Abstract
Gamma-aminobutyric acid (GABA) is the major inhibitory neurotransmitter in the central auditory system. Sensory thalamic structures show high levels of non-desensitizing extrasynaptic GABAA receptors (GABAARs) and a reduction in the redundancy of coded information. The present study compared the inhibitory potency of GABA acting at GABAARs between the inferior colliculus (IC) and the medial geniculate body (MGB) using quantitative in vivo, in vitro, and ex vivo experimental approaches. In vivo single unit studies compared the ability of half maximal inhibitory concentrations of GABA to inhibit sound-evoked temporal responses, and found that GABA was two to three times (P < 0.01) more potent at suppressing MGB single unit responses than IC unit responses. In vitro whole cell patch-clamp slice recordings were used to demonstrate that gaboxadol, a δ-subunit selective GABAAR agonist, was significantly more potent at evoking tonic inhibitory currents from MGB neurons than IC neurons (P < 0.01). These electrophysiological findings were supported by an in vitro receptor binding assay which used the picrotoxin analog [3H]TBOB to assess binding in the GABAAR chloride channel. MGB GABAARs had significantly greater total open chloride channel capacity relative to GABAARs in IC (P < 0.05) as shown by increased total [3H]TBOB binding. Finally, a comparative ex vivo measurement compared endogenous GABA levels and suggested a trend towards higher GABA concentrations in MGB than in IC. Collectively, these studies suggest that, per unit GABA, high affinity extrasynaptic and synaptic GABAARs confer a significant inhibitory GABAAR advantage to MGB neurons relative to IC neurons. This increased GABA sensitivity likely underpins the vital filtering role of auditory thalamus.
Keywords: gamma-aminobutyric acid, GABAA receptor, inferior colliculus, medial geniculate body
gamma-aminobutyric acid (GABA) is considered the major inhibitory neurotransmitter of the mammalian central nervous system, including sensory systems, where it functions to control gain, improve signal-to-noise, localize environmental cues, and, in general, shape the ascending acoustic message. The inferior colliculus (IC; auditory midbrain) and the medial geniculate body (MGB; auditory thalamus) are key structures of auditory neuraxis. Previous studies demonstrated that acoustic information about stimulus identity is further refined/reduced in single unit recordings from MGB relative to similar IC units recordings (Chechik et al. 2006).
The auditory midbrain (IC) is rich in sources of GABAergic neurotransmission. IC receives ascending GABAergic inputs from the dorsal, intermediate, and ventral nucleus of the lateral lemniscus and the superior paraolivary nucleus of the superior olivary complex (Kulesza et al. 2003; Zhang et al. 1998). In addition, diverse GABAergic neurons form collaterals within IC (Oliver et al. 1994). From a functional perspective, studies in chinchilla cochlear nucleus and IC suggested that glycine and/or GABA could selectively alter near or below best modulation frequency (BMF) responses changing band-pass responses into more low-pass responders. A study by Koch and Grothe (1998) concluded that GABA inhibition sharpened frequency modulation tuning for the majority of neurons in IC of the big brown bat. In addition, GABAA receptor (GABAAR) blockade alters responses to sinusoidal amplitude modulated stimuli (SAM) in IC of rats and bats by exerting a gain control effect on temporal and rate modulation (Burger and Pollak 1998; Caspary et al. 2002). Dynamic control of discharge rate near best frequency was found as the major role for GABA inhibition in IC of chinchilla, guinea pig, and bats (Le Beau et al. 1996; Palombi and Caspary 1996; Park and Pollak 1993). Furthermore, GABAARs have a role in controlling gain of stimulus-specific adaptation in IC (Perez-Gonzalez et al. 2012). In general, the role of GABAergic inhibition in IC involves shaping and controlling gain of responses to a variety of simple and complex acoustic stimuli.
Compared to IC, rat MGB has few intrinsic inhibitory interneurons (∼1%) (Bartlett and Smith 1999; Winer and Larue 1996). The two major GABAergic inputs to MGB are from IC (Ito and Oliver 2012; Peruzzi et al. 1997; Winer and Larue 1996) and the thalamic reticular nucleus (Rouiller et al. 1985). Recently, Saldaña (2013) described unexpectedly large inhibitory projections from subcollicular sources to non-lemniscal auditory thalamus. Descending auditory corticothalamic projections terminate on MGB neurons in a region-specific manner, with each auditory cortical region projecting to specific MGB subnuclei (Andersen et al. 1980; Bajo et al. 1995; Diamond et al. 1969; Kelly and Wong 1981; Lee et al. 2004; Lee and Winer 2005; Pandya et al. 1994; Pontes et al. 1975; Rouiller and de Ribaupierre 1985; Sousa-Pinto and Reis 1975; Winer et al. 2001; Wong and Kelly 1981). A recent series of detailed studies by Bartlett and Wang (2007, 2011) suggest that MGB neurons display unique, complex responses to modulated and click train stimuli compared with neurons in IC. Based on the disparate nature of inputs and known differences in the nature of GABAARs between IC and MGB, it is reasonable to expect distinct differences in functional GABAergic neurotransmission between these two auditory structures. Few studies have directly compared the potency of GABAergic inhibition between these two structures in response to acoustic stimuli in vivo. Based on differences between these structures in the expression and distribution of GABAAR subtypes, a direct IC and MGB comparison of GABA efficacy could provide insights into coding characteristics of these structures.
GABAARs are heteromeric pentamers made up of 19 possible subunits (α1–6, β1–3, γ1–3, δ, ε, θ, π, and ρ1–3). A limited number of GABAAR constructs are prevalent and are regionally distributed in different proportions throughout different brain structures where they display differences in ligand binding affinity, receptor kinetics, Cl− conductance, and subcellular location (Belelli et al. 2009; Brickley and Mody 2012; Bright and Brickley 2008; Semyanov et al. 2004; Walker and Semyanov 2008). Wild-type (2α1, 2β2, and γ2) GABAARs make up as much as 70% of the distribution of GABAAR constructs and represents the overwhelming majority of GABAAR constructs in IC (McKernan and Whiting 1996; Pirker et al. 2000). Immunocytochemical and quantitative receptor binding studies showed that α4- and δ-subunits coexist and are prevalent in significant numbers in sensory thalamic nuclei, including MGB (Belelli et al. 2009; Cope et al. 2005; Richardson et al. 2011). They have not been functionally described in IC. Thalamocortical neurons show unique tonic inhibitory properties mediated by extrasynaptic, α4- and δ-subunit containing, non-desensitizing GABAAR constructs (Cope et al. 2005; Richardson et al. 2011). The survey study by Pirker et al., of GABAAR subunit protein expression, describes high levels of α4- and δ-subunits in MGB, and also found low levels of the GABAAR δ-subunit in IC (Pirker et al. 2000). The nature of the GABAAR subunit construct determines affinity and efficacy for a given ligand. GABAARs with a γ2-subunit mediating rapidly desensitizing inhibitory postsynaptic currents is characterized by a relatively low affinity (EC50 ∼ 6–14 μM) for the endogenous agonist GABA (Farrant and Nusser 2005). In contrast, δ-subunit containing GABAARs (δGABAAR) show slow desensitization kinetics and high affinity (EC50 ∼ 0.3–0.7 μM) for ambient GABA and a higher affinity (EC50 ∼ 30–50 nM) for the selective δGABAAR agonist gaboxadol (GBX) (Farrant and Nusser 2005; Meera et al. 2011).
Based on its critical role in sensory gating and the presence of high-affinity extrasynaptic GABAARs, one might predict an enhanced sensitivity to GABA inhibitory neurotransmission in the sensory thalamus relative to other auditory structures. To test this hypothesis, four distinct sets of experiments were designed as follows: 1) In vivo iontophoretic unit recordings comparing GABA potency in IC and MGB; 2 and 3) In vitro whole cell patch-clamp and receptor binding-assessing agonist evoked Cl− currents and channel activations in IC and MGB slices; and 4) Ex vivo proton magnetic resonance spectra (1H-MRS) comparing endogenous GABA levels.
MATERIALS AND METHODS
All experiments were completed using Fischer Brown Norway (FBN) or Long-Evans (LE) male rats maintained on an ad libitum diet and reversed light-dark cycle. Procedures were done in accordance with protocols (No. 41-10-002 and 41-09-024) approved by the Laboratory Animal Care and Use Committee of Southern Illinois University School of Medicine.
The FBN and LE rats were 4–10 mo of age and considered adult rats, based on average life span (Schroeder et al. 1965; Turner and Caspary 2005).
Iontophoresis.
Thirty-seven adult male FBN rats (4–6 mo) were initially anesthetized with injection (1.4 ml/kg im) of a 3:1 mixture of ketamine-HCl (100 mg/ml) and xylazine (20 mg/ml). Anesthesia was maintained with intraperitoneal injections of 100% urethane initially 1.3 ml/kg, and then maintained at one-third of the initial amount (booster doses). Urethane was chosen as the anesthetic agent because its actions are on multiple neurotransmitter systems rather than simply potentiating the effects of inhibitory systems, thus it has less net effect on GABAergic neurotransmission than barbiturates or other anesthetic agents (Hara and Harris 2002). Rats were placed in a modified stereotaxic frame in an IAC sound-attenuating booth. For MGB, a 2 × 2-mm craniotomy was drilled, exposing the dorsal surface of the cortex (−5.5 mm, bregma; 3.5-mm lateral from midline). The IC was approached dorsally by exposing the calvarium just rostral to the lambda and lateral to the midline (2 mm) at a 15∼20° angle (Caspary et al. 2002). A carbon fiber electrode attached to a five-barrel iontophoretic electrode, Carbostar-6 (Kation Scientific, Minneapolis, MN), was coupled to the headstage, then to a preamplifier, and controlled by a PC-based Multichannel Acquisition Processor (MAP) system running MAP software (Plexon, Dallas, TX). Spikes were visualized using Sort Client (Plexon) for real-time spike sorting. A piezoelectric driver (David Kopf Instruments, Tujunga, CA) advanced the electrode to the dorsal aspect of MGB or IC using a broadband noise (BBN) search signal. Single units (3:1 SNR) were discriminated based on waveform morphology and/or principal component analysis. In a few cases, small clusters were studied. Stimulus presentation, real-time data display, and analysis used Auditory Neurophysiology Experiment Control Software (ANECS; Ken Hancock, Blue Hills Scientific, Boston, MA) coupled to TDT System III hardware. Acoustic signals were amplified (ED1), transduced (EC1), and juxtaposed to the right ear canal using polypropylene tubing. The sound system was calibrated offline using a quarter-inch Bruel & Kjaer model 4938 microphone (Naerum, Denmark) into a simulated rat ear (2–46 kHz ± 2 dB) (Palombi and Caspary 1996). SAM carrier frequency was set at the unit's characteristic frequency (CF) or BBN; rate modulation transfer functions (rMTFs) were determined for each unit at 30 dB above CF threshold in response to 2-s SAM stimuli (4-ms raise-fall time, 100% depth) with modulated frequency (fm) stepped between 2 Hz and 512/1,024 Hz. Stimuli were 450 ms in duration (presented randomly across the trial among different fms) with spikes collected over a 500-ms period following stimulus onset (10 stimuli/envelope frequency). Multi-barrel iontophoretic electrodes were coupled to a constant current system (BH-2 Neuro Phore System). The balancing barrel was filled with KAc (2 M); other barrels were filled with GABA (500 mM, pH 4.0; Sigma-Aldrich, St. Louis, MO) and GBX (10 mM, Sigma-Aldrich, St. Louis, MO). Retaining currents were set at −15 nA with ejection currents between 0 and 100 nA. A reversible change greater than 15% of control was considered a positive drug effect. Neurons reported here showed full baseline recovery following cessation of drug application. Repeated runs were frequently used to confirm small effects.
Rats were cardiac perfused with phosphate-buffered saline (0.1 M, pH 7.4) followed by paraformaldehyde (4%). Brains were removed, placed in paraformaldehyde (1–2 h), transferred to sucrose (20%) overnight, sectioned at 50 μm, and stained with fast thionin for localization of recording sites (Palombi and Caspary 1996).
Patch clamp.
Patch-clamp experiments were conducted using procedures and equipment previously described (Kalappa et al. 2010; Richardson et al. 2011). Briefly, adult male FBN rats (4–6 mo) were anesthetized with 2.5–3.0% isoflurane gas and decapitated. Their brains were rapidly removed and transferred to ice-cold sucrose-rich solution (in mM): 250 sucrose, 3 KCl, 1.23 NaH2PO4, 5 MgCl2, 0.5 CaCl2, 26 NaHCO3, 10 glucose (pH 7.4). Horizontal sections, 200–300 μm, containing either the central nucleus of IC or the ventral division of MGB were prepared using a Vibratrome 1000 Plus (Leica Microsystems, Wetzlar, Germany). Postsectioning, slices were transferred to a storage chamber, perfused (30 min) at 30°C with artificial cerebrospinal fluid (ACSF) (in mM): 125 NaCl, 3 KCl, 1.26 NaH2PO4, 2 CaCl2, 1 MgCl2, 26 NaHCO3, 10 glucose, and maintained at room temperature for up to ∼8 h. For patch-clamp recordings, slices were transferred to the recording chamber and perfused with ACSF at a rate of 1 ml/min. A MultiClamp 700B amplifier, with a Digidata 1440A A/D converter (Molecular Devices, Sunnyvale, CA) was used to sample data (10 kHz) with a 2.2-kHz low-pass filter. Patch pipettes were pulled using Sutter P-97 horizontal puller (Sutter Instruments, Novato, CA) with a resistance of ∼4–6 MΩ when filled with the internal solution. Following the formation of a stable gigaohm seal (>2 GΩ), whole cell configuration was established. To measure the membrane capacitance (Cm), membrane resistance (Rm), and access resistance (Ra), a 10-mV depolarizing step voltage command was applied using the membrane test function integrated in pClamp10 software (Molecular Devices). Voltage-clamp recordings were made at room temperature with a −10-mV holding potential to enhance our ability to detect Cl−-mediated outward currents. To reduce noise induced by K+-mediated currents, a Cs-methanesulfonate-based internal solution was used (in mM): 140 CsMeSO3, 6 NaCl, 2 MgCl2, 2 Mg-ATP, 0.3 Na-GTP, 10 HEPES, 0.3 CsOH (pH 7.4). GBX and gabazine (GBZ) (Sigma-Aldrich, St. Louis, MO) were bath applied. Membrane voltages were not corrected for the liquid junction potentials: VLJ (CsMeSO3) = 9.8 mV (Kalappa et al. 2010). Access and series resistance was not compensated for in these voltage-clamp experiments. Analysis of data was conducted offline using Clampfit 10.2.
[3H]TBOB binding.
[3H]t-butylbicycloorthobenzoate ([3H]TBOB) binding used increasing concentrations of GABA (0 nM to 5 μM) to modulate picrotoxin sites within the GABAAR chloride channel in MGB and IC. Concentrations based on Milbrandt et al. (1996) were centered near the Kd, which optimized the potential to quantify binding differences in these two structures. In brief, adult male FBN rats (4–6 mo) were decapitated; brains were rapidly removed and frozen. Brain slices (16-μm thick) were cut using a Leica CM1850 cryostat (Leica Microsystems, Buffalo Grove, IL) at −18°C and stored at −20°C. Sections were prewashed in a buffer containing 50 mM Tris-HCl and 1 mM EDTA (pH 7.4) and placed in the incubation buffer (50 mM Tris-HCl, and 120 mM NaCl, pH 7.4) with [3H]TBOB and GABA (concentration from 0 nM to 5 μM) for 90 min at room temperature. Cold picrotoxin (20 μM) was added as a displacer. Autoradiograms were generated by apposing the slides to a phosphor screen, subsequently scanned using a Cyclone phosphor system (Packard BioScience, PerkinElmer, MA). Images were collected at 600 DPI, and the area of MGB and IC was identified and outlined. Binding intensity was analyzed using OptiQuant image analysis software, which provides tools for grayscale quantification in digital light units (DLU). DLU were converted into fmol/mg protein using a standard curve generated from coexposed 14C-embedded plastic standards (ARC, St. Louis, MO). For detailed methods see Milbrandt et al. 1996.
Spectroscopy.
Seven adult (mean age 10 mo) male LE rats (Harlan, Indianapolis, IN) were used in ex vivo spectroscopy studies. Volume-limited 1H-MRS were obtained using a vertical bore Varian Unity/Inova 600 mHz NMR spectrometer with a 14.1 T magnet. Calibration spectra were determined for GABA standards (10 mM) dissolved in sterile normal saline. Volume of interest (VOI) GABA levels were determined using the integrated area of the spectral peak in closest approximation to 2.2 ppm (i.e., the calibration standard). Animals were treated 10 min prior to euthanasia with 10 mg/kg ip 3-mercaptopropionic acid (product M5801; Sigma-Aldrich, St. Louis, MO) to arrest postmortem GABA inflation (van der Heyden and Korf 1978). Immediately before acquisition, animals were given a lethal dose of anesthetic (Euthasol; Virbac, Ft. Worth, TX), decapitated, the mandible removed, and excess muscle tissue dissected away from the skull. The head was placed in a polyethylene holder along with a 1-mm-diameter glass capillary filled with CuSO4 (3 mM). The CuSO4 image phantom unambiguously indexed laterality. For each animal, an initial MRI brain scan was used to locate the VOI for 1H-MRS. Contiguous transverse (i.e., coronal) slices, 0.5 mm thick (26-μm planar resolution), were obtained, extending 13 mm caudally from bregma (26 slices total). VOI for 1H-MRS were determined in ventral MGB (vMGB), dorsal MGB (dMGB), and IC. Spectra, as TIFF images, were imported into Image J (ver. 1.44p, http://imagej.nih.gov/ij). Peaks in closest approximation to the calibration peak for GABA were outlined, and the area under each curve (AUC) was determined. AUCs were expressed in spectrum baseline units (i.e., curve lower bound) to correct for image gain. For detailed methods see Brozoski et al. 2012.
RESULTS
GABAAR endogenous and selective agonists alter SAM responses in MGB.
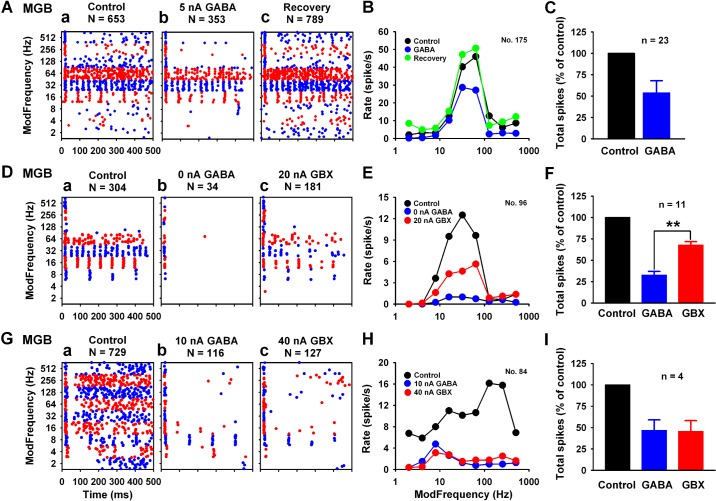
SAM stimuli were used to elicit auditory responses in extracellular recordings from MGB units in anesthetized rats. Figure 1 displays examples of the iontophoretic paradigm used to assess potency of GABAARs. A representative unit showing a bandpass response pattern (BP) (30% of MGB response types) displayed a 46% [(653−353)/(653) × 100%] reduction of SAM-evoked activity with low-dose GABA application (Fig. 1A). A significant decrease in spike rate was evident during drug application near BMF in both the dot raster and the rMTF (Fig. 1, Ab and B). Application of GABA onto 23 MGB BP units (average GABA dose 8.17 ± 1.57 nA) resulted in a 46.24 ± 2.97% decrease of SAM responses from rMTFs at 30 dB above threshold (Fig. 1C). GABA dose was calculated as the smallest dose that produced a greater than 15% change in response. Another MGB SAM response type termed “Mixed” type (Fig. 2C, 35% of the MGB response types) showed a similar reduction in discharge rate of 48.15 ± 3.71% for 31 Mixed units at an average GABA dose of 10.97 ± 2.02 nA (data not shown here). Together, MGB neurons showed exquisite sensitivity to iontophoretic application of GABA.
Fig. 1.
In vivo: effects of the endogenous agonist, gamma-aminobutyric acid (GABA), and the selective agonist, gaboxadol (GBX), application onto medial geniculate body (MGB) units. MGB unit is sensitive to application of the endogenous agonist GABA (A–C, Db, Gb). Ab: 5 nA GABA resulted in a near 50% decrease in spike rate, as shown in dot raster display. B: GABA application showed a selective suppression at or near rate best-modulated frequency (rBMF). C: average total spike change by low-dose GABA (less than 10 nA) for 23 units was 46.24 ± 2.97%. Application of the δ-subunit selective agonist GBX onto MGB units showed varying responses. Average total spikes were 32.59 ± 4.86% under GABA application and 67.15 ± 4.62% under GBX application. F: GABA showed greater reduction of spike rate than GBX for majority units (11/15). The representative unit in D showed profound spike suppression with 0 nA (leaking) GABA and a smaller effect with 20 nA GBX onto the same unit. F: group data indicated that applied GABA was almost twice as potent as GBX (**P < 0.01, independent t-test). There was a small number of units (4/15) that had similar levels of suppression with applied GABA and GBX (G–I). In the representative mixed type unit (G), GABA and GBX show a similar pattern of reduction in discharge rate (15.91% vs. 17.42%) across different modulation frequencies (H). I: group data for this response type indicated the same effects for GABA and GBX (P > 0.5, independent t-test). Data shown as means ± SE. N, number of spikes; n, number of units in all figures.
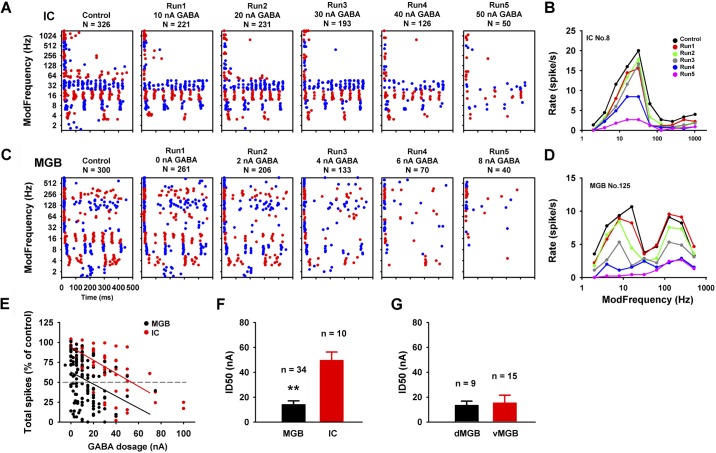
Fig. 2.
In vivo: ability of half-maximal inhibitory concentrations of GABA to inhibit sound-evoked responses in inferior colliculus (IC) and MGB. Dot raster displays sound-evoked responses inhibited by increasing iontophoretic doses of GABA for representative IC (A) and MGB (C) units. Response rates were plotted against modulated frequency (fm) (B and D) for each unit. E: total spikes for each run were calculated as percent of control. Gray dash-line indicates a 50% reduction from the control condition. Higher dosages of GABA were needed to suppress 50% of the responses for IC units (red line). Group ID50 shown in F, mean values 49.56 ± 6.75 nA for IC and 14.01 ± 3.00 nA for MGB, suggested that MGB units were significantly more sensitive than IC units to GABA application (**P < 0.01, independent t-test). No sensitivity difference was found between dorsal and ventral subdivisions of the MGB (G). Data shown as means ± SE.
The selective δGABAAR agonist, GBX, was applied to MGB neurons to test whether more selective activation of extrasynaptic GABAARs would differ from responses to GABA, a less selective endogenous agonist. Responses to both GABA and GBX applications, with recovery to control discharge rates, were obtained from 15 MGB units responding to SAM stimuli. The suppression of SAM responses by GBX was similar but somewhat smaller and less selective than anticipated. The majority of units (11/15) showed greater reduction in total spikes with application of GABA than with application of GBX (Fig. 1, D–F). Average total spikes were 32.59 ± 4.86% under GABA application and 67.15 ± 4.62% under GBX application (Fig. 1F). However, four units showed similar levels of inhibition to the two agonists (Fig. 1, G–I). Due to limitations of the iontophoretic technique, relative sensitivity of GABAARs to different GABA and GBX dose levels is considered difficult to compare (Krogsgaard-Larsen et al. 2004; Mortensen et al. 2004). Based on the present data, both GABA and GBX could inhibit MGB auditory response to a certain extent, but GABA appeared more efficacious than GBX.
GABAAR sensitivity: IC and MGB units, an in vivo comparison.
Experience from prior IC iontophoretic studies (Caspary et al. 2002; Palombi and Caspary 1996), initial MGB iontophoretic studies, and the studies described above suggested that MGB neurons are more sensitive to GABA application than IC neurons. To quantify these suspected GABAAR sensitivity differences, we examined the impact of increasing GABA dose (dose response) using the same electrodes and GABA concentrations to minimize possible experimental design or methods differences in a comparative IC vs. MGB single unit study. Figure 2 compares responses from representative IC and MGB units to GABA application in response to SAM stimuli.
Increasing GABA doses were applied to each unit resulting in increased suppression/inhibition of firing for both units (Fig. 2, A and C). Note that the MGB unit in Fig. 2C responds at very low doses (from leaking, “0” nA) of GABA with increasing suppression of driven activity. A clear decrease in responses at both lower and higher fm could be seen at the 4-nA dose (Fig. 2C). Only onset responses remained relatively unaffected at higher GABA doses. GABA also inhibited SAM responses of IC units but required higher doses of GABA (Fig. 2A). The patterns of suppression of rMTFs in IC units were similarly altered by GABA application with the greatest suppression observed at or near BMF for both structures (Fig. 2, B and D). IC and MGB dose sensitivity to GABA application was quantified by plotting dose against total spikes for each run (normalized) (Fig. 2E). Regression lines showed a dose-dependent response to GABA application. The 50% inhibition dosage (ID50) was calculated based on the normalized spike rate. Higher iontophoretic GABA doses were needed to achieve a 50% reduction of discharge rate for IC units (Fig. 2E). The mean ID50 was significantly lower (14.01 ± 3.00 nA) for MGB units compared with IC units (49.56 ± 6.75 nA) (**P < 0.01, independent t-test) (Fig. 2F). We also compared ID50 for GABA sensitivity between the two major MGB divisions for nine well-localized dMGB and fifteen vMGB units (Fig. 2G). No significant ID50 differences in GABAAR sensitivity were found (P > 0.05, independent t-test).
Tonic currents mediated by extrasynaptic GABAARs: IC and MGB, an in vitro comparison.
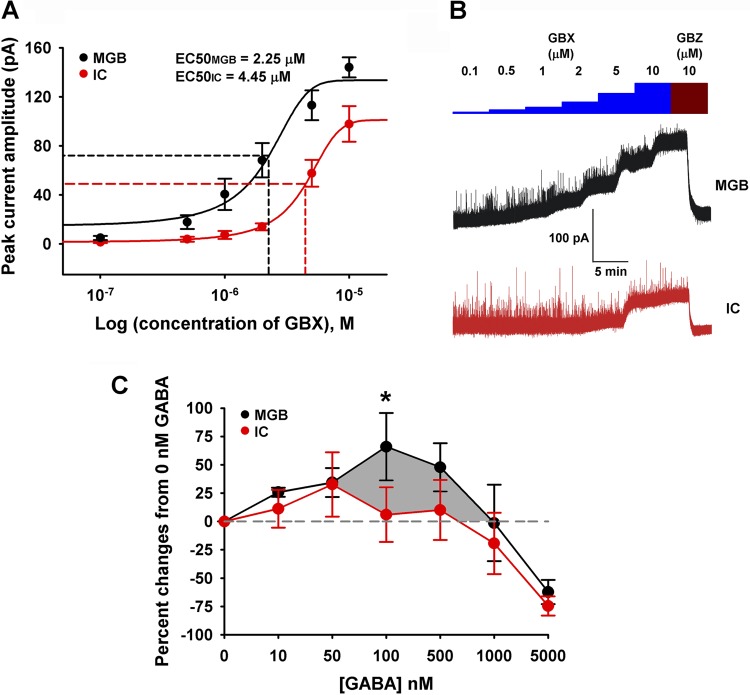
To compare the relative sensitivity of GBX-sensitive extrasynaptic GABAARs in IC and MGB, whole cell voltage-clamp recordings from IC (± SE, n = 5; Cm, 75.5 ± 3.19 pF; Rm, 376.45 ± 59.18 MΩ; Ra, 19.85 ± 1.27 MΩ) and MGB (± SE, n = 5; Cm, 99.75 ± 8.83 pF; Rm, 349.25 ± 36.06 MΩ; Ra, 17.5 ± 1.68 MΩ) neurons from adult slices were conducted with CsMeSO4 internal solution (see materials and methods). Once a stable whole cell configuration was established, neurons were held at −10 mV to maximize visualization of tonic Cl− currents upon activation of extrasynaptic δ-GABAARs. Bath application of increasing doses (0.1–10 μM) of GBX, a δ-GABAARs subunit-selective agonist, evoked tonic currents from both IC and MGB neurons in a dose-dependent manner. The amplitude of GBX-evoked tonic currents were revealed by addition of 10 μM GBZ, a GABAAR antagonist (Fig. 3B). Comparison of dose response curves revealed that GBX was significantly more potent in activating tonic currents in MGB neurons than in IC neurons [EC50MGB = 2.25 μM; EC50IC = 4.45 μM; F (1, 54) = 30.17, P < 0.0001; F-test] (Fig. 3A). Maximal mean peak currents (± SE) for MGB and IC were 144.02 ± 8.15 pA and 97.80 ± 14.51 pA, respectively. These findings are consistent with predictions based on immunocytochemical GABAAR subunit protein distribution studies by Pirker et al. (2000) that suggested higher relative densities of δGABAARs in MGB than in IC.
Fig. 3.
In vitro: GBX induced tonic inhibition and [3H]t-butylbicycloorthobenzoate ([3H]TBOB) binding in brain slices. Whole cell patch-clamp recordings from 5 IC and 5 MGB neurons from adult slices were used to compare the relative sensitivity of extrasynaptic GABAARs in IC and MGB neurons. A: tonic currents were plotted against increasing dosages of GBX. GBX was significantly more potent in activating tonic currents in MGB units than in IC units [EC50MGB = 2.25 μM; EC50IC = 4.45 μM; F (1, 54) = 30.17, P < 0.0001; F-test]. B: bath application of increasing doses (0.1–10 μM) of the δ-GABAAR subunit-selective agonist, GBX, evoked tonic currents in both IC and MGB neurons. Amplitudes of GBX-evoked tonic currents were revealed by addition of 10 μM gabazine (GBZ). C: modulation of [3H]TBOB channel (picrotoxin) binding with increasing concentrations of GABA (0 nM to 5 μM) was performed on IC and MGB slices. 0 nM GABA was set as the control condition and represented the resting/control openings of GABAAR Cl− channels. At low concentrations, both structures showed increased binding indicative of increased GABAAR, Cl− channels openings. Peak percent increase in binding occurred at 100 nM for MGB (*P < 0.05, 2-way ANOVA) and 50 nM for IC, with a significantly larger area (shadow) under the MGB curve (black) suggesting a greater MGB neuronal total chloride flux capacity relative to IC neurons. Both MGB and IC showed desensitization (1,000 nM and 5,000 nM) reflecting a greater percentage of closed GABAAR Cl− channels than in the control condition.
One limitation of this approach is the difference in passive membrane properties of IC and MGB neurons. Although IC and MGB neurons exhibited comparable membrane resistance, IC neurons demonstrated lower membrane capacitance than MGB neurons. However, comparison of EC50 values from normalized dose response curves of GBX-evoked tonic currents (i.e., response net amplitude per unit of membrane capacitance) from IC (EC50IC = 4.91 μM) and MGB (EC50MGB = 1.18 μM) neurons demonstrated that GBX was still significantly more potent [F (1, 64) = 76.68, P < 0.0001; F-test] in activating tonic currents in MGB neurons than in IC neurons (normalized curves not shown). A second limitation of this approach is that the voltage-clamp experiments were conducted under the assumption that space clamp in both MGB and IC neurons were similar and adequate. Although the extent of space clamp may differ between these neurons, it would be safe to assume that IC neurons exhibit better space clamp due to lower membrane capacitance than MGB neurons. Hence, if any discrepancies in peak amplitude estimation were to occur due to inadequate space clamp, the tonic current amplitudes of MGB neurons may be somewhat underestimated compared with IC neurons, which are likely to exhibit better space clamp. Overall, these observations suggested that the differences in adequacy of space clamp and membrane capacitance in IC and MGB neurons may have subtle influence on the absolute values but may not significantly affect the overall conclusion of this voltage-clamp study.
Modulation of binding at the GABAAR picrotoxin site: IC and MGB, in vitro comparison.
An estimate of total GABA-evoked chloride flux was assessed using a ligand that binds in the open GABAAR chloride channel at the picrotoxin binding site. The ability of [3H]TBOB, a picrotoxin competitive analog (Lawrence et al. 1985), to bind at, and be modulated by increasing concentrations of GABA (0 nM-5 μM), was used to compare the maximum number of available GABAARs in IC vs. MGB. Figure 3C compared the [3H]TBOB modulation curves for MGB and IC with 0 nM GABA set as the normalized control condition. This represents the resting/control condition for the open/closed state of GABAAR Cl− channels. At low GABA concentrations, both structures showed increased binding indicative of increased activation/openings of GABAAR Cl− channels (Fig. 3C). Opened channels allow for increased binding at picrotoxin sites in the GABAAR Cl− channel of the originally closed GABAAR Cl− channels. Significant differences between IC GABAARs and MGB GABAARs were seen at 100 nM (*P < 0.05, 2-way ANOVA). The larger area under the MGB curve (from 50 nM to 1,000 nM) suggested that MGB neurons have a greater total chloride flux capacity relative to IC neurons (Fig. 3C). Both MGB and IC showed desensitization at higher GABA concentrations following peak values that reflected a high percentage of closed GABAAR Cl− channels at 1,000 nM and 5,000 nM.
GABA level by 1H-MRS: IC and MGB, an ex vivo comparison.
Improvements in magnetic resonance spectroscopy enabled the acquisition of well-resolved spectra from small tissue volumes, as documented in a recent study (Brozoski et al. 2012). Absolute GABA tissue levels were obtained from IC and MGB using high-resolution point-resolved proton magnetic resonance spectroscopy (1H-MRS) (Fig. 4A). IC, vMGB, and dMGB were selected separately in each rat, and data from left and right hemispheres were combined. Mean GABA concentrations for each structure were dMGB = 3.72 ± 1.13, vMGB = 3.05 ± 0.86, and IC = 1.50 ± 0.29 (Fig. 4B, presented as mM, means ± SE). A nonsignificant trend toward higher GABA levels was found for both vMGB and dMGB compared with GABA levels in IC. An independent t-test showed a P value of 0.05107 (IC vs. dMGB) and 0.0809 (IC vs. vMGB) between IC and MGB. A similar comparison (Brozoski et al. 2012) found GABA levels in MGB overall were significantly higher than in IC (P = 0.011). Both results suggest elevated GABA level in the auditory thalamus relative to the IC.
Fig. 4.
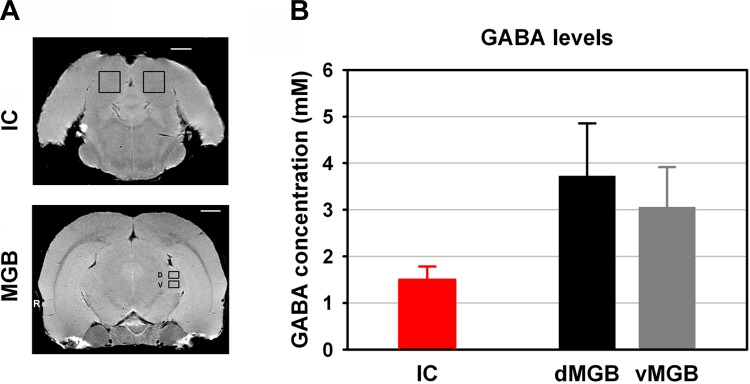
Ex vivo: GABA concentrations of IC and MGB. A: absolute GABA levels were obtained from IC and MGB using 1H-MRS. IC, ventral MGB (vMGB), and dorsal MGB (dMGB) were carefully selected separately in each rat. B: combined values of left and right hemispheres were shown in average group data. Mean GABA concentrations for each structure were IC = 1.50 ± 0.29, dMGB = 3.72 ± 1.13, and vMGB = 3.05 ± 0.86. A nonsignificant but clear trend toward higher GABA levels was found for both vMGB and dMGB compared with GABA levels in IC. An independent t-test showed a P value of 0.05107 (IC vs. dMGB) and 0.0809 (IC vs. vMGB) between IC and MGB. Scale bar = 2 mm.
DISCUSSION
The present series of studies sought to compare the pharmacology of GABA neurotransmission in IC and MGB and found: 1) significantly increased ability of lower dose GABA to suppress SAM-evoked unit responses of MGB units compared with IC units; 2) significantly larger GABAAR-mediated tonic currents evoked by the selective GABAAR agonist, GBX, in voltage-clamped adult MGB neurons compared with similarly examined IC neurons; 3) significantly increased [3H]TBOB binding following GABA application, in MGB compared with IC; and 4) a clear trend towards increased GABA tissue concentrations in MGB compared with IC using 1H-MRS imaging. Collectively, data from these four studies suggest that GABA levels in MGB may be higher than in IC and that activation of synaptic and extrasyanptic GABAARs by GABA and GBX is enhanced in MGB compared with IC.
GABAAR composition and efficacy in IC and MGB.
In IC and MGB, wild-type GABAARs (2α1, 2β2, and γ2) are thought to mediate fast GABAergic inhibition and are activated by release of the inhibitory neurotransmitter GABA (McKernan and Whiting 1996; Milbrandt et al. 1997; Pirker et al. 2000). In addition, other heteromic GABAAR constructs are highly expressed in sensory thalamic structures. α4- and δ-subunit containing GABAAR show high affinity for GABA and the subunit selective agonist GBX (Brown et al. 2002; Mortensen et al. 2010). They are localized to extrasynaptic postsynaptic sites and show tonic non-desensitizing, hyperpolarizing chloride currents (Cope et al. 2005; Richardson et al. 2011). The discussion below suggests that our findings of increased GABA sensitivity in MGB neurons compared with IC neurons may reflect the existence of higher levels of extrasynaptic GABAAR in MGB. By contrast, these GABAAR constructs are not prominent in IC, since α4 and δ subunit protein levels were low, and α4 and δ message levels were below the level of detection (Pirker et al. 2000; Wisden et al. 1992). We probed this using the methodology of Richardson et al. (2011) adapted from Cope et al. (2005) and found, for the first time, that tonic currents could be evoked by GBX application in IC neurons, in a manner similar to what has been seen in thalamus. However, as predicted, the ability of the subunit-selective agonist GBX to evoke tonic currents was reduced by 50% in IC neurons compared with MGB neurons.
A recent series of studies on marmoset suggest that MGB neuronal responses to modulated and click stimuli display unique, more complex responses than do neurons in IC (Bartlett and Wang 2007, 2011). In addition, sensory thalamic neurons are able to switch their discharge pattern from the so-called “tonic” mode to “burst” mode, depending on thalamocortical rhythmicity regulating sleep and attention and depending on the strength and nature of the stimulus (Steriade et al. 1993). These transformations are likely critical to the way sensory pathways process information (see reviews: Sherman 2001; Sherman and Guillery 1996). We hypothesized that these thalamic attributes were due to unique GABA circuits and receptors within MGB. While examining GABA's role in coding modulated signals in a separate study, it became evident that response suppression by GABA application onto MGB units responding to SAM stimuli was far more potent than observed previously in IC (Burger and Pollak 1998; Caspary et al. 2002; Koch and Grothe 1998). We initiated a parallel dose response comparison using the exact same methodology between structures (electrodes, anesthesia, and animal) to compare GABA efficacy. GABA was two to three times (P < 0.01) more potent at suppressing SAM-evoked MGB unit responses than SAM-evoked IC unit responses, further supporting the enhanced GABA sensitivity of sensory thalamic neurons.
GABA concentration in IC and MGB.
GABA is the major inhibitory neurotransmitter in the central auditory system. The extrasynaptic GABAARs in sensory thalamus suggest that their presence and regulation of this GABAAR construct might reflect ambient GABA concentrations. In the present study, a trend toward higher GABA levels in MGB relative to IC was detected using ex vivo 1H-MRS. These findings are consistent with our previously published findings showing significantly higher MGB GABA levels relative to IC, using an earlier MRS data set (Brozoski et al. 2012). 1H-MRS ex vivo GABA levels include all GABA compartments, including astrocytic (Papp et al. 2004; Sperlagh et al. 2002) and other non-vesicular stores (Demarque et al. 2002). Reports of GABA levels/concentrations by others used different methods to quantify GABA in IC and MGB with varying results. A recent HPLC study in hamster found relatively lower GABA levels in MGB subdivisions (7.6 ∼ 8.2 mmol/kg dry wt) than IC subdivisions (8.3 ∼ 12.4 mmol/kg dry wt) (Godfrey et al. 2012). In HPLC studies in human tissue, it was found that there were lower GABA levels in MGB (3.31) than in IC (5.20) (Banay-Schwartz et al. 1989), while these same authors working with rats found similar GABA levels between these two structures (MGB: 85 vs. IC: 83) (Banay-Schwartz et al. 1993). The above-cited studies showing either similar GABA levels/concentration between the two structures or somewhat higher IC GABA levels were carefully conducted but differ significantly in methodology from the present study. The differences between these studies are likely due to use of indirect measures, using different sample/tissue treatments, and species differences. The present 1H-MRS studies used direct measures of GABA concentration against a known standard and found a trend toward higher MGB GABA concentrations relative to IC GABA concentrations, in agreement with our previously published study (Brozoski et al. 2012). The present findings support the notion that elevated GABA levels in MGB could underpin the unique distribution of GABAAR high-affinity constructs found in thalamus which include extrasynaptic α4δ GABAAR constructs that are thought to be regulated by endogenous local GABA concentrations (Belelli et al. 2009; Cope et al. 2005; Richardson et al. 2011).
Acoustic information processing through IC and MGB.
The presence of extrasynaptic GABAAR, elevated GABA concentrations, and unique response modes to inhibition make the thalamus a distinctive auditory structure. The present study used parallel methods in IC and MGB to reveal more potent GABAergic inhibition in MGB relative to IC. Previous study showed that acoustic representation in the IC differs from that in MGB and primary auditory cortex (A1), which suggests a change in coding strategy, from a mixed rate and temporal code to a more refined rate code in MGB (Joris et al. 2004; Liang et al. 2002). Studies in cat also demonstrated coding changes as acoustic information ascended from IC through MGB to A1. Information redundancy presented in IC was thought to be reduced in MGB (Chechik et al. 2006; Las et al. 2005). In support of unit studies in animals, fMRI studies in humans found trends showing a population-based neural representation of the beginning and end of distinct perceptual events that is weak or absent in IC but emerges at the level of MGB (Harms and Melcher 2002). A recent review reported a more efficient temporal coding strategy at sensory thalamocortical levels relative to midbrain coding strategies (“multiplexed temporal processing scales”) (Panzeri et al. 2010), which operates multiple neural codes simultaneously at different temporal scales. Together with previous studies, the hypothesis that stimulus coding diverges in MGB compared with IC is plausible and is supported by neurophysiological data. Thus, we assume that the existence of synaptic/extrasynaptic receptors, with properties of fast/slow decay time and low/high agonist sensitivity, may enable MGB to be a multiplexed router.
Summary.
The present study shows enhanced GABA sensitivity in MGB compared with IC, and this enhancement is likely mediated by both high-affinity extrasynaptic and synaptic components. This increased GABA sensitivity may serve as a basis of the vital filtering role of the auditory thalamus in processing ascending acoustic information.
GRANTS
These studies were supported by National Institute on Deafness and Other Communication Disorders Grants DC-000151 and DC-008532 (to D. M. Caspary), DC-009669 (to C. Bauer and T. J. Brozoski), and Office of Naval Research Grant N000141210214 (to D. M. Caspary).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
R.C. and D.M.C. conception and design of research; R.C., B.I.K., T.J.B., and L.L.L. performed experiments; R.C., B.I.K., T.J.B., and L.L.L. analyzed data; R.C., T.J.B., and L.L.L. interpreted results of experiments; R.C., B.I.K., T.J.B., and L.L.L. prepared figures; R.C. and B.I.K. drafted manuscript; R.C., B.I.K., T.J.B., L.L.L., and D.M.C. approved final version of manuscript; D.M.C. edited and revised manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Brandon C. Cox for reading and editing an earlier version of this manuscript and Dr. Ben D. Richardson and Daniel D. Duque for help with the experiments and valuable discussions.
REFERENCES
- Andersen RA, Knight PL, Merzenich MM. The thalamocortical and corticothalamic connections of AI, AII, and the anterior auditory field (AAF) in the cat: evidence for two largely segregated systems of connections. J Comp Neurol 194: 663–701, 1980 [DOI] [PubMed] [Google Scholar]
- Bajo VM, Rouiller EM, Welker E, Clarke S, Villa AE, de Ribaupierre Y, de Ribaupierre F. Morphology and spatial distribution of corticothalamic terminals originating from the cat auditory cortex. Hear Res 83: 161–174, 1995 [DOI] [PubMed] [Google Scholar]
- Banay-Schwartz M, Lajtha A, Palkovits M. Changes with aging in the levels of amino acids in rat CNS structural elements. I. Glutamate and related amino acids. Neurochem Res 14: 555–562, 1989 [DOI] [PubMed] [Google Scholar]
- Banay-Schwartz M, Palkovits M, Lajtha A. Heterogeneous distribution of functionally important amino acids in brain areas of adult and aging humans. Neurochem Res 18: 417–423, 1993 [DOI] [PubMed] [Google Scholar]
- Bartlett EL, Smith PH. Anatomic, intrinsic, and synaptic properties of dorsal and ventral division neurons in rat medial geniculate body. J Neurophysiol 81: 1999–2016, 1999 [DOI] [PubMed] [Google Scholar]
- Bartlett EL, Wang X. Correlation of neural response properties with auditory thalamus subdivisions in the awake marmoset. J Neurophysiol 105: 2647–2667, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett EL, Wang X. Neural representations of temporally modulated signals in the auditory thalamus of awake primates. J Neurophysiol 97: 1005–1017, 2007 [DOI] [PubMed] [Google Scholar]
- Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic GABAA receptors: form, pharmacology, and function. J Neurosci 29: 12757–12763, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Mody I. Extrasynaptic GABA(A) receptors: their function in the CNS and implications for disease. Neuron 73: 23–34, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright DP, Brickley SG. Acting locally but sensing globally: impact of GABAergic synaptic plasticity on phasic and tonic inhibition in the thalamus. J Physiol 586: 5091–5099, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human alpha(4)beta(3)delta GABA(A) receptors. Br J Pharmacol 136: 965–974, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski T, Odintsov B, Bauer C. Gamma-aminobutyric acid and glutamic acid levels in the auditory pathway of rats with chronic tinnitus: a direct determination using high resolution point-resolved proton magnetic resonance spectroscopy (H-MRS). Front Syst Neurosci 6: 9, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger RM, Pollak GD. Analysis of the role of inhibition in shaping responses to sinusoidally amplitude-modulated signals in the inferior colliculus. J Neurophysiol 80: 1686–1701, 1998 [DOI] [PubMed] [Google Scholar]
- Caspary DM, Palombi PS, Hughes LF. GABAergic inputs shape responses to amplitude modulated stimuli in the inferior colliculus. Hear Res 168: 163–173, 2002 [DOI] [PubMed] [Google Scholar]
- Chechik G, Anderson MJ, Bar-Yosef O, Young ED, Tishby N, Nelken I. Reduction of information redundancy in the ascending auditory pathway. Neuron 51: 359–368, 2006 [DOI] [PubMed] [Google Scholar]
- Cope DW, Hughes SW, Crunelli V. GABAA receptor-mediated tonic inhibition in thalamic neurons. J Neurosci 25: 11553–11563, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarque M, Represa A, Becq H, Khalilov I, Ben-Ari Y, Aniksztejn L. Paracrine intercellular communication by a Ca2+- and SNARE-independent release of GABA and glutamate prior to synapse formation. Neuron 36: 1051–1061, 2002 [DOI] [PubMed] [Google Scholar]
- Diamond IT, Jones EG, Powell TP. The projection of the auditory cortex upon the diencephalon and brain stem in the cat. Brain Res 15: 305–340, 1969 [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci 6: 215–229, 2005 [DOI] [PubMed] [Google Scholar]
- Godfrey DA, Kaltenbach JA, Chen K, Ilyas O, Liu X, Licari F, Sacks J, McKnight D. Amino acid concentrations in the hamster central auditory system and long-term effects of intense tone exposure. J Neurosci Res 90: 2214–2224, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Harris RA. The anesthetic mechanism of urethane: the effects on neurotransmitter-gated ion channels. Anesth Analg 94: 313–318, table of contents, 2002 [DOI] [PubMed] [Google Scholar]
- Harms MP, Melcher JR. Sound repetition rate in the human auditory pathway: representations in the waveshape and amplitude of fMRI activation. J Neurophysiol 88: 1433–1450, 2002 [DOI] [PubMed] [Google Scholar]
- Ito T, Oliver DL. The basic circuit of the IC: tectothalamic neurons with different patterns of synaptic organization send different messages to the thalamus. Front Neural Circuits 6: 48, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris PX, Schreiner CE, Rees A. Neural processing of amplitude-modulated sounds. Physiol Rev 84: 541–577, 2004 [DOI] [PubMed] [Google Scholar]
- Kalappa BI, Gusev AG, Uteshev VV. Activation of functional alpha7-containing nAChRs in hippocampal CA1 pyramidal neurons by physiological levels of choline in the presence of PNU-120596. PLoS One 5: e13964, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JP, Wong D. Laminar connections of the cat's auditory cortex. Brain Res 212: 1–15, 1981 [DOI] [PubMed] [Google Scholar]
- Koch U, Grothe B. GABAergic and glycinergic inhibition sharpens tuning for frequency modulations in the inferior colliculus of the big brown bat. J Neurophysiol 80: 71–82, 1998 [DOI] [PubMed] [Google Scholar]
- Krogsgaard-Larsen P, Frolund B, Liljefors T, Ebert B. GABA(A) agonists and partial agonists: THIP (Gaboxadol) as a non-opioid analgesic and a novel type of hypnotic. Biochem Pharmacol 68: 1573–1580, 2004 [DOI] [PubMed] [Google Scholar]
- Kulesza RJ, Jr, Spirou GA, Berrebi AS. Physiological response properties of neurons in the superior paraolivary nucleus of the rat. J Neurophysiol 89: 2299–2312, 2003 [DOI] [PubMed] [Google Scholar]
- Las L, Stern EA, Nelken I. Representation of tone in fluctuating maskers in the ascending auditory system. J Neurosci 25: 1503–1513, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence LJ, Palmer CJ, Gee KW, Wang X, Yamamura HI, Casida JE. t-[3H]butylbicycloorthobenzoate: new radioligand probe for the gamma-aminobutyric acid-regulated chloride ionophore. J Neurochem 45: 798–804, 1985 [DOI] [PubMed] [Google Scholar]
- Le Beau FE, Rees A, Malmierca MS. Contribution of GABA- and glycine-mediated inhibition to the monaural temporal response properties of neurons in the inferior colliculus. J Neurophysiol 75: 902–919, 1996 [DOI] [PubMed] [Google Scholar]
- Lee CC, Schreiner CE, Imaizumi K, Winer JA. Tonotopic and heterotopic projection systems in physiologically defined auditory cortex. Neuroscience 128: 871–887, 2004 [DOI] [PubMed] [Google Scholar]
- Lee CC, Winer JA. Principles governing auditory cortex connections. Cereb Cortex 15: 1804–1814, 2005 [DOI] [PubMed] [Google Scholar]
- Liang L, Lu T, Wang X. Neural representations of sinusoidal amplitude and frequency modulations in the primary auditory cortex of awake primates. J Neurophysiol 87: 2237–2261, 2002 [DOI] [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci 19: 139–143, 1996 [DOI] [PubMed] [Google Scholar]
- Meera P, Wallner M, Otis TS. Molecular basis for the high THIP/gaboxadol sensitivity of extrasynaptic GABA(A) receptors. J Neurophysiol 106: 2057–2064, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbrandt JC, Albin RL, Turgeon SM, Caspary DM. GABAA receptor binding in the aging rat inferior colliculus. Neuroscience 73: 449–458, 1996 [DOI] [PubMed] [Google Scholar]
- Milbrandt JC, Hunter C, Caspary DM. Alterations of GABAA receptor subunit mRNA levels in the aging Fischer 344 rat inferior colliculus. J Comp Neurol 379: 455–465, 1997 [DOI] [PubMed] [Google Scholar]
- Mortensen M, Ebert B, Wafford K, Smart TG. Distinct activities of GABA agonists at synaptic- and extrasynaptic-type GABAA receptors. J Physiol 588: 1251–1268, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen M, Kristiansen U, Ebert B, Frolund B, Krogsgaard-Larsen P, Smart TG. Activation of single heteromeric GABA(A) receptor ion channels by full and partial agonists. J Physiol 557: 389–413, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver DL, Winer JA, Beckius GE, Saint Marie RL. Morphology of GABAergic neurons in the inferior colliculus of the cat. J Comp Neurol 340: 27–42, 1994 [DOI] [PubMed] [Google Scholar]
- Palombi PS, Caspary DM. GABA inputs control discharge rate primarily within frequency receptive fields of inferior colliculus neurons. J Neurophysiol 75: 2211–2219, 1996 [DOI] [PubMed] [Google Scholar]
- Pandya DN, Rosene DL, Doolittle AM. Corticothalamic connections of auditory-related areas of the temporal lobe in the rhesus monkey. J Comp Neurol 345: 447–471, 1994 [DOI] [PubMed] [Google Scholar]
- Panzeri S, Brunel N, Logothetis NK, Kayser C. Sensory neural codes using multiplexed temporal scales. Trends Neurosci 33: 111–120, 2010 [DOI] [PubMed] [Google Scholar]
- Papp L, Vizi ES, Sperlagh B. Lack of ATP-evoked GABA and glutamate release in the hippocampus of P2X7 receptor−/− mice. Neuroreport 15: 2387–2391, 2004 [DOI] [PubMed] [Google Scholar]
- Park TJ, Pollak GD. GABA shapes a topographic organization of response latency in the mustache bat's inferior colliculus. J Neurosci 13: 5172–5187, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Gonzalez D, Hernandez O, Covey E, Malmierca MS. GABA(A)-mediated inhibition modulates stimulus-specific adaptation in the inferior colliculus. PLoS One 7: e34297, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peruzzi D, Bartlett E, Smith PH, Oliver DL. A monosynaptic GABAergic input from the inferior colliculus to the medial geniculate body in rat. J Neurosci 17: 3766–3777, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience 101: 815–850, 2000 [DOI] [PubMed] [Google Scholar]
- Pontes C, Reis FF, Sousa-Pinto A. The auditory cortical projections onto the medial geniculate body in the cat. An experimental anatomical study with silver and autoradiographic methods. Brain Res 91: 43–63, 1975 [DOI] [PubMed] [Google Scholar]
- Richardson BD, Ling LL, Uteshev VV, Caspary DM. Extrasynaptic GABA(A) receptors and tonic inhibition in rat auditory thalamus. PLoS ONE 6: e16508, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouiller EM, Colomb E, Capt M, De Ribaupierre F. Projections of the reticular complex of the thalamus onto physiologically characterized regions of the medial geniculate body. Neurosci Lett 53: 227–232, 1985 [DOI] [PubMed] [Google Scholar]
- Rouiller EM, de Ribaupierre F. Origin of afferents to physiologically defined regions of the medial geniculate body of the cat: ventral and dorsal divisions. Hear Res 19: 97–114, 1985 [DOI] [PubMed] [Google Scholar]
- Saldaña E. Ascending projections to the non-leminiscal auditory thalamus. In: 36th ARO Midwinter Meeting Baltimore, MD, 2013 [Google Scholar]
- Schroeder HA, Balassa JJ, Vinton WH., Jr Chromium, cadmium and lead in rats: effects on life span, tumors and tissue levels. J Nutr 86: 51–66, 1965 [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABA A receptors: modulating gain and maintaining the tone. Trends Neurosci 27: 262–269, 2004 [DOI] [PubMed] [Google Scholar]
- Sherman SM. Tonic and burst firing: dual modes of thalamocortical relay. Trends Neurosci 24: 122–126, 2001 [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. Functional organization of thalamocortical relays. J Neurophysiol 76: 1367–1395, 1996 [DOI] [PubMed] [Google Scholar]
- Sousa-Pinto A, Reis FF. Selective uptake of (3H)leucine by projection neurons of the cat auditory cortex. Brain Res 85: 331–336, 1975 [DOI] [PubMed] [Google Scholar]
- Sperlagh B, Kofalvi A, Deuchars J, Atkinson L, Milligan CJ, Buckley NJ, Vizi ES. Involvement of P2X7 receptors in the regulation of neurotransmitter release in the rat hippocampus. J Neurochem 81: 1196–1211, 2002 [DOI] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science 262: 679–685, 1993 [DOI] [PubMed] [Google Scholar]
- Turner JG, Caspary DM. Comparison of two rat models of aging. In: Plasticity and Signal Representation in the Auditory System, edited by Syka J, Mezenich MM. New York: Springer, 2005 [Google Scholar]
- van der Heyden JA, Korf J. Regional levels of GABA in the brain: rapid semiautomated assay and prevention of postmortem increase by 3-mercapto-propionic acid. J Neurochem 31: 197–203, 1978 [DOI] [PubMed] [Google Scholar]
- Walker MC, Semyanov A. Regulation of excitability by extrasynaptic GABA(A) receptors. Results Probl Cell Differ 44: 29–48, 2008 [DOI] [PubMed] [Google Scholar]
- Winer JA, Diehl JJ, Larue DT. Projections of auditory cortex to the medial geniculate body of the cat. J Comp Neurol 430: 27–55, 2001 [PubMed] [Google Scholar]
- Winer JA, Larue DT. Evolution of GABAergic circuitry in the mammalian medial geniculate body. Proc Natl Acad Sci USA 93: 3083–3087, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci 12: 1040–1062, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong D, Kelly JP. Differentially projecting cells in individual layers of the auditory cortex: a double-labeling study. Brain Res 230: 362–366, 1981 [DOI] [PubMed] [Google Scholar]
- Zhang DX, Li L, Kelly JB, Wu SH. GABAergic projections from the lateral lemniscus to the inferior colliculus of the rat. Hear Res 117: 1–12, 1998 [DOI] [PubMed] [Google Scholar]