Bernstein LR, Trahiotis C. Detection of interaural delay in high-frequency sinusoidally amplitude-modulated tones, two-tone complexes, and bands of noise. J Acoust Soc Am 95: 3561–3567, 1994 [DOI] [PubMed] [Google Scholar]
- Bernstein LR, Trahiotis C. Enhancing sensitivity to interaural delays at high frequencies by using “transposed stimuli.” J Acoust Soc Am 112: 1026–1036, 2002 [DOI] [PubMed] [Google Scholar]
- Bonham BH, Lewis ER. Localization by interaural time difference (ITD): effects of interaural frequency mismatch. J Acoust Soc Am 106: 281–290, 1999 [DOI] [PubMed] [Google Scholar]
- Boudreau JC, Tsuchitani C. Binaural interaction in the cat superior olive S segment. J Neurophysiol 31: 442–454, 1968 [DOI] [PubMed] [Google Scholar]
- Brughera A, Dunai L, Hartmann WM. Human interaural time difference thresholds for sine tones: the high-frequency limit. J Acoust Soc Am 133: 2839–2855, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caird D, Klinke R. Processing of binaural stimuli by cat superior olivary complex neurons. Exp Brain Res 52: 385–399, 1983 [DOI] [PubMed] [Google Scholar]
- Colburn HS, Chung Y, Zhou Y, Brughera A. Models of brainstem responses to bilateral electrical stimulation. J Assoc Res Otolaryngol 10: 91–110, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colburn HS, Esquissaud P. An auditory-nerve model for interaural time discrimination of high-frequency complex stimuli. J Acoust Soc Am 59, Suppl 1: S23, 1976 [Google Scholar]
- Culling JF, Colburn HS, Spurchise M. Interaural correlation sensitivity. J Acoust Soc Am 110: 1020–1029, 2001 [DOI] [PubMed] [Google Scholar]
- Day ML, Koka K, Delgutte B. Neural encoding of sound source location in the presence of a concurrent, spatially separated source. J Neurophysiol 108: 2612–2628, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day ML, Semple MN. Frequency-dependent interaural delays in the medial superior olive: implications for interaural cochlear delays. J Neurophysiol 106: 1985–1999, 2011 [DOI] [PubMed] [Google Scholar]
- Devore S, Delgutte B. Effects of reverberation on the directional sensitivity of auditory neurons across the tonotopic axis: influences of interaural time and level differences. J Neurosci 30: 7826–7837, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson PG, Caspary DM. Synaptic potentials of chinchilla lateral superior olivary neurons. Hear Res 38: 221–228, 1989 [DOI] [PubMed] [Google Scholar]
- Gai Y, Doiron B, Rinzel J. Slope-based stochastic resonance: how noise enables phasic neurons to encode slow signals. PLoS Comput Biol 6: e1000825, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JM, Brown PB. Functional organization of the dog superior olivary complex: an anatomical and electrophysiological study. J Neurophysiol 31: 639–656, 1968 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Hernandez T, Mantolan-Sarmiento B, Gonzalez-Gonzalez B, Perez-Gonzalez H. Sources of GABAergic input to the inferior colliculus of the rat. J Comp Neurol 372: 309–326, 1996 [DOI] [PubMed] [Google Scholar]
- Griffin SJ, Bernstein LR, Ingham NJ, McAlpine D. Neural sensitivity to interaural envelope delays in the inferior colliculus of the guinea pig. J Neurophysiol 93: 3463–3478, 2005 [DOI] [PubMed] [Google Scholar]
- Helfert RH, Aschoff A. Superior olivary complex and nuclei of the lateral lemniscus. In: The Central Auditory System, edited by Ehret G, Romand R. New York: Oxford Univ. Press, 1997, p. 193–258 [Google Scholar]
- Henning GB. Detectability of interaural delay in high-frequency complex waveforms. J Acoust Soc Am 55: 84–90, 1974 [DOI] [PubMed] [Google Scholar]
- Hines ML, Carnevale NT. The NEURON simulation environment. Neural Comput 9: 1179–1209, 1997 [DOI] [PubMed] [Google Scholar]
- Joris PX. Envelope coding in the lateral superior olive. II. Characteristic delays and comparison with responses in the medial superior olive. J Neurophysiol 76: 2137–2156, 1996 [DOI] [PubMed] [Google Scholar]
- Joris PX. Interaural time sensitivity dominated by cochlea-induced envelope patterns. J Neurosci 23: 6345–6350, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris PX, Van de Sande B, Louage DH, van der Heijden M. Binaural and cochlear disparities. Proc Natl Acad Sci USA 103: 12917–12922, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris PX, Yin TC. Responses to amplitude-modulated tones in the auditory nerve of the cat. J Acoust Soc Am 91: 215–232, 1992 [DOI] [PubMed] [Google Scholar]
- Joris PX, Yin TC. Envelope coding in the lateral superior olive. I. Sensitivity to interaural time differences. J Neurophysiol 73: 1043–1062, 1995 [DOI] [PubMed] [Google Scholar]
- Joris PX, Yin TC. Envelope coding in the lateral superior olive. III. Comparison with afferent pathways. J Neurophysiol 79: 253–269, 1998 [DOI] [PubMed] [Google Scholar]
- Kiang NY, Moxon EC. Tails of tuning curves of auditory-nerve fibers. J Acoust Soc Am 55: 620–630, 1974 [DOI] [PubMed] [Google Scholar]
- Klumpp RG, Eady HR. Some measurements of interaural time difference thresholds. J Acoust Soc Am 28: 859–860, 1956 [Google Scholar]
- Kumoi K, Saito N, Tanaka C. Immunohistochemical localization of gamma-aminobutyric acid- and aspartate-containing neurons in the guinea pig superior olivary complex. Hear Res 68: 173–179, 1993 [DOI] [PubMed] [Google Scholar]
- Langford TL. Responses elicited from medial superior olivary neurons by stimuli associated with binaural masking and unmasking. Hear Res 15: 39–50, 1984 [DOI] [PubMed] [Google Scholar]
- Loftus WC, Bishop DC, Oliver DL. Differential patterns of inputs create functional zones in central nucleus of inferior colliculus. J Neurosci 30: 13396–13408, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus WC, Bishop DC, Saint Marie RL, Oliver DL. Organization of binaural excitatory and inhibitory inputs to the inferior colliculus from the superior olive. J Comp Neurol 472: 330–344, 2004 [DOI] [PubMed] [Google Scholar]
- Mathews PJ, Jercog PE, Rinzel J, Scott LL, Golding NL. Control of submillisecond synaptic timing in binaural coincidence detectors by Kv1 channels. Nat Neurosci 13: 601–609, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden D, Pasanen EG. Lateralization of high frequencies based on interaural time differences. J Acoust Soc Am 59: 634–639, 1976 [DOI] [PubMed] [Google Scholar]
- Nuetzel JM, Hafter ER. Lateralization of complex waveforms: effects of fine structure, amplitude, and duration. J Acoust Soc Am 60: 1339–1346, 1976 [DOI] [PubMed] [Google Scholar]
- Oliver DL, Beckius GE, Shneiderman A. Axonal projections from the lateral and medial superior olive to the inferior colliculus of the cat: a study using electron microscopic autoradiography. J Comp Neurol 360: 17–32, 1995 [DOI] [PubMed] [Google Scholar]
- Rakerd B, Aaronson NL, Hartmann WM. Release from speech-on-speech masking by adding a delayed masker at a different location. J Acoust Soc Am 119: 1597–1605, 2006 [DOI] [PubMed] [Google Scholar]
- Recio-Spinoso A, Temchin AN, van Dijk P, Fan YH, Ruggero MA. Wiener-kernel analysis of responses to noise of chinchilla auditory-nerve fibers. J Neurophysiol 93: 3615–3634, 2005 [DOI] [PubMed] [Google Scholar]
- Rothman JS, Manis PB. Kinetic analyses of three distinct potassium conductances in ventral cochlear nucleus neurons. J Neurophysiol 89: 3083–3096, 2003a [DOI] [PubMed] [Google Scholar]
- Rothman JS, Manis PB. The roles potassium currents play in regulating the electrical activity of ventral cochlear nucleus neurons. J Neurophysiol 89: 3097–3113, 2003b [DOI] [PubMed] [Google Scholar]
- Sanes DH. An in vitro analysis of sound localization mechanisms in the gerbil lateral superior olive. J Neurosci 10: 3494–3506, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackleton TM, McAlpine D, Palmer AR. Modelling convergent input onto interaural-delay-sensitive inferior colliculus neurones. Hear Res 149: 199–215, 2000 [DOI] [PubMed] [Google Scholar]
- Tollin DJ, Yin TC. Interaural phase and level difference sensitivity in low-frequency neurons in the lateral superior olive. J Neurosci 25: 10648–10657, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Colburn HS. A modeling study of the responses of the lateral superior olive to ipsilateral sinusoidally amplitude-modulated tones. J Assoc Res Otolaryngol 13: 249–267, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin TC, Chan JC. Interaural time sensitivity in medial superior olive of cat. J Neurophysiol 64: 465–488, 1990 [DOI] [PubMed] [Google Scholar]
- Yin TC, Kuwada S. Binaural interaction in low-frequency neurons in inferior colliculus of the cat. III. Effects of changing frequency. J Neurophysiol 50: 1020–1042, 1983 [DOI] [PubMed] [Google Scholar]
- Yin TC, Kuwada S, Sujaku Y. Interaural time sensitivity of high-frequency neurons in the inferior colliculus. J Acoust Soc Am 76: 1401–1410, 1984 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Carney LH, Colburn HS. A model for interaural time difference sensitivity in the medial superior olive: interaction of excitatory and inhibitory synaptic inputs, channel dynamics, and cellular morphology. J Neurosci 25: 3046–3058, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilany MS, Bruce IC, Nelson PC, Carney LH. A phenomenological model of the synapse between the inner hair cell and auditory nerve: long-term adaptation with power-law dynamics. J Acoust Soc Am 126: 2390–2412, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]